Abstract
Urinary tract infections (UTIs) occur predominantly in females but also affect substantial male patient populations; indeed, morbidity in complicated UTI is higher in males. Because of technical obstacles, preclinical modeling of UTI in male mice has been limited. We devised a minimally invasive surgical bladder inoculation technique that yields reproducible upper and lower UTI in both male and female mice, enabling studies of sex differences in these infections. Acute uropathogenic Escherichia coli (UPEC) cystitis in C57BL/6 and C3H/HeN males recapitulated the intracellular bacterial community pathway previously shown in females. However, surgically infected females of these strains exhibited more robust bladder cytokine responses and more efficient UPEC control than males. Compared with females, C3H/HeN males displayed a striking predilection for chronic cystitis, manifesting as persistent bacteriuria, high-titer bladder bacterial burdens, and chronic inflammation. Furthermore, males developed more severe pyelonephritis and 100% penetrant renal abscess (a complication that is rare in female mice). These phenotypes were sharply abrogated after castration but restored with exogenous testosterone, suggesting that male susceptibility to UTI is strongly influenced by androgen exposure. These data substantiate the long-standing presumption that anatomic differences in urogenital anatomy confer protection from UTI in males; however, as clinically observed, male sex associated with more severe UTI once these traditional anatomic barriers were bypassed. This study introduces a highly tractable preclinical model for interrogating sex differences in UTI susceptibility and pathogenesis, and illuminates an interplay between host sex and UTI that is more complex than previously appreciated.
Keywords: pyelonephritis, sex differences, urinary tract infection, testosterone
Community-onset urinary tract infection (UTI) disproportionally affects females1; however, routine and complicated UTIs in male patients are neither nonexistent nor benign. Males at the extremes of age, namely infants and the very elderly, exhibit increased incidence of UTI. Further, complicated UTI commonly manifests in males with urodynamic problems (e.g., spinal cord injury or neurogenic bladder), indwelling urinary catheters, immunosuppression, and/or structural abnormalities. Importantly, while female cases of complicated and upper-tract UTI (pyelonephritis) outnumber male cases overall, males carry an increased risk of mortality from these infections.2–5 Millions of males also suffer from acute and chronic bacterial prostatitis. In total, UTIs comprise debilitating diseases with substantial morbidity and appreciable mortality specific to males.6,7 Of note, uropathogenic Escherichia coli (UPEC) is the predominant cause of UTI in both sexes, causing >80% of community onset UTI, approximately 25% of hospital-acquired UTI, and >70% of infectious prostatitis.1,8–10
Sex differences in UTI epidemiology have heretofore been attributed almost entirely to anatomic and hygienic factors, including the permissiveness of vaginal and perineal environments to microbial colonization, shorter urethral length, and shorter distance from anus to the urethral meatus in females.9,11,12 However, these hypotheses have not been stringently proven, and substantial deficiencies persist in our understanding of the interplay between host sex and uropathogens. Recent advances in our translational view of UTIs and in the molecular details of UPEC pathogenesis have been developed in an exclusively female murine model where pathogens are delivered into the bladder lumen by transurethral catheterization13,14; however, the bladders of male mice cannot be reliably catheterized. Upon delivery to the female bladder, UPEC are rapidly internalized into superficial facet cells lining the stratified transitional epithelium,15–26 subsequently replicating in the cytoplasm to form biofilm-like masses of bacteria, termed intracellular bacterial communities (IBCs).21 Murine evidence for this IBC cascade has been corroborated by detection of exfoliated IBCs in the urine of females with cystitis.27,28 Female mice either resolve acute cystitis or develop chronic bacterial cystitis, while very few develop lasting kidney infection or renal abscess.29,30 While chronic cystitis as described in murine hosts may not be common clinically with routine antibiotic treatment, increasing evidence suggests this to be a relevant model for severe and recurrent UTI.31,32
Previous studies have instilled uropathogens into the urethra of male mice,33,34 inducing infection of the prostate but failing to reproducibly infect the bladders and kidneys. The lack of a tractable male model of UTI has precluded detailed examination of host and microbial mechanisms underlying the sex discrepancies observed in UTI. In response to this deficit, we developed a minimally invasive technique that bypasses simple anatomic sex differences to permit infection of the urinary tract in both male and female mice. Acute cystitis utilized an IBC pathway in males which mirrored acute bladder infection in females. While most surgically infected female mice controlled UPEC effectively, males exhibited a sharply higher prevalence of chronic cystitis. More striking, male mice exhibited marked susceptibility to renal abscess and pyelonephritis, mirroring the increased morbidity reported in males who develop these complicated UTIs. These male UTI phenotypes were reversed by gonadectomy but complemented via exogenous testosterone administration, implicating androgens in susceptibility to severe UTI. Collectively, this study illuminates sex-based influences on susceptibility and host response to UTI, and establishes a novel platform for modeling the pathogenesis and sequelae of cystitis, pyelonephritis, and ascending renal abscesses.
Results
Direct Bladder Inoculation Reliably Infects the Urinary Tract of C57BL/6 Mice
We developed a highly reproducible, minimally invasive surgical approach that allows direct male-female comparisons in UTI pathogenesis. We first employed our minimally invasive inoculation model to induce UTI in C57BL/6 male and female mice with the cystitis-derived UPEC strain UTI89, monitoring bladder and kidney colonization at three time points representing acute and chronic stages of UTI29,30,35 (Figure 1). Organ titers in surgically infected female C57BL/6 were similar to published bacterial burdens in catheter-infected females,22,29,36–38 indicating that our minimally invasive method recapitulates the widely used female model.13 Mice displayed no signs of systemic infection or peritonitis, and blood cultures were sterile at 6 and 24 hours postinfection (hpi).
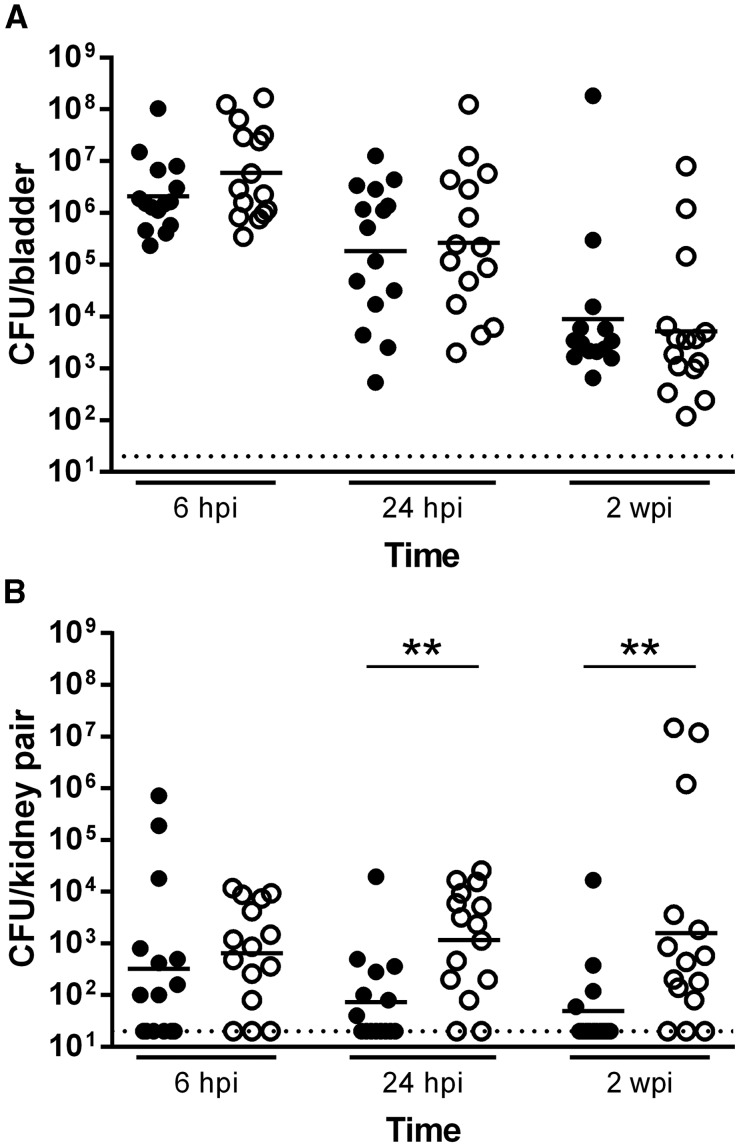
Figure 1.
