Abstract
C3 glomerulopathy is a recently described form of CKD. C3GN is a subtype of C3 glomerulopathy characterized by predominant C3 deposits in the glomeruli and is commonly the result of acquired or genetic abnormalities in the alternative pathway (AP) of the complement system. We identified and characterized the first mutation of the C3 gene (p. I734T) in two related individuals diagnosed with C3GN. Immunofluorescence and electron microscopy studies showed C3 deposits in the subendothelial space, associated with unusual deposits located near the complement receptor 1 (CR1)-expressing podocytes. In vitro, this C3 mutation exhibited decreased binding to CR1, resulting in less CR1-dependent cleavage of C3b by factor 1. Both patients had normal plasma C3 levels, and the mutant C3 interacted with factor B comparably to wild-type (WT) C3 to form a C3 convertase. Binding of mutant C3 to factor H was normal, but mutant C3 was less efficiently cleaved by factor I in the presence of factor H, leading to enhanced C3 fragment deposition on glomerular cells. In conclusion, our results reveal that a CR1 functional deficiency is a mechanism of intraglomerular AP dysregulation and could influence the localization of the glomerular C3 deposits.
Keywords: immunology and pathology, C, glomerular disease
The newly recognized entity, C3 glomerulopathy (C3G), is a rare kidney disease characterized by the predominance of C3 deposits in glomeruli.1 The C3 deposits are localized to the mesangium, within or along the glomerular basement membrane (GBM). Based on the pattern of deposits observed by immunofluorescence (IF) and electron microscopy, two subtypes of C3G have been described: dense deposit disease (DDD) and C3GN.2,3 DDD and C3GN result from dysregulation of the Complement alternative pathway (AP).4,5 The AP is continuously activated at a low level by spontaneous hydrolysis of the internal thioester bond of C3, resulting in generation of C3(H2O).6,7 This pathway is amplified in the setting of activating surfaces such as bacteria or apoptotic and necrotic cells. To avoid excessive or undesirable autoamplification, the AP is tightly regulated in the fluid phase by the plasma regulatory protein factor H (FH) and on cell surfaces by three membrane proteins: membrane cofactor protein (MCP; CD46), complement receptor 1 (CR1; CD35), and decay accelerating factor (DAF; CD55). Together, these regulators act by preventing the formation of and dissociating the AP C3 convertase (FH, CR1, and DAF) and by serving as cofactors for factor I (FI)-mediated inactivation of C3b to iC3b (FH, MCP, and CR1).
DDD and C3GN are associated with low plasma C3 levels in more than 50% of cases.5,8,9 AP dysregulation is mainly acquired, induced by the presence of C3 Nephritic Factor (C3Nef), which is an autoantibody against the AP C3 convertase that stabilizes the enzymatic complex,10 or anti-FH autoantibodies.9–11 Less frequently, C3G has a genetic background, associated with mutations in the FH, FI, or CFHR5 genes.9,12 The sole reported mutation in the C3 gene was in DDD and revealed that fluid phase–restricted AP dysregulation, which caused continuous generation of C3 cleavage fragments in plasma, plays a major role in DDD pathogenesis.13
Here, we present for the first time the functional characterization of a C3 mutation identified in two related patients with C3GN. The mutation leads to a combined FH/CR1 functional deficiency causing a tissue-restricted AP dysregulation in the patients, and provides unique insights into the pathogenesis of C3GN. Moreover, in view of podocyte CR1 expression, this mutation provides clues for a role for CR1 in the specific intraglomerular localization of C3 deposits.
Results
Patients
The clinical presentations of the two related patients (patient II-1 and II-2; Figure 1A) are detailed in the Concise Methods section. In brief, patient II-1, a 45-year-old man, was referred to a nephrology department in December 1999 with nephrotic syndrome (proteinuria 3.37 g/day) associated with microscopic hematuria and ARF with serum creatinine level of 156 μmol (eGFR 32 ml/min per 1.73 m2).
Figure 1.
Pedigree with familial C3G and microscopy of kidney biopsies of patient II-1. (A) Pedigree demonstrating that two members of generation II carry the C3 mutation I734T (II-1 and II-2, black squares). (B) By light microscopy, patient II-1 presented with a membranoproliferative GN pattern characterized by hypertrophy of the mesangial matrix with hypercellularity secondary to infiltration by neutrophils and macrophages, and mesangial and subendothelial deposits. (C) Immunofluorescence staining was positive for C3. Staining for Ig was negative (not shown). Deposits were granular and localized in the mesangium and along the GBM, as well as in the subepithelial or subendothelial spaces. (D) Electron microscopy (original magnification, ×3000) showed subendothelial and voluminous extramembranous deposits (humps) (*) (E) Electron microscopy (original magnification, ×10,000) showed microvesicular structures (single arrow) and laminated thread-like structures (double arrow). (F, G) Recurrence of C3GN with a membranoproliferative GN pattern was observed after kidney transplantation in patient II-1 by light microscopy. (H) Immunofluorescence staining was positive only for C3. (I) Deposits were predominantly localized in the mesangium, as detected by electron microscopy.
The initial renal biopsy showed hypertrophy of the mesangial matrix and hypercellularity associated with mesangial, subendothelial, and big extramembranous deposits (humps), consistent with the diagnosis of membranoproliferative GN Figure 1B). Immunofluorescence staining was positive for C3 (Figure 1C). Staining for immunoglobulins was negative. By electron microscopy, deposits were granular, nonorganized, and localized to the mesangium, along the GBM, in the subendothelial space and associated with voluminous humps (Figure 1D). Moreover, microvesicular structures and laminated thread-like structures were observed in the extramembranous side of the GBM (Figure 1E). Interstitial fibrosis involved approximately 30% of the cortical area. Arterial vessels were normal.
This patient progressed to ESRD 23 months after diagnosis. Patient II-1 was transplanted. Kidney biopsies were performed at 3 and 34 months post-transplantation because of proteinuria and renal failure. They demonstrated recurrence of the disease with the same pattern of a C3GN (Figure 1, F–I).
His 43-year-old brother (patient II-2) had a kidney biopsy in November 2005 due to nephrotic syndrome. Results of his kidney biopsy showed similar findings by light microscopy and IF to his brother’s biopsy.
