Abstract
Evidence suggests that autophagy promotes the development of cellular senescence. Because cellular senescence contributes to renal aging and promotes the progression from AKI to CKD, we investigated the potential effect of tubular autophagy on senescence induction. Compared with kidneys from control mice, kidneys from mice with conditional deletion of autophagy-related 5 (Atg5) for selective ablation of autophagy in proximal tubular S3 segments (Atg5Δflox/Δflox) presented with significantly less tubular senescence, reduced interstitial fibrosis, and superior renal function 30 days after ischemia/reperfusion injury. To correlate this long-term outcome with differences in the early injury process, kidneys were analyzed 2 hours and 3 days after reperfusion. Notably, compared with kidneys of control mice, Atg5Δflox/Δflox kidneys showed more cell death in outer medullary S3 segments at 2 hours but less tubular damage and inflammation at day 3. These data suggest that the lack of autophagy prevents early survival mechanisms in severely damaged tubular cells. However, if such compromised cells persist, then they may lead to maladaptive repair and proinflammatory changes, thereby facilitating the development of a senescent phenotype and CKD.
Keywords: acute renal failure, ischemia/reperfusion, proximal tubule, fibrosis, pathophysiology of renal disease and progression, progression of renal failure
Progression from AKI to CKD is a multifactorial process that is incompletely understood.1 Among the involved mechanisms, cellular senescence has emerged as an important factor disturbing renal regeneration and promoting kidney aging.2–5 Cellular senescence is a state of irreversible growth arrest resulting from telomere dysfunction or various cellular stresses that activate cyclin–dependent kinase inhibitors, particularly p16INK4a. We have previously shown that ischemia/reperfusion (I/R) –related cell stress enhances the expression of p16INK4a, which hampers renal repair and contributes to the transition from AKI to CKD.3
Another cellular mechanism that seems to be important in the outcome of AKI is autophagy. Autophagy is an evolutionarily conserved pathway that plays a central role in turnover of proteins and degradation of damaged organelles.6 Under stress conditions, such as I/R, autophagic activity dramatically increases in tubular cells, and experimental data suggest that this upregulation may serve a protective role.7 Although autophagy and senescence are two discrete cellular stress responses, they have overlapping functions in cancer control and possibly, the aging process.8 Importantly, activation of both processes might be functionally linked: autophagy can facilitate senescence induction under various circumstances and in various cell types.9–11 The underlying molecular mechanism explaining this prosenescent nature might be the need of autophagic degradation of an inhibitory isoform of p53 to suppress replicative senescence.12
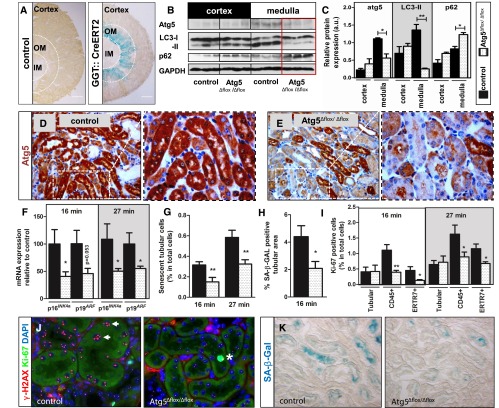
To test the potential interdependence of autophagy and cellular senescence, autophagy-related 5 (Atg5) protein was deleted in tubular epithelial cells. To this end, Atg5flox/flox mice were crossed with GGT::Cre-ERT2 mice carrying a transgene with tamoxifen–dependent Cre recombinase expression selectively in the proximal tubular S3 segment (Figure 1A).13 This segment, which is the most affected part of the nephron during I/R injury, is located in the outer stripe of the outer medulla and shows rapid activation of autophagy after I/R (Supplemental Figure 1). Cre recombinase activation with tamoxifen resulted in a significant reduction in outer medullary Atg5 expression and suppressed autophagic activity (Figure 1, B–E, Supplemental Figure 2, A–D). Histologically, this was accompanied by a lack of LC3 punctae and accumulation of p62 (Supplemental Figure 2, E–H). Ultrastructural analysis confirmed the loss of autophagosomes, the occurrence of multilamellar structures and concentric membrane clusters, and an increase in altered mitochondria (Supplemental Figure 2, I and J). A similar ultrastructural pattern has previously been described in autophagy–deficient tubular cells by Liu et al.14
Figure 1.
