Abstract
Endothelial progenitor cells (EPCs) may be relevant contributors to endothelial cell (EC) repair in various organ systems. In this study, we investigated the potential role of EPCs in renal EC repair. We analyzed the major EPC subtypes in murine kidneys, blood, and spleens after induction of selective EC injury using the concanavalin A/anti-concanavalin A model and after ischemia/reperfusion (I/R) injury as well as the potential of extrarenal cells to substitute for injured local EC. Bone marrow transplantation (BMTx), kidney transplantation, or a combination of both were performed before EC injury to allow distinction of extrarenal or BM-derived cells from intrinsic renal cells. During endothelial regeneration, cells expressing markers of endothelial colony-forming cells (ECFCs) were the most abundant EPC subtype in kidneys, but were not detected in blood or spleen. Few cells expressing markers of EC colony-forming units (EC-CFUs) were detected. In BM chimeric mice (C57BL/6 with tandem dimer Tomato-positive [tdT+] BM cells), circulating and splenic EC-CFUs were BM-derived (tdT+), whereas cells positive for ECFC markers in kidneys were not. Indeed, most BM-derived tdT+ cells in injured kidneys were inflammatory cells. Kidneys from C57BL/6 donors transplanted into tdT+ recipients with or without prior BMTx from C57BL/6 mice were negative for BM-derived or extrarenal ECFCs. Overall, extrarenal cells did not substitute for any intrinsic ECs. These results demonstrate that endothelial repair in mouse kidneys with acute endothelial lesions depends exclusively on local mechanisms.
Keywords: endothelial injury, endothelial progenitor cell, endothelial repair, thrombotic, glomerulonephritis
Primary endothelial lesions occur in several types of human kidney disease such as thrombotic microangiopathy (TMA), vasculitis, lupus nephritis, and glomerulonephritis. Recent clinical trials in patients with renal TMA have shown that primary endothelial lesions lead to a clinically remarkable repair response providing full restitution of kidney function.1 Experimentally, the relevance of endothelial repair for healing of glomerular lesions had already been proven many years ago.2 While our pathophysiologic understanding of primary endothelial injury in TMAs has markedly improved in recent years,3,4 little is known about the orchestration of subsequent endothelial repair mechanisms. Following the landmark publication by Asahara and colleagues,5 in which they provided the first evidence that circulating bone marrow (BM)-derived endothelial progenitor cells (EPCs) may participate in processes of vascular postnatal angiogenesis, a huge amount of literature appeared to support this view in various organ systems.6–9 Based on these findings, the so far prevalent theory of endothelial self-renewal was questioned.
Numerous studies investigated the role of extrarenal EPCs following renal injury. However, many of those studies demonstrated rather associative data and widely relied on the detection of such cells in the circulation.10–12 In an effort to functionally dissect the heterogeneously defined EPCs, two subtypes of EPCs, the (early) endothelial cell colony-forming units (EC-CFUs) and (late) endothelial outgrowth cells (EOC, or ECFCs) were described.13–15 Therefore, CD34+ or mononuclear cells were isolated from blood or BM and cultured in colony-forming assays.5,11,16–19 Isolated EC-CFU cells were characterized by expression of the endothelial markers CD34, VEGFR2, CD31, CD146, and CD105, and also hematopoietic surface markers such as CD133, CD45, CD14, and CD115. In contrast, ECFCs were negative for hematopoietic surface markers and showed clonal proliferative potential and vessel formation capability in vitro and in vivo.14,15,17,20
To investigate the orchestration of endothelial injury and repair mechanisms, we established an experimental mouse model of selective endothelial injury leading to subsequent TMA in the kidney.21 This model is induced by renal arterial perfusion with concanavalin A followed by anti-conA antibodies, subsequently inducing acute and severe EC injury. In a previous study, we have shown that following endothelial injury a relevant number of cells carrying markers of EPCs could be detected in injured kidneys, suggesting that these cells contributed to the subsequent healing of endothelial lesions.22 We now wanted to further explore and clarify the functional relevance and origin of such cells for endothelial regeneration in the kidney.
To address this issue, the present study investigated distinct, previously defined EPC subtypes in the mouse kidney and the hematopoietic system (blood and spleen) following selective endothelial and also renal ischemia/reperfusion injury. To distinguish circulation-derived cells from tissue-resident cells, we used bone marrow transplantation (BMTx), kidney transplantation (KTx) and combined BMTx and KTx, and evaluated these cells by multicolor fluorescent activated cell sorting (FACS) and histology.
Results
Extent of EC injury and Endothelial Repair Response Following Selective Endothelial Injury in Mouse Kidneys
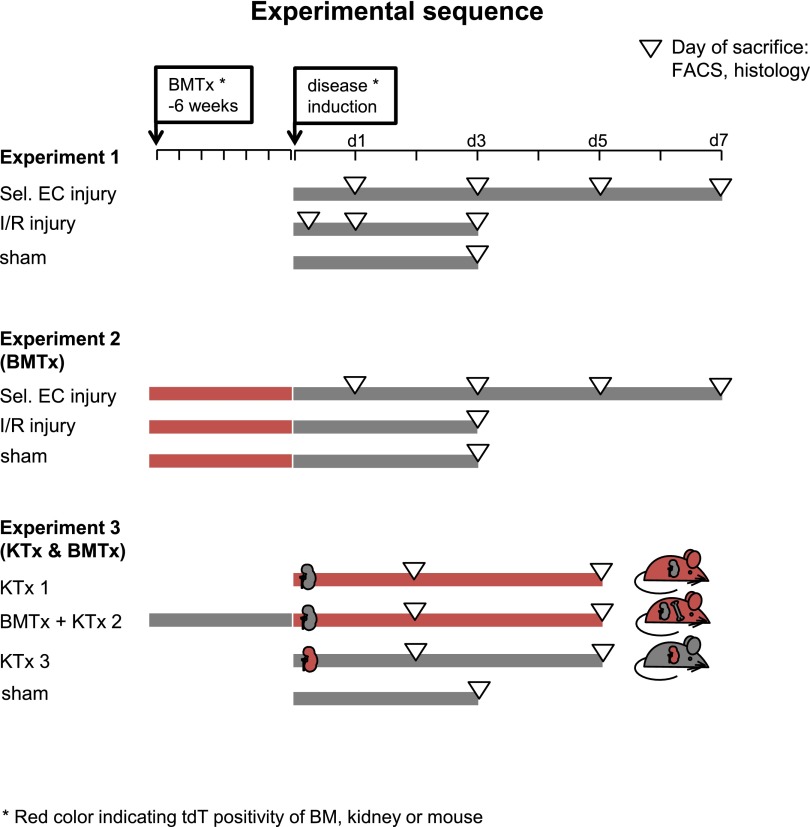
In a first step (experiment 1, Figure 1), we characterized the extent of the endothelial injury, the local repair response, and inflammation in C57BL/6 mice following either site-selective renal endothelial injury or ischemia/reperfusion (I/R) injury induced by a warm ischemia time of 25 min.
Figure 1.
Experimental sequence. Three central experiments were used to investigate the role of EPCs for EC repair. In experiment 1, site-selective endothelial injury (sel. EC injury) and I/R injury were used and combined with prior BMTx in experiment 2. For experiment 3, KTx and combined BMTx and KTx with a predefined ischemia time were used.
