Abstract
CKD is the gradual, asymptomatic loss of kidney function, but current tests only identify CKD when significant loss has already happened. Several potential biomarkers of CKD have been reported, but none have been approved for preclinical or clinical use. Using RNA sequencing in a mouse model of folic acid-induced nephropathy, we identified ten genes that track kidney fibrosis development, the common pathologic finding in patients with CKD. The gene expression of all ten candidates was confirmed to be significantly higher (approximately ten- to 150-fold) in three well established, mechanistically distinct mouse models of kidney fibrosis than in models of nonfibrotic AKI. Protein expression of these genes was also high in the folic acid model and in patients with biopsy-proven kidney fibrosis. mRNA expression of the ten genes increased with increasing severity of kidney fibrosis, decreased in response to therapeutic intervention, and increased only modestly (approximately two- to five-fold) with liver fibrosis in mice and humans, demonstrating specificity for kidney fibrosis. Using targeted selected reaction monitoring mass spectrometry, we detected three of the ten candidates in human urine: cadherin 11 (CDH11), macrophage mannose receptor C1 (MRC1), and phospholipid transfer protein (PLTP). Furthermore, urinary levels of each of these three proteins distinguished patients with CKD (n=53) from healthy individuals (n=53; P<0.05). In summary, we report the identification of urinary CDH11, MRC1, and PLTP as novel noninvasive biomarkers of CKD.
Keywords: CKD, fibrosis, transcriptional profiling
CKD, the gradual degradation of renal excretory function, is increasingly recognized as a major public health problem, affecting 10%–16% of the adult population globally,1 with approximately 26 million cases in the United States.2 The socioeconomic impact of CKD is high, with 27.6% of total Medicare costs being used to treat it, and it is third only to diabetes and heart failure.3 It is estimated that only approximately 11% of patients with at least moderate decrease in kidney function eventually progress to kidney failure and become dependent on dialysis or transplantation treatments.4 However, even the patients that do not progress are at increased risk of cardiovascular disease and death.5 The disease is usually asymptomatic until at least two-thirds of the functional capacity of the kidneys is already lost.6 As a result, most people are unaware that they have it and are diagnosed only in late stages of CKD.2
Current guidelines from the Kidney Disease Improving Global Outcome CKD Work Group recommend the definition, classification, and prognosis of CKD based on the eGFR (using formulas that rely on serum creatinine measurements and cystatin C), and albuminuria.7 Due to renal compensatory mechanisms, serum creatinine levels only show alterations when more than half of the kidney function is already lost, and is also affected by many other factors like muscle mass, hydration, medications, age, and gender. Similarly, significant renal damage is needed for measurable proteinuria or even microalbuminuria, whereas only low levels of protein in the urine are detected when the cause of CKD is hypertension or tubulointerstitial disease.8 While new biomarkers are being proposed, many are still in the early stages of testing and none are currently approved for clinical use.9
There still is a great need for new biomarkers in CKD to help diagnosis, prognosis, and facilitation of preclinical studies and clinical trials for the development of new, curative therapies. Ideally these biomarkers should reflect kidney pathology rather than generalized processes. Since fibrosis is the common histologic finding that evolves with CKD,6 the objective of this study was to identify translational biomarkers of CKD using a mouse model of progression to kidney fibrosis induced by folic acid (FA) administration. This model was chosen because of our previous experience with temporal characterization of injury and fibrosis in FA nephropathy, and the need for using a mouse model where the severity of injury/fibrosis can be manipulated using pharmacologic approaches.10 Using RNA sequencing (RNA-seq) in the fibrotic kidneys, we identified and confirmed increased expression for a panel of ten genes. Increased protein expression for these candidates was also confirmed in animal models and humans with kidney fibrosis. We also developed selected reaction monitoring (SRM) assays for these ten proteins and report that three of them, cadherin 11 (CDH11), mannose receptor C1 (MRC1), and phospholipid transfer protein (PLTP), were significantly increased in the urine of CKD patients (n=53) as compared to patients without kidney disease (n=53).
Results
Identification of Candidate Genes for Biomarkers of Kidney Fibrosis through RNA-seq
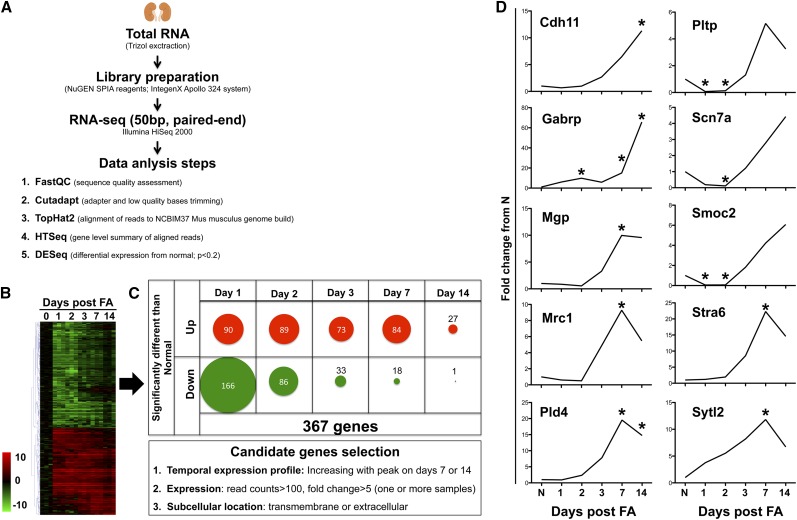
In order to identify differentially expressed genes in progressive kidney fibrosis, RNA-seq (50 bp, paired end) was performed in mouse kidneys obtained at days 0, 1, 2, 3, 7, and 14 after a single intraperitoneal injection of 250 mg/kg FA (n=3/time-point, Figure 1). The validity of the RNA-seq experiment was verified through expression changes in predicted genes10 at the appropriate time-points for acute injury [kidney injury molecule-1 (Kim-1) and fibrinogen-beta (Fgβ)] and fibrosis (collagen 1a1 [Col1a1], fibronectin 1 [Fn1]) (Supplemental Figure 1). Three hundred sixty seven genes showed significant changes from normal at least at one time-point when the expression in the injured/fibrotic kidneys was compared with normal for all time-points using DESeq analysis with a cutoff of P<0.2 (Figure 1, A–C, Supplemental Table 1). Hierarchic clustering of data demonstrated a dynamic and temporal pattern of gene expression changes with a subset of genes showing differential expression during injury phase (days 1–3), with a return to normal for most by day 14; and another subset of genes showing upregulation only as the kidneys progressed to fibrosis, particularly at days 7 and 14 (Figure 1C). To select genes that are indicative of fibrosis we considered candidates from this later group that had robust upregulation (>five-fold with >100 read counts at peak) over time as fibrosis progressed. Another important criteria was the location of the protein product of the gene, either in extracellular matrix or transmembrane (information obtained from the UniProt database), to increase the chance of detecting it in the urine for usage as a biomarker.11 We selected for further validation ten candidates that fit the selection criteria, with their individual temporal expression profiles from RNA-seq shown in Figure 1D.