Targeted Atg5 deletion impairs activation of autophagy and diminishes development of cellular senescence after I/R in S3 segments of kidney proximal tubules. (A) GGT::Cre-ERT2 mice expressing tamoxifen–inducible Cre recombinase were crossed with ROSA26 (R26R) reporter mice and analyzed for LacZ expression. GGT::Cre-ERT2 mice were crossed with Atg5flox/flox mice to generate animals with deletion of the Atg5 gene in the S3 segment. Three weeks after tamoxifen induction, Atg5Δflox/Δflox mice underwent 16- or 27-minute renal I/R injury. Scale bar, 500 µm. (B and C) Representative immunoblots and corresponding densitometry show a reduction of Atg5, a loss of LC3 lipidation, and an accumulation of p62 in the outer medulla of Atg5Δflox/Δflox kidneys at 16 hours after 16 minutes of renal clamping. (D and E) Representative immunohistochemistry of Atg5 in the outer medulla revealing reduced Atg5 expression in Atg5Δflox/Δflox kidneys versus control kidneys. (F) Quantitative real-time PCR showing relative expression of established senescence markers 30 days after I/R. (G) Quantification of γ-H2AX+/Ki67− cells. (H) Quantification of SA-β-GAL–positive area. (I) Quantification of Ki67–positive tubular and interstitial cells (fibroblast marker ERTR7 and leukocyte marker CD45) showing significantly fewer proliferating interstitial cells in Atg5Δflox/Δflox kidneys. (J) Representative coimmunostaining from outer medulla for γ-H2AX (red) and Ki67 (green) reveals fewer senescent tubular cells without Ki67 and five or more γ-H2A.X foci (arrows) in Atg5Δflox/Δflox kidneys. A Ki67–positive proliferating tubular cell is marked with an asterisk. (K) Representative pictures from the outer medulla showing diminished SA-β-GAL staining in Atg5Δflox/Δflox kidneys after 16 minutes of clamping. Data are presented as mean values±SEMs (n=4 in C, n=10 in F–I). Original magnifications, ×200 in D, E, and K; ×630 in J. *P<0.05; **P<0.01. DAPI, 4′,6-diamidino-2-phenylindole; IM, inner medulla; OM, outer medulla.
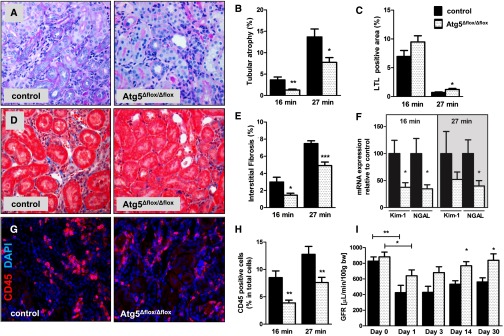
At 30 days after 16 or 27 minutes of transient renal ischemia, Atg5Δflox/Δflox kidneys had reduced expression of senescence markers p19ARF and p16INK4a (Figure 1F), fewer phenotypically senescent tubular cells with γ-H2AX+/Ki67− nuclei (Figure 1, G and J), and reduced senescence–associated β-galactosidase (SA-β-GAL) in the outer medullary region (Figure 1, H and K). Although there was no difference in the proliferation rate of tubular cells, Atg5Δflox/Δflox kidneys displayed significantly fewer proliferating CD45+ leukocytes and ER-TR7+ fibroblasts as judged by Ki67 (Figure 1I). This was paralleled by less tubular atrophy, more Lotus tetragonolobus lectin (LTL) staining for intact brush borders, and markedly reduced fibrotic matrix expansion (Figure 2, A–E). Atg5Δflox/Δflox kidneys also showed reduced expression of NGAL and Kim-1 as markers for sustained tubular injury and a reduction in peritubular CD45–positive leukocytes (Figure 2, F–H). Concomitantly, GFR recovery was significantly better at 14 and 30 days after I/R in Atg5Δflox/Δflox mice after bilateral renal ischemia for 16 minutes (Figure 2I).
