Abstract
Renovascular disease (RVD) induces renal microvascular (MV) rarefaction that drives progressive kidney injury. In previous studies, we showed that renal vascular endothelial growth factor (VEGF) therapy attenuated MV damage, but did not resolve renal injury at practical clinical doses. To increase the bioavailability of VEGF, we developed a biopolymer-stabilized elastin-like polypeptide (ELP)-VEGF fusion protein and determined its in vivo potential for therapeutic renal angiogenesis in RVD using an established swine model of chronic RVD. We measured single-kidney blood flow (RBF) and GFR and established the degree of renal damage after 6 weeks of RVD. Pigs then received a single stenotic kidney infusion of ELP-VEGF (100 μg/kg), a matching concentration of unconjugated VEGF (18.65 μg/kg), ELP alone (100 μg/kg), or placebo. Analysis of organ distribution showed high renal binding of ELP-VEGF 4 hours after stenotic kidney infusion. Therapeutic efficacy was determined 4 weeks after infusion. ELP-VEGF therapy improved renal protein expression attenuated in RVD, restoring expression levels of VEGF, VEGF receptor Flk-1, and downstream angiogenic mediators, including phosphorylated Akt and angiopoietin-1 and -2. This effect was accompanied by restored MV density, attenuated fibrogenic activity, and improvements in RBF and GFR greater than those observed with placebo, ELP alone, or unconjugated VEGF. In summary, we demonstrated the feasibility of a novel therapy to curtail renal injury. Recovery of the stenotic kidney in RVD after ELP-VEGF therapy may be driven by restoration of renal angiogenic signaling and attenuated fibrogenic activity, which ameliorates MV rarefaction and improves renal function.
Keywords: renal artery stenosis, renal protection, VEGF, angiogenesis, drug, transporter, microcirculation
Renal vascular disease (RVD), usually caused by renal artery stenosis, can lead to CKD and ESRD. RVD increases cardiovascular morbidity and mortality, hospitalization, shortens life expectancy, and is increasing at a sustained pace in the United States.1 Moreover, renal function does not improve or even deteriorates in almost half of the patients with RVD despite treatment. Recent clinical studies suggest that patients undergoing current therapeutic strategies, which include drugs and renal angioplasty, do not show differences in outcomes that could demonstrate distinct benefits of one treatment over the other when compared side by side.2,3 Moreover, renal function does not improve or even deteriorates in almost half of patients with RVD despite treatment, highlighting a pressing need for novel therapeutic strategies for the growing population of patients suffering from RVD.
Using a clinically relevant swine model of chronic RVD that mimics several of the pathologic features and the progressive nature of human RVD,4,5 we showed that a key pathologic feature accompanying the loss of renal function and the progression of renal injury in the stenotic kidney is a progressive microvascular (MV) rarefaction. We demonstrated that the latter is paralleled by defective renal angiogenesis, which is likely driven by a progressive decrease in the renal availability of vascular endothelial growth factor (VEGF), since intrarenal VEGF therapy significantly attenuated MV damage and loss and improved renal function.6,7 However, these therapeutic effects were still insufficient to resolve renal injury. Potential reasons for the incomplete resolution of renal damage may be related to VEGF’s short t1/2 or its susceptibility to degradation in vivo. A potential alternative to overcome these limitations is to increase the frequency of treatment. However, repeated intrarenal administrations to enhance the effects are impractical for clinical use and may increase the risk of adverse events. Therefore, we sought a means to prolong the plasma t1/2 of exogenously administered VEGF and improve its renal targeting and efficacy.
Elastin-like polypeptides (ELP) are a class of bioengineered proteins with great potential as drug delivery vectors due to their long plasma t1/2, low immunogenicity, and adaptability to be fused to nearly any therapeutic peptide, protein, or small molecule drug. Because ELPs are protein-based, their sequence is genetically encoded. This facilitates their modification for fusion with peptides and protein-based therapeutics prolonging their t1/2 and tissue residence time, protecting them from proteolysis, and providing an excellent opportunity for developing tailored treatments.8–11 ELPs naturally accumulate at high levels in kidney and liver tissues. We have recently generated and characterized in vitro an ELP fusion with human VEGF. This protein construct is highly active in vitro, the ELP fusion did not adversely affect the function of VEGF, and the addition of the ELP carrier reduced the plasma clearance rate and extended the t1/2 compared with unconjugated VEGF.12 However, whether ELP-VEGF constructs could serve as therapeutic tools to protect the kidney has not to our knowledge been previously investigated.
We aim to determine the potential therapeutic application of ELP-VEGF constructs to renal therapy. We hypothesize that stabilization and kidney accumulation achieved by fusing VEGF to the biopolymer carrier will lead to therapeutic efficacy for renal recovery in RVD.
Results
In vitro Characterization of ELP-VEGF Activity
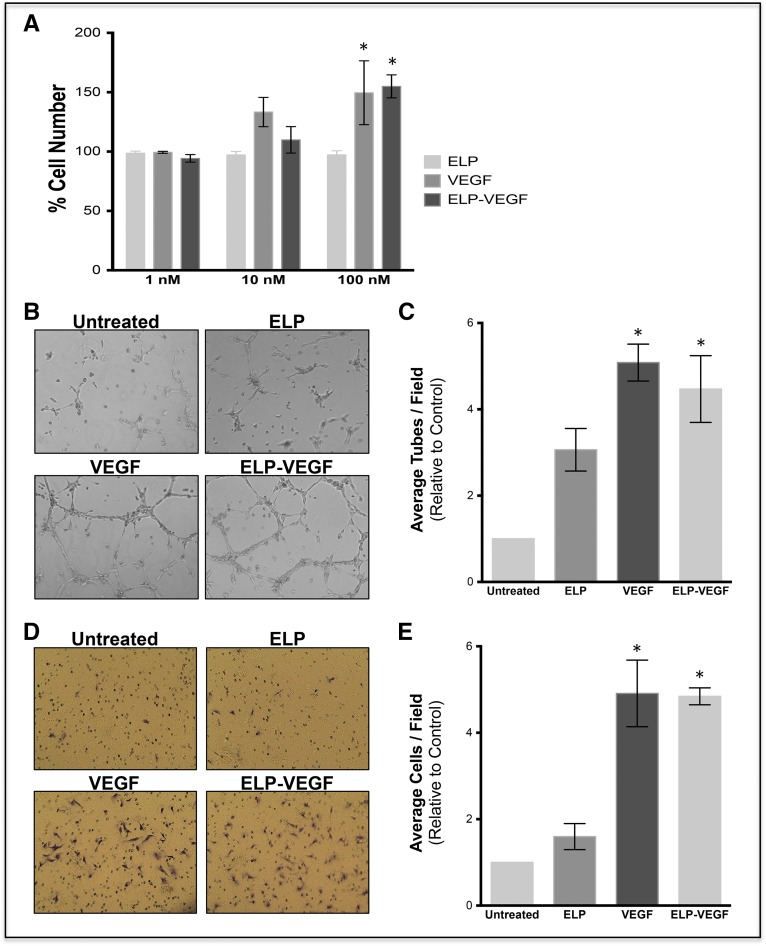
Using primary human glomerular microvascular endothelial (HGME) cells, proliferation, tube formation, and migration were determined. The ELP-VEGF construct shows similar potency, inducing cell proliferation, migration, and tube formation (Figure 1, A–E). For details, please see Supplemental Material.