Figure 1.
Selection of ten candidate genes as potential biomarkers of kidney fibrosis development. (A) Schematic of the steps taken for the RNA-seq analysis from retrieval of kidney tissue to generation of lists of genes with expression significantly different from that in normal mice. (B) Hierarchic clustering grouped the temporal profiles of gene expression variation. Data are shown as the average fold change from normal with three samples for each gene and each time-point (log2 scale). Only the 367 genes that showed significant variation at the P<0.2 level at least for one time-point are shown. (C) Breakdown of numbers of significantly up- or down-regulated genes at each time-point and selection criteria for kidney fibrosis biomarker candidate genes. (D) Fold changes from normal for the ten candidates selected for follow-up. Data are shown as the mean for the three samples included in RNA-seq at each time-point. *Indicates time-points when P<0.2 for the fold change from normal.
Confirmation of the Ten Candidate Genes in Mouse Models of Kidney Fibrosis
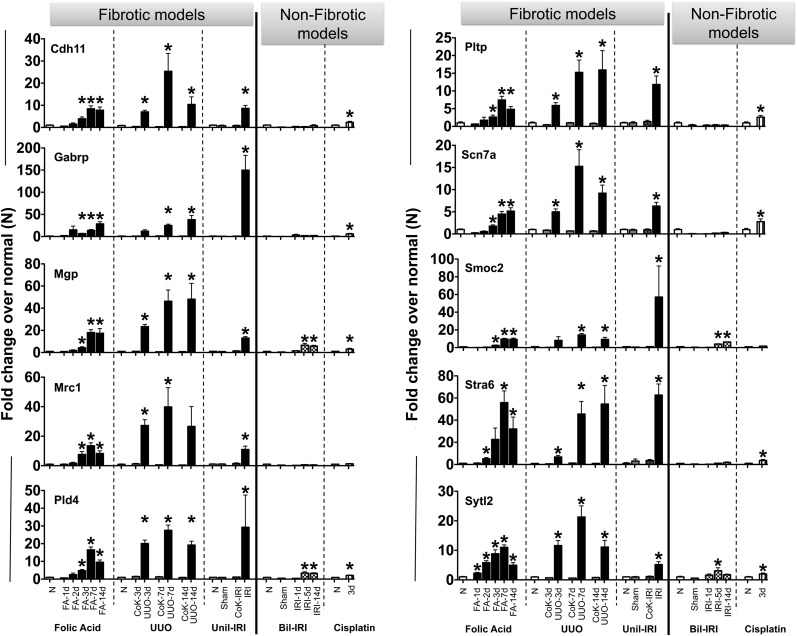
To generalize the utility of the candidate genes as biomarkers of fibrosis irrespective of the animal model used, and to further test if their expression associates more with fibrosis rather than AKI, we performed quantitative real-time (qRT)-PCR for all the candidate genes in three well established and mechanistically distinct mouse models that result in kidney fibrosis, as well as two that cause AKI (Supplemental Figure 2). The Pearson correlation coefficient between quantitative expression of the ten candidate genes measured by RNA-seq and qRT-PCR was found to be above 0.7 for all, indicating a good correlation between techniques (Supplemental Figure 3). The mRNA expression of all ten genes was significantly high (approximately ten- to 150-fold compared with normal) in the kidneys of mice with developing fibrosis irrespective of whether it was initiated by FA administration or unilateral ureteral obstruction (UUO) or unilateral ischemia reperfusion injury (IRI) (Figure 2). Generally, expression was highest in UUO mice, who developed a more robust fibrotic phenotype encompassing the whole kidney rather than patchy fibrosis as in the FA model,10 though Gabrp and Smoc2 reached their highest levels following unilateral IRI. Very modest (<five-fold) increases in many of the genes were seen in AKI models. These results indicate that the kidney expression of these ten genes is more robust for fibrosis development than for AKI.
Figure 2.
Expression levels for the ten candidate genes in mouse models of kidney fibrosis and AKI. The mRNA expression was assessed by qRT-PCR in the following mouse models: FA nephropathy model (n=6/group), UUO (n=5/group), unilateral IRI (Unil-IRI; n=4 for sham groups and n=5 for Unil-IRI groups, samples collected at 42 days post-Unil-IRI), bilateral IRI (Bil-IRI) and cisplatin-induced AKI (n=5/group). Data were normalized to GAPDH and are presented as mean±SEM of the fold change from normal (N) group in each model. For Unil-IRI, N were the contralateral (CoK) kidneys from the sham-operated mice. *P<0.05 when compared with N.