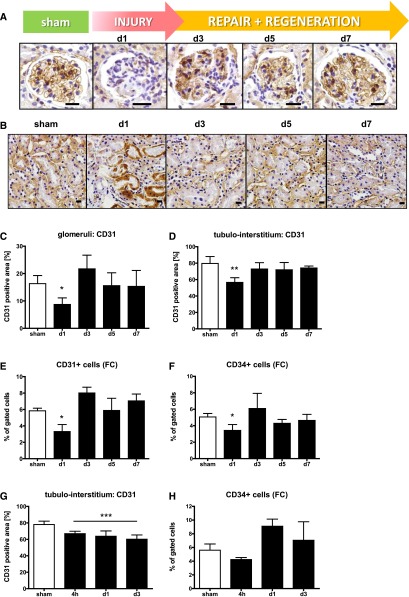
Endothelial lesions and their subsequent repair were evaluated after staining of tissue sections for CD31. As depicted in Figure 2, A and B, extensive loss of CD31-positive (CD31+) ECs in glomeruli (A) and peritubular capillaries (B) could be detected following selective endothelial injury. By histology, CD31+ glomerular (8.7±2.4 versus 16.3±2.9% per glomerular cross-section; P<0.01; Figure 2C) and peritubular EC were decreased (56.6±5.7 versus 79.6±8.4%; Figure 2D; P<0.01) after 24 hours compared with controls. This could also be verified by a reduced number of CD31+ cells (Figure 2E) and CD34+ cells (Figure 2F) by FACS analysis (CD31: 3.3±0.8% of gated cells [n=5319±1363 cells per measurement] 24 h after initial EC injury versus 5.83±0.8% [n=9373±1533 cells] in sham operated mice). Thus, a 43% reduction was observed after 24 h. Using both methods, the amount of CD31+ cells and CD34+ ECs markedly increased on day 3 and was back at baseline levels after 5–7 days. By histology, an almost complete reconstitution of the glomerular and peritubular endothelium (Figure 2, B and C) occurred until day 7 (glomerular CD31+ area d7: 15.3±5.9% versus 16.3±2.9% in ctrl).
Figure 2.
Endothelial injury and regeneration. Representative pictures of zinc fixed sections stained with anti-CD31 in glomeruli (A) and the tubulo-interstitium (B) (large scale=10 μm). Assessment of the glomerular (C) or peritubular endothelium (D) after staining for CD31 positive area measured using densitometry. Reduction of CD31 (E) and CD34 (F) positive cells measured by flow cytometry (fc) following selective EC injury or I/R (G, H).
To assess the role of EC proliferation for the repair process, proliferating cells were stained by repetitive twice-daily injection of the proliferation marker EdU (5-ethynyl-2′-deoxyuridine) until day 7 after selective EC injury and analyzed by flow cytometry and histology. Thereby, 5±1.5% of assessed ECs were found to be EdU+ 7 days after initial injury (sh: 1.5±0.8%). Histologic analysis supported these findings. Glomeruli of injured kidneys contained more ERG/EdU+ cells (mean sh: 0.23±0.16; d7: 1.22±0.6; P<0.01). Furthermore 8.2±3% of the ERG+ cells in the peritubular endothelium were EdU+ (sham 1.2±0.8).
Following I/R injury, a remarkable reduction of CD31+ peritubular ECs was observed by histology (4 h: 66.9±2.9%; d1: 63.7±6.4%; d3: 60.1±5.2% versus 78±4.1% in sham ctrl, Figure 2G) and verified by a reduction of CD34+ cells 4 h after reperfusion by FACS analysis (Figure 2H).
ECFCs Represent the Major EPC Subtype After Endothelial Injury
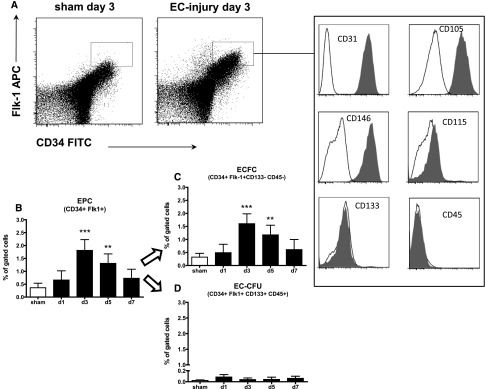
We have previously shown increased numbers of CD34+/Flk-1+ EPCs following site-selective renal EC injury in mice.22 To further dissect these cells, we now established an expanded set of surface markers to detect either CD34+ Flk1+ CD133+ CD115+ CD146+ CD31+ CD45– CD133– CD14– CD105– ECFC or CD34+ Flk1+ CD133+ CD45+ EC-CFU (Figure 3A).
Figure 3.
EPCs detected in the kidney mainly express surface markers of ECFC. CD34+/Flk-1 positive cells (measured using flow cytometry) were increased 3 days following endothelial selective injury (A, B). The major part of CD34+/Flk1+ cells express endothelial and lack hematopoietic surface markers (A). ECFCs (C) represented the major and EC-CFUs (D) the minor type of EPC in the murine kidney following selective EC injury.
Using this set of surface markers and the gating provided in Supplemental Figure 1, EC-CFUs were rarely found in peripheral blood samples (0.002±0.001%, Supplemental Table 1) or splenic tissues (0.005±0.002%, Supplemental Table 1) of sham-operated controls and no differences could be detected between diseased mice and controls (Supplemental Table 1). Cells expressing surfaces markers of ECFCs were absent in blood and spleen.
In kidneys that underwent selective EC injury, increased numbers of CD34+/Flk-1+ EPCs were detected on day 3 (1.84±0.4%; P<0.001 of gated cells; Supplemental Figure 1) and day 5 (1.31±0.4%; P<0.01 of gated cells) compared with baseline (Figure 3B). The majority of CD34+/Flk-1+ cells could be further defined as CD34/Flk1/CD31/CD146/CD115 positive and CD45/CD133/CD14/CD105 negative cells, thereby showing all characteristics of ECFCs (90.9–98.2% mean: 95.2±2.3% of EPCs; sham n=Ø 520; disease d3 n=Ø 2290 cells), while a very small number of cells could be defined as CD34/Flk1/CD133/CD45 positive EC-CFUs (<1.8%; 1.4±1.5%; n=1–47 cells; Figure 3, A, C, and D).
Because renal I/R represents the most frequently used AKI model for the investigation of EPCs in existing literature, we also induced I/R injury in a group of mice and compared them to sham-operated controls 4 h, 1 and 3 days after I/R. Thereby, we were able to show analogous effects on EPC numbers and characteristics in blood, spleen, and kidney (Supplemental Table 2), closely resembling the findings subsequent to selective renal endothelial injury.