Direct bladder inoculation reliably infects the urinary tract of C57BL/6 mice. Female (filled circle) and male (empty circle) C57BL/6 mice were surgically infected with 107 CFU UTI89. (A) Bladders were homogenized at the indicated time points, serially diluted, and CFU enumerated. Each point represents one mouse; bars indicate geometric mean. Data comprise three independent experiments of five mice per group. Dotted line represents the limit of detection. (B) Total bacteria present in both kidneys were enumerated. Aggregate data from three independent experiments with five mice per group are shown. Statistically significant differences between sexes are indicated (**P<0.01).
Bladder bacterial burdens in C57BL/6 males and females were equivalent at all time points examined (Figure 1A). However, C57BL/6 males developed significantly higher kidney titers at 24 hpi (P=0.004) and 2 weeks postinfection (wpi) (P=0.003) compared with females (Figure 1B). Male and female C57BL/6 mice expressed similar levels of bladder cytokines and chemokines at 6 hpi. By 24 hpi, females displayed modest but statistically significant increases in bladder IL-1α, IL-10, IL-12 (p40), granulocyte colony-stimulating factor (G-CSF), macrophage inhibitory protein 1 alpha (MIP-1α), and TNF-α content relative to males (Supplemental Figure 1).
Cystitis in Male C57BL/6 Mice Follows the IBC Pathway
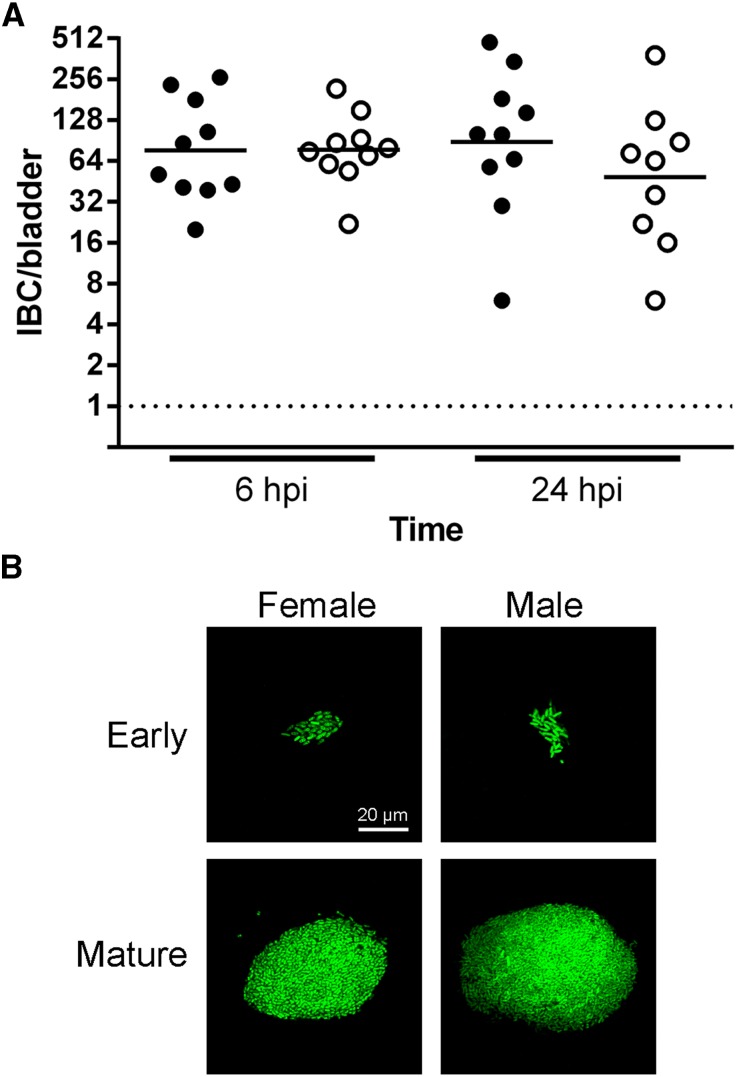
The importance of the IBC pathway in cystitis has not been interrogated in male hosts. By β-galactosidase (LacZ) staining,39 IBCs were observed in all infected bladders of both sexes, with no significant differences in IBC number between males and females at 6 or 24 hpi (Figure 2A). Confocal microscopy revealed no differences in bacterial morphology, size, or structure in early or mature IBCs between sexes (Figure 2B). Thus, acute cystitis in male hosts proceeds through an IBC pathway analogous to that previously observed in females.
Figure 2.
Cystitis in male C57BL/6 mice follows the IBC pathway. Female (filled circle) and male (empty circle) C57BL/6 mice were surgically infected with 107 CFU UTI89. (A) IBCs present in bisected bladders at 6 or 24 hpi were stained with X-gal and enumerated. Data from two independent experiments with five mice per group are shown; bars indicate geometric mean. Dotted line represents the limit of detection. There were no statistically significant differences between sexes at either time point. (B) The morphology of IBCs in female (left) and male (right) C57BL/6 mice were observed at 16 hpi with green fluorescent protein-expressing UTI89 via confocal microscopy. In both sexes, early IBCs appeared as small (20-μm diameter) collections of intracellular bacteria with bacillary morphology, while mature IBCs comprised coccoid UPEC in >100-μm masses. Representative images of early (top) and mature (bottom) IBCs are shown.