Figure 2.
Atg5 deletion in S3 segments leads to better recovery of GFR, diminished tubular atrophy, less interstitial fibrosis, and reduced inflammation at day 30 after I/R. Control and Atg5Δflox/Δflox mice underwent 16 or 27 minutes of renal I/R and were analyzed at day 30. (A) Representative pictures from the outer medulla and (B) corresponding analyses showing reduced tubular atrophy in periodic acid–Schiff staining, (C) a higher percentage of intact LTL–positive proximal tubules, and (D and E) less interstitial matrix expansion by Masson Trichrome staining in Atg5Δflox/Δflox kidneys. (F) Quantitative real-time PCR showing reduced expression of established renal damage markers Kim-1 and NGAL in Atg5Δflox/Δflox kidneys. (G and H) Representative immunostaining and quantification from the outer medulla showing reduced numbers of CD45-positive cells in Atg5Δflox/Δflox kidneys. (I) Transcutaneous GFR measurements of control and Atg5Δflox/Δflox animals at indicated time points after 16 minutes of bilateral renal clamping. Data are presented as mean values±SEMs (n=10). Original magnifications, ×400 in A, D, and G. *P<0.05; **P<0.01; ***P<0.001. DAPI, 4′,6-diamidino-2-phenylindole.
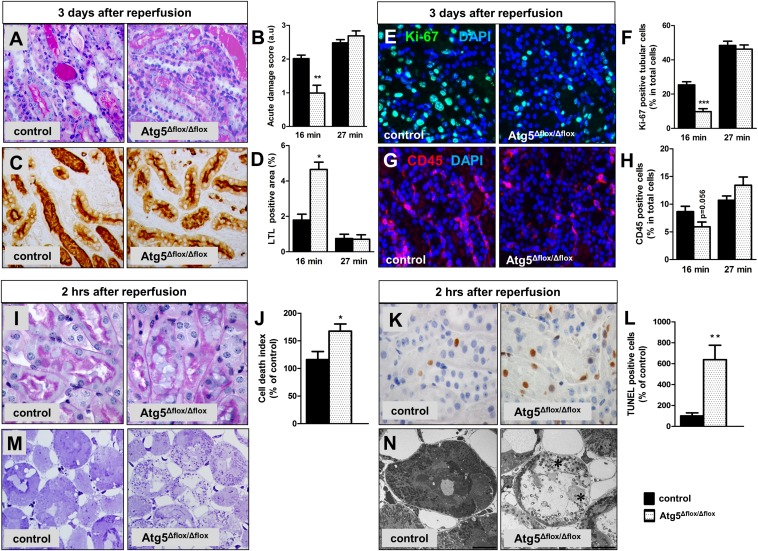
Harmful effects of tubular autophagy have already been described in experimental urinary tract obstruction and kidneys of mice with transgenic TGF-β overexpression.15,16 In the context of AKI, our data are in contrast to previous reports that suggested a nephroprotective role of autophagy.7,14,17,18 Methodologically, we used a similar approach as two previous studies, in which mice with transgenic deletion of tubular Atg5 underwent I/R.14,17 In these studies, suppression of tubular autophagy was detrimental and resulted in increased damage and more severe loss of renal function at 2 and 7 days after I/R.14,17 To assess early postischemic injury, we evaluated additional groups of mice 3 days and 2 hours after I/R. Kidneys that had undergone 27 minutes of ischemia showed massive damage without significant differences in tubular injury, proliferation, or inflammation at day 3 (Figure 3, A–H), whereas exposure to only 16 minutes of ischemia resulted in less tubular damage in Atg5Δflox/Δflox kidneys as shown by more intact brush borders, reduced tubular cell proliferation, and a strong trend for fewer infiltrating leukocytes (Figure 3, A–H). Surprisingly, an opposite pattern was found when mice were analyzed in the very early reperfusion phase at 2 hours. At this early time, Atg5Δflox/Δflox kidneys showed significantly greater damage, with abundant necrosis in outer medullary tubules (Figure 3, I, M, and N). Although kidneys of both groups displayed minimal numbers of apoptotic cells detected by cleaved caspase-3 (data not shown), outer medullary tubules of Atg5Δflox/Δflox kidneys showed significantly more terminal deoxynucleotidyl transferase–mediated digoxigenin–deoxyuridine nick–end labeling (TUNEL) –positive cells (Figure 3, K and L).