Figure 1.
In vitro activity of the ELP-VEGF construct shows similar potency compared with unconjugated VEGF in HGME cells. (A) Stimulation of HGME cell proliferation was determined by exposing HGME cells to ELP control, unconjugated VEGF, or ELP-VEGF for 72 hours, and viable cells were detected using the MTS cell proliferation assay. (B, C) Tube formation in HGME: cells were plated on growth factor reduced Matrigel, the media was supplemented with the indicated proteins and tube formation was assessed after 5 hours. Original magnification, ×10. (D, E) HGME migration: cells were plated in the top well of Matrigel-coated Boyden chambers, and media in the bottom well was supplemented with the test proteins. Migration was quantified by crystal violet staining (original magnification, ×10) after 16–24 hours of protein exposure. *Levels are significantly higher than untreated cells as assessed by a one-way ANOVA and post hoc Bonferroni multiple comparison.
In vivo Pharmacokinetics and Biodistribution of Fluorescently Labeled ELP-VEGF following Single Kidney Intrarenal Administration
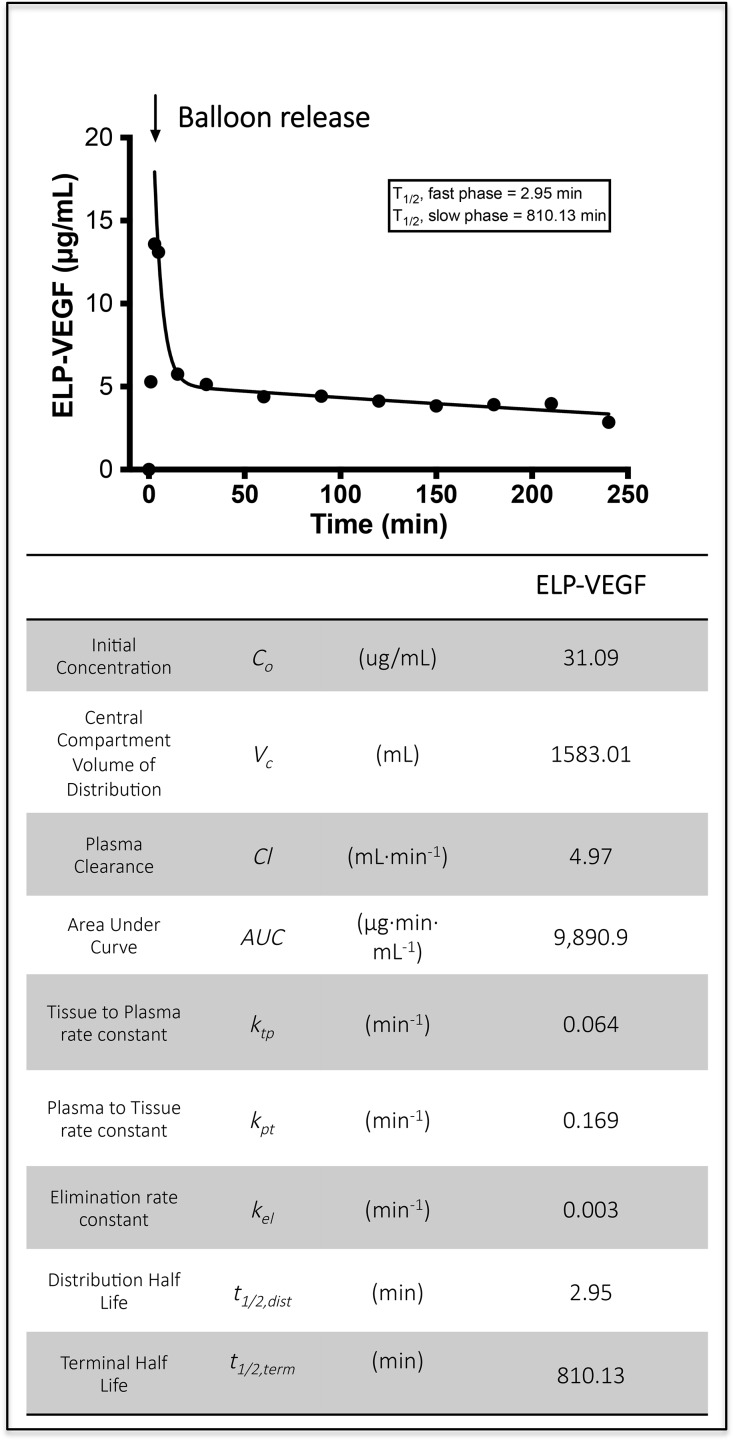
Blood was sampled at fixed time-points and plasma fluorescence measurements were taken to monitor ELP-VEGF levels. Distribution phase t1/2 was 2.95 minutes and the terminal plasma t1/2 was 810.1 minutes (Figure 2).
Figure 2.
Pharmacokinetics of ELP-VEGF in pigs show a long terminal t1/2 after single intrarenal administration. Three pigs (average weight 49.2 kg) were given fluorescently labeled ELP-VEGF by direct intrarenal administration. A balloon was inflated to block blood flow in and out of the injected kidney for 3 minutes. The balloon was released, and plasma was sampled to determine ELP-VEGF levels. Plasma levels were determined by direct detection of fluorescence and fit to a two compartment pharmacokinetic model.
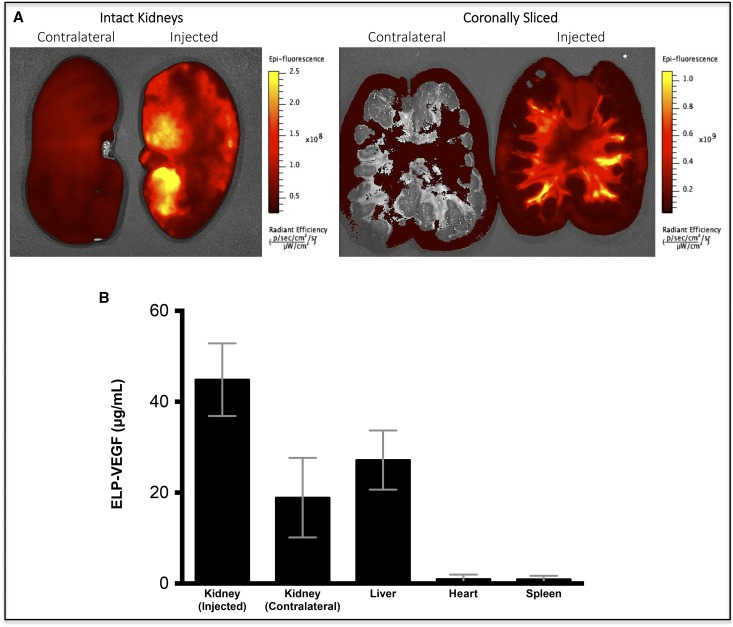
Organ biodistribution was determined 4 hours after injection. Retention of ELP-VEGF in the injected kidney was a 2.4-fold higher than the contralateral kidney (Figure 3A), although some protein did reach systemic circulation and other organs (Figure 3B). These results demonstrate that most ELP-VEGF is retained in the injected kidney, suggesting that intrarenal administration is a viable route for delivery of ELP-VEGF for therapy of RVD. For details, please see Supplemental Material.