Protein Expression of the Candidates after Kidney Fibrosis in Mice and Humans
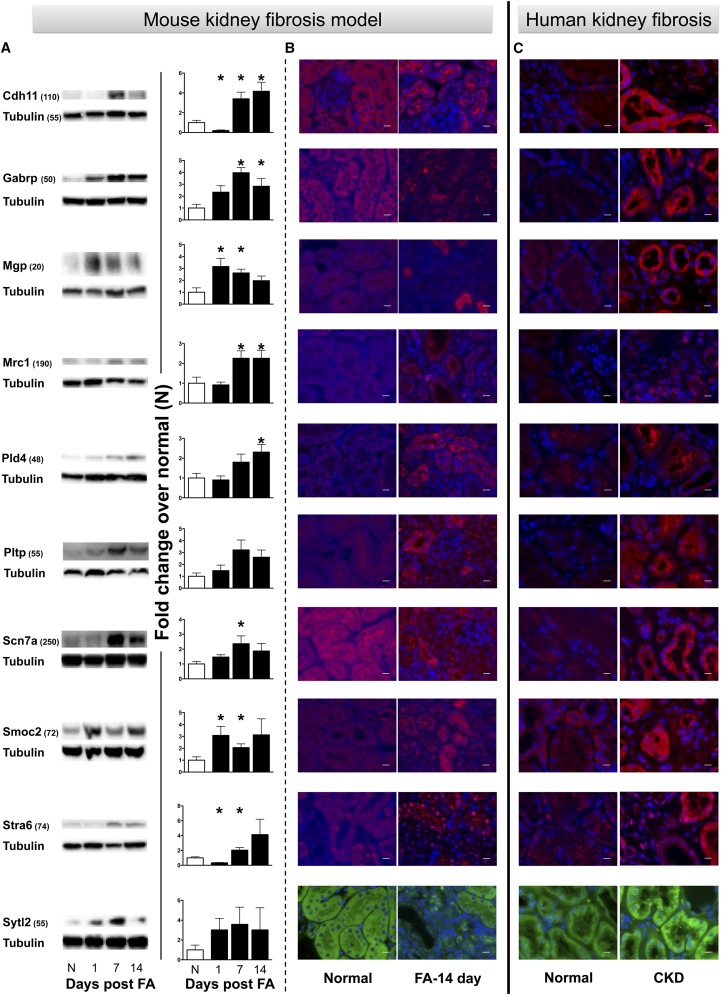
We next verified if increased gene expression translates into protein expression in fibrotic kidneys, which would open the possibility for these proteins or degradation fragments to reach the urine where their detection could lead to their use as fibrosis biomarkers. We first tested them by immunoblot in the mouse kidneys at various time-points during the development of fibrosis in the FA model (Figure 3A). Most of the proteins reached statistically significant (P<0.05) increased kidney expression at least at one time-point. The temporal profiles for protein expression generally matched those for gene expression for the FA model. Notable exceptions were MGP and SMOC2, with an earlier increase in protein expression than that for the gene expression (Figures 2 and 3). The peak fold change over normal for the protein expression was also generally lower than for the gene expression, but this is expected considering that gene expression does not always translate stoichiometricaly to protein expression. For a better picture of their increased expression and localization, immunofluorescence was performed in normal and fibrotic mouse kidneys (day 14 in the FA model). Representative pictures in Figure 3B, as well as localization patterns (Table 1), show increased presence for all proteins in the kidney, particularly in relation to the tubular structures. To evaluate the expression pattern of these ten proteins in human kidneys, immunofluorescence staining was performed in human kidney tissues (n=5) that were severely fibrotic (80% fibrotic), and compared with normal human kidneys (<5% fibrosis) (Supplemental Figure 4). Representative pictures from a normal kidney and a fibrotic kidney in Figure 3C show increased protein presence for the ten candidates similar to what was found in the FA model, with a similar location pattern (Table 1).
Figure 3.
Significantly increased protein expression for the ten candidates in fibrotic kidney samples from mice and humans. (A) Protein levels detected by immunoblot following FA injection in mice. Data were normalized to tubulin and are presented as mean±SEM of the fold change from Normal (N), n=5/group. *P<0.05 when compared with N. Approximate molecular mass in kDa indicated in parentheses. (B) Representative immunofluorescence images show increased protein expression and localization in mouse fibrotic kidney samples at 14 days after FA injection when compared with N. (C) Increased protein expression was also detected in fibrotic human kidney tissue when compared with normal tissue. Images were taken at 63× magnification and scale bars represent 10 μm.
Table 1.
Localization of candidate proteins in normal and fibrotic kidneys from mice and humans
| Normal | Fibrotic | |
|---|---|---|
| CDH11 | Very fine granular subtle cytoplasmic staining in PT and DT | More reactivity along the brush border and in cellular debris within the tubule lumens |
| GABRP | Very mild/minimal cytoplasmic staining in both PT and DT | Strong reactivity along the apical surface, brush border, and in cellular debris within the tubule lumens |
| MGP | Very minimal and subtle cytoplasmic staining in both PT and DT | More reactivity along the brush border and in cellular debris within the tubule lumens in mice. Coarse granular reactivity in cytoplasm of some PT, but also along TBM in some tubules |
| MRC1 | Very minimal, subtle fine granular cytoplasmic staining in PT and DT | Mild reactivity along the apical surface of PT |
| PLD4 | Very minimal fine granular cytoplasmic staining, slightly stronger in DT than PT | Granular cytoplasmic reactivity, stronger in DT than PT in mice. In humans much stronger granular cytoplasmic reactivity in PT, particularly along apical surface |
| PLTP | Very mild cytoplasmic staining in both PT and DT | Very mild cytoplasmic staining along the apical surface of PT and also in the DT |
| SCN7a | Minimal fine granular cytoplasmic staining | Minimal fine granular cytoplasmic staining plus strong staining in some tubules with irregular granular staining that is stronger along the apical surface as well in the cellular debris in lumens |
| SMOC2 | Very fine granular subtle cytoplasmic staining in both PT and DT | Stronger cytoplasmic reactivity in the DT when compared with PT in mice. More reactivity in both PT and DT, but stronger in DT with irregular granular cytoplasmic pattern in humans |
| STRA6 | Fine granular cytoplasmic staining, slightly more prominent in PT than DT | Stronger staining in general with nuclear staining in PT |
| SYTL2 | Very minimal cytoplasmic staining in both PT and DT | Slight irregular reactivity in some PT |
PT, proximal tubule; DT, distal tubule; TBM, tubular basement membrane.
Response of Candidate Genes to Increased Severity or Therapeutic Reversibility of Fibrosis
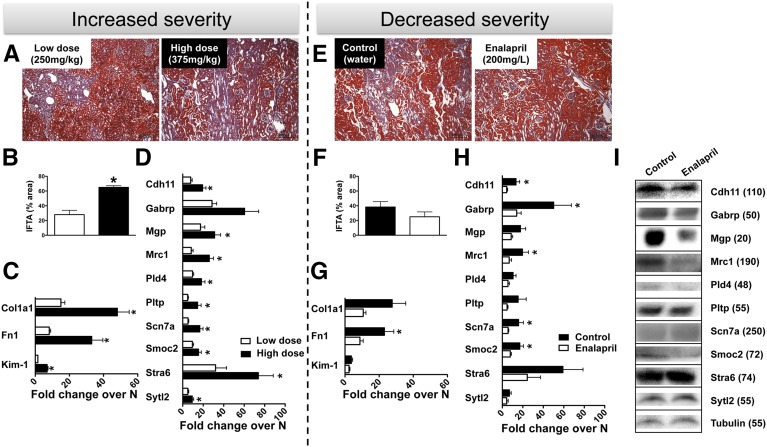
For a biomarker to be truly useful in managing patients with CKD, it will not only have to detect the disease but also give information on prognosis, indicating if a patient responds to treatment as evidenced by declining fibrosis or whether the patient is progressing toward ESRD as evidenced by increasing fibrosis. To test this, we injected mice with a higher dose of FA (375 mg/kg) that resulted in increased kidney fibrosis at day 14 compared with the previously used 250 mg/kg dose (low dose), as indicated by histology, with more than double the fibrotic area in the high-dose group (Figure 4, A and B), as well as by an approximate three-fold (P<0.05, Figure 4C) greater increase in Col1a1 expression and an approximate four-fold greater increase in Fn1 and Kim-1 (P<0.05). Nine out of ten candidate fibrosis markers showed significantly (P<0.05) higher mRNA levels in the high-dose group compared with the low-dose group (Figure 4D).