Assays for Complement Components, Genetic Analyses, and Protein Structure
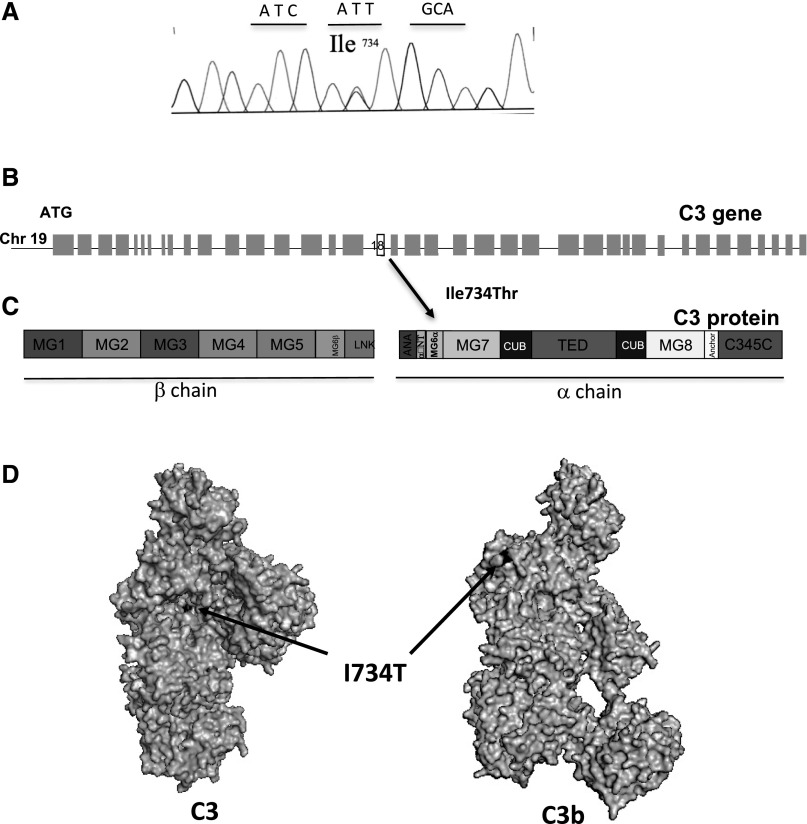
Both patients had normal plasma C3 and FB levels. The screening for C3Nef, anti-FH, anti-FB, and anti-C3 autoantibodies was negative. Patients II-1 and II-2 carry the same c.2327T>C heterozygous mutation in the C3 gene, leading to p.I756T (I734T, mature protein numbering) substitution on the surface of the MG6α domain of the protein (Figure 2). The mutation was not detected in the single nucleotid polymorphism databases (http://www.1000genomes.org/ and http://evs.gs.washington.edu/EVS/). No mutations were found in the other screened genes (FH, CFHR5, MCP, FB, and FI).
Figure 2.
Localization of C3 I734T mutation. (A) A chromatogram corresponding to the DNA sequence surrounding the mutated nucleotides in C3 of a patient carrying I734T. (B) Position within the C3 gene and (C) the primary structure of C3. (D) Position of the residue on the protein surface. Mapping of I734T on the surface of C3 and C3b was performed using Pymol software.
The mutation was not detected in the healthy father (I-1) and no sample was available for the mother, who died in 2006 from colorectal cancer (Figure 1A).
Functional Characterization of the I734T C3 Mutation
The I734T Mutation Leads to Complement AP Activation on the Cell Surface
Both patients had normal plasma C3 and FB levels. To assess complement activation on the cell surface, a GEnC cell line was used.14,15 GEnC expressed MCP, DAF, and CD59 but not CR1.16 Incubation of resting cells with 30% serum from normal healthy donors (NHS) led to physiologic C3 deposition. C3 deposition was higher after incubation with patients’ serum (Figure 3, A and B), similar to the increase observed with the FH-depleted (FH-dpl) serum.16–19 Incubation of apoptotic-necrotic cells with patient sera led to a significantly increased C5b-9 deposition as compared with NHS (Figure 3C). To determine whether the increase in C3 deposition was dependent on FH dysregulation, increasing doses of purified FH were added to patient sera and compared with FH-dpl serum and serum of patient with C-terminal mutation in FH. Addition of purified FH resulted in decreased C3 deposition on the cell surface in the same experimental conditions (Figure 3D).
Figure 3.
Complement AP activation on resting and apoptotic-necrotic glomerular endothelial cells. Cells were incubated with sera from C3 I734T patients. (A) C3 fragment deposition on resting GEnC in the presence of sera from NHS, serum depleted in FH (FH-dpl), and C3 I734T patient II-2. (B) C3 fragment deposition after incubation of apoptotic-necrotic GEnC with NHS or patient sera. (C) C5b-9 deposition on apoptotic-necrotic GEnC in the presence of NHS and patient sera. These analyses were carried out three times using patient II-1 serum. In every experiment the deposition was compared with a control NHS as well as a total of four NHS, which were very similar to the control NHS (not shown). A representative flow cytometry histogram is shown. C3 deposition obtained with patient II-2 and FH-dpl were normalized to the C3 deposition from the reference NHS on cells to compare the fold increase. (D) C3 deposition on apoptotic-necrotic GEnC in the presence of patients’ sera, FH-dpl serum, and serum of a patient with C-terminal mutation in FH (W1183R), supplemented with increasing concentrations of purified FH. The C3 fragments deposition in absence of FH was taken as 100% and the decrease at any dose of FH was calculated as a percentage of this basal level. Iso, isotope; RFI, relative fluorescent intensity.
The C3 deposits on kidney biopsy of our patients were localized to the vicinity of the podocytes that express CR1, a receptor and regulatory protein for C3 (Figure 4A). Therefore, complement activation on the podocyte surface was tested. An increase in deposits containing C3c and C3d was observed when utilizing the patients’ serum compared with normal serum (Figure 4, B and C). The increase was stronger with the anti-C3b/iC3b antibody compared with the anti-C3d antibody. The patients’ serum augmented C3 activation fragment deposition on the surface of the cells and on extracellular matrix between the cells.
Figure 4.
Complement AP activation on podocytes. (A) Podocytes express CR1 on their surface. Labeling of CR1 with anti–CR1-FITC was positive compared with the isotype control. (B, C) The deposition of C3 activation fragments on podocytes was compared after incubation with NHS and serum from patient II-1. (B) C3c staining (recognizing C3b/iC3b deposition). Stronger staining on the cell surface and in the extracellular matrix was observed with the patient serum (arrow). (C) C3d staining (recognizing primarily C3dg/C3d and to a smaller extent C3b/iC3b). Similar staining was detected on the cell surface but, in the presence of patient serum, deposits in the extracellular matrix were also observed (double arrow). Experiments were performed two times with same results.
C3I734T Binding to FB was Normal and Led to the Formation of a C3 Convertase Comparable to WT Control
The affected residue I734 is in a close proximity to the FB binding site of C3b, particularly in the loading form of the C3bB complex (Figure 5A).20–22 The interaction between recombinant C3 I734T and purified FB with or without FD was normal and no difference in C3 convertase formation was observed when comparing the C3 I734T mutant and the WT (Figure 5, B and C).
Figure 5.
AP C3 convertase formation by C3 I734T and cleavage of C3 I734T by the C3 convertase. (A) I734 position on the structures of C3b with FB in closed (refractory to cleavage by FD) and open (prone to cleavage by FD) conformations and the active convertase C3bBb. C3b is colored light gray and FB is dark gray. (B) The binding of FB to recombinant WT or C3 I734T protein bound to an anti-C3d mAb on the biosensor chip studied by SPR. Binding of FB was analyzed by flowing recombinant C3 WT (gray line) or C3 I734T (black line) over an anti-C3d antibody coated chip, followed by FB. Binding of FB to the mutant protein was similar to WT. (C) Formation of C3 convertase by injection of recombinant C3 WT (gray line) or C3 I734T (black line) on anti-C3d antibody coated chip surface, followed by injection of FB plus FD. Protein deposition on the chip was followed over time. No difference was observed in the two cases. (D) Hemolytic assay for the C3 convertase forming capacity of normal plasma and patient II-1 plasma on rabbit erythrocytes. Lysis was similar in the three different experiments. (E) Recombinant C3 WT and C3 I734T were tested for their capacity to be cleaved to C3b in the presence of a preformed solid-phase C3 convertase. Both α-chains of C3 WT and C3 I734T were cleaved to α′. The experiment was repeated twice with identical results.