Figure 3.
The effect of S3 segment–specific Atg5 deletion on early I/R injury is dependent on the duration of I/R. Control and Atg5Δflox/Δflox mice underwent 16 or 27 minutes of renal I/R and were analyzed at day 3. (A) Representative periodic acid–Schiff staining images from the outer medulla of 16-minute clamped kidneys and (B) morphologic damage scoring showing reduced acute injury in Atg5Δflox/Δflox kidneys. (C) Representative LTL staining images from the outer medulla of 16-minute clamped kidneys and (D) quantification showing a higher percentage of LTL-positive area in Atg5Δflox/Δflox kidneys. (E) Representative Ki67 immunostaining images from the outer medulla of 16-minute clamped kidneys and (F) quantification showing reduced tubular proliferation in Atg5Δflox/Δflox kidneys. (G) Representative CD45 immunostaining images from the outer medulla of 16-minute clamped kidneys and (H) quantification showing reduced immune infiltration in Atg5Δflox/Δflox kidneys. Control and Atg5Δflox/Δflox mice underwent 16 minutes of renal I/R and were analyzed at 2 hours after reperfusion. (I) Representative periodic acid–Schiff staining images from the outer medulla and (J) stereologic quantification of dead tubular cells showing enhanced cell death in Atg5Δflox/Δflox kidneys after 16 minutes of clamping. (K) Representative TUNEL assay images from the outer medulla and (L) quantification showing a significant increase in the number of apoptotic tubular cells in Atg5Δflox/Δflox kidneys after 16 minutes of clamping. (M) Representative Toluidine blue–stained Epon sections and (N) electron microscopy images from control and Atg5Δflox/Δflox kidneys after 27 minutes of clamping indicating more severe damage in Atg5Δflox/Δflox kidneys as reflected by dead lysing tubular epithelial cells (asterisks). Data are presented as mean values±SEMs (n=7–8 in day 3 I/R samples, and n=5 in 2-hour I/R samples). Original magnifications, ×400 in A, C, E, G, and M; ×630 in I and K. Scale bar, 10 µm in N. *P<0.05; **P<0.01; ***P<0.001. DAPI, 4′,6-diamidino-2-phenylindole.
The pattern of TUNEL positivity in the absence of cleaved caspase-3 is in agreement with the recently highlighted minor role of apoptosis in renal I/R and points to the involvement of other forms of regulated cell death, such as necroptosis or ferroptosis.19 Our data cannot define the specific cell death pathway involved but clearly indicate that the accelerated loss of severely injured S3 segment cells was associated with a favorable subsequent outcome, suggesting that the persistence of compromised tubular cells led to maladaptive changes in repair and inflammation as observed on day 3. Additional research will be needed to dissect the underlying interdependence between autophagy, cell death dynamics, and secondary changes in the postinjurious tubular milieu.