Figure 4.
Expression of ten candidate genes in the kidney increases with increased severity and responds to treatment. (A) Representative images of Masson trichrome-stained slides. (B) Estimation of the tissue area presenting interstitial fibrosis and tubular atrophy (IFTA) and (C) measurement of gene expression by qRT-PCR for Col1a1, Fn1, and Kim-1 after a high dose (375 mg/kg) or low dose (250 mg/kg) of FA. (D) Kidney mRNA expression for the ten candidate genes in high-dose versus low-dose FA-injected mice. For both (C) and (D), qRT-PCR data were normalized to GAPDH and are represented as mean±SEM of the fold change from normal; n=6/group. *P<0.05 when compared with low-dose group for each gene. (E) Representative kidney tissue stained with Masson trichrome and (F) area of IFTA estimation indicating decreased kidney fibrosis 14 days after FA injection in mice receiving enalapril 200 mg/L of drinking water compared with control (plain water). Scale bars indicate 200 μm. (F) Decreased kidney expression of Col1a1 and Fn1 by qRT-PCR and (G) the ten candidate genes in enalapril-treated mice compared with control. For (F) and (G), qRT-PCR data were normalized to GAPDH and are represented as mean±SEM of the fold change from normal; n=6/group. *P<0.05 when compared with enalapril group for each gene. (I) Protein levels of the ten candidates detected by immunoblot in the kidney 14 days after FA injection in control and enalapril treatment-receiving mice. Approximate molecular mass in kDa indicated in parentheses.
Conversely, to test if the markers reflect recovery from fibrosis we took advantage of the fact that interventions on the renin-angiotensin system represent at the moment the best treatment for CKD, preventing the progression of renal damage as well as reducing proteinuria, and even resulting in regression of glomerulosclerosis, tubulointerstitial fibrosis, and vascular lesions in humans and animal models.12 Hence, mice were treated with 200 mg/L of the angiotensin converting enzyme inhibitor enalapril13 continuously in the drinking water after FA injection. This resulted in reduced kidney fibrosis development by >33% at day 14, as indicated by histologic assessment (Figure 4, E and F) and an approximate three-fold reduction of Col1a1 and Fn1 mRNA expression (Figure 4G). Most candidate markers also showed gene expression levels matching this reduced fibrosis development with enalapril treatment, with significant (P<0.05) decreases for Cdh11, Gabrp, Mrc1, Scn7a, and Smoc2 (Figure 4H). Reduced protein expression of CDH11, GABRP, MGP, MRC1, PLD4, and SMOC2 was also observed in enalapril-treated mice kidneys as compared with vehicle-treated mice (Figure 4I). Together, these results indicate good correlation with fibrosis severity and response to treatment for the selected candidate biomarkers.
Testing the Specificity of Candidate Markers in a Mouse Model and Patients with Liver Fibrosis
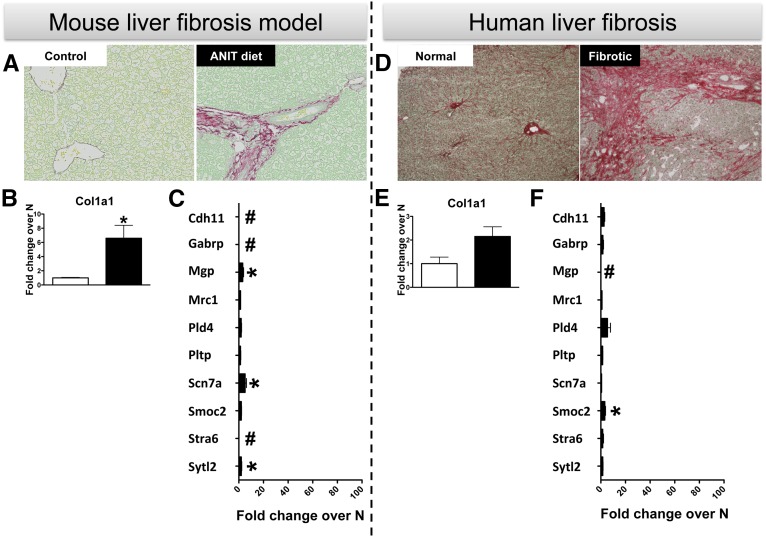
Fibrosis is a universal pathologic finding for chronic diseases in any organ,14 and it would be useful to identify biomarkers of fibrosis that are specific to an organ or are able to indicate their origin. While some of our candidates have already been linked to fibrosis in other organs, with Cdh11 found in lungs15 and arthritis16 and Mgp in vascular fibrosis,17 we decided to test the kidney specificity for our panel in samples from a mouse model and human patients with liver fibrosis. Using Picrosirius red staining in Figure 5A, α-naphthylisothiocyanate (ANIT) exposure (an established model to induce liver fibrosis in mice18) significantly increased peribiliary fibrosis when compared with mice fed a control diet. Specifically, fibrosis was indicated by a robust deposition of collagen around intrahepatic bile ducts. This was reflected in significantly increased Col1a1 gene expression for the ANIT diet group (Figure 5B). Although some candidate markers showed significantly higher (P<0.05) gene expression levels in the ANIT diet group (Mgp, Scn7a, and Sytl2), fold changes were generally much lower than those in the kidney fibrotic models (Figure 5C). Like the experimental setting, robust peribiliary and bridging fibrosis was observed in livers from patients with primary sclerosing cholangitis as shown by Picrosirius red staining (Figure 5D) and an increase in Col1a1 mRNA expression (Figure 5E). Except for Smoc2, there were no significant gene expression increases for the members of the candidate biomarkers panel (Figure 5F). This indicates that at least in liver fibrosis the selected biomarkers will not exhibit increases to confound results for coexisting kidney fibrosis.