Figure 3.
Biodistribution of ELP-VEGF in pigs show that fluorescently labeled ELP-VEGF accumulates higher in the kidney, suggesting enhanced renal preference. Three pigs (average weight 49.2 kg) were given fluorescently labeled ELP-VEGF by direct intrarenal administration. Organ distribution was determined 4 hours after injection by (A) ex vivo whole-organ fluorescence imaging and (B) quantification.
In vivo Efficacy of ELP-VEGF
We then sought to determine whether a single intrarenal dose of ELP-VEGF was efficacious for improving renal function in the swine RVD model.
General Characteristics
Body weight, blood pressure, and degree of stenosis were similar among RVD- and RVD+ELP-VEGF–treated pigs (Tables 1 and 2).
Table 1.
General characteristics in normal, RVD, and RVD pigs before treatment with ELP/ELP-VEGF
| Parameter | Normal | RVD | RVD+ELP-VEGF |
|---|---|---|---|
| Body weight (kg) | 50.2±3.8 | 49.5±2.2 | 51.2±3.4 |
| Degree of stenosis (%) | 0.0±0.0 | 73.3±5.1a | 74.8±11.1a |
| MAP (mmHg) | 110.0±2.0 | 143.3±8.3a | 135.8±5.1a |
| Plasma creatinine (μmol/l) | 78.6±7.5 | 105.4±7.2 | 111.9±8.1 |
| Albuminuria (μg/ml) | 7.9±0.3 | 128.0±28.5a | 108.2±44.5a |
| RVR (mmHg/ml per min) | 0.18±0.05 | 0.56±0.1 | 0.46±0.1 |
| Cortical volume (cc) | 114.8±8.1 | 62.5±6.7a | 74.8±4.8a |
| Medullary volume (cc) | 35.6±2.1 | 18.1±2.2a | 18.1±1.9a |
Data are presented as mean±SEM, n=7 per group. Parameters were obtained after 6 weeks of observation. MAP, mean arterial pressure; RVR, renal vascular resistance.
P<0.05 versus Normal.
Table 2.
General characteristics and renal concentration (ELISA, stenotic kidney) of TNF-α in normal, RVD, and RVD pigs 4 weeks after treatment with ELP/ELP-VEGF
| Parameter | Normal | RVD | RVD+ELP-VEGF |
|---|---|---|---|
| Body weight (kg) | 56.8±4.8 | 59.2±2.3 | 59.5±2.7 |
| Degree of stenosis (%) | 0.0±0.0 | 75.3±4.6a | 76.8±9.2a |
| MAP (mmHg) | 111.0±2.5 | 145.2±11.4a | 135.8±2.0a |
| Plasma creatinine (μmol/l) | 83.8±6.9 | 142.4±13.4ab | 129.4±10.2a |
| Albuminuria (μg/ml) | 7.6±0.4 | 145.9±39.6a | 57.9±6.6ab |
| Nephrin (μg/ml urine) | 0.3±0.16 | 1.5±0.3a | 0.1±0.05b |
| RVR (mmHg/ml per min) | 0.19±0.01 | 0.54±0.07 | 0.32±0.06abc |
| Cortical volume (cc) | 124.1±7.0 | 64.3±8.7a | 96.8±5.0abc |
| Medullary volume (cc) | 37.2±5.3 | 18.4±2.1a | 20.2±2.5a |
| TNF-α (pg/mg tissue) | 9.8±1.4 | 19.4±0.6a | 13.4±3.2ab |
Data are presented as mean±SEM, n=7 per group. Parameters were obtained at 10 weeks. MAP, mean arterial pressure; RVR, renal vascular resistance.
P<0.05 versus Normal.
P<0.05 versus RVD.
P<0.05 versus pretreatment.
Multidetector Computed Tomography-Derived Renal Hemodynamics and Function
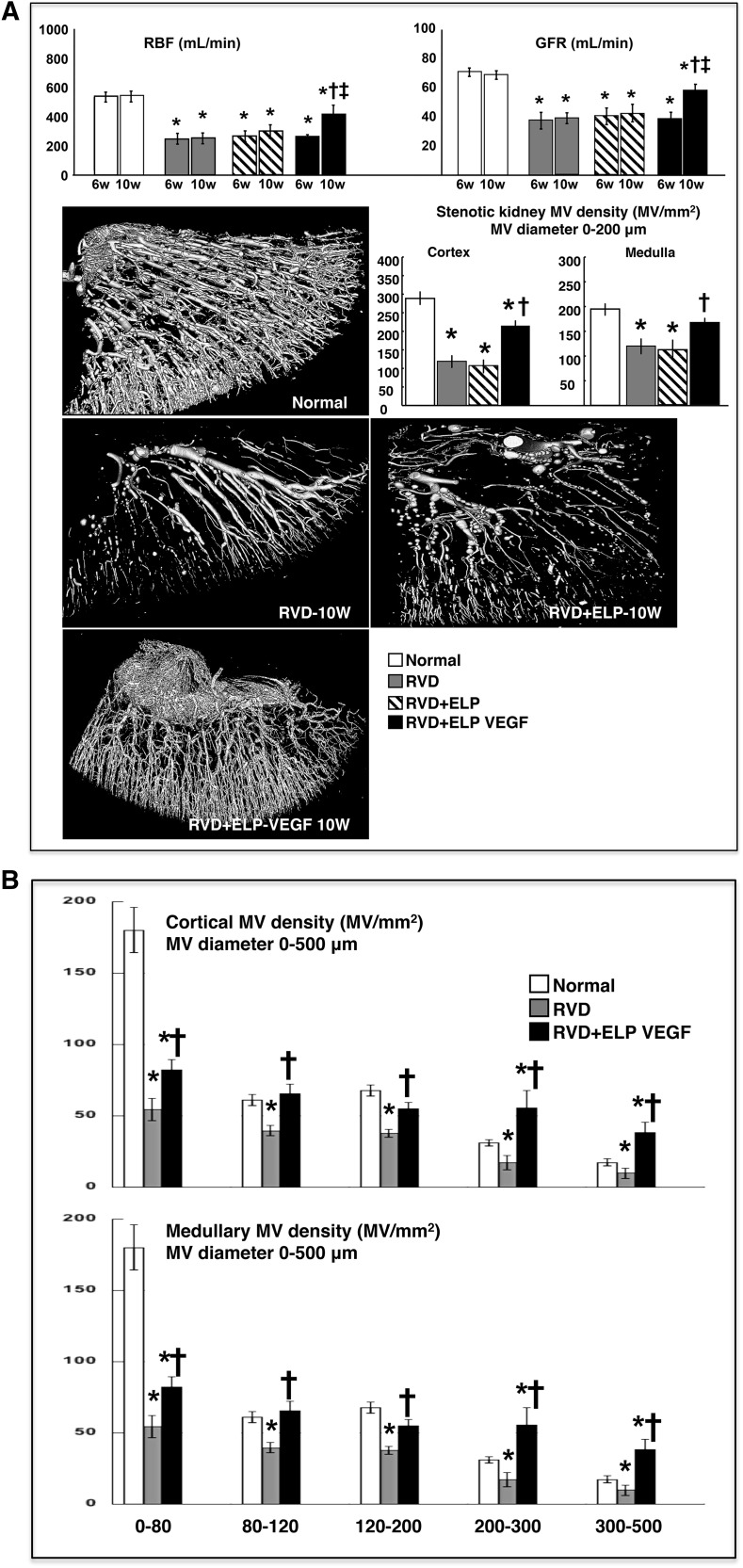
As measured by multidetector computed tomography (MDCT), renal blood flow (RBF) and GFR were similarly attenuated in all pigs with RVD after 6 weeks of observation, which correlated with a significant increase in renal vascular resistance of the stenotic kidney and were accompanied by increased plasma creatinine (Table 1). Blunted RBF and GFR remained unchanged in RVD at 10 weeks, but were dramatically improved after ELP-VEGF therapy (around 70%) compared with 6 weeks pretreatment values (Figure 4A, top). Improvements in proteinuria, renal vascular resistance, and a plateau in plasma creatinine accompanied the improvements in renal function (Table 2), suggesting slower or halted progression of renal damage after ELP-VEGF therapy.