EPCs Detected in the Kidney During Endothelial Repair are not of Bone Marrow Origin
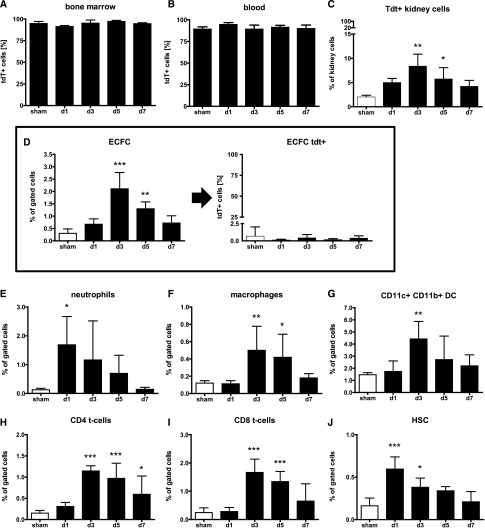
To clarify the origin of these putative EPCs in the kidney, we next generated BM chimeras by using tdTomato (tdT+) transgenic mice, in which expression of tdT is driven by the β-actin promoter, as donors and C57BL/6 mice as recipients (experiment 2, Figure 1). This allowed us to detect all BM-derived cells inside the kidney via their positivity for tdT+. Chimerism was verified 5 weeks after BMTx and selective endothelial injury or I/R was induced 1 week later. Overall, BM-transplanted C57BL/6 mice demonstrated a high degree of reconstitution with tdT+ cells in BM (94.8±2.8%; Figure 4A), peripheral blood (90.6±3.8%; Figure 4B), and spleens (75.4±5.3%, Figure 4C). Following EC injury, the proportions of tdT+ cells recruited to the kidney (Figure 4C) were increased on days 3 (8.3±2.5%; P<0.01) and 5 (5.7±2.7%; P<0.05) compared with controls (1.98±0.4%).
Figure 4.
Renal cells expressing EPC surface makers are not bone marrow derived. Most bone marrow (A) and blood cells (B) of chimeric mice (D: tdT+; R: C57BL/6) mice were positive for tdT (donor derived). Significantly more tdT+ cells were recruited to the kidney (C). Cells expressing ECFC surface markers (D, left) were negative for tdT (D, right). Selective EC injury was accompanied by a significant inflammatory response as depicted by influx of neutrophils (E), macrophages (F), dendritic cells (G), CD4+ T cells (H), CD8+ T cells (I), and HSC (J).
Rarely, tdT+ cells expressing surface markers of the EC-CFU subtype were found in blood (0.002±0.002%) or spleen samples (0.004±0.006%) and no cells carrying ECFC markers were detected in these organs (Supplemental Table 3). Very few hematopoietic stem cells (HSCs) could be found in blood samples, but no differences occurred over time (data not shown).
Following selective EC injury, cells expressing ECFC markers were significantly increased on days 3 (2.1±0.6%; P<0.01) and 5 (1.3±0.28%; P<0.05) compared with controls (0.30±0.18%) following selective endothelial injury (Supplemental Table 3) as well as on day 3 after I/R (Supplemental Table 4). The numbers of EC-CFUs were unchanged (Supplemental Tables 3 and 4). In both injury models, only a very small number of renal ECFCs were tdT+ (EC: 0.143±0.21%; I/R 0.178±0.07%) while more than 98% of all ECFCs were tdT– and thereby not of bone marrow origin (Figure 4D, Supplemental Tables 3 and 4). The numbers of renal (sca-1+;c-kit+;lin-) HSCs were increased on days 1 and 3 (Supplemental Table 3) after selective EC injury (Figure 4J) as well as day 3 after I/R (Supplemental Table 4), but only a small proportion was tdT+ (EC: 14.74±10.97%; I/R: 24.15±8.193%; Supplemental Tables 3 and 4).
The inflammatory response to selective EC injury was evaluated by measuring neutrophils, macrophages, T cells, and dendritic cells extracted from the kidneys and quantified by FACS analysis. Compared with controls, a rapid accumulation of neutrophils could be detected starting 24 h after selective EC injury (Figure 4E, Supplemental Table 3). On day 3, macrophages (Figure 4F), dendritic cells (DCs; Figure 4G), and T cells (Figure 4, H and I) showed a maximum increase. Enhanced numbers of macrophages as well as CD4+ and CD8+ T cells could also be detected on day 5, while the inflammatory response resolved on day 7 (data in Supplemental Table 3).
Following I/R, a similar influx of inflammatory cells was observed in injured kidneys (Supplemental Figure 2) including neutrophils, macrophages, DCs, CD4+ and CD8+ T cells (Supplemental Table 4).
In contrast, B cells were unchanged in both injury models. Cells expressing hematopoietic stem cell markers in the kidney were increased 3 days after disease induction (Supplemental Table 4). In contrast to populations of putative progenitor cells, these BM-derived cells were widely tdT+ in both disease models, including neutrophils (mean values of all experimental time points: EC: 99.65±0.44%; IR: 100±0%), macrophages (EC: 94.4±6.25%; IR: 91.45±12.14%), DCs (EC: 96.55±1.5%; I/R: 97.57±2.5%), and B cells (tdT+: EC: 99.21±0.7%; I/R 98.05±1.2%) (Supplemental Table 3). In inverse BM chimera (D: C57BL/6 R: tdT+), corresponding results were found (Supplemental Table 5). Congruously, almost all cells expressing ECFC surface markers were tdT+ in these mice (tdT+: 99.63±0.5%).
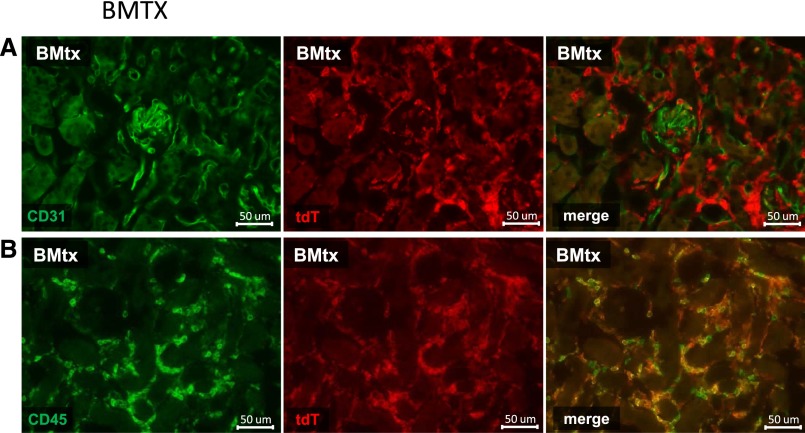
Kidney tissue sections from all mice were also systematically evaluated for double positive (tdT+/CD31+ or tdT+/CD34+) endothelial or hematopoietic cells (tdT+/CD45+) using the same antibody clones that were used for FACS analysis. Thereby, no tdT+/CD31+ or tdT+/CD34+ cells (Figure 5A) were found in glomerular or peritubular capillaries, while numerous tdT+/CD45+ hematopoietic cells could be detected in all kidneys (Figure 5B).
Figure 5.
Histology following EC injury in tdT BM chimeric C57BL/6 mice. Co-staining of tdT+ cells (red) with CD31 (green; A) and CD45 (green; B) using the Alexa flour 488 dye on formalin-fixed frozen sections.