Figure 5.
Modest increases in some of the candidate ten genes in mice and human following liver fibrosis. (A) Increased peribiliary fibrosis was detected by Picrosirius red staining in liver tissue from mice fed with the ANIT diet for 4 weeks when compared with those receiving control chow diet (n=5/group). Original magnification, ×200. qRT-PCR for (B) Col1a1 and (C) ten candidate genes in ANIT versus control diet mice at the 4 week time-point. qRT-PCR data were normalized to GAPDH and are represented as mean±SEM of the fold change from control. *P<0.05 compared with control. # indicates that a fold change could not be calculated due to low expression. (D) Picrosirius red staining indicating increased fibrosis in patients with primary sclerosing cholangitis compared with Normal (N). Original magnification, ×100. qRT-PCR for (E) Col1a1 and (F) ten candidate genes in liver tissue from primary sclerosing cholangitis patients compared with N. qRT-PCR data were normalized to GAPDH and are represented as mean±SEM of the fold change from N. n=6 for N and 15 for fibrotic patients. *P<0.05 when compared with N. # indicates that a fold change could not be calculated due to low expression in one or both groups. Note: same x axis scale from 0 to 100 is used for (C) and (F) for comparisons with Figure 4.
Development of SRM Assays and Evaluation of Biomarker Potential in Human Urine
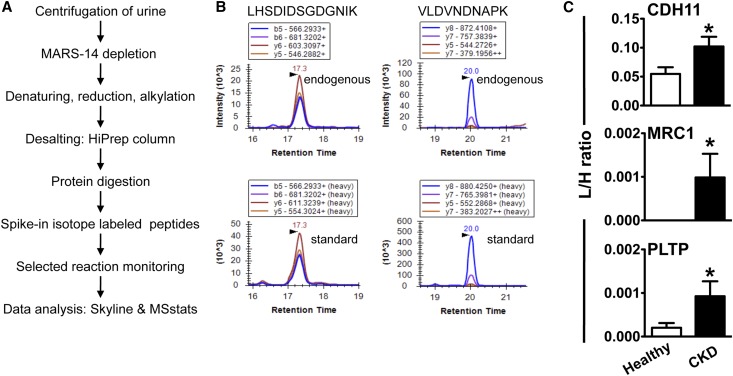
To test if the ten candidate proteins are expressed at a level that can be detected in urine and begin assessing their biomarker potential in CKD, we carried out SRM, a sensitive and quantitative targeted mass spectrometry approach, and analyzed urine from 53 CKD patients and 53 healthy individuals (Table 2). After affinity chromatography depletion of the 14 most abundant plasma proteins that are likewise present in urine at lower concentration, the urine samples were reduced, alkylated, desalted, and trypsin-digested (Figure 6A). Proteotypic peptides that uniquely represent each protein (Supplemental Table 2) were measured by SRM. To allow for relative quantification, each sample was spiked with isotope-labeled peptides, heavy analogs of the light sequences, which function as an internal standard. Three out of ten targeted proteins were detected in urine by SRM at low fmol to amol range. Figure 6B shows an example of peptide LHSDIDSGDGNIK and VLDVNDNAPK from CDH11 detected in urine from CKD patients, the transitions of the endogenous light and labeled spiked-in peptide elute at the same retention time and in the same rank order, confirming their identification. CDH11 and PLTP were detected in the urine from CKD patients as well as in the control samples, while MRC1 was only detected in urine from the CKD patient sample cohort. The relative abundance of these proteins is presented as ratios of endogenous peptide to internal standard across individual samples (Figure 6C). CDH11 showed the highest expression levels of the three proteins. PLTP and MRC1 were observed at similar levels to each other but considerably lower abundance compared with CDH11. MRC1 might be expressed at a very low level in healthy samples but below the limit of detection of this approach. Of the three detectable proteins, group comparison indicates significant upregulation (P<0.05) for CDH11 (approximately two-fold), MRC1 (infinite fold due to no detectable expression in healthy controls), and PLTP (approximately five-fold) in CKD patients. The low amount of starting material and low expression levels have resulted in protein abundances below the limit of detection, and did not allow us to detect the remaining proteins in this study by SRM.
Table 2.
Demographic and clinical characteristics of patients with CKD and healthy volunteers
| Variables | Biopsy-Confirmed Fibrosis (n=30) | CKD Stages III–V (n=23) | Healthy (n=53) |
|---|---|---|---|
| Sex, female % | 30.0 | 34.8 | 54.7 |
| Race, white % | 60.0 | 65.2 | 24.5 |
| Age, years | 58.1±13.6 | 62.0±12.6 | 35.3±12.5 |
| eGFR, ml/min per 1.73 m2 | 29.1±19.1 | 31.9±11.3 | – |
| DM % | 36.7 | 39.1 | – |
| HTN % | 86.7 | 30.4 | – |
| Etiology | |||
| DN % | 20.0 | 47.8 | – |
| Vascular/HTN % | 43.3 | 13.0 | – |
| Othera % | 36.7 | 39.1 | – |
| Treatment with ACEi % | 50.0 | 73.9 | – |
| Treatment with diuretic % | 53.3 | 39.1 | – |
Results are mean±SD or percentages. DM, diabetes mellitus; HTN, hypertension; DN, diabetic nephropathy; ACEi, ace-inhibitor.
Patients with calcineurin-inhibitor toxicity, glomerulonephritis, or advanced fibrosis with unknown underlying etiology.
Figure 6.
SRM assay indicates increased protein levels of CDH11, MRC1, and PLTP in urine samples from CKD patients when compared with those from healthy individuals. (A) Workflow describing sample processing and mass spectrometry analysis. (B) SRM chromatogram of peptides LHSDIDSGDGNIK and VLDVNDNAPK from CDH11 (as a representative example) detected in diseased urine. Transitions of the endogenous light (top) and spiked-in isotope-labeled heavy peptide (bottom) elute at the same retention time and rank order. (C) Detection of CDH11, MRC1, and PLTP in patients with CKD (n=53) compared with healthy controls (n=53). Relative abundance in individual samples is based on the area ratio of endogenous peptide to internal standard. *P<0.05 when compared with healthy controls. L/H, abundance of light over heavy sequence detected in individual sample.