In agreement with previous studies, we observed that transgenic Atg5 deletion promotes postischemic tubular cell death. However, the beneficial outcome in our model is different from findings by other groups.14,17 In contrast to previous studies, Atg5 was selectively deleted in the S3 segment in our mice. Transgenic targeting of Atg5 in the other studies encompassed much larger tubular portions (i.e., the S1-S2-S3 segment17 or the entire tubule14). Our findings suggest that the heterogeneous distribution of tubular Atg5 deletion and cell injury caused the different outcome of our study. There is increasing evidence that cell integrity and activation status determine whether autophagy is protective or detrimental.20,21 Indeed, Decuypere et al.22 recently suggested a double-edged role for autophagy in renal I/R, highlighting the possibility that the effect of autophagy might directly depend on the severity of cellular damage. Although cells in the S3 segment are most severely affected by I/R injury, the epithelium in other tubular portions is less disturbed or remains healthy.23 Our S3 segment–specific Atg5 deletion comprised primarily cells that suffered from maximum damage and drove them into accelerated cell death. At day 3, a large portion of these cells was also lost in control kidneys, indicating an overwhelming damage, which blocks successful regeneration. In the studies using more ample tubular Atg5 deletion, cells from less damaged segments were also lost, encompassing potentially viable cells that would normally contribute to adequate tubular repair.14,17 This concept can also explain the detrimental effects of globally inactivating autophagy using pharmacologic approaches.18,24
Our data strongly suggest that antagonizing autophagy can improve renal functional outcome and delay senescence. I/R damage was ameliorated in Atg5Δflox/Δflox kidneys at day 3 in the 16-minute ischemia group. Although this early difference might have affected the subsequent development of cellular senescence, there was no discernable advantage in the more heavily damaged kidneys after 27-minute ischemia at early time points. The fact that these Atg5Δflox/Δflox kidneys still had a significantly reduced load of senescence at day 30 argues for a direct involvement of autophagy in establishing senescence, which is consistent with findings by others.9–12
In summary, the observation that autophagy facilitates the establishment of a senescence phenotype and prevents clearance of potentially harmful cells is crucial in light of potential novel therapies to treat AKI by unselective enhancement of autophagy.7 Additional research should focus on strategies for modulating autophagy that can be adapted for varied conditions and individual cellular stress levels.
Concise Methods
Transgenic Animals
Mice expressing tamoxifen–inducible Cre recombinase [B6N;D2-Tg(Ggt1-cre/ERT2)1Pepe/Cnrm or GGT::Cre-ERT2] were crossed with Atg5flox/flox mice (B6.129S-Atg5tm1Myok) to generate strains with deletion of the Atg5 gene in the S3 segment of proximal tubules (Atg5Δflox/Δflox). Three weeks before I/R injury, tamoxifen (Sigma-Aldrich, St. Louis, MO) was injected (0.1 mg/40 g body wt) in 10- to 12-week-old male mice for 5 consecutive days. Genotyping was performed using mouse tail genomic DNA as previously described.13,25 To analyze the specificity and efficiency of Cre expression, GGT::Cre-ERT2 mice were crossed with the ROSA26 (R26R) reporter mice, and the double transgenics were analyzed for LacZ expression. Follow-up of unstressed Atg5Δflox/Δflox mice until 9 months after tamoxifen injection confirmed a reduction in renal Atg5 protein without a significant phenotype (Supplemental Figure 3). All experimental methodologies were in agreement with institutional guidelines for animal research and approved by the state authorities.
I/R
Renal I/R injury was performed in 15- to 16-week-old mice by unilateral or bilateral clamping of the renal pedicles as previously described.26 The mice were clamped for 16 (bilateral clamping) or 27 minutes (unilateral clamping). Mice were euthanized at 2 hours, 3 days, or 30 days postsurgery. Representative kidney tissues were snap frozen for RNA and protein analysis. Alternatively, tissues were fixed in 4% buffered formalin for paraffin embedding or frozen in Tissue-Tek OCT (Sakura, Zoeteroude, Holland) compound for cryosections.