UPEC More Effectively Colonizes the Male C3H/HeN Bladder
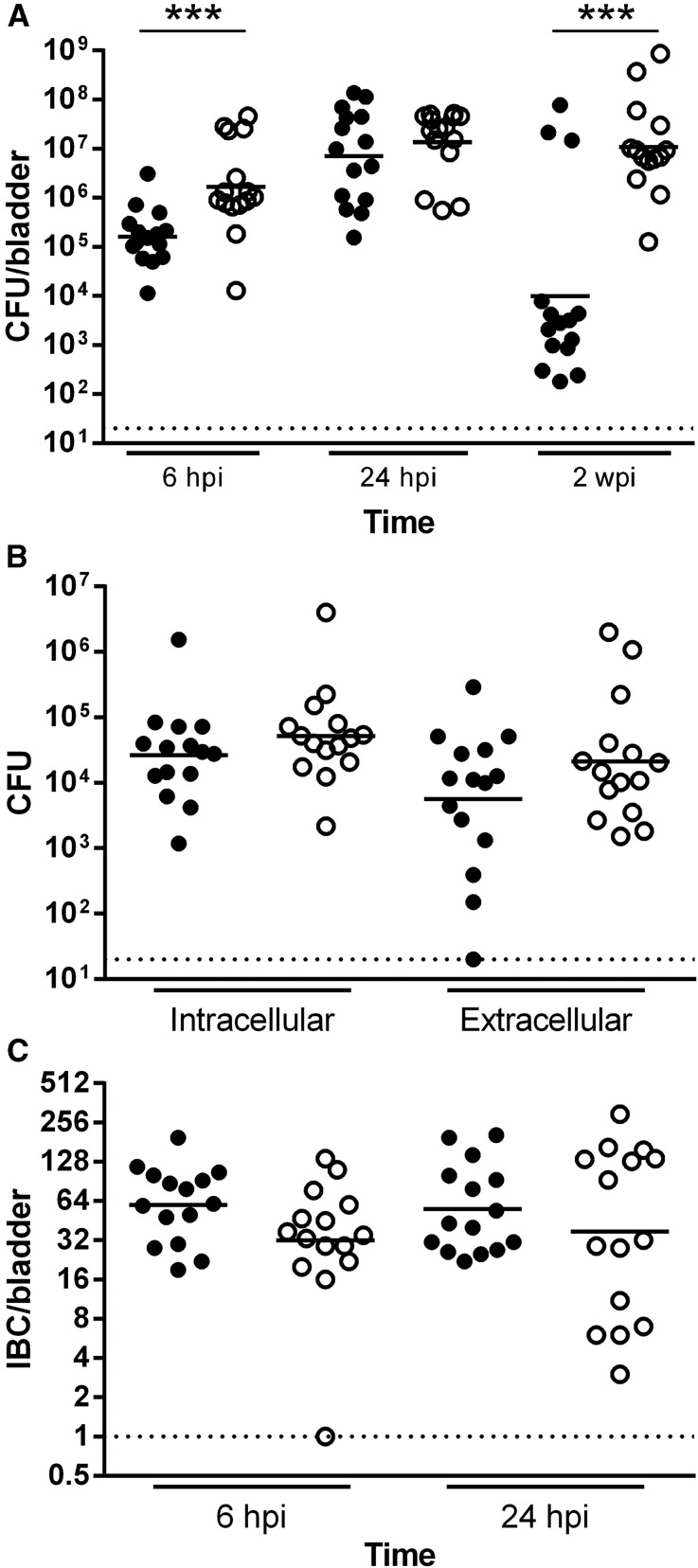
We next applied our minimally invasive method in C3H/HeN mice (a host strain in which a minority of infected females develop chronic cystitis29). Surgically infected male C3H/HeN developed robust acute cystitis, with bladder bacterial loads significantly higher than those in females at 6 hpi (P<0.001; Figure 3A). Using an ex vivo gentamicin protection assay,40 we found modest increases in both the intracellular and extracellular bladder compartments of C3H/HeN males compared with females (Figure 3B). As in the C57BL/6 background, we observed no differences between sexes in IBC numbers in C3H/HeN hosts at 6 or 24 hpi (Figure 3C). We found only modest sex differences in bladder cytokine content during acute cystitis in the C3H/HeN background (Supplemental Figure 2).
Figure 3.
UPEC more readily colonizes the male C3H/HeN bladder. Female (filled circle) and male (empty circle) C3H/HeN mice were surgically infected with 107 CFU of UTI89. (A) Bladders were homogenized at the indicated time points, serially diluted, and CFU enumerated. Each point represents one mouse; bars indicate geometric mean. Data comprise three independent experiments with five mice per group. Dotted line represents the limit of detection. Mice were classified as chronically infected if 2-wpi bladder titers were >104 CFU/bladder. Statistically significant differences between males and females are indicated (***P<0.001). (B) Bacteria present in the intracellular (gentamicin-protected; P=0.18) or extracellular (gentamicin-sensitive; P=0.38) compartments of bladders from female (filled circle) and male (empty circle) C3H/HeN mice at 6 hpi were enumerated. Aggregate data from three independent experiments with five mice per group are shown. (C) IBCs present in bisected bladders at 6 and 24 hpi in female (filled circle) and male (empty circle) C3H/HeN mice were enumerated by microscopy following X-gal staining. Data from three independent experiments with five mice per time point are shown.
Male C3H/HeN Mice are Highly Susceptible to Chronic Cystitis
In catheter-infected C3H/HeN females, a bimodal distribution of bladder UPEC titers develops by 2–4 wpi; a minority (20%–40%) exhibit chronic cystitis, while most females resolve bacteriuria.29,39 As previously defined, chronic cystitis comprises persistent, high-titer bacteriuria (>104 CFU/ml), high bladder bacterial load at 2–4 wpi, ongoing robust inflammation, and persistent inability of the bladder to regenerate the terminally differentiated superficial facet cell layer.29,30 Elevation in serum IL-5, IL-6, G-CSF, and keratinocyte-derived chemokine (KC; CXCL1) at 24 hpi constitutes a biomarker signature that predicts development of chronic cystitis in female mice.29
C3H/HeN females surgically infected with 107 CFU UTI89 exhibited a phenotypic split as seen in the catheterization model, with 20% of females showing high bladder CFU at 2 wpi (Figure 3A). Strikingly, male bladders demonstrated significantly higher bacterial burdens than female bladders at 2 wpi (P<0.001; Figure 3A), with 100% of males developing the high titers typical of chronic cystitis (P<0.001 versus female proportion).