The lysis of rabbit erythrocytes in the presence of either patient’s plasma was similar to that observed with normal plasma. Thus, patients with this C3 mutation are able to form a functional C3 convertase equivalent to WT (Figure 5D).
C3 I734T is Cleaved by the C3 Convertase to C3b I734T
To test if C3 I734T can be cleaved to the C3b form by a C3 convertase, recombinant C3 WT or C3 I734T were incubated in presence of preformed surface-bound C3 convertases in an ELISA plate. The cleavage pattern revealed a nearly identical time-dependent appearance of the α′ proteolytic band by Western blotting for C3WT and C3I734T (Figure 5E). Cleavage of C3 to C3b was measured by the generation of the α′ fragment. The experiment was repeated twice. We did not observe differences between WT and I734T (data not shown).
C3 I734T Binding to FH is Normal and Led to Normal Regulation of the C3 Convertase but is Associated with Lower FH-Mediated Cofactor Activity
The affected residue I734 is in close proximity to the FH binding site of C3b (Figure 6A)23 By surface plasmon resonance (SPR), C3(H20) I734T binding to FH was weaker than WT but the difference did not reach statistical significance (Figure 6B).
Figure 6.
Interaction of C3 I734T with FH was normal with normal regulation of C3 convertase on sheep erythrocytes, but was associated with decreased inactivation by FI. (A) The location of I734 on the structure of C3b in a complex with FH CCP1–4. I734 is close to the binding site of FH-CCP1. The arrow shows the position of the mutated residue, which is hidden by FH. C3b is shown in light gray and FH in dark gray. (B) The real-time binding of purified recombinant WT or C3 I734T to immobilized FH was studied by SPR. FH was coupled to the biosensor chip followed by injection of recombinant C3 WT (gray line) or C3 I734T (black line) over the chip. The affinity for FH was determined by fitting the sensorgrams with the ProteON Manager software. The affinity of recombinant C3 I734T and C3 WT for FH were similar. Results were also compared with those obtained with the mutant R139W, which has known functional consequences. (C) Hemolytic assays with sheep erythrocytes showed similar lysis of cells in the presence of plasma of patient II-1 compared with normal plasma (n=3). (D) Inactivation of C3(H2O) I734T to iC3 I734T by FI was decreased compared with C3(H2O) WT. Recombinant C3(H2O) I734T and C3(H2O) WT were incubated with FI and FH. Cleavage of C3b to iC3 was indicated by generation of α43 product and decrease of the α-chain. Commercially available plasma-derived C3, treated identically to the recombinant proteins to obtain C3(H2O) and incubated in presence of FH and FI, served as a positive control for these experiments. The experiment was repeated three times with statistically significant results.
Lysis of sheep erythrocytes was similar, when they were exposed to patient or normal plasma. (Figure 6C). Analysis of FH cofactor activity revealed that the cleavage of C3(H2O) I734T to iC3 I734T was significantly decreased compared with the WT, as measured by the appearance of the cleavage fragment α43 (Figure 6D).
C3I734T Binding to MCP was Normal and Associated with Normal MCP-Mediated Cofactor Activity
The affected residue I734 is removed from the putative MCP binding site of C3b (Figure 7A).24 Similar binding of C3 I734T and C3 WT to MCP (Figure 7B) and normal cleavage of C3(H2O) I734T to iC3 I734T were observed (Figure 7C).
Figure 7.
Interactions of C3 WT and C3 I734T was normally inactivated by FI in the presence of MCP. (A) Position of I734T on the structure of C3b in a complex with MCP. I734 is remote from the binding site for MCP. C3b is colored in light gray and MCP in dark gray. (B) The real-time binding of recombinant C3 WT or mutant C3 I734T to MCP was studied by SPR. MCP was coupled to the biosensor chip followed by injection of recombinant C3 WT (gray line) or C3 I734T (black line). The affinity for MCP was determined by fitting the sensorgrams with Langmuir 1:1 model by ProteON Manager software. The affinity of recombinant C3 I734T for MCP was similar compared with the C3 WT. (C) Cofactor activity of MCP for the inactivation of recombinant C3(H2O) to iC3 by FI. Inactivation of C3 I734T to iC3 I734T by FI and MCP was similar to that of C3 WT. Recombinant C3(H2O) I734T or C3(H2O) WT was incubated with FI and MCP. Cleavage of C3(H2O) to iC3 was measured by the generation of α43 fragment and decrease of the α-chain. The experiment was repeated three times with similar results.
C3 I734T Binding to CR1 was Decreased and Associated with Lower CR1-Mediated Cofactor Activity
The location of the CR1 binding site is not well characterized, but at least part of it was mapped to the C3b peptide 727–767 to which I734 belongs (Figure 8A).25–27 Kinetic analysis by SPR indicated more than two-fold reduction in the apparent binding affinity (Figure 8B).
Figure 8.
C3 binding to CR1 and cofactor activity of CR1 were decreased. (A) I734 position on the structure of C3b in a complex with CR1 (putative binding site in dark gray). I734 is close to the binding site of CR1. C3b is colored in light gray. (B) The real-time binding of purified recombinant C3WT or C3 I734T to CR1 was studied by SPR. CR1 was coupled to the biosensor chip and C3WT (gray line) or C3 I734T (black line) was flowed over the surface. The affinity for CR1 was determined by fitting the sensorgrams. The affinity of recombinant C3 I734T for CR1 was significantly decreased compared with C3 WT. (C) Cofactor activity of CR1 for the inactivation of recombinant C3(H2O) to iC3 by FI. Inactivation of C3(H20) I734T by FI in the presence of CR1 was decreased compared with inactivation of C3 WT. Recombinant C3 I734T and C3 WT were incubated with FI and FH. Cleavage of C3(H2O) to iC3 was indicated by generation of α43 fragment and decrease of the α-chain. The experiment was repeated four times with similar results.
To study the functional consequences of this lower binding to CR1, the capacity of FI to cleave C3 I734T in presence of CR1 was tested by Western blotting. The results demonstrate a dose-dependent appearance of the 43-kDa (α43) fragment and a dose-dependent decrease of the α-chain. In the presence of CR1, cleavage of C3(H2O) I734T to iC3 I734T was significantly decreased, pointing to a defect in cofactor activity of CR1 (Figure 8C).