Renal Function
Transcutaneous measurement of GFR was performed in conscious mice as previously described.27 Briefly, the mice were anesthetized under short isoflurane exposure, and fluorescent renal marker FITC-sinistrin was injected (7.5 mg/100 g body wt dissolved in 0.25 ml 0.9% NaCl; Mannheim Pharma & Diagnostics GmbH, Mannheim, Germany). A miniaturized fluorescent detector enabled with an internal memory device was fixed on the epilated back of the mice, which measured and stored the elimination kinetics of FITC-sinistrin over 60 minutes. GFR was calculated from FITC-sinistrin plasma clearance as established previously.27
Ultrastructural Analyses
Mouse kidneys were perfusion fixed through the left ventricle with a mixture of 150 mM Hepes (pH 7.35) containing 1.5% formaldehyde and 1.5% glutaraldehyde. Tissue blocks (2-mm3 blocks) were postfixed in 1% OsO4 and 4% aqueous uranyl acetate. Samples were dehydrated in acetone and embedded in Epon (Agar 100 resin; Agar Scientific). Ultrathin sections (50 nm) were poststained with uranyl acetate and lead citrate and observed in a Morgagni transmission electron microscope (FEI, Eindhoven, The Netherlands). Images were procured with a side–mounted Veleta CCD camera. Profiles of S3 segments were identified among profiles of other proximal tubules by being exactly cross-sectioned while the section was taken transversally to the axis of the elongated part of the straight tubules. Additional criteria were least developed endocytic apparatus, no large and elongated mitochondria, and the characteristic microvilli in the brush border.
Renal Histology and Immunohistochemistry
Three-micrometer sections were used for Periodic acid–Schiff staining to evaluate acute and chronic kidney damage. Acute tubular injury was semiquantitatively graded from zero to four on the basis of tubular dilation, basement membrane denudation, intraluminal casts, cell flattening, and loss of brush-bordered membrane using an established protocol.28 Epon–embedded semithin sections (1 µm) were stained with Toluidine blue to visualize cell damage in 2-hour I/R samples. Cell death at 2 hours of reperfusion was stereologically quantified using a test grid composed of 25 points per image, which was superimposed on digital images of Periodic acid–Schiff-stained sections. The numbers of points that were localized on dead tubular cells were counted and divided by the total points that were localized on tubules. Chronic tubular atrophy was defined as loss of tubular nuclei, reduced tubular diameter, and thickened tubular basement membrane. Chronic damage was quantified by normalizing the area of damaged tubules to the total tubular area using ImageJ software (National Institutes of Health, Bethesda, MD). Tubular damage was also assessed by quantifying tubular area staining positive for LTL, which identifies intact brush border in renal proximal tubules using ImageJ software.29 Interstitial fibrosis was determined using Masson Trichrome staining, where the blue–stained fibrotic area was normalized against the total tubulointerstitial area using the QWin V3 software.3 All of the aforementioned analyses were performed by blinded observation of 12 random nonoverlapping visual fields (200× magnification). Glomeruli and vessels larger than the size of adjacent tubules were not included in the analysis. Immunostainings were performed using the following primary antibodies: anti-Ki67 (SP6; Thermo Fisher Scientific), anti-CD45 (BD Pharmingen, Franklin Lakes, NJ), anti–γ-H2AX (JBW301; EMD Millipore), anti-LC3 (5F10; Nanotools, Teningen, Germany), anti-Atg5 (NB110–53818; Novus Biologicals), and anti-p62 (p0067; Sigma-Aldrich). The staining was visualized using either the Envision Monoclonal DAB System (Dako) or Alexa 488/555 fluorescent secondary antibodies (Invitrogen). Fluorescein-labeled LTL was used as a brush border marker to identify damaged proximal tubules (Vector Laboratories). ApopTag In Situ Apoptosis Detection Kit was used according to the manufacturer’s instructions for TUNEL assay (S7100; EMD Millipore). Quantitative analysis of positive cells was performed in 12 random nonoverlapping visual fields (400× magnification) in each sample. For analysis of senescence phenotype, Ki67–negative and γ-H2A.X–positive (five or more foci) tubular nuclei were considered senescent as previously described.3 Data are presented as percentage ratio of positive cells versus total tubular cells or total number of cells per vision field.
SA-β-GAL and X-GAL Staining
SA-β-GAL staining was performed as described previously with minor modifications.29 Briefly, 8 µM kidney cryosections were fixed in fixation solution (2% formaldehyde and 0.2% glutaraldehyde in PBS) at room temperature for 10 minutes. The sections were washed with PBS and incubated at 37°C at night with freshly prepared staining solution (pH 6.0; 40 mM citric acid/Na phosphate buffer, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM sodium chloride, 2 mM magnesium chloride, and 1 mg/ml X-GAL prepared in dimethylformamide). The stained sections were visualized by light microscopy. Quantitative analysis was performed in 12 random nonoverlapping visual fields (400× magnification) in each sample. SA-β-GAL–positive area was determined by normalizing the blue-stained area against the total tubular area using ImageJ software.