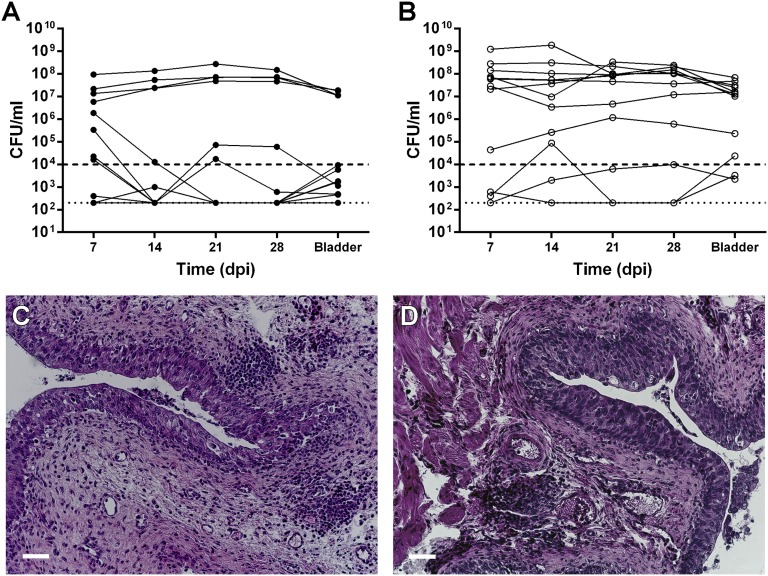
To confirm that these high bacterial loads in male bladders indeed reflected the chronic cystitis phenotype, we surgically infected male and female C3H/HeN mice and enumerated bacterial CFU in weekly urine samples and bladder homogenates at 4 wpi. Consistent with prior studies29,39,41 and our initial 2-wpi data (Figure 3A), a minority (24%) of C3H/HeN females exhibited persistent bacteriuria at 4 wpi, compared with 76% of males (P<0.01 versus female proportion; Figure 4, A and B). Histopathologic analysis of bladders from female or male mice with persistent bacteriuria demonstrated a hyperplastic transitional cell layer with complete exfoliation of superficial facet cells, severe edema, and chronic, follicle-like inflammatory infiltrates in the submucosa (Figure 4, C and D). Bladders from male or female mice that resolved bacteriuria lacked any notable pathology at 4 wpi (data not shown). Bladders of male and female mice with persistent bacteriuria at 2 wpi displayed identical bacterial morphology and luminal colonization by immunofluorescence and confocal microscopy (Supplemental Figure 3). Both male and female C3H/HeN mice with chronic cystitis showed local elevations in multiple proinflammatory cytokines, compared with mice that resolved acute infection (Supplemental Figure 4). Taken together, these data argue that the phenotype observed in high-titer male bladders indeed reflects chronic cystitis as observed in females,29 although male sex is associated with an increased frequency of developing chronic cystitis.
Figure 4.
Male C3H/HeN mice develop chronic cystitis. Female (filled circle) and male (empty circle) C3H/HeN mice were surgically infected with 107 CFU of UTI89 and monitored for 4 wpi. (A and B) Weekly urine bacterial titers and corresponding 4-wpi bladder titers were enumerated in (A) female and (B) male mice following infection. Solid lines connect corresponding urine titer time points and 4-wpi bladder titers (Bladder) from each individual mouse. Dashed horizontal lines represent the cutoff for significant bacteriuria (104 CFU/ml). Dotted horizontal lines show the limit of detection. Data were compiled from two independent experiments. (C and D) Paraffin-embedded bladder sections taken at 4 wpi from persistently bacteriuric (C) female and (D) male C3H/HeN mice were examined by hematoxylin and eosin and light microscopy. Scale bars approximate 50 μm.
Surgically infected females that developed chronic cystitis demonstrated inflammatory cytokine signatures at 24 hpi (i.e., elevated serum IL-5, IL-6, G-CSF, and KC) comparable to those reported in catheter-infected females (Supplemental Figure 5).29 Males demonstrating persistent bacteriuria had significantly higher serum IL-6 at 24 hpi compared with resolved males (P=0.03); increases in serum IL-5, G-CSF, and KC trended similarly (Supplemental Figure 5). These findings suggest that male C3H/HeN hosts, like females, feature an acute inflammatory checkpoint that influences chronic infection outcome.
Male C3H/HeN Mice Develop Severe Pyelonephritis and Renal Abscess
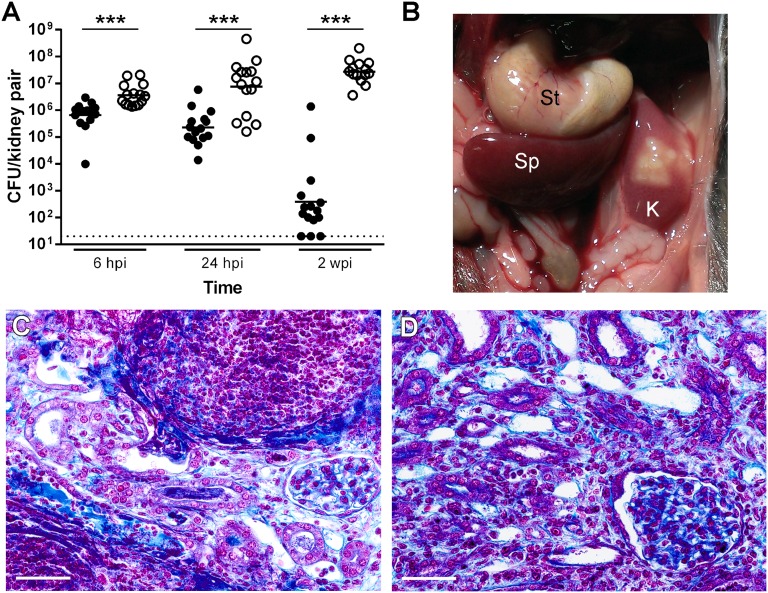
We hypothesized that the amplified renal infection observed in C57BL/6 males (Figure 1B) would be recapitulated more strongly in the C3H/HeN background, a host with increased propensity for vesicoureteral reflux.37,42 Indeed, surgically infected male C3H/HeN displayed significantly higher renal bacterial loads compared with females (P<0.001; Figure 5A). Moreover, while females largely resolved kidney infection over time, renal bacterial loads in males rose substantially from 6 hpi to 2 wpi, with no males able to resolve kidney infection (Figure 5A). These excessive bacterial loads in males correlated with grossly evident renal abscess ar 2 wpi (Figure 5B); 100% of males demonstrated gross abscess formation in at least one kidney, with most (87%) harboring bilateral abscess. In contrast, only 7% of females developed unilateral abscess (P<0.001 versus female proportion). Males surgically infected with UPEC urosepsis isolate CFT073 likewise exhibited abscess and significantly higher kidney bacterial titers than females at 2 wpi (P<0.001; Supplemental Figure 6).
Figure 5.
Male C3H/HeN mice develop severe pyelonephritis and 100% penetrant renal abscess. Female (filled circle) and male (empty circle) C3H/HeN mice were surgically infected with 107 CFU UTI89. (A) Kidney pairs were homogenized, serially diluted, and CFU enumerated at the indicated time points. Each point represents one mouse; bars indicate geometric mean. The dotted horizontal line represents the limit of detection. Data comprise three independent experiments of five mice per group. Statistically significant differences between sexes are indicated (***P<0.001). (B) At autopsy, gross renal abscesses were observed in male, but not female, C3H/HeN mice at 2 wpi. A representative image of the stomach (St), spleen (Sp) and abscessed left kidney (K) of a male C3H/HeN mouse at 2 wpi is shown. (C and D) Paraffin-embedded kidney sections at 2 wpi from male C3H/HeN mice were examined by Gomori trichrome stain and light microscopy. Representative images illustrate (C) areas of necrotic abscess and (D) diseased renal cortex; areas of collagen deposition appear blue (scale bars, 50 μm).