Discussion
We report the initial identification and functional characterization of a C3 mutation in a familial case of C3GN. This mutation resulted in cell surface AP dysregulation, induced by a combined FH/CR1 functional deficiency. FH activity was mildly decreased whereas CR1 binding and function were markedly impaired. This mutation was identified in C3GN patients with subendothelial and subepithelial C3 deposits associated with unusual accumulation of debris of cellular membranes in the subepithelial space near the CR1-expressing podocytes. Therefore these deposits may be accounted for by the functional CR1 deficiency.
In silico analysis revealed that the C3 mutation I734T may not be associated with major structural changes. The mutant recombinant C3 was produced with preserved overall integrity in a mammalian expression system and both patients had normal plasma C3 and Bb levels. In addition, binding studies and hemolytic assays indicated that the mutant C3 protein binds FB normally and forms a functional C3 convertase equivalent to WT. Our findings made it unlikely that there was a dysregulation of the AP in the fluid phase. However, cellular models of Complement activation performed with plasma and sera of the patients demonstrated that, even in the heterozygous state, the C3 I734T mutation was associated with overactivation of the AP on the endothelial cell surface, leading to increased C3 deposits. Further, in one tested patient, elevated soluble C5b-9 levels were noted, highlighting the overactivation of the terminal pathway. Increased levels of C5b-9 were deposited also on apoptotic-necrotic cells incubated with patients’ serum. Since cellular debris of cell membranes was detected in the subepithelial space on the patients’ biopsies along with C3 fragments, the Complement system may have been overactivated locally in the glomeruli in these patients.
The central Complement component C3 interacts with multiple ligands, in particular the regulatory proteins of complement, including FH, MCP, and CR1. We showed that MCP binding and cofactor activity were intact. These results suggest that the mutated residue is not involved in the binding site of MCP, and that a functional MCP deficiency is not playing a role in the disease pathogenesis in this family. In contrast, the MG6α domain of C3, in which the mutation is located, is thought to be involved in interaction with FH.23 In addition, various studies, mainly based on site-directed mutagenesis, have shown that residues 727–767, which are located in the α′NT and MG6α domains of C3b engage CR1.25,27–29 Binding of the C3 mutant protein to purified CR1 was significantly decreased compared with the WT, leading to a marked decrease in cofactor activity of CR1. This result strengthens the notion that I734 in the C3 protein sits within the binding interface with CR1 and that C3 I734T induces a functional CR1 deficiency, which could contribute to disease pathophysiology. Moreover, the mutated residue is at the FH CCP1 binding site, according to the crystal structure of the C3b-FH1–4 complex.23 In vitro, the interaction of the mutant C3 protein with FH was near-normal, with preserved capacity of FH to regulate the mutant C3 convertase, suggesting that I734 is not a residue critical for the regulation of the C3 convertase formed with the C3 mutant. The C3 deposition from patients’ serum was increased on GEnC cells compared with NHS sera, but was efficiently controlled by addition of purified FH. These cells express MCP but not CR1 and Complement control is dependent on the presence of functional FH.16,19,30 In addition, the cofactor activity of FH was moderately decreased in vitro. The mutation is located at the area of the putative FI binding site, during the formation of the trimolecular complex of C3b-FH-FI.31 Therefore it could be suggested that the mutation does not alter FH binding, but alters the binding of FI to C3b in the presence of FH, which could contribute to disease pathophysiology. Taken together, these results indicate that the C3 mutation induced a perturbation of the FH function but that it could be corrected by excess FH.
C3 mutations have been described in another renal disease associated with AP dysregulation, atypical hemolytic uremic syndrome (aHUS), and commonly result in alteration of C3 interactions with FH and MCP.19,32–35 However, the location and the functional consequences of the C3 mutations in aHUS are different from the mutation reported here. The aHUS mutations affect mainly the binding of MCP and FH but not CR1,33,35 while the mutation found here in C3GN affected mainly the binding and function of CR1, moderately affected FH, and did not affect MCP.33
Martinez-Barricarte et al. reported the first C3 mutation (C3923ΔDG) associated with DDD.13 Despite the close proximity between I734T and 923ΔDG on the C3 and C3b protein surface, their functional consequences were very different. While both C3 I734T and C3 923ΔDG were resistant to proteolysis by FI in the presence of FH and not MCP, C3 923ΔDG generated an active AP C3 convertase, resistant to decay by FH, and resulting in fluid-phase C3 consumption. However, mutant C3 923ΔDG could not be cleaved by a WT C3 convertase, leading to its accumulation in plasma, in contrast to C3I734T, which was cleaved comparable to WT. Results of functional characterization of both C3 mutations published in DDD and found here in C3GN highlight that DDD is associated with fluid-phase AP dysregulation, while cell membrane AP dysregulation is seen in C3GN. However, as in DDD, C3 I734T mutation remains a unique observation in C3GN. Only the I734T mutation in the C3 gene was associated with a familial form of C3GN in the French C3GN cohort, which includes 174 patients.
The inability of CR1 to regulate the AP has not been reported in the context of C3G. CR1 is expressed on most circulating cells including erythrocytes, neutrophils, B-lymphocytes, and monocytes, but not on endothelial cells. Interestingly, the only site of CR1 expression in the kidney is on podocytes.36–39 An assessment of complement AP activation on podocytes demonstrated overactivation in the presence of patients’ serum compared with NHS with the accumulation of iC3b fragments on the cell surface and on the extracellular matrix. However, interestingly, labeling for C3d showed a similar signal in the presence of patients’ serum on the surface of the cells compared with the normal serum, but the labeling was more increased in the extracellular matrix with patients' sera compared with NHS. Since CR1 is the only Complement cofactor protein able to assist FI for the cleavage of iC3b to C3dg, the defect in the interaction of the mutant iC3b with CR1 could result by the inefficient conversion of iC3b to C3dg/C3d and therefore accumulation of iC3b on podocytes. Taken together, these results indicate that a functional CR1 deficiency may result in C3 fragment deposition on the podocyte side of the GBM.
In C3G, C3 deposits are composed of C3b or C3b metabolites resulting from proteolysis of C3b by FI and its cofactors, iC3b, C3c, and C3dg.40,41 Results obtained from experimental models of FH and FI deficiency in mice demonstrated that the nature of the C3 activation products (iC3b, C3d, C3dg, or C3b) is an important determinant for the localization of the C3 deposits.42 The functional consequences of the C3 I734T mutation suggest that the predominant form of C3 protein in the deposits might be C3b and not iC3b, having the capacity to amplify the C3 deposits at the tissue level.
Moreover, both patients presented with voluminous extramembranous C3 deposits (humps) and debris from the GBM in the subepithelial space. We propose that lower cofactor activity of CR1 on podocytes could predispose to inefficient clearance and accumulation of C3 deposits and debris in the subepithelial space. The mutant C3 protein is able to form initial functional C3 convertase in fluid phase leading to C3 deposits in the glomerular endothelial cells. Due to the impaired FH cofactor activity the amplification of the AP leads to increased C3 deposits along the subendothelial space, with diffusion toward the subepithelial space in the area of lacking functional CR1. Therefore, there may be a limited cleavage of C3b/iC3b to C3 fragments with lower molecular weight, which can be eliminated in the urine (Figure 9). Unfortunately rodents lack a human CR1 ortholog, which precludes in vivo analysis to confirm the role of CR1 in C3GN.