Extrarenal Cells do not Serve as Progenitor Cells During Endothelial Repair in the Mouse Kidney
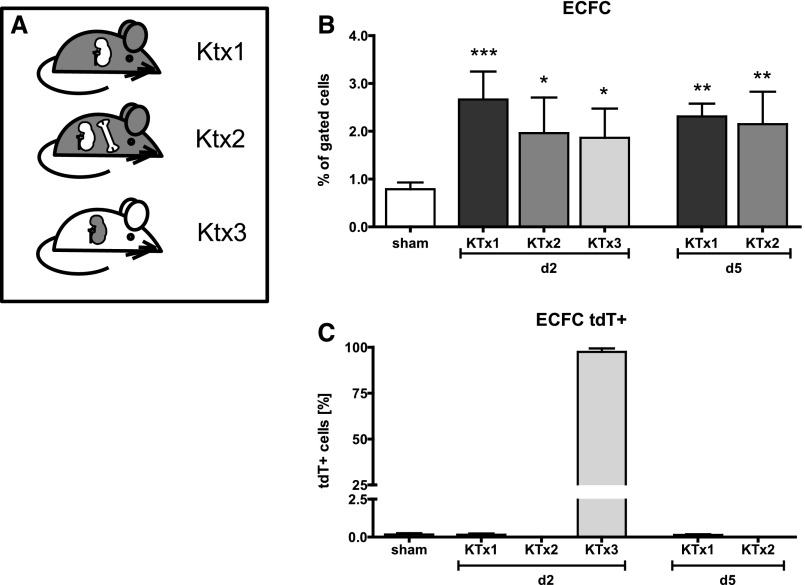
After excluding the bone marrow origin of renal EPCs, we next aimed to clarify the potential contribution of any extrarenal cell type as a source of ECFCs and direct substitute of local EC during microvascular repair (experiment 3, Figure 1). Based on the finding that selective EC injury and I/R caused the same increase of putative progenitor cells inside injured kidneys, we decided to perform isogenic KTx between C57BL/6 mice as donors and tdT+ mice as recipients (KTx1; Figure 6A), thereby inducing a well-defined degree of I/R injury. In pilot KTx experiments, ischemia times of donor kidneys were adjusted to closely resemble the degree of tissue injury from our I/R experiments by histology. By combining KTx and BMTx we aimed to identify any potential extrarenal stem cell niche aside from bone marrow (KTx2; Figure 6A).
Figure 6.
Extrarenal progenitor cells do not contribute to EC repair in the mouse kidney. Following renal transplantation in various constellations (A), putative EPCs were increased in all groups and time points (B). ECFCs were negative for tdT (C) (not of extrarenal origin). Inverse transplantation shown in KTx group 3.
As detected in our prior experiments, EC-CFUs were rarely found in blood (0.002±0.003%) and spleens (0.006±0.006%) following KTx, and ECFCs were completely absent at each time point (Supplemental Table 6).
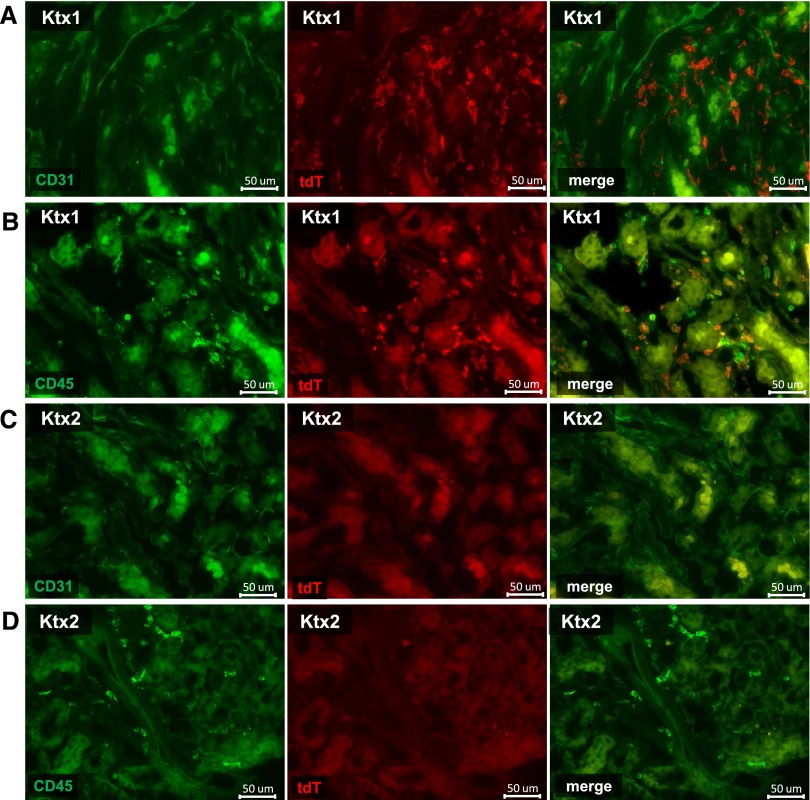
In transplanted kidneys, the extent of ECFC (Figure 6B) and HSC (Supplemental Figure 3, Supplemental Table 6) was equivalent to the first set of our experiments. Most inflammatory cells were tdT+ (neutrophils: 99.84±0.18%; macrophages: 76.81±16.13%; DCs: 72.84±12.4%; CD4+ T cells: 70.8±7.7%; CD8+ T cells: 75.5±9.0%). Extrarenal (tdT+) putative progenitor cells carrying markers of ECFCs were virtually absent in transplanted kidneys (0.15±0.05%; Figure 6C). In contrast, a massive recruitment of tdT+ inflammatory cells could be detected in kidney transplants, including neutrophils, macrophages, DCs and T cells (Supplemental Figure 3, Supplemental Table 6). Remarkably, only approximately 11.6% of cells expressing HSC surface markers were tdT+. Again, we were not able to detect tdT+ cells with a positive double staining for CD31 (Figure 7A) or CD34 by histology, while virtually all tdT+ cells were CD45+ (Figure 7B). Crossover KTx (Ktx3; Figure 6A) with tdT+ donors and tdT– recipients demonstrated identical results (Supplemental Table 6).
Figure 7.
Histology following experimental kidney transplantation in mice. Co-staining of tdT+ cells (red) with CD31 (green; A, day 2) and CD45 (green; B, day 2) using the Alexa flour 488 dye on formalin-fixed frozen sections. Figure 7C depicts the absence of tdT+/CD31+ EC following combined BMTx with KTx and Figure 7D depicts the BM origin if invading CD45+ extrarenal by their tdT negativity. Virtually no other extrarenal cells can be detected.
Following the generation of tdT+ mice with chimeric tdT– BM, we then transplanted kidneys from C57BL/6 donors into these BM chimeras (Ktx2). This allowed us to evaluate the potential participation of any putative progenitor cell from outside the bone marrow niche. Again, cells with ECFC characteristics were reproducibly detectable in transplanted kidneys (d2: 1.96±0.7%; P<0.05; d5 2.15±0.7%; P<0.05; sham 0.8±0.1%), but all of them were tdT-negative. Commensurate with FACS findings no CD31, respectively CD34 tdT+ positive ECs were found by histologic analysis (Figure 7, C and D).