Histologic examination of male C3H/HeN kidneys demonstrated extensive abscess formation in subcapsular, cortical, and medullary locations (Supplemental Figure 7). These abscesses featured a dense neutrophilic infiltrate with areas of necrosis (Figure 5C). The surrounding renal parenchyma was abnormal throughout, exhibiting interstitial infiltrates, edema and fibrosis, mesangial sclerosis, and tubular atrophy with visible thyroidization suggesting active, progressive renal disease (Figure 5D, Supplemental Figure 7).
Testosterone Mediates UTI Severity in Male C3H/HeN Mice
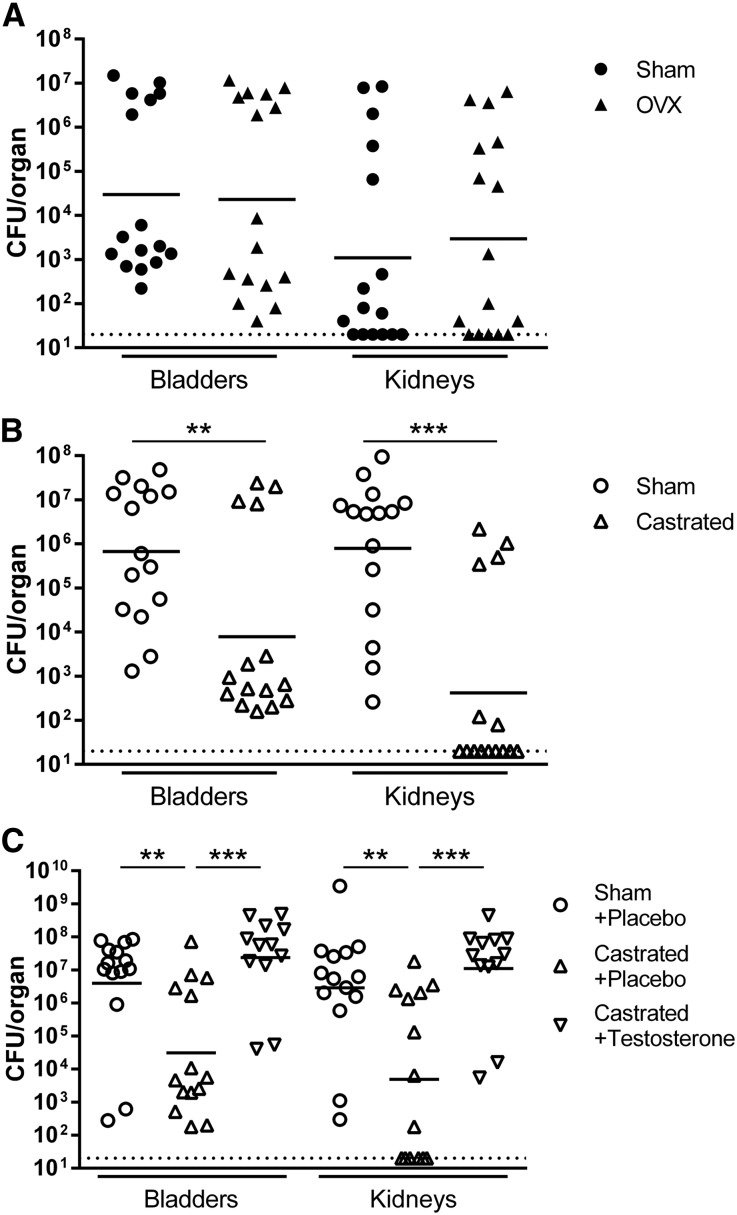
The observed susceptibility of male mice to chronic cystitis, pyelonephritis, and renal abscess could be attributable to a number of sex-specific factors. We adapted our model to examine the potential influences of sex steroid hormones. Females and males were gonadectomized or sham-operated on at 5 weeks of age, and allowed to recover completely over 4 weeks before minimally invasive UTI was introduced. We found no observable differences in bladder or kidney colonization at 2 wpi between ovariectomized and sham-operated C3H/HeN females, suggesting that estrogens do not influence chronic cystitis risk (Figure 6A). However, bladder bacterial burdens in castrated males at 2 wpi were significantly lower than in sham-operated controls (P<0.01; Figure 6B). The majority (87%) of sham-operated C3H/HeN males developed chronic cystitis, compared with only 27% of castrated males (P=0.003 versus castrated proportion). Further, castrated males exhibited a bimodal distribution remarkably similar to C3H/HeN females. Castration similarly attenuated renal infection, reflected in significantly lower kidney bacterial titers in castrated animals compared with sham-operated controls (P<0.001; Figure 6B).
Figure 6.
Testosterone enhances susceptibility to chronic UTI. (A) Sham-operated (filled circle) and ovariectomized (OVX; filled triangle) C3H/HeN female mice were surgically infected with 107 CFU of UTI89 at 4 weeks postoperatively (at 9 weeks of age). Bladders (left) and kidney pairs (right) were homogenized, serially diluted, and CFU enumerated at 2 wpi. Each point represents one mouse; bars indicate geometric mean. Data comprise three independent experiments of five mice per group. (B) Sham-operated (empty circle) and castrated (empty triangle) C3H/HeN male mice were surgically infected with 107 CFU of UTI89 at 4 weeks postoperatively (9 weeks of age). Bladders (left) and kidney pairs (right) were homogenized, serially diluted, and CFU enumerated at 2 wpi. Each point represents one mouse; bars indicate geometric mean. Data comprise three independent experiments of five mice per group. The dotted horizontal line shows the limit of detection. (C) Sham-operated male C3H/HeN mice (empty circle) were subcutaneously implanted with slow-release placebo pellets 5 days postoperatively, and castrated males were implanted with placebo (empty triangle) or testosterone (empty inverted triangle) pellets. Mice were surgically infected 4 weeks postgonadectomy (at 9 weeks of age). Bladders (left) and kidney pairs (right) were homogenized, serially diluted, and CFU enumerated at 2 wpi. Each point represents one mouse; bars indicate geometric mean. Data comprise three independent experiments of four to five mice per group. The limit of detection is indicated with the dotted horizontal line. Statistically significant differences between experimental groups are indicated (**P<0.01; ***P<0.001).
To specify whether male susceptibility to severe UTI is mediated by androgens or by another testicular factor, we implanted slow-release testosterone or placebo pellets 5 days after castration or sham operation. Consistent with the above results, castration attenuated severe UTI in placebo-treated males (Figure 6C). However, treatment of castrated males with exogenous testosterone reversed the protective effects of gonadectomy; significantly higher bladder (P<0.001) and kidney (P<0.001) bacterial burdens were present in testosterone-treated castrated males compared with placebo-treated castrated males (Figure 6C). Testosterone-complemented castrated males were statistically indistinguishable from placebo-treated sham-operated males. Collectively, these data indicate that androgens mediate male susceptibility to chronic cystitis and severe pyelonephritis.