Figure 9.
Schematic diagram of AP dysregulation caused by the C3 I734T mutation leading to glomerular C3 deposits. Generated C3b and its cleavage fragments from the capillary lumen (CL) cross the fenestrated glomerular endothelium (GEc) and reach the subendothelial space and the mesangium (M) in the vicinity of mesangial cells (Mc), where C3b is only partially regulated by FH, leading to the accumulation of C3b and iC3b in these areas. C3b and iC3b fragments cross the GBM toward the subepithelial space. They cannot be appropriately regulated by CR1 expressed on the foot podocytes, resulting in iC3b/C3d accumulation (humps). US, urinary space.
Immunosuppressive treatment of the two patients was unsuccessful, as they rapidly reached ESRD. Eculizumab is an approved monoclonal anti-C5 antibody which blocks the activity of the terminal Complement pathway.43 Eculizumab seems to be associated with better efficacy in cases with high levels of plasma sC5b-9 and may be used in the case of mutations such as the one described here. Our results suggest that soluble CR1, which was tested in DDD,44 would be inefficient in our patients due to impaired interaction of the mutant C3b with CR1.
In conclusion, the functional consequences of the C3 mutation reported here are distinct from those previously reported for aHUS and DDD. Two mechanisms are implicated in the C3 deposits observed in this case of C3GN. An FH defect of the C3b degradation by FI on the endothelial cells, and a functional CR1 deficiency on podocytes provide an explanation for the C3 deposits in the subendothelial and subepithelial spaces. These defects participate in the disease pathogenesis. In view of the broader spectrum of anti-C therapeutics that may be available to the clinicians in the near future (especially soluble CR1),44 our results highlight the importance of studying CR1 function in C3G.
Concise Methods
Patients
Between 2000 and 2014, 174 patients were referred to the Immunology Laboratory at Hôpital Georges Pompidou, Paris, France, for Complement exploration in the context of C3G. Two related patients (patient II-1 and II-2, Figure 1A) who were diagnosed with C3GN were recruited from two French departments of nephrology and carried the same heterozygous mutation in the C3 gene. Patients gave their informed consent.
Patient II-1, a 45-year-old man, was referred to a nephrology department in December 1999 with nephrotic syndrome (proteinuria 3.37 g/day) associated with microscopic hematuria and ARF, with a serum creatinine level of 156 μmol (eGFR 32 ml/min per 1.73 m2). Testing for hepatitis B virus and hepatitis C virus, antinuclear antibodies, anti–double-stranded DNA antibodies, monoclonal gammopathy, and cryoglobulinemia was negative. Patient II-1 was transplanted. Kidney biopsies were performed at 3 and 34 months post-transplantation because of proteinuria and renal failure. He died in 2010 from lung cancer.
His 43-year-old brother (patient II-2) was referred to a department of nephrology in November 2005 because of nephrotic syndrome (proteinuria 3.24 g/day) associated with hematuria, high BP, and renal failure, with a serum creatinine of 204 μmol/l (eGFR 43 ml/min per 1.73 m2). Testing for hepatitis B virus, hepatitis C virus, antinuclear antibodies, double-stranded DNA antibodies, monoclonal gammopathy, and cryoglobulinemia was negative. This patient progressed to ESRD 20 months after diagnosis. In 2009, he was transplanted. C3GN recurrence was determined by kidney biopsy 11 months after transplantation.
Assays for Complement Components and Genetic Screening
All immunologic and genetic analyses were performed in a reference laboratory for the investigation of the Complement system (European Hospital Georges Pompidou, France). EDTA plasma samples were obtained from both patients. Plasma concentrations of C3, FB, FH, and FI were measured as previously described.14 C3Nef activity was determined by assessing the ability of purified plasma IgG to stabilize the membrane-bound C3bBb convertase.45 Anti-FH, anti-C3, and anti-FB antibodies were looked for using ELISA as previously described.46,47 Genomic DNA for direct sequencing was obtained from peripheral blood leukocytes. Direct sequencing of all FH, FB, FI, MCP, CFHR5, and C3 exons was performed as previously described.9,32
Structure Analysis
The crystal structures of C3,26 C3b,48 C3bBD,20 C3bBb,49 and C3bFH1–423 were obtained from the Protein Data Bank. Molecular graphic imaging and analysis were produced using a Pymol and UCSF Chimera package. For this study, the numbering will be according to the mature protein sequence (without the 22-amino acid long leader peptide).
Assays Utilizing Patients’ Plasma and Sera
Hemolytic Assays
Rabbit erythrocytes and sheep erythrocytes were used in hemolytic assays to evaluate the functionality of the C3 convertase formed from patients’ plasma. Plasma samples from NHS and the patients were diluted in veronal buffer (7 mM MgCl2 and 10 mM EGTA, pH 7.4) and incubated with 100 μl 108 erythrocytes/ml at 37°C for 30 minutes. Hemolysis was detected by determining the OD at 414 nm. Control experiments were performed in the presence of EDTA.
The sheep erythrocyte surface is highly sialylated, allowing for FH binding and protecting the surface from C3 convertase formation.50,51 However, in the presence of certain FH mutations or FH blockade by anti-FH antibodies in the plasma, a dose-dependent lysis is seen.46,50 Therefore plasma from an aHUS patient with a mutation in CCP20 of FH, W1183R, was used as a positive control in the hemolytic test with sheep erythrocytes as described elsewhere46,50. This mutation has been identified in several cohorts in the context of aHUS46,50. This mutation is localized to CCP20 of FH that is implicated in the binding of FH to C3b, C3d, and glycosaminoglycans52.
Endothelial Cell Assays
Conditionally immortalized cells from a GEnC cell line, described in detail by Satchell et al.,15 were used for this study. GEnC were grown in 24-well plates. They were allowed to proliferate at 33°C until reaching confluency and then transferred to 37°C for 7 days. The cells were cultured in complete endothelial cells growth medium 2 (Lonza). The experiments were performed as described previously for analysis of aHUS patients’ sera.16–19
Briefly, after washing with PBS, adherent cells were incubated with NHS or patient serum (diluted at 1:3 with M199 medium to a final volume of 300 μl) for 30 minutes at 37°C, with 5% CO2. At the end of the incubation, the cells were washed again with PBS and the adherent GEnC were detached by a nonenzymatic procedure using PBS-5 mM EDTA and 5 mg/ml lidocaine.