Figure 4.
Intrarenal administration of ELP-VEGF improved renal function, and cortical and medullary vascular density in the stenotic kidney. Effect of intrarenal ELP-VEGF on renal function (top) and MV architecture (three-dimensional micro-CT reconstruction, bottom) and quantification in normal, RVD-, RVD+ELP-, and RVD+ELP-VEGF–treated kidneys. *P<0.05 versus Normal; †P<0.05 versus RVD/RVD+ELP; ‡P<0.05 versus 6 weeks. (B) Cortical and medullary quantification of MV density divided by MV diameter in normal, RVD-, and RVD+ELP-VEGF–treated kidneys. *P<0.05 versus Normal; †P<0.05 versus RVD.
Micro Computed Tomography Quantification and Morphometric Analysis of the Renal Microcirculation
As shown by micro computed tomography (CT), the stenotic kidney showed a significant reduction in cortical and medullary MV density accompanied by substantial MV remodeling compared with normal controls (Figure 4A, bottom). Notably, intrarenal ELP-VEGF significantly improved both cortical and medullary MV density and remodeling of small and large microvessels (0–500 μm in diameter), which was evident throughout the renal parenchyma (Figure 4B).
Effects of ELP Alone on Renal Function and MV Architecture
General characteristics were similar to untreated RVD (Table 3), and no improvements in RBF, GFR, or MV rarefaction (Figure 4A) were observed after administration of ELP, suggesting that the carrier did not have therapeutic effects and renal improvements were due to the polymer-stabilized VEGF construct.
Table 3.
General characteristics in RVD pigs before and 4 weeks after intrarenal administration of free ELP (not bound to VEGF)
| Parameter | RVD (6 Weeks) | RVD+ELP (10 Weeks) |
|---|---|---|
| Body weight (kg) | 47.4±6.6 | 48.4±7.5 |
| Degree of stenosis (%) | 74.2±8.1a | 74.9±7.3a |
| MAP (mmHg) | 133.1±4.5a | 133.3±6.3a |
| RVR (mmHg/ml per min) | 0.52±0.1a | 0.45±0.1a |
| Cortical volume (cc) | 75.6±9.0a | 79.8±9.3a |
| Medullary volume (cc) | 27.1±6.8a | 23.1±2.5a |
Parameters were obtained at 6 and 10 weeks. MAP, mean arterial pressure; RVR, renal vascular resistance.
P<0.05 versus Normal.
Effects of Intrarenal Administration of Unconjugated VEGF on Renal Function and MV Architecture
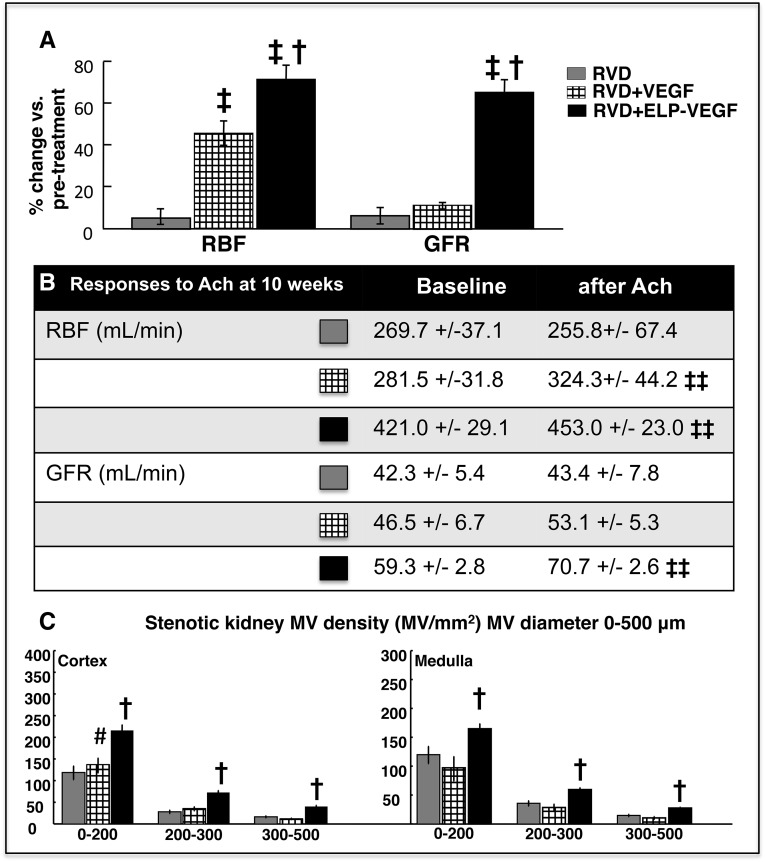
A single intrarenal administration of unconjugated (free) VEGF121 significantly improved stenotic RBF but not GFR (P<0.05 and P=NS, respectively, versus pretreatment values) and the magnitude of those changes was significantly less compared with ELP-VEGF therapy (Figure 5A). Furthermore, intrastenotic kidney infusion of acetylcholine (quantified at 10 weeks) improved RBF but not GFR in unconjugated VEGF-treated kidneys, whereas both RBF and GFR were improved after ELP-VEGF (Figure 5B). Finally, unconjugated VEGF therapy improved MV density only in those cortical microvessels under 200 μm in diameter and not in larger microvessels (200–500 μm in diameter, Figure 5C), which matches our previous work using VEGF165.6 Overall, these findings strongly support a superior efficacy of ELP-VEGF therapy over unconjugated VEGF.
Figure 5.