Finally, we investigated whether a relevant number of so far unclassified extrarenal cells might have been recruited to the kidneys and therefore potentially contribute to EC repair via replacement. Thereby, the vast majority of evaluated cells could be clearly assigned to inflammatory cells (mean 91%; Figure 4B) of the following subtypes: neutrophils: 1.32±0.54%; macrophages: 0.29±0.14%; DCs: 1.69±2.98%; CD4+: 51.80±9.19%; CD8+: 29.26±9.60% and B cells 5.85±6.11%. Further evaluation demonstrated that approximately 87.42–98.00% of tdT+ (extrarenal) cells could be assigned to inflammatory cell types. Virtually all tdT+ cells (98.11±1.32%) preferentially expressed lineage markers, while only 1.90±1.02% (mean cell number: 43.6 cells out of 150,000 FACS events) were tdT+ and negative for lineage markers.
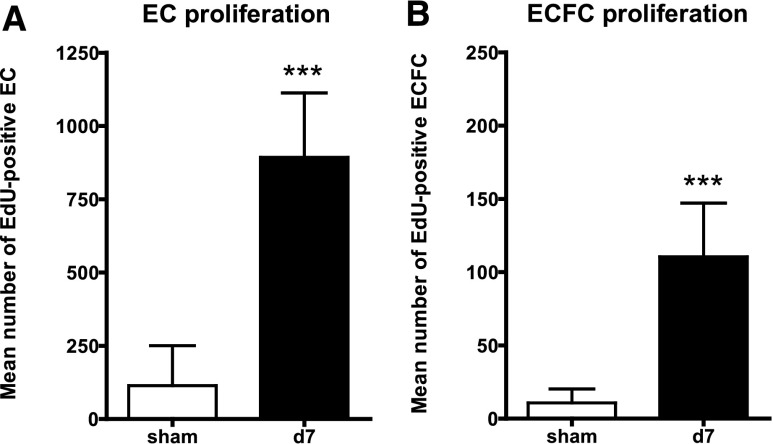
Following these experiments we wanted to evaluate whether the putative progenitor cells detected by markers of ECFCs and residing in the kidney profusely contributed to the endothelial repair process. Therefore, we counted the amount of proliferating, EdU+ ECFCs following a continuous, twice-daily labeling of cells between 1 and 7 days after selective EC injury. Compared with mature ECs, among which the number of EdU+ cells increased from n=114±136 cells (in sham controls) to 893±219 (Figure 8A) after 7 days, ECFCs showed the same extent of proliferation (3.7±2.2%; n=110±37 cells) after 7 days, but in the presence of a much lower baseline cell number (Figure 8B), thereby rebutting the expected exceptional proliferative capacity of local progenitor cells.
Figure 8.
Limited EC and ECFC proliferation following endothelial injury. Proliferating ECs (A) and ECFCs (B) measured with EdU proliferation assay (flow cytometry) 7 days following endothelial specific injury. Significantly more ECs as well as ECFCs could be detected in injured kidneys.
Discussion
Stimulated by the initial description of a role of EPCs as contributors to endothelial repair, numerous studies appeared, describing and investigating their potential role or therapeutic use for endothelial repair in acute and chronic kidney disease.23–25 While it has never been shown that EPCs integrate into the renal microvasculature, thereby facilitating rapid endothelial repair, the conclusion that extrarenal EPCs directly contribute to the repair of endothelial lesions is frequently drawn and used as a justification to measure such cells in the circulation.
The present study aimed to explore in depth the origin and potential role of EPCs at the basis of previously defined distinct EPC subtypes under pathophysiological conditions in the kidney. Using two different forms of endothelial injury, (1) inducible selective endothelial injury, and (2) more frequently used, but less endothelial selective I/R injury, we performed extensive analysis by flow cytometry matched with histology by using identical antibody clones. Based on our previously published work,21,22,26 we carefully evaluated the extent of endothelial injury in both types of acute EC injury and the subsequent repair response. Following the rapid loss of ECs in our selective injury model, ECs reappeared on day 3, leading to ample restoration of glomerular and peritubular capillaries 1 week after onset of disease. In line with the hypothesis that mechanisms apart from local EC proliferation might contribute to EC repair in our disease model, we found only a limited amount of local EC proliferation. The acute reduction of ECs following I/R injury was less pronounced, but clearly detectable. Acute endothelial injury was always accompanied by a remarkable inflammatory response. In concurrence with previous work by ourselves22 and others,27 we detected a relevant increase of cells carrying EPC markers in the kidney following both selective EC and I/R injury. We were able to show that these putative progenitors inside the kidney carried a large variety of markers that had been used to define and detect EPCs in various organ systems and the circulation.14 We showed that ECFCs represent the major (approximately 95%) and EC-CFUs the minor EPC subtype in the kidney (<1.8%). A minor number of EC-CFUs were present in the peripheral blood and spleen, a finding in line with the report by Sonomi et al.28
In the absence of unique antigens to identify EPCs and to define their extrarenal origin,14,29,30 we generated tdT+ bone marrow chimeric C57BL/6 mice. This allowed us to detect almost all BM-derived cells by their tdT positivity. Unexpectedly, in the presence of a clear increase in ECFCs in all injured kidneys, all ECFCs were tdT negative and thereby not of BM origin. In our study, these tdT negative ECFCs could also be identified after I/R injury, verifying our findings in a second and very frequently used experimental approach.11,27,31 While the renal microvascular endothelium underwent a remarkable repair response (Figure 1), no EPCs of BM origin were recruited and histologic work-up of kidney sections revealed no tdT+ ECs inside the kidney. Identical findings were reproduced in control experiments using inverse chimeras or isogenic controls (Supplemental Table 5). EC-CFUs, the minor subtype, were of BM origin and could be detected in the circulation, spleen and kidneys in both types of EC injury. However, these cells did not increase during the repair process and did not give rise to local ECs. This rare presence of circulating BM-derived EC-CFUs is also consistent with existing literature.13,32–34
To evaluate BM chimerism and provide a control for further transplantation-induced side effects, we decided to perform the assessment of the inflammatory response in parallel. Many BM-derived inflammatory cells (macrophages, neutrophils, DCs, B cells and T cells) were recruited to the kidney and this could be found to the same extent in all chimeric mice, demonstrating that the BM and BM originating cells were functional. Indirectly the similar inflammatory response in our experiments also indicates the uniform extent of EC injury. To further define the niche of recruited EPCs we performed isogenic kidney transplantation of tdT negative kidneys (C57BL/6) into tdT+ recipients and subsequently combined this approach with prior generation of C57BL/6 BM chimeric tdT+ mice to further increase sensitivity for FACS analysis and histology. This approach was also a prerequisite to analyze the origin of the cells and to dissect the relevance of BM versus extrarenal versus intrarenal cell types for EC repair.
In our initial experiments we showed equal potency of selective EC and I/R injury in regard to the EPC recruitment. Considering the technical and analytical issues of experiments combining KTx and selective EC injury after selective renal arterial perfusion, we decided to use a well-defined I/R injury model in combination with kidney transplantation to investigate the contribution of extrarenal progenitor cells. Hereby, we detected the same numbers of EPCs, ECFCs, and EC-CFU subtypes in the transplanted kidneys, while these cells were not of extrarenal (and also not of bone marrow) origin (tdT negative). KTx into BM chimeric mice generated mice carrying tdT solely in not BM-derived, extrarenal cells. This approach was used to gain further insight into the relevance of other extrarenal cell types that might potentially contribute to renal repair via direct substitution of injured renal ECs. Using this combined (KTx into BM chimeras) approach, the total number of tdT+ cells coming from outside the transplanted kidneys was very low. Using our broad set of markers we were able to identify approximately 95% of all recruited cells either directly as inflammatory cells or as cells of the hematopoietic lineage (lin+ cells). Considering the number of cell events we had measured by FACS, we assume that we only left 0.002% of all cells in our experiments unidentified, practically ruling out any relevant contribution of extrarenal cells as a substitute for lost microvascular ECs in the kidney. This raises not only doubt about a functional role of such “unidentified” cells as relevant bystanders, but also questions both the terminology of EPCs and their current definitions.