The cells that spontaneously detached overnight from the confluent monolayer were shown to be apoptotic-necrotic (100% annexin V–positive and over 70% propidium iodide–positive). The apoptotic-necrotic cells were transferred to FACS tubes and incubated with 150 μl of serum diluted at 1:3. To test the effect of FH on the regulation of C3 deposition from normal or patients’ sera, apoptotic-necrotic GEnC were incubated in the presence of increasing concentrations of purified FH (Complement Technologies, Tyler TX; 0–100 μg/ml). In this experiment, we used serum from a patient with aHUS carrying an FH mutation W1183R with well characterized functional consequences as a control52. Apoptotic-necrotic cells are less protected from Complement attack and allow activation of Complement until the activation of the terminal pathway with formation of the C5 convertase and sublytic C5b-9; sublytic C5b-9 is not formed on the surface of resting endothelial cells. Adherent cells and apoptotic-necrotic cells were labeled with an anti-C3c mAb (Quidel), or an anti–C5b-9 mAb (kind gift from Prof. Paul Morgan, Cardiff, UK) or a control IgG1, followed by phycoerythrin-labeled secondary antibody (Beckman Coulter, Marseille, France). Cells were analyzed by flow cytometry on a Becton Dickinson FACS Calibur using CellQuest software. The results are expressed in relative fluorescent intensity, where the mean fluorescent intensity of every sample was divided by the mean fluorescent intensity of the isotype control.
Podocyte Assays
A conditionally immortalized human podocyte cell line, described in detail by Saleem et al.53, was used for this study. Podocytes were grown on collagen I-coated slides, allowed to proliferate at 33°C until reaching approximately 70% confluence and then transferred to 37°C for 14 days. The cells were cultured in RPMI 1640 medium supplemented with penicillin, streptomycin, glutamine, insulin, transferrin, and sodium selenite (Sigma Chemicals, Dorset, UK) and 10% FCS. Podocytes were fixed with paraformaldehyde and labeled with an anti–CR1-FITC antibody (BioLegend) or an isotype control (BioLegend). Alternatively, the cells were incubated for 30 minutes at 37°C with NHS or serum of patient II-1 diluted 1:4 in RPMI 1640, washed with PBS, and fixed with 4% paraformaldehyde. Cells were then stained with rabbit anti-human C3c (Dako) or biotinylated monoclonal mouse anti-C3d (Quidel) followed by a secondary labeling with goat anti-rabbit Alexa fluor 488 (Invitrogen) or streptavidin-Alexa fluor 488 (Invitrogen). Images were acquired with a fluorescent Zeiss Axioskop 2 plus microscope and a QImaging Retiga 3000 camera (original magnification, ×20), using the QImaging software.
The anti-C3c antibody recognized the C3 activation fragments C3(H2O), C3b, and iC3b on the cell surface. The specificity of the anti-C3d was tested by a sandwich ELISA. It strongly recognized C3d, weakly recognized C3b and iC3b, and only gave background signal for C3c and C3 (data not shown). Therefore, this antibody was considered to label primarily C3d and, to a lesser extent, C3b and iC3b.
Assays Using Recombinant Proteins
Recombinant C3 Production
The I734T mutant C3 (C3 I734T) was obtained by site-directed mutagenesis of the WT C3 plasmid as described previously.19,33,35 The generation of the R139W C3 mutant was performed as previously described.19 Briefly, the I734T mutation was introduced in WT C3 cloned into the pKG5-En vector using site-directed mutagenesis (QuickChange II XL, Stratagene, La Jolla, CA). The constructs were sequenced to confirm that no additional mutations had been introduced. The expression level of the mutant plasmid was compared with WT after three independent transient transfections of CHO-K1 cells using Lipofectamine (Invitrogen) and three days of culture in complete DMEM medium (DMEM, D-glucose, pyruvate, penicillin, streptomycin, and 10% FCS). The plasmid was introduced into CHO-K1 cells via stable transfection using selection with G418. Recombinant C3 proteins were obtained from the stably transfected cells after 3 days of culture in DMEM without FCS. The quantity of C3 was assessed by a sandwich ELISA, using an anti-human C3 antibody for capture and biotinylated anti-human C3, followed by streptavidin-horseradish peroxidase for detection. The integrity of the recombinant C3 proteins was assessed by Western blot analysis under reducing conditions and showed a migration pattern indistinguishable from the WT.
Recombinant C3 proteins were purified by ion-exchange chromatography (diethylaminoethanol) from five independent productions. Every experiment employing recombinant protein utilized material from at least three independent productions.
To test the interaction of the recombinant proteins with the Complement regulators, the C3 proteins were converted to C3(H2O) by three freeze-thaw cycles. The efficacy of the conversion was confirmed by the ability of FH and FI to induce complete cleavage of the recombinant proteins. C3(H2O), despite the presence of the anaphylatoxin domain (C3a), is very similar to C3b as it binds to the same ligands and is regulated in the same fashion.54–59 Therefore, we used these recombinant proteins to characterize C3(H2O) and C3b interactions.
SPR Analysis
Interactions with Complement Regulators
The interaction of purified C3 WT and C3 I734T with its ligands, FH, MCP, and CR1, was analyzed using SPR technology with ProteON XPR36 equipment (BioRad), as described previously.19,35 Purified recombinant C3 was in the form of C3(H2O) and was able to bind to Complement regulators. FH, MCP, CR1, and a monoclonal anti-C3d antibody (Quidel) were coupled to individual flow channels of a GLC biosensor chip using standard amine-coupling, according to the manufacturer’s instructions. The recombinant C3 WT and C3 I734T were used as analytes at concentrations starting from 1 μg/ml. Five concentrations and a running buffer were injected at 30 μl/min in HEPES buffer (10 mM HEPES, 25 mM NaCl, Tween 0.005%, pH 7.4) for 300 seconds across the four immobilized ligands and an empty, activated/deactivated channel as a control. The injection of equal amounts of C3 WT and C3 I734T was confirmed by identical binding to the anti-C3d antibody.
Data were analyzed using ProteON Manager software and the data from the blank channel were subtracted. Kinetic parameters were calculated by fitting the obtained sensorgrams into a two-state interaction model.
Recombinant C3 R139W was used as a control. This mutation has been identified in 14 patients with sporadic aHUS from the French cohort.19 This mutation is associated with normal binding to FH but reduced binding to MCP. The recombinant C3 R139W was produced and tested in parallel with C3 I734T.
Interaction with FB and Formation of C3 Convertase
Immobilized FB does not interact efficiently with C3 proteins in fluid phase. Therefore, C3 proteins were immobilized on the chip to test FB binding and convertase formation. To this end, anti-C3d antibody was immobilized on three different channels of the chip and loaded with supernatant containing equal amounts of C3 WT or C3 I734T proteins (concentration 1 μg/ml). Five different concentrations of purified FB, diluted in Mg-containing running buffer, and running buffer alone (10 mM HEPES, 25 mM NaCl, 10 mM Mg2+, Tween 0.005%, pH 7.4) were injected simultaneously over these surfaces. A mix of FB (3 μg, Complement Technologies), and FD (0.1 μg, Complement Technologies) was injected onto these surfaces to assess C3 convertase formation.