Discussion
In this study, we report the first preclinical UTI model to permit direct elucidation of sex differences in susceptibility and host response to infection, using a novel inoculation technique that bypasses anatomic differences in the lower urinary tracts of male and female mice. Acute cystitis in male mice proceeded through the IBC cascade in a fashion similar to females, but the incidence of chronic cystitis was strikingly higher in C3H/HeN males. Further, these males developed more advanced pyelonephritis and highly prevalent abscess formation compared with females. These phenotypes were mitigated in males who underwent gonadectomy prior to infection, but were complemented with exogenous testosterone. Our findings indicate that the influences of sex on susceptibility to multiple forms of UTI are complex and not limited to traditionally cited anatomic differences. In addition, our minimally invasive model provides a new preclinical platform for translatable studies of upper- and lower-tract UTI pathogenesis in both male and female hosts.
To successfully colonize the urinary tract, UPEC must circumvent formidable host defenses, which are mechanic, biochemical, and immunologic in nature. In ascending UTI, UPEC evades these defenses, in part, by following a well documented pathogenic cascade keyed by the formation of IBCs in the bladder.15–21,23–26 Our data offer the first evidence of the IBC pathway in males and indicate that the events comprising acute cystitis are fundamentally similar in both sexes. Males that resolved acute infection also maintained a small population of persisting UPEC within bladder tissue, likely representing the quiescent intracellular reservoirs believed to seed same-strain recurrent infection in females.36
Male C3H/HeN mice also showed greater susceptibility to chronic cystitis than females, with chronically infected animals of both sexes displaying similar histopathology and inflammatory profiles. The chronic cystitis phenotype has proven to be an important outcome in female models investigating the natural history of UTI and host responses to repeated UPEC inoculation.29,30,35 Our data support previous research in catheter-infected female mice demonstrating that infection outcome is determined within 24 hpi via an acute host inflammatory checkpoint.29 Thus, in both female and male hosts, the nature and degree of early host inflammatory responses may specify risk for severe or recurrent UTI.
Sex differences in immune response have been demonstrated in a number of human infections and animal models; in many of these studies, females display more robust resistance to pathogens.43–45 Among other hypotheses, this enhanced defense has been attributed to an evolutionary need for pregnant females to protect their fetuses from infection,46 and has been linked to a higher incidence of autoimmune and chronic inflammatory diseases in females.47,48 Germane to the present study, UTI are the most common bacterial infections of human females9 and females of other animal species,49–51 and UPEC strains isolated from female humans and animals share considerable phylogenetic similarity and parallel virulence profiles.50,51 Heightened defenses in the female urinary tract may thus have evolved in response to continual exposure to bacterial pathogens (e.g., following sexual intercourse11). Consistent with this paradigm, female mice more aptly controlled both lower and upper UTI compared with males in our model. Additionally, females displayed higher local proinflammatory cytokines at 24 hpi (when bladder UPEC burdens were similar between sexes), suggesting that a more pronounced acute inflammatory response may be raised in the female urinary tract to better control infection.
In addition to potential evolutionary pressure to repel urinary pathogens, some evidence has suggested that estrogen may impact immune response to UTI, although the influence of male androgens on UTI outcome has not been explored. The incidence of UTI in females increases following menopause,52 and studies in ovariectomized C57BL/6 mice suggest that diminished estrogen may favor bacterial persistence in the bladder.53,54 Here, we found that ovariectomy did not affect chronic infection outcome in C3H/HeN females, but androgens potentiated the development of chronic cystitis and severe pyelonephritis in males. The exact organizational or activational influences of androgens on UTI severity in both males and females represent areas of ongoing investigation. Notably, our findings suggest that clinical modulation of androgens may represent a potential therapeutic route to combat recalcitrant or recurrent UTI.
At a glance, our findings in this new model may appear to represent a stark contradiction to the female predominance in human UTI epidemiology. However, minimally invasive inoculation bypasses anatomic sex differences below the bladder. Thus, our data are in fact consistent with the long-standing presumption that UTI risk in females is potentiated by anatomic features. Conversely, anatomy represents a key defense against UTI in males, and the evolutionarily “naïve” urinary tract of males may provide a more hospitable environment for UPEC and other uropathogens once these male anatomic barriers are bypassed. This paradigm is consonant with clinical data reflecting increased morbidity and mortality in males who develop pyelonephritis and complicated UTI, compared with females with these conditions.2–5 While acute cystitis was equivalent in male and female mice, chronic cystitis progression was much higher in males and associated with marked renal infection, leading us to speculate that severe UTI in the male mouse is driven primarily by testosterone-induced susceptibility within the kidney.
In addition to facilitating the study of sex differences, our minimally invasive model fills another substantial gap in the basic investigation of UTI. No published approaches in experimental UTI result in more than a very small minority of wild-type animals developing an ascending renal abscess.29,55,56 Consequently, the field has lacked ability to model ascending abscess development and the resolution of severe renal infection with treatment. In the present model, most C3H/HeN males developed severe pyelonephritis and exhibit renal abscesses, judged to arise via the ascending route (rather than hematogenous, as we detected no bacteria in blood cultures). As noted above, C3H mice feature enhanced vesicoureteral reflux compared with C57BL/6 mice,37,42 reflecting a risk factor for pyelonephritis that is also commonly present in the human population. Our work therefore opens a new avenue into modeling the development, therapy, resolution, and sequelae of severe pyelonephritis and ascending renal abscess. Beyond these niches, our method also induces visible infection of the prostate, making the model potentially useful for the study of bacterial prostatitis.
In summary, the work described here will accelerate fundamental investigation into the mechanisms of UTI initiation, progression, and persistence in male hosts, as well as expanding studies of pyelonephritis, ascending renal abscess, and the treatment of complicated UTI. These advances also represent a timely response to recent calls by the National Institutes of Health (NIH)57 and in the basic and clinical literature58–60 for sex-based research approaches to infectious and other diseases, including a specific focus on UTI.61,62 Continued work in this model promises to address these initiatives at a mechanistic level and to generate translatable advances in prevention and treatment of these common infections.
Concise Methods
Bacterial Strains and Growth Conditions
UPEC strain UTI89 was isolated from a patient with cystitis.63 UPEC strain CFT073 was isolated from the blood of a patient with acute pyelonephritis.64 For surgical infections, bacteria were grown statically in Luria-Bertani broth for 16 hours at 37°C to induce type 1 piliation. The cultures were centrifuged at 7500g for 10 minutes at 4°C before resuspension in sterile PBS to a final density of approximately 4×108 CFU/ml.
Surgical Murine Model of UTI
Animal care and use protocols received prior approval from the Washington University Animal Studies Committee. Experiments were conducted on inbred C57BL/6 (The Jackson Laboratory, Bar Harbor, ME) or C3H/HeN (Harlan Laboratories, Indianapolis, IN) murine backgrounds. Mice were housed under constant temperature and humidity, with a 12-hour light/dark cycle. The female murine model of cystitis with transurethral inoculation via catheter has been described in methodologic detail.13,65 For minimally invasive infection, mice aged 8–9 weeks were maintained under inhalation anesthesia with 3% isoflurane via vaporizer and nose cone. Anesthetized mice were positioned supine, and the ventral abdomen was shaved and disinfected with 70% alcohol and 2% chlorhexidine solution. A 2–3 mm, vertical, midline incision was made directly overlying the bladder, first through the abdominal skin and then the peritoneum. The bladder was exposed through the incision using gentle bilateral pressure on the abdomen, and aseptically emptied. The inoculum was prepared in a 1-ml tuberculin syringe adapted to a 30-gauge, 0.5-inch needle. The needle was introduced to the bladder lumen near the neck of the bladder at 45°. The lumen was inoculated with 50 μl of bacterial suspension (containing 1–2×107 CFU) over a period of 10 seconds. The bladder was allowed to expand for an additional 10 seconds before withdrawing the needle; the injection site sealed immediately with no evident leakage. The bladder was gently replaced, the peritoneum and skin were closed separately with simple, interrupted sutures, and the animal was awakened in fresh air. Infections were allowed to proceed for durations from 6 hours to 4 weeks.