This is the first in-depth study with thorough assessment of the role of EPCs following selective injury of the renal microvascular endothelium. Although other groups have noticed the lack of recruitment of BM-derived cells to the renal endothelium,35–37 no other study evaluated the relevance of existing definitions of EPCs for kidney disease and no comparable disease models of selective EC injury, reliably providing a high extent of EC injury, have been used. Frequently, the methods used to generate such data were criticized and existing definitions of EPCs were not addressed. Studies focused either on eGFP expression of single cell markers during I/R35,36 or chronic endothelial injury37 with subsequent histologic analysis. Others failed to integrate sufficient markers to dissect the BM origin of ECs.15 In the absence of a detectable number of EPCs, the misleading conclusion of an “EPC insufficiency” has been frequently drawn in recent years.13,27,30 Using a rat model of selective EC injury, our own group has recently shown that the number of ECs that might arise from extrarenal cells must be extremely low, being consistent with the results obtained in this mouse.26 While these rat experiments solely relied on co-localization by histology and evaluated EC replacement in the long run, the present experimental design focused on the immediately necessary endothelial repair response. Likely, substitution of single ECs by circulating mature ECs is a comprehensible event in the transplant situation and especially in the long term. Consistent with this, data from FISH analysis38 in humans suggest that in patients that underwent prior BM or kidney transplantation, ECs can be replaced by the recipient’s cells.39,40 This special allogenic situation is also associated with increased numbers of circulating mature ECs,41–43 which might become integrated into the renal microvasculature.
So far, very few experiments have aimed to define the role of putative progenitor cells under pathophysiologic conditions. Mainly the group of Ingram and Yoder contributed to the definition of EPC subtypes such as ECFCs and EC-CFUs.44 They have shown that ECFCs might be integrated into the vasculature, but never demonstrated that this occurred to a relevant extent upon kidney injury.24 A variety of studies also suggest that this might occur in vascular beds outside the kidney,45–48 but always using cultured cells.
For acute endothelial lesions, the in vivo relevance of such definitions had never been tested in concise experiments before. We now demonstrate that these definitions do not apply under pathophysiologic conditions. While most studies also failed to depict the necessity of immediate EC repair, we defined the extent of injury and demonstrated the repair in both models, which is a clear strength of our data.
Numerous studies investigated the therapeutic transfer of cultured BM-derived “progenitor” cells protecting the murine kidney from I/R injury.12,49 In contrast, the integration of isolated but uncultured ECFCs into vessels has never been shown.24 Our present data clearly demonstrate that normal pathophysiology neither relies on the EC substitution from an extrarenal EPC niche nor can these conclusions be drawn for the efficacy of therapeutic application of cells. Considering the detection of such ECFC marker carrying, putative progenitors were also assessed for their proliferative potential. Given the proliferative response of these cells in our experiments in comparison to mature ECs, they do not seem to have the capability to replenish lost ECs in vivo to a relevant extent.
Clearly, acute renal endothelial repair does not depend on the substitution by extrarenal cells. Cell therapy will therefore not provide a so-called EPC giving rise to later mature ECs inside the kidney. Nevertheless such cells can directly or indirectly (via angiogenic cytokines) interact with the endothelial layer and enhance endothelial repair. Therefore, we suggest calling these cells pro-angiogenic cells (PACs) instead of using the misleading terminology of EPCs. In contrast to EPCs, PACs might also include hematopoietic inflammatory cells interacting with local ECs and efforts should also focus on the understanding of such cell–cell interactions. Of note, we also addressed the recruitment of HSCs, demonstrating that the majority of cells carrying HSC cell markers reside inside the kidney (Supplemental Tables 3 and 4). While this study further supports the relevance of local repair mechanisms, it does not address the relevance of circulating hematopoietic cells for microvascular repair.
In summary, we showed that extrarenal cells, including so-called EPCs and their subtypes, do not contribute to the endothelial repair of primary endothelial lesions in the kidney and that there is no relevant extrarenal cellular source providing new ECs in two models of acute EC injury, calling the definition of such cells as EPCs into question.
In regard to the further understanding of renal repair mechanisms, future research should focus on the regenerative potential of a possibly existing renal endothelial precursor cell niche and additionally on the paracrine signaling of circulation cells triggering those processes.
Concise Methods
Animal Experiments
101 C57BL/6 (wt) (Janvier Laboratories; Strasburg; France) and 51 tandem dimer Tomato (tdTomato; tdT)\β-actin mice (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) (The Jackson Laboratories and Charles River Laboratories, Sulzfeld, Germany) were obtained for this study. All animal studies were conducted according to German laws on welfare of animals and approved by the local authorities (Landesdirektion Sachsen AZ: 24-9168-11-1/2011-31, AZ: 24-9168-11-1/2011-32 and AZ: 24-9168-11-1/2013-41).
Site-Selective Endothelial Injury
The anti-concanavalin A (anti-con A) serum was generated in New Zealand white rabbits by Seqlab (Sequence Laboratories GmbH, Goettingen, Germany) and tested for antibody concentration to selectively induce the injury model in the wt mice as previously described.21 Briefly, direct perfusion of the left kidney with concanavalin A was followed by anti-con A serum perfusion. A further five mice served as NaCl perfused controls. Mice were sacrificed 24 h, 3, 5, and 7 days after renal arterial perfusion. On the day of sacrifice, mice were anesthetized using inhaled isoflurane (2%), blood was collected via puncture of the inferior caval vein, and mice were then perfused via the heart with 0.9% (w/v) NaCl solution to remove blood components from both kidneys. The left kidneys and the spleen were harvested and processed either for histology or FACS as described below.
Ischemia-Reperfusion Injury
Unilateral ischemia reperfusion was induced in the left kidneys of 35 mice. Under general anesthesia induced by 2% isoflurane, a flank incision was made. The renal artery of the left kidney was localized and separated from the vein. A thread was carefully pulled around the artery. Two thread endings fixed with weights of 1 g were carefully put on a stand. Thereby, the artery was slightly lifted and the blood flow was interrupted for 25 min at 37°C. After this procedure thread and weights were removed from the stand, blood flow was restored allowing renal reperfusion. Five control mice underwent sham operation without interrupting the arterial blood flow. Mice were sacrificed 4 h, 24 h, and 3 days after I/R injury, and blood, spleen, and kidneys were harvested as described above.
5-Ethynyl-2′-Deoxyuridine (EdU) Cell Proliferation Assay
EdU (15 µg/g) was injected starting 24 h after injury induction every 12 h intraperitoneally. Kidneys were harvested on day 7 and EdU+ cells were analyzed using flow cytometry and histology. ECs and EPC subtypes were stained as described before. EdU+ ECs were detected as anti-ERG (erythroblast transformation specific-related gene; Abcam, Inc., Cambridge, UK)/EdU double positive cells.