Intrarenal administration of ELP-VEGF improved stenotic kidney hemodynamics and MV rarefaction more efficiently than unconjugated VEGF. Comparisons between intrarenal unconjugated VEGF versus ELP-VEGF therapy on: (A) Basal stenotic kidney RBF and GFR, expressed as %change compared with pretreatment values; (B) RBF and GFR responses to intrarenal infusion of acetylcholine (Ach); (C) Effects of the treatments on cortical and medullary MV density (three-dimensional micro-CT reconstruction, divided by MV diameter) in RVD+VEGF- and RVD+ELP-VEGF–treated kidneys. †P<0.05 versus RVD/RVD+VEGF; ‡P<0.05 versus 6 weeks; ‡‡ P<0.05 versus baseline; #P=0.09 versus RVD.
Renal Protein Expression in the Stenotic Kidney
Angiogenic Factors
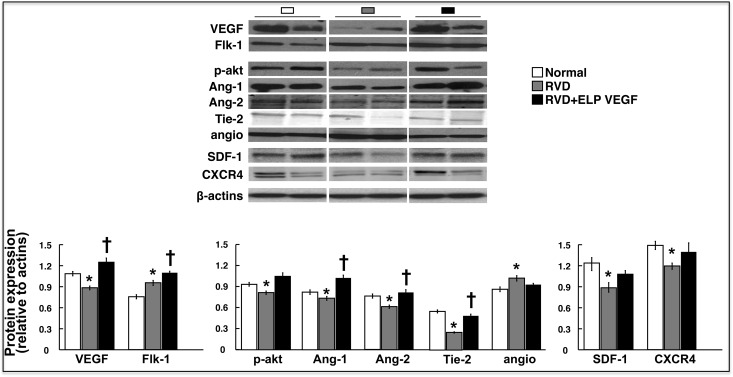
Expression of VEGF, the receptor Flk-1, angiopoietin-1 and -2 (Ang-1 and Ang-2), and the Tie-2 receptor were significantly reduced in RVD but largely restored and accompanied by improved expression of phosphorylated Akt (p-Akt), stromal-derived factor (SDF)-1, and the CXCR4 receptor, and attenuated expression of antiangiogenic angiostatin after ELP-VEGF therapy, suggesting a proangiogenic milieu in the stenotic kidney of ELP-VEGF–treated pigs (Figure 6).
Figure 6.
Intrarenal administration of ELP-VEGF improved the expression of angiogenic factors and promoters of mobilization and homing of progenitor cells in the stenotic kidney. Representative renal protein expression (two bands per group) and quantification of VEGF, its receptor Flk-1, p-Akt, Ang-1 and Ang-2, Tie-2, angiostatin (angio), SDF-1 and its receptor CXCR4, and quantification (bottom) in normal, RVD-, and RVD+ELP-VEGF–treated kidneys. *P<0.05 versus Normal; †P<0.05 versus RVD.
Inflammatory and Fibrotic Factors and Podocyte Damage
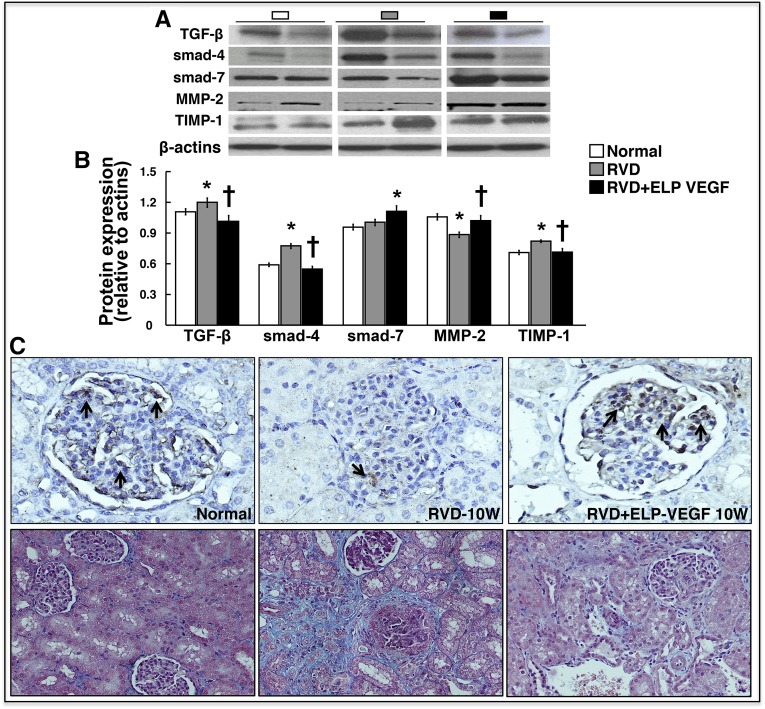
ELP-VEGF therapy decreased the renal concentration of TNF-α (Table 2), and attenuated the expression of profibrotic TGF-β, smad-4, and tissue inhibitor of matrix-metalloproteinase (TIMP)-1, whereas improved smad-7 and matrix metalloproteinase-2 (MMP-2) compared with untreated RVD, suggesting a potential decrease in proinflammatory, profibrotic, and tissue remodeling activity (Figure 7A). Furthermore, ELP-VEGF therapy improved glomerular expression of podocin (Figure 7B) and reduced nephrinuria (Table 2), suggesting protection on podocytes.
Figure 7.
Intrarenal administration of ELP-VEGF reduced renal fibrogenic activity and attenuated podocyte damage and fibrosis in the stenotic kidney. (A) Representative renal protein expression (two bands per group) and quantification of TGF-β, smads 4–7, MMP-2 and its inhibitor TIMP-1 in normal, RVD-, and RVD+ELP-VEGF–treated kidneys. (B) Representative pictures (from stenotic kidneys) of the glomeruli (original magnification, ×40), shown as examples to illustrate podocin immunoreactivity (black arrows). (C) Representative trichrome pictures (from stenotic kidneys) of the glomeruli and tubules, and tubule-interstitial regions (original magnification, ×20, shown as examples to illustrate renal damage). *P<0.05 versus Normal; †P<0.05 versus RVD.
Stenotic Kidney Morphology and MV Remodeling
Glomerulosclerosis and tubule-interstitial fibrosis (7.3±0.01% and 9.3±0.04%, respectively, P<0.05 versus Normal) were significantly reduced (2.3±0.04% and 3.4±0.1%, respectively, P<0.05 versus RVD and Normal) after ELP-VEGF therapy (Figure 7C). Similarly, MV media-to-lumen ratio (0.34±0.01, P<0.05 versus Normal) was improved after ELP-VEGF therapy (0.18±0.005, P<0.05 versus RVD, P=NS versus Normal), suggesting attenuated MV remodeling in addition to the improvements in MV rarefaction.
Discussion
The current study focused on the design, characterization, and potential application of a novel ELP-VEGF construct for renal therapy. Our study showed that the construct is active in vitro and effective in vivo, since it stimulated proliferation, migration, and tube formation of cells, it accumulated in the injected kidney, significantly reduced renal injury, and improved renal function in the stenotic kidney. Notably, this novel compound showed a distinct protective effect on the stenotic kidney microvasculature by reducing MV remodeling and rarefaction and promoting generation of new cortical and medullary microvessels, which likely led the recovery of RBF and GFR. These findings suggest an exciting new therapeutic application of a compound that has not, to our knowledge, been previously tested in renal disease. The enhancement of the therapeutic actions without decreasing the potency or inducing collateral effects of VEGF therapy may represent a breakthrough in targeted approaches for the kidney that could go beyond RVD. Thus, our study provides a first step for pharmacologic validation of a novel drug candidate that could promote the translation of this technology toward the ultimate goal of clinical application.