Determination of Urine and Tissue Bacterial Loads
Postinfection, clean-catch urine samples were collected using gentle suprapubic pressure, serially diluted and plated to Luria-Bertani agar to enumerate CFU. For organ titers, mice were euthanized by CO2 asphyxiation, and bladders and kidney pairs were aseptically removed, homogenized in 1 ml or 0.8 ml (respectively) of sterile PBS, and serially diluted and plated. Cystitis in C3H/HeN mice was termed “chronic” if end point bladder and all urine titers were >104 CFU/ml, and “resolved” if end point bladder burdens or at least one urine titer were <104 CFU/ml.29,30,32
Tissue and Serum Cytokine Analysis
The concentrations of 23 mouse cytokines in whole-bladder homogenates or sera were analyzed with a multiplex bead array platform (Bio-Plex; Bio-Rad, Hercules, CA) as described previously.29,66 Data represent the mean and SD from five individual mice across two biologic replicates per time point, each analyzed in technical duplicate.
IBC Enumeration and Confocal Microscopy
For IBC quantification, bladders were bisected, splayed, fixed, and stained for bacterial β-galactosidase as previously described.39 IBCs were counted under a dissecting light microscope. For IBC confocal microscopy, splayed bladders of C57BL/6 male and female mice were examined at 16 hpi with green fluorescent protein-expressing UTI89, as described previously.39 Bacteria present at chronic time points were visualized by immunofluorescence. Splayed bladder halves were fixed in 2.5% paraformaldehyde, blocked in 1% BSA and 0.3% Triton X-100 in PBS for 1 hour, incubated with rabbit anti-E. coli (E3500–06C; US Biologic, Salem, MA) and secondary AlexaFluor 488-conjugated goat anti-rabbit IgG (Life Technologies, Grand Island, NY) antibodies, and stained with SYTO 61 red fluorescent nucleic acid stain (Molecular Probes, Eugene, OR). Images were acquired on a Zeiss LSM 510 META confocal laser scanning microscope (Carl Zeiss Inc., Thornwood, NY).
Ex vivo Gentamicin Protection Assay
Quantification of intracellular and extracellular bacteria in the murine bladder at 6 hpi was performed by a modified ex vivo gentamicin protection assay, as previously described.40 Bladders were aseptically harvested at 6 hpi, bisected, and washed vigorously in PBS, which was plated to enumerate extracellular bacteria. Following 90 minutes of incubation at 37°C in PBS containing gentamicin (100 μg/ml), bladders were washed with PBS, then homogenized and plated to determine intracellular CFU.
Bladder and Kidney Histopathology
Bladder and kidneys were bisected and fixed in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid). Fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin or Gomori trichrome stain.
Serum Collection and Storage
Venous blood was collected by submandibular puncture using 5-mm steel lancets (MediPoint, Mineola, NY) into BD Microtainer serum separation tubes (Becton Dickinson, Franklin Lakes, NJ). Samples were allowed to clot for 1 hour and were clarified by centrifugation at 15,000g for 5 minutes before storage at –80°C.
Gonadectomy and Testosterone Implantation
Bilateral orchiectomy (castration), bilateral ovariectomy, or sham operations were performed at Harlan Laboratories (Indianapolis, IN) following standard procedures at 5 weeks of age. Animals were allowed to recover 1 week before shipment. Where indicated, 60-day continuous release pellets containing 25 mg testosterone or placebo (Innovative Research of America, Sarasota, FL) were implanted subcutaneously at the nape of the neck 5 days following castration or sham operation. Mice were surgically infected at 9 weeks of age (i.e., 4 weeks following gonadectomy or sham surgery).
Statistical Analyses
Statistical tests for significance were performed using Prism 6 software (GraphPad Software Inc., La Jolla, CA). Observed differences in bacterial CFU, IBC numbers, and cytokine levels were analyzed with the unpaired, two-tailed, nonparametric Mann–Whitney U test. To compare proportions of mice developing persistent bacteriuria and chronic cystitis or renal abscess, a two-tailed Fisher exact test was used. P values <0.05 were deemed significant.
Disclosures
D.A.H. serves on the Scientific Advisory Board of BioVersys AG, Basel, Switzerland. P.D.O. and K.A.H. have no potential conflicts to declare.
Supplementary Material
Acknowledgments
We thank K. Tiemann and W. Beatty for technical assistance, and S. Hultgren for critical review of the manuscript.
This work was supported by NIH grants R01-DK080752 and R01-DK082546 (to D.A.H.), P50-DK064540 (Specialized Centers of Research sponsored by the NIH Office of Research on Women’s Health), F30-DK104446 (to P.D.O.), and T32-AI007172.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030327/-/DCSupplemental.