Discussion
Recent estimates place the lifetime risk of developing CKD with at least a moderate decrease in kidney function to >50%, second only to the risk for hypertension for chronic diseases, which stands at 83%–90%.19 Yet, while the diagnosis and treatment of hypertension has become routine in clinical practice, for CKD there is an almost complete lack of diagnostic, prognostic, and predictive biomarkers, as well as specific therapies. Regardless of etiology, CKD is characterized structurally by excessive accumulation of extracellular matrix in glomeruli and tubular interstitium, leading to a progressive loss in renal function.6 Related to the structural alterations, histologic assessment of tissue biopsy is considered the gold standard for diagnosis, but it is rarely performed due to risks of hemorrhage, pain, and death.20 For functional biomarkers, the formation of ultrafiltrate by glomeruli is the process that has received the most attention. While the gold standard for the eGFR is the timed urinary inulin clearance, it is difficult to perform and the formulas based on serum creatinine or cystatin C concentration that are standard in clinical practice do have shortcomings. Together with measurements of proteinuria/albuminuria that are also used for the diagnosis and prognosis of CKD, they form the basis for the current definition of the disease.7
However, all these functional biomarkers have reduced sensitivity and specificity and they generally reflect late stages, when significant damage has already happened.8 The current study was designed to discover novel biomarkers associated with kidney fibrosis progression, the common structural alteration in CKD. We present here, to the best of our knowledge, the first report of gene expression profiling over time in the development of kidney fibrosis through the use of RNA-seq. Through this technology we have identified several candidates with a temporal expression profile matching the development of fibrosis in the FA nephropathy model. This was confirmed in additional models for ten of these candidates for whom increased kidney protein expression was also detected in the FA model and CKD patients. Most importantly, we were able to detect the presence of three candidates in the urine through the use of SRM technology. Urinary levels of proteins CDH11, MRC1, and PLTP were found to be significantly higher in CKD patients compared with healthy individuals. As urine is an easily accessible, stable biologic sample whose proteome reflects in large part, the kidney structure and function,11 our results should be the basis for further validation of urinary measurement of these proteins as noninvasive, translational biomarkers of CKD.
Some of the ten candidates identified in our study, like MGP and SMOC2, are known to be present in the extracellular matrix. For example, MGP is involved in vascular fibrosis and calcification, where measurements of its circulatory forms are being tested as markers for this occurrence even in the context of ESRD.17 Considering the impaired lipid metabolism in CKD, particularly as it relates to triglycerides and HDL, plasma PLTP was investigated as marker of ESRD, with no detectable changes.21 Some of the transmembrane proteins that we have identified, like CDH11 and MRC1, have been previously shown to be involved in fibrosis. In particular for CDH11, a transmembrane glycoprotein that mediates cell-cell adherens junctions, it was described to be involved in the development of lung fibrosis through regulation of TGF-β production, both in humans and in animal models.15 It was also shown to be involved in the secretion of inflammatory mediators by synovial fibroblasts, leading to rheumatoid arthritis.16 MRC1 is a marker of alternatively activated M2 macrophages that is involved in collagen uptake, indicating its role in adaptive and maladaptive fibrosis.22 All these results indicate that our candidates are not merely bystanders, with a random change in expression that just associates with the disease, but they are involved in pathophysiologic mechanisms that are important for the development of kidney fibrosis. It remains to be tested if some of the other candidates identified by our study play a mechanistic role in the development of kidney fibrosis and could offer future therapeutic targets, even as the lack of detection by SRM indicates that they probably do not have urinary biomarker potential.
The fold change for urinary levels in CKD patients versus healthy controls were not particularly high for CDH11 (approximately two-fold) but reached approximately five-fold for PLTP in the small patient cohort available for this study. However, the detection of MRC1 only in CKD patients could suggest a better discriminatory potential even in the absence of a high fold change. To our knowledge, this is the first report of MRC1 and PLTP measurement in the urine. In a different clinical situation, ureteropelvic junction obstruction, CDH11 was reported before to show a two-fold increase in the urine compared with healthy controls, a level that is similar to that found in our study for CKD patients.23 Furthermore, even if the number of patients in our study was small, there was no association of higher values for CDH11, MRC1, or PLTP with certain etiologies presumed to have led to CKD for the patients in our cohort. This could suggest that these biomarkers reflect common disease processes, and further support for it comes from the preclinical data where there was significant upregulation in three distinct animal models. Additionally, while we did not detect an increase in Cdh11, Mrc1, or Pltp gene expression in liver fibrosis, as previously indicated, they could reflect fibrosis development in other organs. It is possible that their release in the plasma from those organs followed by urinary excretion could confound their use for kidney disease prediction. Future studies should detect the situations when other fibrotic diseases lead to urinary expression, and test if the levels reached in urine are generally lower than for kidney fibrosis, or if the plasma-to-urine ratio of their concentration could distinguish renal versus prerenal origin.
In summary, there is great interest in developing new biomarkers for CKD. While many markers are currently being evaluated, none have been preclinically or clinically approved yet.9 The present study identifies CDH11, MRC1, and PLTP as novel urinary biomarkers for kidney fibrosis that characterizes CKD. Further evaluation in multicentered, large, longitudinal human cohorts of CKD is needed to evaluate their early diagnostic, predictive, and prognostic value as biomarkers of CKD.
Concise Methods
Animal Studies
All animal studies were performed in compliance with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health and were approved by the Harvard Medical School Animal Care and Use Committees. Mice were purchased from Charles River Laboratories and were acclimated to our animal facility for at least one week prior to experimentation. Mouse models of AKI,24 kidney fibrosis,10 and liver fibrosis18 were used as previously described by our group.
FA nephropathy model: male Balb/c mice (25–29 g) received a single intraperitoneal (ip) injection of 250 mg/kg FA dissolved in a 0.3 M sodium bicarbonate solution.10 Mice were euthanized at 1, 2, 3, 7, and 14 days following administration. For the experiments testing increased kidney fibrosis, mice received a single ip injection of 375 mg/kg FA and they were euthanized at 14 days. For the experiments testing decreased kidney fibrosis following FA injection, all mice received a 250 mg/kg FA ip injection and cages were randomized to receive either a 200 mg/L solution of enalapril in the water bottle for the treatment group or regular water for the control group throughout the duration of the experiment. All mice were euthanized at 14 days.
Cisplatin-induced AKI model: male Balb/c mice (25–29 g) received a single ip injection of 20 mg/kg cisplatin dissolved in normal saline or vehicle alone. These mice were euthanized and samples were collected 72 hours after the injection.