CKD is a progressive disorder affecting almost 14% of the general population. It is an independent risk factor for cardiovascular morbidity and mortality, as patients with diagnosed cardiovascular disease show a staggering 40.8% prevalence of CKD, a number that has doubled in less than 20 years.13 Chronic RVD can progressively deteriorate renal function and lead to CKD and ESRD. It affects between 9% and 11% of the general population, but this number goes up in older patients or those with diagnosed coronary artery or peripheral vascular disease.1,3,14
Although renal arterial stenosis and the resultant decrease in blood flow is the initial and possibly main instigator of renal injury in RVD, therapeutic strategies that aim to resolve the vascular obstruction such as renal angioplasty and stenting are effective in recovering renal function in less than half of the cases. The disparity between technical success and outcomes has served as the impetus for numerous trials to assess the efficacy of current medical therapy (that includes renin-angiotensin blockers, lipid-lowering drugs, and so on) versus renal angioplasty. Currently, the bulk of evidence shows no significant advantages of renal angioplasty compared with standard medical therapy that could justify the risk of undergoing revascularization procedures.2 These observations emphasize a need for development of new treatments and strategies to improve the renal recovery prospects and outcomes in RVD.
Previous studies (including ours) showed that the progressive damage of the stenotic kidney MV architecture in RVD is accompanied (and likely mediated) by decreased expression and availability of renal VEGF and downstream effectors, suggesting blunted renal angiogenesis.7,15–17 It is possible that disruption of VEGF signaling in the kidney initiates MV rarefaction or alternatively, the absence of VEGF exacerbates the progressive damage of the renal microvasculature. VEGF is a pivotal angiogenic cytokine that maintains MV networks everywhere in the body and is crucial for MV repair and proliferation via increasing recruitment of progenitor cells.18,19 Furthermore, VEGF in the kidney is crucial for the health, integrity, and function of podocytes, key components of the glomerular filtration barrier.20,21 Thus, it is clear that VEGF plays an important role in the kidney that goes beyond the angiogenic effects.
Recent studies from our laboratory as well as others have shown that administration of exogenous VEGF protects the kidney.6,7,22,23 We showed that improvement of renal VEGF signaling by a single administration of recombinant-human VEGF165 into the stenotic kidney6,7 expanded the MV architecture, improved MV rarefaction and remodeling, and decreased fibrosis, accompanied by improved hemodynamics and filtration function. Our results support the feasibility of therapeutic angiogenesis by VEGF therapy to recover renal function in RVD. However, while these data were promising, there were limitations that prevent it from being practical in the clinical setting (e.g., short t1/2 of VEGF, ease of delivery, rapid degradation, and tissue targeting). Such limitations may have played a role in the persistence of some of the renal damage despite VEGF therapy being administered at an early stage of RVD. Therefore, novel strategies to enhance the capability of VEGF therapy may have a significant impact for a rapid application of this new treatment into clinical practice.
The use of ELP technologies as a therapeutic tool, to our knowledge, has not been previously tested for renal therapy. In the current study we characterized a novel ELP-VEGF construct in vitro and in vivo. The in vitro studies showed that ELP-VEGF could stimulate proliferation, tube formation, and migration of primary human glomerular endothelial cells at doses equivalent to unconjugated VEGF121, and that fusion to ELP did not alter the potency of VEGF. These results are consistent with our recent study showing that both unconjugated VEGF and ELP-VEGF induce such effects in HUVEC cells with equal potency.12 We also observed that injected ELP-VEGF constructs in the swine have a prolonged t1/2 (concurring with our recent data12) and are retained in the kidney in vivo, which supported the feasibility for a following testing of the therapeutic ability of the ELP-VEGF to protect the kidney in our RVD model. We observed that single intrarenal ELP-VEGF treatment improved RBF and GFR of the stenotic kidney, parameters that remained attenuated in those placebo- or ELP-control–treated kidneys. Furthermore, improvements in stenotic kidney RBF and GFR were greater than unconjugated VEGF121 therapy, highlighting a superior efficacy of ELP-VEGF to recover renal function.
The recovered stenotic kidney RBF and GFR were accompanied by a decreased in the MV media-to-lumen ratio and a significant expansion of both cortical and medullary MV density, which was evident in all vessels with diameters under 500 μm. We showed that intrarenal VEGF165 therapy expands the renal microvasculature, but this effect was evident only in those microvessels of smaller diameters (under 200 μm),6,7 which was also observed after intrarenal administration of unconjugated VEGF121 in the current study. The expansion of the smaller renal microvasculature suggests MV sprouting from preexisting vessels (angiogenesis) possibly via VEGF-integrins interactions.7,24 On the other hand, the expansion in renal microvessels of all sizes after ELP-VEGF therapy supports a potential effect on the renal microcirculation possibly driven by both angiogenesis and also by improved repair and remodeling of the preexisting vasculature. However, we believe it is unlikely that our treatment induced proliferation of larger microvessels (e.g., interlobar arteries). Speculatively, augmented MV density of larger vessels may reflect improved MV remodeling possibly driven by increased transmitted pressure from the expanded downstream smaller microvasculature. However, further studies are needed to elucidate this possibility.
The expansion of the renal microvasculature was accompanied and likely mediated by the distinct increased expression of VEGF and the Flk-1 receptor, Ang-1 (which may have improved the maturation and accelerated the functionality of the newly generated vessels25), Ang-2 (which is proangiogenic when tissue levels of VEGF are high26), and the Tie-2 receptor, and augmented p-Akt.27 These are all crucial downstream effectors of the VEGF-mediated angiogenic cascade and suggest a potential restoration of proangiogenic activity. Furthermore, administration of ELP-VEGF improved the expression of SDF-1 and CXCR4. Together with angiopoietins, these are important promoters of mobilization and homing of cell progenitors,28 which are crucial steps to achieve angiogenesis that have been shown to be stimulated by VEGF.29 In addition to the protective effects on the renal MV architecture, intrarenal ELP-VEGF therapy improved glomerular expression of podocin, reduced the excretion of nephrin (both major podocyte slit diaphragm-associated proteins30,31), and reduced albuminuria, implying a reduction in podocyte damage. Since podocytes are both targets and sources of VEGF in the kidney,32–34 ELP-VEGF administration may have in turn stimulated a positive feedback mechanism that could have potentiated podocyte production of VEGF and contributed to renoprotection.