References
- 1.Foxman B: Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 49: 53–70, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Klemstine KL, Brown PD: Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 13: 144–150, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Efstathiou SP, Pefanis AV, Tsioulos DI, Zacharos ID, Tsiakou AG, Mitromaras AG, Mastorantonakis SE, Kanavaki SN, Mountokalakis TD: Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch Intern Med 163: 1206–1212, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Nicolle LE, Friesen D, Harding GK, Roos LL: Hospitalization for acute pyelonephritis in Manitoba, Canada, during the period from 1989 to 1992; impact of diabetes, pregnancy, and aboriginal origin. Clin Infect Dis 22: 1051–1056, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Ki M, Park T, Choi B, Foxman B: The epidemiology of acute pyelonephritis in South Korea, 1997-1999. Am J Epidemiol 160: 985–993, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Wenninger K, Heiman JR, Rothman I, Berghuis JP, Berger RE: Sickness impact of chronic nonbacterial prostatitis and its correlates. J Urol 155: 965–968, 1996 [PubMed] [Google Scholar]
- 7.Benway BM, Moon TD: Bacterial prostatitis. Urol Clin North Am 35: 23–32, v, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Domingue GJ Sr, Hellstrom WJ: Prostatitis. Clin Microbiol Rev 11: 604–613, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foxman B: The epidemiology of urinary tract infection. Nat Rev Urol 7: 653–660, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Ulleryd P, Zackrisson B, Aus G, Bergdahl S, Hugosson J, Sandberg T: Selective urological evaluation in men with febrile urinary tract infection. BJU Int 88: 15–20, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Hooton TM: Recurrent urinary tract infection in women. Int J Antimicrob Agents 17: 259–268, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Hooton TM, Stapleton AE, Roberts PL, Winter C, Scholes D, Bavendam T, Stamm WE: Perineal anatomy and urine-voiding characteristics of young women with and without recurrent urinary tract infections. Clin Infect Dis 29: 1600–1601, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Hung CS, Dodson KW, Hultgren SJ: A murine model of urinary tract infection. Nat Protoc 4: 1230–1243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey AJ, Tan CK, Ipe DS, Sullivan MJ, Cripps AW, Schembri MA, Ulett GC: Urinary tract infection of mice to model human disease: Practicalities, implications and limitations. Crit Rev Microbiol 26: 1–20, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Song J, Bishop BL, Li G, Duncan MJ, Abraham SN: TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe 1: 287–298, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright KJ, Seed PC, Hultgren SJ: Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol 9: 2230–2241, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Eto DS, Gordon HB, Dhakal BK, Jones TA, Mulvey MA: Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimH-mediated bacterial invasion of host cells. Cell Microbiol 10: 2553–2567, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ: Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol 166: 1148–1155, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Berry RE, Klumpp DJ, Schaeffer AJ: Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli. Infect Immun 77: 2762–2772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ: Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19: 2803–2812, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ: Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301: 105–107, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Mulvey MA, Schilling JD, Hultgren SJ: Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69: 4572–4579, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eto DS, Jones TA, Sundsbak JL, Mulvey MA: Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog 3: e100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN: Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem 279: 18944–18951, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN: Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med 13: 625–630, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Eto DS, Sundsbak JL, Mulvey MA: Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol 8: 704–717, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ: Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 4: e329, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robino L, Scavone P, Araujo L, Algorta G, Zunino P, Vignoli R: Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog Dis 68: 78–81, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ: Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6: e1001042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ: Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36: 616–648, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brien VP, Hannan TJ, Schaeffer AJ, Hultgren SJ: Are you experienced? Understanding bladder innate immunity in the context of recurrent urinary tract infection. Curr Opin Infect Dis 28: 97–105, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz DJ, Conover MS, Hannan TJ, Hultgren SJ: Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathog 11: e1004599, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehm BJ, Colopy SA, Jerde TJ, Loftus CJ, Bushman W: Acute bacterial inflammation of the mouse prostate. Prostate 72: 307–317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudick CN, Berry RE, Johnson JR, Johnston B, Klumpp DJ, Schaeffer AJ, Thumbikat P: Uropathogenic Escherichia coli induces chronic pelvic pain. Infect Immun 79: 628–635, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC: Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun 79: 4250–4259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mysorekar IU, Hultgren SJ: Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A 103: 14170–14175, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowen SE, Watt CL, Murawski IJ, Gupta IR, Abraham SN: Interplay between vesicoureteric reflux and kidney infection in the development of reflux nephropathy in mice. Dis Model Mech 6: 934–941, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan CY, St John AL, Abraham SN: Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 38: 349–359, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson TF, Watts KM, Hunstad DA: OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infect Immun 77: 5245–5251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA: Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun 74: 4793–4800, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Totsika M, Kostakioti M, Hannan TJ, Upton M, Beatson SA, Janetka JW, Hultgren SJ, Schembri MA: A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis 208: 921–928, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murawski IJ, Maina RW, Malo D, Guay-Woodford LM, Gros P, Fujiwara M, Morgan K, Gupta IR: The C3H/HeJ inbred mouse is a model of vesico-ureteric reflux with a susceptibility locus on chromosome 12. Kidney Int 78: 269–278, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Robinson DP, Lorenzo ME, Jian W, Klein SL: Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadioglu A, Cuppone AM, Trappetti C, List T, Spreafico A, Pozzi G, Andrew PW, Oggioni MR: Sex-based differences in susceptibility to respiratory and systemic pneumococcal disease in mice. J Infect Dis 204: 1971–1979, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Klein SL: The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev 24: 627–638, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Billington WD: The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol 60: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Gleicher N, Barad DH: Gender as risk factor for autoimmune diseases. J Autoimmun 28: 1–6, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Jacobson DL, Gange SJ, Rose NR, Graham NM: Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84: 223–243, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Whittam TS, Wolfe ML, Wilson RA: Genetic relationships among Escherichia coli isolates causing urinary tract infections in humans and animals. Epidemiol Infect 102: 37–46, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson JR, Johnston B, Clabots CR, Kuskowski MA, Roberts E, DebRoy C: Virulence genotypes and phylogenetic background of Escherichia coli serogroup O6 isolates from humans, dogs, and cats. J Clin Microbiol 46: 417–422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bélanger L, Garenaux A, Harel J, Boulianne M, Nadeau E, Dozois CM: Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol Med Microbiol 62: 1–10, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Stamm WE, Raz R: Factors contributing to susceptibility of postmenopausal women to recurrent urinary tract infections. Clin Infect Dis 28: 723–725, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Wang C, Symington JW, Ma E, Cao B, Mysorekar IU: Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect Immun 81: 733–739, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lüthje P, Brauner H, Ramos NL, Övregaard A, Gläser R, Hirschberg AL, Aspenström P, Brauner A: Estrogen supports urothelial defense mechanisms. Sci Transl Med 5: 190ra80, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Svensson M, Yadav M, Holmqvist B, Lutay N, Svanborg C, Godaly G: Acute pyelonephritis and renal scarring are caused by dysfunctional innate immunity in mCxcr2 heterozygous mice. Kidney Int 80: 1064–1072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaillon S, Moalli F, Ragnarsdottir B, Bonavita E, Puthia M, Riva F, Barbati E, Nebuloni M, Cvetko Krajinovic L, Markotic A, Valentino S, Doni A, Tartari S, Graziani G, Montanelli A, Delneste Y, Svanborg C, Garlanda C, Mantovani A: The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 40: 621–632, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Clayton JA, Collins FS: Policy: NIH to balance sex in cell and animal studies. Nature 509: 282–283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollitzer E: Biology: Cell sex matters. Nature 500: 23–24, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Miller VM: Why are sex and gender important to basic physiology and translational and individualized medicine? Am J Physiol Heart Circ Physiol 306: H781–H788, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cahill L: A half-truth is a whole lie: on the necessity of investigating sex influences on the brain. Endocrinology 153: 2541–2543, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drekonja DM, Rector TS, Cutting A, Johnson JR: Urinary tract infection in male veterans: treatment patterns and outcomes. JAMA Intern Med 173: 62–68, 2013 [DOI] [PubMed] [Google Scholar]
- 62.den Heijer CDJ, Penders J, Donker GA, Bruggeman CA, Stobberingh EE: The importance of gender-stratified antibiotic resistance surveillance of unselected uropathogens: a Dutch Nationwide Extramural Surveillance study. PLoS One 8: e60497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen SL, Hung CS, Pinkner JS, Walker JN, Cusumano CK, Li Z, Bouckaert J, Gordon JI, Hultgren SJ: Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci U S A 106: 22439–22444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW: Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58: 1281–1289, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ: Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282: 1494–1497, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Ingersoll MA, Kline KA, Nielsen HV, Hultgren SJ: G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol 10: 2568–2578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.