Bone Marrow Transplantation
BM transplantation was performed using 8–10-week-old tdT mice as donors and congenic C57BL/6 mice of the same age as recipients. Inverse transplantations as well as transplantations of C57BL/6 into C57BL/6 were performed as controls. For BM transplantation, BM cells were isolated from tibiae and femurs by flushing with DMEM. Two hours after recipient’s irradiation (7,6 Gray for 7 min), BM cells containing 2×106 BM mononuclear cells in 0.2 ml Hanks’ Balanced Salt solution were injected into the lateral tail vein of recipients. After BM transplantation, mice were housed under sterile conditions and received antibiotic treatment with ofloxacin 20 µg/ml in the drinking water for 4 weeks. Chimerism was confirmed by flow cytometric analysis of 50 µl of blood 5 weeks after BM transplantation. In addition, chimerism was verified on the day of sacrifice by FACS analysis of BM cells in all recipients.
Experimental Kidney Transplantation in Mice
Vascularized kidney transplantation from 12 C57BL/6 (donor) mice and 12 tdTomato mice (recipients) was performed as described previously.50,51 Crossover transplantation was performed as a control procedure (four tdT donors and four C57BL/6 recipients). In brief, the animals were anesthetized with isoflurane, and the left donor kidney attached to a cuff of the aorta, the renal vein and the ureter were removed en bloc. After left nephrectomy of the recipient, the vascular anastomosis to the recipient abdominal aorta and vena cava was done, below the level of the native renal vessels. The ureter was directly anastomosed to the bladder. Ischemia times were standardized (30 min cold and 25 min warm ischemia time) to induce ischemic allograft damage.
Flow Cytometry
Flow cytometry was performed using a FACSCanto II from BD with subsequent analysis using the FlowJo data analysis software (FlowJo, Ashland, OR). To quantify peripheral circulating progenitor and stem cells by FACS, mononuclear cells were isolated from peripheral blood or from splenic tissue homogenates as described before.22 Renal tissues were processed in culture dishes (Greiner Bio-One, Frickenhausen, Germany) and cut into small pieces (1–3 mm) immediately after washing with PBS containing 5% FCS. To digest renal tissue, collagenase type IA (1 mg/ml; Darmstadt, Life Technologies) was used in combination with DNAse (Sigma-Aldrich; Taufkirchen, Germany) to prevent the clumping of cells due to DNA release from dead cells at 37°C in 5% CO2 for 45 min. After dissociation, the cell suspensions were filtered through a 40 µm nylon mesh (BD Bioscience, Heidelberg, Germany). Before antibody incubation, nonspecific staining was prevented using anti-mouse CD16/CD32 (FC-block) and apoptotic cells were excluded using 7-AAD staining. Single cell suspensions from kidneys, blood samples, and spleens were incubated with the following specific antibodies for 30 min at room temperature.
Putative progenitor cells were identified through their co-expression of CD34, Flk-1, and seven other surface markers stained with three different ab-cocktails: First, cells were stained with CD34 FITC, Flk-1 APC and CD133 eFluor 710 (Prominin-1) and CD45 V500. The second ab-cocktail consisted of CD34 Fitc Flk-1 APC and the endothelial surface markers CD31 Pecy7; CD105 eFluor 450; CD146 PerCP Cy5.5. In a third approach the cells were stained using CD34 FITC, Flk-1 APC and the hematopoietic surface markers CD115 PerCP 710; CD14 PeCy7.
Hematopoietic stem cells were identified as c-kit+ Sca-1+ and lin– cells. Macrophages, DCs, B cells, and T cells were identified after staining with antibodies against CD11c PE-Cy7, CD11b BV 421, GR1 PerCP Cy5.5, F4/80 APC, CD4 PE-Cy7, CD8 APC, CD19 APC-Cy7, IgM PE-Cy7 and CD45R PE Cy5.5 (B220). IgG2a antibodies served as isotype controls for each procedure. All antibodies were obtained from BD Biosciences or ebioscience (Frankfurt, Germany).
Immunohistochemical and Immunofluorescence Staining
Tissues for immunofluorescent staining were fixed in a 4% formaldehyde solution for 24 h, followed by incubation in 30% sucrose overnight (4°C). Embedding was performed in an optimal cutting temperature compound (Tissue-Tek, Torrance, CA), and samples were stored at –80°C. Frozen samples were cut into 5 µm thick sections. Nonspecific protein binding was blocked by 30 min incubation with 10% PBS-NDS (normal donkey serum). Sections were incubated with the following rat monoclonal antibodies: anti-mouse CD31, anti-mouse CD34, or anti-mouse CD45 (all from BD Biosciences) for 12 h at 4°C followed by a fluorescent secondary antibody (1:500 Alexa Fluor® 488 donkey anti-rat IgG Antibody; Life Technologies, Darmstadt, Germany). Identical antibody clones were used for FACS analysis. Tissues for light microscopy were fixed in zinc fixative solution (BD Pharmingen, Heidelberg, Germany), embedded in paraffin and cut into 2 µm sections for indirect immunoperoxidase staining as previously described.52 Staining for CD31 was performed using a rat anti-mouse CD31/PECAM-1 antibody (BD Biosciences, Heidelberg, Germany). Negative controls for immunostaining included either deletion of the primary antibody or substitution of the primary antibody with equivalent concentrations of an irrelevant murine or rat monoclonal antibodies. The CD31 and CD34 expressions were assessed using computer assisted image analysis as described before21 using ImageJ (National Institutes of Health). The expression was assessed in glomeruli and in peritubular capillaries separately.
Statistical Analysis
All results are presented as mean±SD. Statistical analysis was performed using either one-way ANOVA (with Bonferroni post hoc testing) or the unpaired t test (using GraphPad Prim 4.0 GraphPad Software Inc., San Diego, CA). Statistical significance was defined as P<0.05 (*), P<0.01 (**), and P<0.001 (***).
Disclosures
None.
Supplementary Material
Acknowledgments
The technical assistance of Doreen Weigel and Andrea Wagner is gratefully acknowledged.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to B.H. (HO-2522/6-1), V.T.T. (TO-679/2-1) and C.H. (HU-600/6-1).
Parts of this work were presented during the Kidney Weeks in 2012, 2013 and 2014.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030321/-/DCSupplemental.