Cleavage of Recombinant C3 by a Solid-Phase AP C3 Convertase
A solid-phase C3 convertase, C3bBb, was assembled on a microtiter plate coated with C3b, diluted in PBS to 2.5 μg/ml (Complement Technologies), overnight at 4°C. After blocking with 2% BSA for 30 minutes at 37°C, the wells were washed with 10 mM HEPES and 40 mM NaCl supplemented with 10 mM MgCl2. Afterward, FB (0.5 μg/ml, CompTech), FD (0.01 μg/ml, CompTech), and properdin (0.02 μg/ml Complement Technologies) were added and incubated for 30 minutes at 37°C to form a C3 convertase. Next, recombinant C3 WT or C3 I734T (5 ng) (in 10 mM HEPES, 40 mM NaCl, 10 mM MgCl2) was added to the wells and incubated for 0–60 minutes at 37°C. Unpurified supernatant was used as a source of C3 to assure that the recombinant proteins kept their hemolytically active form, and thus were cleavable by the C3 convertase. The reaction was stopped by adding 10 μl dithiothreitol-containing sample buffer. Samples were boiled at 95°C for 10 minutes followed by electrophoresis on 10% Tris-glycine polyacrylamide gels (Life Technology) and transferred to nitrocellulose membrane by the iBlot system (Life Technology). The cleavage of the recombinant C3 was probed by Western blotting, using the SNAP system (EMD Millipore). After blocking with Tris 10 mM, NaCl 150 mM, 0.1% Tween, and 1% BSA, the blots were probed with a 1:5000 dilution of goat anti-human C3 IgG (Calbiochem) followed by horseradish peroxidase-conjugated rabbit anti-goat IgG (Santa Cruz Biotechnology). The signal was developed by chemiluminescence using an ECL kit (PerkinElmer) and MyECL Imager (Thermo Fisher Scientific).
Cofactor Assays
Recombinant C3 WT or C3 I734T proteins were freeze-thawed three times to generate C3(H2O) and 2.5 ng was incubated at 37°C for 30 minutes with FI (20 ng Complement Technologies) and different doses of FH (0, 31, 125, or 500 ng; Complement Technologies), recombinant soluble MCP (0, 25, 50, or 100 ng), or soluble CR1 (0, 12.5, 50, or 200 ng (R&D Systems) in tris buffered-saline (10 mM Tris, 150 mM NaCl, pH 7.4). Samples were boiled and the cleavage of C3 WT or C3 I734T to iC3b was probed as described above. The cleavage efficiency was evaluated by the appearance of the α43 band and the disappearance of the α-chain and quantitated by densitometry of the scanned images. The ratio between α43 and the β bands was plotted versus the cofactor concentration.
Statistical Analyses
The results were analyzed using GraphPad Prism software. The statistical analysis was performed using the Mann–Whitney U and t tests. P values below 0.05 were considered significant (*P<0.05; **P<0.01; ***P<0.001).
Disclosures
None.
Acknowledgments
We thank Prof. Fadi Fakhouri for advice and critical review of the manuscript.
S.C. receives a fellowship from the Fondation pour la Recherche Médicale (FRM 20130727355). L.T.R. was a recipient of a European Molecular Biology Organization Long-Term Fellowship (ALTF 444-2007). S.B. is supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale (FRM ARF20140129163). This work was supported by grants from Agence Nationale de la Recherche (ANR Genopath 2009-2012 09geno03101I), Assistance Publique-Hôpitaux de Paris Programme Hospitalier de Recherche Clinique (AOM08198), by EU FP7 grant 2012-305608 (EURenOmics), by the Association for Information and Research on Renal Genetic Diseases - France, and by the Institut National de la Santé et de la Recherche Médicale. Research reported in this publication was also supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) under award numbers NIH 5T32-DK007126 (to A.J.), R01-GM099111, and U54-HL112303 (to E.C.S. and J.P.A.), and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the NIH, under award number P30-AR048335. Experimental support (to J.P.A. and E.C.S.) was provided by the Protein Production and Purification Core of the Rheumatic Diseases Core Center.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC: C3 glomerulopathy: a new classification. Nat Rev Nephrol 6: 494–499, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Hou J, Markowitz GS, Bomback AS, Appel GB, Herlitz LC, Barry Stokes M, D’Agati VD: Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int 85: 450–456, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 366: 1119–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, Cramer C, Nester CM, Smith RJ: Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol 6: 1009–1017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT: Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol 6: 262, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT: Complement System Part II: Role in Immunity. Front Immunol 6: 257, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Würzner R, Zipfel PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grünfeld JP, Niaudet P, Lesavre P, Frémeaux-Bacchi V: Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 82: 454–464, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Dragon-Durey MA, Blanc C, Marinozzi MC, van Schaarenburg RA, Trouw LA: Autoantibodies against complement components and functional consequences. Mol Immunol 56: 213–221, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Goodship TH, Pappworth IY, Toth T, Denton M, Houlberg K, McCormick F, Warland D, Moore I, Hunze EM, Staniforth SJ, Hayes C, Cavalcante DP, Kavanagh D, Strain L, Herbert AP, Schmidt CQ, Barlow PN, Harris CL, Marchbank KJ: Factor H autoantibodies in membranoproliferative glomerulonephritis. Mol Immunol 52: 200–206, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y, Voskarides K, Deltas C, Palmer A, Frémeaux-Bacchi V, de Cordoba SR, Maxwell PH, Pickering MC: Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, Tortajada A, Pinto S, Lopez-Trascasa M, Morgan BP, Llorca O, Harris CL, Rodríguez de Córdoba S: Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest 120: 3702–3712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roumenina LT, Loirat C, Dragon-Durey MA, Halbwachs-Mecarelli L, Sautes-Fridman C, Fremeaux-Bacchi V: Alternative complement pathway assessment in patients with atypical HUS. J Immunol Methods 365: 8–26, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O’Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW: Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69: 1633–1640, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Roumenina LT, Jablonski M, Hue C, Blouin J, Dimitrov JD, Dragon-Durey MA, Cayla M, Fridman WH, Macher MA, Ribes D, Moulonguet L, Rostaing L, Satchell SC, Mathieson PW, Sautes-Fridman C, Loirat C, Regnier CH, Halbwachs-Mecarelli L, Fremeaux-Bacchi V: Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood 114: 2837–2845, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Frimat M, Tabarin F, Dimitrov JD, Poitou C, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT: Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 122: 282–292, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Marinozzi MC, Vergoz L, Rybkine T, Ngo S, Bettoni S, Pashov A, Cayla M, Tabarin F, Jablonski M, Hue C, Smith RJ, Noris M, Halbwachs-Mecarelli L, Donadelli R, Fremeaux-Bacchi V, Roumenina LT: Complement factor B mutations in atypical hemolytic uremic syndrome-disease-relevant or benign? J Am Soc Nephrol 25: 2053–2065, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roumenina LT, Frimat M, Miller EC, Provot F, Dragon-Durey MA, Bordereau P, Bigot S, Hue C, Satchell SC, Mathieson PW, Mousson C, Noel C, Sautes-Fridman C, Halbwachs-Mecarelli L, Atkinson JP, Lionet A, Fremeaux-Bacchi V: A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood 119: 4182–4191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forneris F, Ricklin D, Wu J, Tzekou A, Wallace RS, Lambris JD, Gros P: Structures of C3b in complex with factors B and D give insight into complement convertase formation. Science 330: 1816–1820, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torreira E, Tortajada A, Montes T, Rodríguez de Córdoba S, Llorca O: 3D structure of the C3bB complex provides insights into the activation and regulation of the complement alternative pathway convertase. Proc Natl Acad Sci U S A 106: 882–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torreira E, Tortajada A, Montes T, Rodríguez de Córdoba S, Llorca O: Coexistence of closed and open conformations of complement factor B in the alternative pathway C3bB(Mg2+) proconvertase. J Immunol 183: 7347–7351, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Wu YQ, Ricklin D, Janssen BJ, Lambris JD, Gros P: Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol 10: 728–733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson BD, Schmitz NB, Santiago C, Zocher G, Larvie M, Scheu U, Casasnovas JM, Stehle T: Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathog 6: e1001122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becherer JD, Lambris JD: Identification of the C3b receptor-binding domain in third component of complement. J Biol Chem 263: 14586–14591, 1988 [PubMed] [Google Scholar]