For X-GAL staining, 30-µm cryosections were fixed for 10 minutes in fixation solution (0.5% glutaraldehyde in PBS), washed three times with PBS, and subsequently stained overnight in the same staining solution as SA-β-GAL at 37°C.
Quantitative Real–Time PCR
RNeasy Mini Kit (Qiagen, Hilden, Germany) was used to isolate RNA from snap-frozen kidneys as per the manufacturer’s instructions; 1 µg RNA was reverse transcribed using M-MLV-Reverse Transcription (Promega) and random hexamers (Invitrogen). All quantitative real–time PCRs were performed in ABI PRISM 7700 Sequence Detector (Applied Biosystems) using TaqMan assay for Atg5 (Mm00504340_m1; Applied Biosystems) and custom synthesized primers with FAM-labeled probes: p16INK4a forward: GGG CAC TGC TGG AAG CC; p16INK4a reverse: AAC GTT GCC CAT CAT CAT C; p16INK4a probe: CCG AAC TCT TTC GGT CGT A; p19ARF forward: TCG TGA ACA TCT TGT TGA GGC TA; p19ARF reverse: GTT GCC CAT CAT CAT CAT CAC CTG; p19ARF probe: CGG TGC GGC CCT CTT CTC AAG ATC; HPRT forward: TGA CAC TGG TAA AAC AAT GCA AAC T; HPRT reverse: AAC AAA GTC TGG CCT GTA TCC AA; and HPRT probe: TCC ACC AGC AAG CTT GCA ACC TTA ACC. The mRNA of NGAL, Kim-1, and α-smooth muscle actin (α-SMA) was quantified by SYBR Green–based detection using specific primers: NGAL forward: TGA AGG AAC GTT TCA CCC GCT TTG; NGAL reverse: ACA GGA AAG ATG GAG TGG CAG ACA; Kim-1 forward: AAA CAA GAG ATT CCC ACA CG; Kim-1 reverse: GTC GTG GGT CTT CCT GTA GC; α-SMA forward: GTG CTA TGT CGC TCT GGA CTT TGA; and α-SMA reverse: ATG AAA GAT GGC TGG AAG AGG GTC. The threshold cycle (Ct) for each individual PCR was determined by the instrument software, and Ct values obtained were normalized to HPRT gene expression. Quantification of gene expression was done according to the 2−ΔΔCt method.
Immunoblotting
Immunoblotting was performed as previously described.30 Proteins resolved on SDS-PAGE were transferred to PVDF membrane, blocked with 5% milk in Tris-buffered saline with Tween-20, and incubated overnight at 4°C with primary antibodies: anti–LC3-II (L8918; Sigma-Aldrich), anti-Atg5 (8540; Cell Signaling Technology, Danvers, MA), anti-p62 (p0067; Sigma-Aldrich), anti–β-actin (ab82618; Abcam, Cambridge, United Kingdom), and anti-GAPDH (MAB374; EMD Millipore). Postincubation, membranes were incubated with appropriate HRP–conjugated secondary antibodies (Cell Signaling Technology) and visualized by Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore) or Supersignal H West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
Statistical Analyses
Results are expressed as means±SEMs. Statistical significance was determined by unpaired t test (GraphPad Software, San Diego, CA). P<0.05 was considered to be statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Margit Überheide for technical assistance. We appreciate the help of Dr. Noboru Mizushima (University of Tokyo) in providing Atg5-floxed mice. We thank Dr. Andreas Linkermann for helpful discussions. We acknowledge Dr. Manoj Balakrishna Menon for providing reagents and antibodies.
This study was supported by Deutsche Forschungsgemeinschaft Grants Sonderforschungsbereich KFO 201 (to P.P.), OM 6/5 (to P.P.), and SFB 738 (to A.M. and R.S.).