References
- 1.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Iruela-Arispe L, Gordon K, Hugo C, Duijvestijn AM, Claffey KP, Reilly M, Couser WG, Alpers CE, Johnson RJ: Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol 147: 1715–1727, 1995 [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman K, Seldon M, Richards R: Thrombotic microangiopathies, thrombotic thrombocytopenic purpura, and ADAMTS-13. Semin Thromb Hemost 38: 47–54, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, López JA: ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 100: 4033–4039, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Dome B, Timar J, Ladanyi A, Paku S, Renyi-Vamos F, Klepetko W, Lang G, Dome P, Bogos K, Tovari J: Circulating endothelial cells, bone marrow-derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: From biology to therapy. Crit Rev Oncol Hematol 69: 108–124, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Dussault S, Maingrette F, Ménard C, Michaud SE, Haddad P, Groleau J, Turgeon J, Perez G, Rivard A: Sildenafil increases endothelial progenitor cell function and improves ischemia-induced neovascularization in hypercholesterolemic apolipoprotein E-deficient mice. Hypertension 54: 1043–1049, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Goligorsky MS: Endothelial progenitor cells: from senescence to rejuvenation. Semin Nephrol 34: 365–373, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G: Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Di Marco GS, Rustemeyer P, Brand M, Koch R, Kentrup D, Grabner A, Greve B, Wittkowski W, Pavenstädt H, Hausberg M, Reuter S, Lang D: Circulating endothelial progenitor cells in kidney transplant patients. PLoS One 6: e24046, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon O, Miller S, Li N, Khan A, Kadry Z, Uemura T: Bone marrow-derived endothelial progenitor cells and endothelial cells may contribute to endothelial repair in the kidney immediately after ischemia-reperfusion. J Histochem Cytochem 58: 687–694, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS: Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol 291: F176–F185, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T: Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hirschi KK, Ingram DA, Yoder MC: Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: 1584–1595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoder MC, Ingram DA: The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim Biophys Acta 1796: 50–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahrens I, Domeij H, Topcic D, Haviv I, Merivirta RM, Agrotis A, Leitner E, Jowett JB, Bode C, Lappas M, Peter K: Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS One 6: e23210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC: Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104: 2752–2760, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S: Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95: 952–958, 2000 [PubMed] [Google Scholar]
- 19.Yang J, Ii M, Kamei N, Alev C, Kwon SM, Kawamoto A, Akimaru H, Masuda H, Sawa Y, Asahara T: CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PLoS One 6: e20219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C: Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 24: 1806–1813, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hohenstein B, Braun A, Amann KU, Johnson RJ, Hugo CP: A murine model of site-specific renal microvascular endothelial injury and thrombotic microangiopathy. Nephrol Dial Transplant 23: 1144–1156, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hohenstein B, Kuo MC, Addabbo F, Yasuda K, Ratliff B, Schwarzenberger C, Eckardt KU, Hugo CP, Goligorsky MS: Enhanced progenitor cell recruitment and endothelial repair after selective endothelial injury of the mouse kidney. Am J Physiol Renal Physiol 298: F1504–F1514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basile DP: The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Basile DP, Yoder MC: Circulating and tissue resident endothelial progenitor cells. J Cell Physiol 229: 10–16, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W: Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res 94: 230–238, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Schirutschke H, Vogelbacher R, Stief A, Parmentier S, Daniel C, Hugo C: Injured kidney endothelium is only marginally repopulated by cells of extrarenal origin. Am J Physiol Renal Physiol 305: F1042–F1052, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Patschan D, Patschan S, Müller GA: Endothelial progenitor cells in acute ischemic kidney injury: strategies for increasing the cells’ renoprotective competence. Int J Nephrol 2011: 828369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somani A, Nguyen J, Milbauer LC, Solovey A, Sajja S, Hebbel RP: The establishment of murine blood outgrowth endothelial cells and observations relevant to gene therapy. Transl Res 150: 30–39, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA: Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 35: 1109–1118, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Fadini GP, Losordo D, Dimmeler S: Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110: 624–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becherucci F, Mazzinghi B, Ronconi E, Peired A, Lazzeri E, Sagrinati C, Romagnani P, Lasagni L: The role of endothelial progenitor cells in acute kidney injury. Blood Purif 27: 261–270, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Tsukada S, Kwon SM, Matsuda T, Jung SY, Lee JH, Lee SH, Masuda H, Asahara T: Identification of mouse colony-forming endothelial progenitor cells for postnatal neovascularization: a novel insight highlighted by new mouse colony-forming assay. Stem Cell Res Ther 4: 20, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padfield GJ, Tura O, Haeck ML, Short A, Freyer E, Barclay GR, Newby DE, Mills NL: Circulating endothelial progenitor cells are not affected by acute systemic inflammation. Am J Physiol Heart Circ Physiol 298: H2054–H2061, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO: Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry TE, Song M, Despres DJ, Kim SM, San H, Yu ZX, Raghavachari N, Schnermann J, Cannon RO 3rd, Orlic D: Bone marrow-derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunction. Cardiovasc Res 84: 317–325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga Z, Gaspert A, Behnke S, von Teichman A, Fritzsche F, Fehr T: Tubular and endothelial chimerism in renal allografts using fluorescence and chromogenic in situ hybridization (FISH, CISH) technology. Pathol Int 62: 254–263, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Haller H, de Groot K, Bahlmann F, Elger M, Fliser D: Stem cells and progenitor cells in renal disease. Kidney Int 68: 1932–1936, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Rosenberger C, Khamaisi M, Goldfarb M, Shina A, Shilo V, Zilbertrest F, Rosen S, Heyman SN: Acute kidney injury in the diabetic rat: studies in the isolated perfused and intact kidney. Am J Nephrol 28: 831–839, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Woywodt A, Schroeder M, Gwinner W, Mengel M, Jaeger M, Schwarz A, Haller H, Haubitz M: Elevated numbers of circulating endothelial cells in renal transplant recipients. Transplantation 76: 1–4, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Popa ER, Kas-Deelen AM, Hepkema BG, Van Son WJ, The TH, Harmsen MC: Donor-derived circulating endothelial cells after kidney transplantation. Transplantation 74: 1320–1327, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Mohamed AS, Thomson J, McDonald KJ, Hillyard DZ, Mark PB, Elliott HL, Jardine AG: Circulating endothelial cells in renal transplant recipients. Transplant Proc 37: 2387–2390, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA: Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS: Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 112: 1618–1627, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P, Vandenwijngaert S, Dépelteau H, Streb W, Chaothawee L, Maes F, Gheysens O, Debyser Z, Gillijns H, Pellens M, Vandendriessche T, Chuah M, Collen D, Verbeken E, Belmans A, Van de Werf F, Bogaert J, Janssens S: Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol 55: 2232–2243, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Jung HS, Kim MJ, Hong SH, Lee YJ, Kang S, Lee H, Chung SS, Park JS, Park KS: The potential of endothelial colony-forming cells to improve early graft loss after intraportal islet transplantation. Cell Transplant 23: 273–283, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, Stitt AW: Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 3: 18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchimura H, Marumo T, Takase O, Kawachi H, Shimizu F, Hayashi M, Saruta T, Hishikawa K, Fujita T: Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol 16: 997–1004, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Rong S, Lewis AG, Kunter U, Haller H, Gueler F: A knotless technique for kidney transplantation in the mouse. J Transplant 2012: 127215, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rong S, Hueper K, Kirsch T, Greite R, Klemann C, Mengel M, Meier M, Menne J, Leitges M, Susnik N, Meier M, Haller H, Shushakova N, Gueler F: Renal PKC-ε deficiency attenuates acute kidney injury and ischemic allograft injury via TNF-α-dependent inhibition of apoptosis and inflammation. Am J Physiol Renal Physiol 307: F718–F726, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Hohenstein B, Renk S, Lang K, Daniel C, Freund M, Léon C, Amann KU, Gachet C, Hugo CP: P2Y1 gene deficiency protects from renal disease progression and capillary rarefaction during passive crescentic glomerulonephritis. J Am Soc Nephrol 18: 494–505, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.