Surgical models of AKI and kidney fibrosis: all surgical procedures were performed under general anesthesia (50 mg/kg pentobarbital sodium, ip administration) and mice received lost fluid replacement (1 ml normal saline, heated at 37°C, administered subcutaneously immediately after surgery) as well as pain medication (buprenorphine, 0.05 mg/kg, administered subcutaneously every 12 hours for the first 2 days; first dose in the 1 ml of normal saline administered immediately after surgery and three additional doses in 50 μl normal saline).10 UUO was performed in male Balb/c mice as previously described and mice were euthanized for sample collection at 3, 7, and 14 days after the intervention. Bilateral renal IRI was performed in male Balb/c mice by applying Roboz microclips (70 g pressure) on both renal arteries for 30 minutes, and animals were euthanized at 1, 7, and 14 days. Sham surgery, with animals that received the laparotomy and renal pedicle manipulation but not the hemostasis, was performed in additional mice and these were euthanized 1 day after the intervention. Unilateral ischemia reperfusion as well as sham surgery was performed similarly in C57BL/6 female mice, and these mice were euthanized 41 days after the intervention.
Liver fibrosis model: male C57BL/6 mice (25–29 g) were fed chow (AIN-93M) containing 0.025% ANIT for 4 weeks prior to euthanizing. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International–accredited facility at Michigan State University and all procedures were approved by the Michigan State University Institutional Animal Care and Use Committee. Biospecimen collection is described in the Supplemental Material.
Human Studies
All participants were patients or healthy volunteers recruited at Brigham and Women’s Hospital (BWH), Boston. The Institutional Review Board approved the protocols for recruitment and sample collection, which was performed with written informed consent of the participants. Urine samples from patients with CKD were obtained from the BWH ambulatory nephrology clinic. Inclusion criteria included a diagnosis of CKD under the care of a nephrologist at BWH; eGFR 15–60 ml/min per 1.73 m2 with any degree of proteinuria or eGFR>60 ml/min per 1.73 m2 with ≥1 gm proteinuria. Patients were excluded if they had any of the following: a recent hospitalization or episode of AKI (>50% rise in serum creatinine over a 1-week period) within 3 months; active glomerulonephritis; reported or suspected urinary tract infection within the past 3 weeks; or planned change in the dose of a diuretic and/or antihypertensive medication during the study period. Presumed etiology was diabetic kidney disease (n=11), hypertensive or vascular disease (n=3), or other or unknown causes (n=9). Additional urine samples from patients with biopsy confirmed CKD were obtained from the Boston Kidney Biopsy Cohort, a prospective observational study of individuals undergoing native kidney biopsy at three tertiary care hospitals in Boston, Massachusetts. Urine samples were obtained on the day of kidney biopsy. Urine samples were selected from patients with CKD (eGFR<60 ml/min per 1.73 m2) with confirmed interstitial fibrosis and tubular atrophy >50%, excluding those with autoimmune diseases, idiopathic glomerular diseases, and AKI. Clinicopathologic diagnoses were diabetic kidney disease (n=6), hypertensive or vascular disease (n=13), or advanced fibrosis or secondary glomerulosclerosis of other or unknown cause (n=11). Urine samples from healthy volunteers were obtained from the PhenoGenetics Project, a study of the impact of genetic variation in healthy individuals. Participants 18–65 years of age were recruited from the Boston area through advertisements in local media and flyers. Inclusion criterion was willingness to provide 120 ml of blood four times per year for five years. Exclusion criteria were the presence of self-reported inflammatory diseases (e.g., asthma or psoriasis), autoimmune diseases (e.g., lupus of multiple sclerosis), chronic metabolic diseases (e.g., thyroid disease or diabetes), or chronic infections (e.g., Hepatitis B or C, or HIV).
Deidentified human kidney tissue samples from patients with severe kidney fibrosis were obtained from the Department of Pathology at BWH. Deidentified liver tissue samples from patients with primary sclerosing cholangitis undergoing liver transplant were obtained through the University of Kansas Medical Center Liver Center Tissue Bank.
Immunofluorescence, Immunoblottting, and qRT-PCR
All the techniques were performed using standard protocols established in the laboratory.10 Primary antibodies used are indicated in Supplemental Material and primer pairs used are listed in Supplemental Tables 3 and 4.
RNA-seq
RNA samples (n=3 mice/time-point) were sequenced at the Biopolymers Facility at the Harvard Medical School. The quantity and quality of mRNA were assayed on an Agilent 2200 TapeStation instrument and by SYBR qRT-PCR assay. Libraries were prepared from 10 ng total RNA per sample using the IntegenX Apollo 324 system and NuGEN SPIA reagents. Libraries were multiplexed in groups of three per lane of a flowcell, and 50 cycle, paired-end sequencing was performed on an Illumina HiSeq2000 instrument. Illumina sequence quality was surveyed with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to ensure suitability of library generation and sequencing for further analysis. Adapters and lower-quality bases were trimmed with Cutadapt (https://code.google.com/p/cutadapt/), using a Phred quality cutoff of 20, and reassessed with FastQC. Trimmed reads were aligned to the Ensembl NCBIM37 Mus musculus genome build with TopHat2. Aligned reads were summarized at the gene level against the Illumina iGenomes (http://tophat.cbcb.umd.edu/igenomes.html) NCBIM37 Mus musculus gene annotations using HTSeq (http://www.huber.embl.de/users/anders/HTSeq/doc/overview.html). Normalized read counts at all time-points after FA injection were tested with DESeq for differential gene expression against normal samples using an adjusted P value cutoff of 0.2. The full dataset is available in the National Center for Biotechnology Information Gene Expression Omnibus database with the accession number GSE65267. Raw data are accessible at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE65267.
SRM Assays
Urine from control and CKD patient samples were randomized prior to sample preparation and mass spectrometry analysis. Urine was centrifuged at 10,000 g for 10 minutes at room temperature, and the pellet was discarded. A total of 1 ml supernatant was depleted from the 14 most abundant plasma proteins using the multiple affinity removal system (MARS Hu-14, 4.6 × 100 mm, Agilent Technologies) according to the manufacturer’s protocol. Thirty-nine proteotypic peptides that are unique to the ten proteins studied and their respective SRM assays were obtained from SRMAtlas (www.srmatlas.org)25 (Supplemental Table 2). For each peptide sequence the heavy isotope–labeled analog with the C-terminal arginine as R[13C6; 15N4] or lysine as K[13C6; 15N2] and carbamidomethylated cysteine residues were synthesized as crude product (Thermo Fisher Scientific) to allow for relative quantification. A total of 2 μg urine digest spiked with an aliquot of the mixed standard peptides at a concentration between 45 fmol and 600 fmol for each individual peptide were analyzed on a QTRAP 5500 LC-MS system equipped with a Nano Spray Source III and an Eksigent Nano LC 2D (AB Sciex). Peptides were analyzed in scheduled SRM mode with Q1 and Q3 set to unit resolution, a 2-second cycle time, a declustering potential of 70, and a retention time window of 360 seconds. SRM data and the transition list are available in the PeptideAtlas SRM Experiment Library data repository http://www.peptideatlas.org/PASS/PASS00647. SRM data were analyzed with Skyline26 and MSstats.27 The relative protein expression level is reported as the ratio of endogenous light to the heavy standard.