Additional benefits of renal ELP-VEGF therapy include a potential attenuation in proinflammatory and fibrogenic activity. Indeed, the renal concentration of TNF-α was reduced in the stenotic kidney after ELP-VEGF treatment, suggesting a potential reduction in the renal inflammatory milieu. Furthermore, we also observed a distinct reduction in the renal expression of TGF-β/smad-4, TIMP-1, and augmented MMP-2 after ELP-VEGF therapy, which are pivotal mediators for extracellular-matrix accumulation and turnover that may determine the expansion of the MV networks.35,36 The TGF-β pathway is an important promoter of renal fibrosis often involved in renal disease from different etiologies.37 In addition, TGF-β can also lead to renal fibrosis by promoting endothelial-to-mesenchymal transition and blunting angiogenesis,38 an effect that may have contributed to MV rarefaction and the subsequent improvement following treatment. Therefore, an improvement in MMP-2/TIMP-1 coupled with an augmented MV proliferation may explain the expansion of the renal MV architecture after ELP-VEGF therapy. Consequently, the ELP-VEGF–treated kidney showed a reduction of renal fibrosis at both the glomerular and tubule-interstitial level.
It is possible that the greater renoprotective effects of ELP-VEGF therapy were mediated by a combined increased in tissue binding and extended t1/2 (or alternatively a slow plasma clearance) of the construct.11 These properties of ELP-VEGF may have extended the time for VEGF to bind to its receptors widely distributed in vascular endothelial cells, glomerular cells, and podocytes,32,39 and amplified autocrine/paracrine actions of VEGF in the kidney,40,41 as in turn may explain the superior efficacy over unconjugated VEGF therapy. However, a potential limitation of our study may be that the model may represent an early stage of RVD and that only one time-point was evaluated, since RVD in humans is a slowly progressive disease and intrinsic kidney damage develops over months and years. Future studies are needed to determine whether the protective actions of ELP-VEGF may persist for a longer term or could also be effective when applied at more advanced stages of RVD. Such studies may contribute for the understanding and translation of the findings to human RVD. We are also aware that the improvements in renal expression of angiogenic or fibrogenic factors after ELP-VEGF therapy are associations and a cause-effect relationship or in-depth mechanisms were not determined. However, the clear improvements in stenotic kidney outcomes after ELP-VEGF therapy are major strengths of this preclinical study that support the potential application of ELP technology to enhance the renal therapeutic potential of VEGF supplementation. Application of ELP-VEGF therapy for the kidney may help many patients who have intermediate renal artery stenosis that, if untreated, could progress to more severe RVD and subsequent development of CKD with the added increase in cardiovascular risk.42,43 Furthermore, although the scope of the current manuscript was on the renal therapeutic effects of the constructs in RVD, it is possible that the implications of our study may go beyond renal injury and open new avenues for potential application of our VEGF delivery system to target vascular injury in other tissues. Achieving high levels of the construct in renal tissue without decreasing the activity of the therapeutic factor (VEGF) may provide the basis for new studies to determine the use of ELP in other models of acute and/or chronic renal diseases.
Concise Methods
In vitro Studies
Generation of Constructs and Purification of Polypeptides
The coding sequence for human VEGF121 was fused in frame with the ELP coding sequence, and the chimeric protein was recombinantly expressed and purified, as recently described.12 For in vitro comparison studies, recombinant human VEGF121 was used (ProSpec, East Brunswick, NJ).
Cell Culture
Culture studies were performed in primary HGME cells. Cells in passage 4–13 were used for all performed experiments. For details, please see Supplemental Material.
HGME Proliferation Assay
HGME cells were seeded at 10,000 cells/well in 96-well plates. Viable cells were detected after 72 hours of exposure to test agents using the MTS cell proliferation assay (Promega, Madison, WI). For details, please see Supplemental Material.
HGME Tube Formation Assay
Cells were seeded in 24-well plates (sterile and nontissue culture treated) coated with growth factor reduced Matrigel (BD Biosciences). After a 5-hour incubation with test agents, five nonoverlapping fields per well were imaged, and the tubes between two cell nodes were counted for each field, averaged for each well, and expressed relative to untreated wells. For details, please see Supplemental Material.
HGME Migration Assay
Corning BioCoat growth factor reduced Matrigel Invasion Chambers (Corning) were used to quantify HMGE migration. Membranes were photographed using an inverted microscope and ×10 magnification objective on five independent fields per membrane. The number of cells per field were counted and averaged for each well, and the data are expressed relative to untreated wells. For details, please see Supplemental Material.
Labeling ELP-VEGF with Fluorescent Probes
ELP-VEGF was labeled with Alexa Fluor 633 succinimidyl ester (Life Technologies), as recently described.12
In vivo determination of Pharmacokinetics and Biodistribution after Intrarenal Administration in the Swine Model
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all studies, and all animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals. Three pigs were anesthetized and intrarenally injected with Alexa Fluor 633-labeled ELP-VEGF to achieve a dose of 1 mg/kg body wt. Blood was sampled from a previously placed venous catheter (jugular vein) at 1, 3, 5, 15, and 30 minutes after injection and every 30 minutes thereafter for 4 hours, and plasma was collected and frozen after centrifugation. At 4 hours, the pigs were euthanized by an overdose injection of sodium pentobarbital (100 mg/kg), and the organs were removed for analysis. For details, please see Supplemental Material.
In vivo Renal Functional Studies
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all the studies. Twenty-nine prejuvenile domestic pigs (Sus scrofa domestica) were used for the study, which lasted a total of 10 weeks. In 22 pigs, unilateral renal artery stenosis was induced and blood pressure continuously measured by telemetry, as described.4,7,44 Six weeks after induction of RVD, the degree of renal artery stenosis was quantified in all pigs by renal angiography, as described.4,7,44 In vivo helical MDCT flow studies were then performed for quantification of basal single-kidney RBF, perfusion, and GFR. CT-derived quantifications of renal hemodynamics were repeated during suprarenal infusion of acetylcholine to test endothelium-dependent renal MV endothelial function responses, as described.4 Immediately after completion of the MDCT studies, and while still under anesthesia, all RVD animals were treated with a single intrarenal (stenotic kidney) infusion of vehicle (RVD, n=7), ELP (100 μg/kg, RVD+ELP, n=5), or ELP-VEGF (100 μg/kg, RVD+ELP-VEGF, n=7). In addition, a smaller group of pigs were treated with a single intrarenal administration of unconjugated VEGF121 (at a dose of 18.65 μg/kg, which is an equimolar dose that matches the concentration of VEGF in the ELP-VEGF construct) and serve as treated controls to determine the differences in therapeutic efficacy between ELP-VEGF and unconjugated VEGF (RVD+VEGF, n=3). Blood and urine were collected (at 6 and 10 weeks) to measure plasma creatinine, nephrin in urine (suggestive of podocyte damage), and albuminuria, following vendors’ instructions. Pigs were then observed for 4 additional weeks and then MDCT in vivo studies repeated. After completion of all the in vivo studies, the pigs were euthanized, kidneys removed, and ex vivo studies performed, as shown.6,45,46 For details, please see Supplemental Material.
High-Resolution CT Imaging
MDCT analysis was used to calculate single-kidney RBF (ml/min), GFR (ml/min), and renal perfusion (ml/minute per cc tissue), using previously validated methods.4,47,48 For details, please see Supplemental Material. Micro-CT reconstruction and quantification of renal MV density was performed as extensively described.7,16 For details, please see Supplemental Material.
Ex vivo Studies
Protein expression and renal morphology were assessed in Normal, RVD, and RVD+ELP-VEGF pigs.