Parts of this work have been presented at the American Society of Nephrology Renal Week, November 11–16, 2014 in Philadelphia, PA and the Experimental Biology Conference, March 28–April 1, 2015 in Boston, MA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111059/-/DCSupplemental.
References
- 1.Belayev LY, Palevsky PM: The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens 23: 149–154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt R, Melk A: New insights on molecular mechanisms of renal aging. Am J Transplant 12: 2892–2900, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A: Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol 23: 1467–1473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements ME, Chaber CJ, Ledbetter SR, Zuk A: Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8: e70464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westhoff JH, Schildhorn C, Jacobi C, Hömme M, Hartner A, Braun H, Kryzer C, Wang C, von Zglinicki T, Kränzlin B, Gretz N, Melk A: Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol 21: 327–336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klionsky DJ, Emr SD: Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takabatake Y, Kimura T, Takahashi A, Isaka Y: Autophagy and the kidney: Health and disease. Nephrol Dial Transplant 29: 1639–1647, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Gewirtz DA: Autophagy and senescence in cancer therapy. J Cell Physiol 229: 6–9, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y: Autophagy mediates the process of cellular senescence characterizing bile duct damages in primary biliary cirrhosis. Lab Invest 90: 835–843, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y: Autophagy may precede cellular senescence of bile ductular cells in ductular reaction in primary biliary cirrhosis. Dig Dis Sci 57: 660–666, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavaré S, Arakawa S, Shimizu S, Watt FM, Narita M: Autophagy mediates the mitotic senescence transition. Genes Dev 23: 798–803, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horikawa I, Fujita K, Jenkins LM, Hiyoshi Y, Mondal AM, Vojtesek B, Lane DP, Appella E, Harris CC: Autophagic degradation of the inhibitory p53 isoform Δ133p53α as a regulatory mechanism for p53-mediated senescence. Nat Commun 5: 4706, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dworniczak B, Skryabin B, Tchinda J, Heuck S, Seesing FJ, Metzger D, Chambon P, Horst J, Pennekamp P: Inducible Cre/loxP recombination in the mouse proximal tubule. Nephron, Exp Nephrol 106: e11–e20, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB: Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8: 826–837, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Li L, Zepeda-Orozco D, Black R, Lin F: Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol 176: 1767–1778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ, Kriz W: Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 177: 632–643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y: Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902–913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z: Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z: Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Levine B: Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ 22: 367–376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gewirtz DA: The four faces of autophagy: Implications for cancer therapy. Cancer Res 74: 647–651, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Decuypere JP, Pirenne J, Jochmans I: Autophagy in renal ischemia-reperfusion injury: Friend or foe? Am J Transplant 14: 1464–1465, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang M, Liu K, Luo J, Dong Z: Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 176: 1181–1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N: Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Sörensen I, Rong S, Susnik N, Gueler F, Shushakova N, Albrecht M, Dittrich AM, von Vietinghoff S, Becker JU, Melk A, Bohlmann A, Reingruber S, Petzelbauer P, Haller H, Schmitt R: Bβ(15-42) attenuates the effect of ischemia-reperfusion injury in renal transplantation. J Am Soc Nephrol 22: 1887–1896, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schock-Kusch D, Geraci S, Ermeling E, Shulhevich Y, Sticht C, Hesser J, Stsepankou D, Neudecker S, Pill J, Schmitt R, Melk A: Reliability of transcutaneous measurement of renal function in various strains of conscious mice. PLoS One 8: e71519, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broekema M, Harmsen MC, Koerts JA, Petersen AH, van Luyn MJ, Navis G, Popa ER: Determinants of tubular bone marrow–derived cell engraftment after renal ischemia/reperfusion in rats. Kidney Int 68: 2572–2581, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Berkenkamp B, Susnik N, Baisantry A, Kuznetsova I, Jacobi C, Sörensen-Zender I, Broecker V, Haller H, Melk A, Schmitt R: In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS One 9: e88071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt R, Marlier A, Cantley LG: Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol 19: 2375–2383, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.