Statistical Analyses
Statistical analyses (t-tests and Pearson correlations) were performed using GraphPad Prism 5 (GraphPad Software Inc.). Hierarchic clustering was performed using the MeV software.28
Disclosures
None.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Dr. Philip De Jager for providing urine samples from the PhenoGenetics Project at BWH.
J.P.L. was supported by the National Institutes of Health (NIH)/National Institute of Environmental Health Sciences (R01-ES017537). R.L.M. was funded by the American Recovery and Reinvestment Act funds through NIH/National Human Genome Research Institute (RC2-HG005805), National Institute of General Medical Sciences (2P50 GM076547), and Center for Systems Biology (S10RR027584 and GM087221).
The Boston Kidney Biopsy Cohort led by S.S.W. is supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (DK093574). Resources were made available from NIH grant UH3TR000504 (to J.H.). Work in the Vaidya laboratory was supported by an Outstanding New Environmental Scientist award from NIH/National Institute of Environmental Health Sciences (ES017543) and an Innovation in Regulatory Science Award from Burroughs Wellcome Fund (BWF-1012518).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015020225/-/DCSupplemental.
References
- 1.Matsushita K, Ballew SH, Astor BC, Jong PE, Gansevoort RT, Hemmelgarn BR, Levey AS, Levin A, Wen CP, Woodward M, Coresh J Chronic Kidney Disease Prognosis Consortium : Cohort profile: the chronic kidney disease prognosis consortium. Int J Epidemiol 42: 1660–1668, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Stenvinkel P: Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med 268: 456–467, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Hoerger TJ, Wittenborn JS, Segel JE, Burrows NR, Imai K, Eggers P, Pavkov ME, Jordan R, Hailpern SM, Schoolwerth AC, Williams DE Centers for Disease Control and Prevention CKD Initiative : A health policy model of CKD: 1. Model construction, assumptions, and validation of health consequences. Am J Kidney Dis 55: 452–462, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 6.López-Novoa JM, Rodríguez-Peña AB, Ortiz A, Martínez-Salgado C, López Hernández FJ: Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications. J Transl Med 9: 13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens PE, Levin A Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Devarajan P: The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis 17: 469–479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE: Biomarkers in chronic kidney disease: a review. Kidney Int 80: 806–821, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Craciun FL, Ajay AK, Hoffmann D, Saikumar J, Fabian SL, Bijol V, Humphreys BD, Vaidya VS: Pharmacological and genetic depletion of fibrinogen protects from kidney fibrosis. Am J Physiol Renal Physiol 307: F471–F484, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konvalinka A, Scholey JW, Diamandis EP: Searching for new biomarkers of renal diseases through proteomics. Clin Chem 58: 353–365, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Cravedi P, Remuzzi G: Mechanisms and treatment of CKD. J Am Soc Nephrol 23: 1917–1928, 2012 [DOI] [PubMed] [Google Scholar]
- 13.El Chaar M, Chen J, Seshan SV, Jha S, Richardson I, Ledbetter SR, Vaughan ED Jr, Poppas DP, Felsen D: Effect of combination therapy with enalapril and the TGF-beta antagonist 1D11 in unilateral ureteral obstruction. Am J Physiol Renal Physiol 292: F1291–F1301, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Friedman SL, Sheppard D, Duffield JS, Violette S: Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5: 167sr1, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Schneider DJ, Wu M, Le TT, Cho SH, Brenner MB, Blackburn MR, Agarwal SK: Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. FASEB J 26: 503–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noss EH, Chang SK, Watts GF, Brenner MB: Modulation of matrix metalloproteinase production by rheumatoid arthritis synovial fibroblasts after cadherin 11 engagement. Arthritis Rheum 63: 3768–3778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewé RB, Brandenburg VM, Bekers O, Vermeer C: Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 104: 811–822, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Joshi N, Kopec AK, Towery K, Williams KJ, Luyendyk JP: The antifibrinolytic drug tranexamic acid reduces liver injury and fibrosis in a mouse model of chronic bile duct injury. J Pharmacol Exp Ther 349: 383–392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grams ME, Chow EK, Segev DL, Coresh J: Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis 62: 245–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manno C, Strippoli GF, Arnesano L, Bonifati C, Campobasso N, Gesualdo L, Schena FP: Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 66: 1570–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Pahl MV, Ni Z, Sepassi L, Moradi H, Vaziri ND: Plasma phospholipid transfer protein, cholesteryl ester transfer protein and lecithin:cholesterol acyltransferase in end-stage renal disease (ESRD). Nephrol Dial Transplant 24: 2541–2546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushiyama T, Oda T, Yamada M, Higashi K, Yamamoto K, Sakurai Y, Miura S, Kumagai H: Alteration in the phenotype of macrophages in the repair of renal interstitial fibrosis in mice. Nephrology (Carlton) 16: 522–535, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Mesrobian HG, Mitchell ME, See WA, Halligan BD, Carlson BE, Greene AS, Wakim BT: Candidate urinary biomarker discovery in ureteropelvic junction obstruction: a proteomic approach. J Urol 184: 709–714, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini KL, Han T, Bijol V, Saikumar J, Craciun FL, Chen WW, Fuscoe JC, Vaidya VS: MicroRNA-155 deficient mice experience heightened kidney toxicity when dosed with cisplatin. Toxicol Sci 141: 484–492, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusebauch U, Deutsch EW, Campbell DS, Sun Z, Farrah T, Moritz RL: Using PeptideAtlas, SRMAtlas, and PASSEL: Comprehensive Resources for Discovery and Targeted Proteomics. Curr Protoc Bioinformatics 46: 13.25.11–13.25.28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ: Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26: 966–968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, Vitek O: MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30: 2524–2526, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J: TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.