Western Blotting
Standard blotting protocols were followed, as described,5,46 to determine renal expression of VEGF, the specific receptor Flk-1, proangiogenic Ang-1 and Ang-2, and the Tie-2 receptor, p-Akt, SDF-1, and its receptor CXCR4. Furthermore, antiangiogenic angiostatin, tissue-remodeling factors TGF-β and mediators smads-4 and -7, and MMP-2 and its inhibitor TIMP-1 (all obtained from Santa Cruz Biotechnology, CA) were also measured. For details, please see Supplemental Material.
Histology
Midhilar 5-μm cross-sections of each kidney (one per animal) were examined to quantify tubule-interstitial fibrosis, glomerulosclerosis, and media-to-lumen ratio as described.4,7 Additional cross-sections were used to determine glomerular expression of podocin. For details, please see Supplemental Material.
Statistical Analyses
Results are expressed as mean±SD or SEM as indicated. Comparisons within groups were performed using paired t test, and among groups using one-way ANOVA, with Bonferroni correction for multiple comparisons. Statistical significance was accepted for P≤0.05.
Disclosures
A.R.C. serves as a consultant for Actelion Pharmaceuticals US, Inc. G.L.B. is owner of Leflore Technologies LLC, a private company working to commercialize ELP-based technologies in several disease areas.
Supplementary Material
Acknowledgments
This work was supported by grants HL095638, HL51971, and GM104357 (A.R.C.), and HL121527 (G.L.B.) from the National Institutes of Health, and by grant 18490005 from the American Heart Association (A.R.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040346/-/DCSupplemental.
References
- 1.Textor SC, Lerman LO: Reality and renovascular disease: when does renal artery stenosis warrant revascularization? Am J Kidney Dis 63: 175–177, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr, Dworkin LD CORAL Investigators : Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA: High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO: Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO: Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chade AR, Kelsen S: Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am J Physiol Renal Physiol 302: F1342–F1350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR: Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant 25: 1079–1087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidwell GL: Peptides for cancer therapy: a drug-development opportunity and a drug-delivery challenge. Ther Deliv 3: 609–621, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Bidwell GL 3rd, Davis AN, Fokt I, Priebe W, Raucher D: A thermally targeted elastin-like polypeptide-doxorubicin conjugate overcomes drug resistance. Invest New Drugs 25: 313–326, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Bidwell GL 3rd, Raucher D: Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv Drug Deliv Rev 62: 1486–1496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George EM, Liu H, Robinson GG, Bidwell GL: A polypeptide drug carrier for maternal delivery and prevention of fetal exposure. J Drug Target 22: 935–947, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George EM, Liu H, Robinson GG, Mahdi F, Perkins E, Bidwell GLI 3rd: Growth factor purification and delivery systems (PADS) for therapeutic angiogenesis. Vasc Cell 7: 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2012 [Google Scholar]
- 14.Textor SC, Misra S, Oderich GS: Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int 83: 28–40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J 20: 1706–1708, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO: Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol 24: 1854–1859, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR: Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO: Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eirin A, Zhu XY, Li Z, Ebrahimi B, Zhang X, Tang H, Korsmo MJ, Chade AR, Grande JP, Ward CJ, Simari RD, Lerman A, Textor SC, Lerman LO: Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol 33: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ollero M, Sahali D:. Inhibition of the VEGF signalling pathway and glomerular disorders [published online ahead of print December 4, 2014]. Nephrol Dial Transplant 30: 1449–1455, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Chade AR, Kelsen S: Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv 3: 376–383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard EC, Friedrich JL, Basile DP: VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE: Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol 28: 1703–1713, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Qin D, Trenkwalder T, Lee S, Chillo O, Deindl E, Kupatt C, Hinkel R: Early vessel destabilization mediated by Angiopoietin-2 and subsequent vessel maturation via Angiopoietin-1 induce functional neovasculature after ischemia. PLoS One 8: e61831, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobov IB, Brooks PC, Lang RA: Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A 99: 11205–11210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dellinger MT, Brekken RA: Phosphorylation of Akt and ERK1/2 is required for VEGF-A/VEGFR2-induced proliferation and migration of lymphatic endothelium. PLoS One 6: e28947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO: Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells 28: 1039–1047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY: VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res 91: 402–411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tufro A, Veron D: VEGF and podocytes in diabetic nephropathy. Semin Nephrol 32: 385–393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Dong J, Yuan L, Liang C, Ren K, Zhang W, Fang F, Shen J: Nephrin and podocin loss is prevented by mycophenolate mofetil in early experimental diabetic nephropathy. Cytokine 44: 85–91, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Bertuccio C, Veron D, Aggarwal PK, Holzman L, Tufro A: Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem 286: 39933–39944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feliers D, Gorin Y, Ghosh-Choudhury G, Abboud HE, Kasinath BS: Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species. Am J Physiol Renal Physiol 290: F927–F936, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Foster RR, Satchell SC, Seckley J, Emmett MS, Joory K, Xing CY, Saleem MA, Mathieson PW, Bates DO, Harper SJ: VEGF-C promotes survival in podocytes. Am J Physiol Renal Physiol 291: F196–F207, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Cauchard JH, Robinet A, Poitevin S, Bobichon H, Maziere JC, Bellon G, Hornebeck W: UVA-mediated down-regulation of MMP-2 and MT1-MMP coincides with impaired angiogenic phenotype of human dermal endothelial cells. Biochem Biophys Res Commun 345: 681–687, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Kargiotis O, Chetty C, Gondi CS, Tsung AJ, Dinh DH, Gujrati M, Lakka SS, Kyritsis AP, Rao JS: Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene 27: 4830–4840, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Böttinger EP, Bitzer M: TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, Chander PN, Goligorsky MS: Curtailing endothelial TGF-beta signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol 26: 817–829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas S, Vanuystel J, Gruden G, Rodríguez V, Burt D, Gnudi L, Hartley B, Viberti G: Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol 11: 1236–1243, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Veron D, Villegas G, Aggarwal PK, Bertuccio C, Jimenez J, Velazquez H, Reidy K, Abrahamson DR, Moeckel G, Kashgarian M, Tufro A: Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts alphaVbeta3 integrin signaling in the glomerulus. PLoS One 7: e40589, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villegas G, Lange-Sperandio B, Tufro A: Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int 67: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Rump LC, Stegbauer J: Coronary artery stenosis: a new risk factor for chronic kidney injury? Kidney Int 87: 676–677, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Sun D, Eirin A, Zhu XY, Zhang X, Crane JA, Woollard JR, Lerman A, Lerman LO: Experimental coronary artery stenosis accelerates kidney damage in renovascular hypertensive swine. Kidney Int 87: 719–727, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC: Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Chade AR, Stewart NJ, Peavy PR: Disparate effects of single endothelin-A and -B receptor blocker therapy on the progression of renal injury in advanced renovascular disease. Kidney Int 85: 833–844, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelsen S, He X, Chade AR: Early superoxide scavenging accelerates renal microvascular rarefaction and damage in the stenotic kidney. Am J Physiol Renal Physiol 303: F576–F583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO: Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 243: 405–412, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO: Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.