- 26.Janssen BJ, Gros P: Structural insights into the central complement component C3. Mol Immunol 44: 3–10, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Oran AE, Isenman DE: Identification of residues within the 727-767 segment of human complement component C3 important for its interaction with factor H and with complement receptor 1 (CR1, CD35). J Biol Chem 274: 5120–5130, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Farries TC, Seya T, Harrison RA, Atkinson JP: Competition for binding sites on C3b by CR1, CR2, MCP, factor B and factor H. Complement Inflamm 7: 30–41, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Lambris JD, Lao Z, Oglesby TJ, Atkinson JP, Hack CE, Becherer JD: Dissection of CR1, factor H, membrane cofactor protein, and factor B binding and functional sites in the third complement component. J Immunol 156: 4821–4832, 1996 [PubMed] [Google Scholar]
- 30.Bruneau S, Neel M, Roumenina LT, Frimat M, Laurent L, Fremeaux-Bacchi V, Fakhouri F: Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood 125: 1038–1046, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Roversi P, Johnson S, Caesar JJ, McLean F, Leath KJ, Tsiftsoglou SA, Morgan BP, Harris CL, Sim RB, Lea SM: Structural basis for complement factor I control and its disease-associated sequence polymorphisms. Proc Natl Acad Sci U S A 108: 12839–12844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, Moulin B, Servais A, Provot F, Rostaing L, Burtey S, Niaudet P, Deschênes G, Lebranchu Y, Zuber J, Loirat C: Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 8: 554–562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frémeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault de Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G: Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5: 1844–1859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schramm EC, Roumenina LT, Rybkine T, Chauvet S, Vieira-Martins P, Hue C, Maga T, Valoti E, Wilson V, Jokiranta S, Smith RJH, Noris M, Goodship T, Atkinson JP, Fremeaux-Bacchi V: Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood 125: 2359–2369, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazatchkine MD, Fearon DT, Appay MD, Mandet C, Bariety J: Immunohistochemical study of the human glomerular C3b receptor in normal kidney and in seventy-five cases of renal diseases: loss of C3b receptor antigen in focal hyalinosis and in proliferative nephritis of systemic lupus erythematosus. J Clin Invest 69: 900–912, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moll S, Lange S, Mihatsch MJ, Dragic Z, Schifferli JA, Inal JM: CRIT is expressed on podocytes in normal human kidney and upregulated in membranous nephropathy. Kidney Int 69: 1961–1968, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Moll S, Miot S, Sadallah S, Gudat F, Mihatsch MJ, Schifferli JA: No complement receptor 1 stumps on podocytes in human glomerulopathies. Kidney Int 59: 160–168, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Ichida S, Yuzawa Y, Okada H, Yoshioka K, Matsuo S: Localization of the complement regulatory proteins in the normal human kidney. Kidney Int 46: 89–96, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ: C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 82: 465–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR 3rd, Zipfel PF, Dogan A, Smith RJ: Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int 75: 952–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT, Botto M, Pickering MC: Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest 118: 608–618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V French Study Group for aHUS/C3G : Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 8: 643–657, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Nester CM, Holanda DG, Marsh HC, Hammond RA, Thomas LJ, Meyer NC, Hunsicker LG, Sethi S, Smith RJ: Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J Am Soc Nephrol 24: 1820–1829, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frémeaux-Bacchi V, Weiss L, Demouchy C, May A, Palomera S, Kazatchkine MD: Hypocomplementaemia of poststreptococcal acute glomerulonephritis is associated with C3 nephritic factor (C3NeF) IgG autoantibody activity. Nephrol Dial Transplant 9: 1747–1750, 1994 [PubMed] [Google Scholar]
- 46.Blanc C, Roumenina LT, Ashraf Y, Hyvärinen S, Sethi SK, Ranchin B, Niaudet P, Loirat C, Gulati A, Bagga A, Fridman WH, Sautès-Fridman C, Jokiranta TS, Frémeaux-Bacchi V, Dragon-Durey MA: Overall neutralization of complement factor H by autoantibodies in the acute phase of the autoimmune form of atypical hemolytic uremic syndrome. J Immunol 189: 3528–3537, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Chen Q, Müller D, Rudolph B, Hartmann A, Kuwertz-Bröking E, Wu K, Kirschfink M, Skerka C, Zipfel PF: Combined C3b and factor B autoantibodies and MPGN type II. N Engl J Med 365: 2340–2342, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Janssen BJ, Christodoulidou A, McCarthy A, Lambris JD, Gros P: Structure of C3b reveals conformational changes that underlie complement activity. Nature 444: 213–216, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Rooijakkers SH, Wu J, Ruyken M, van Domselaar R, Planken KL, Tzekou A, Ricklin D, Lambris JD, Janssen BJ, van Strijp JA, Gros P: Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol 10: 721–727, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roumenina LT, Roquigny R, Blanc C, Poulain N, Ngo S, Dragon-Durey MA, Frémeaux-Bacchi V: Functional evaluation of factor H genetic and acquired abnormalities: application for atypical hemolytic uremic syndrome (aHUS). Methods Mol Biol 1100: 237–247, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Sánchez-Corral P, González-Rubio C, Rodríguez de Córdoba S, López-Trascasa M: Functional analysis in serum from atypical Hemolytic Uremic Syndrome patients reveals impaired protection of host cells associated with mutations in factor H. Mol Immunol 41: 81–84, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Ferreira VP, Herbert AP, Cortés C, McKee KA, Blaum BS, Esswein ST, Uhrín D, Barlow PN, Pangburn MK, Kavanagh D: The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol 182: 7009–7018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Isenman DE, Kells DI, Cooper NR, Müller-Eberhard HJ, Pangburn MK: Nucleophilic modification of human complement protein C3: correlation of conformational changes with acquisition of C3b-like functional properties. Biochemistry 20: 4458–4467, 1981 [DOI] [PubMed] [Google Scholar]
- 55.Li K, Gor J, Perkins SJ: Self-association and domain rearrangements between complement C3 and C3u provide insight into the activation mechanism of C3. Biochem J 431: 63–72, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Nishida N, Walz T, Springer TA: Structural transitions of complement component C3 and its activation products. Proc Natl Acad Sci U S A 103: 19737–19742, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pangburn MK, Schreiber RD, Müller-Eberhard HJ: Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med 154: 856–867, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez E, Nan R, Li K, Gor J, Perkins SJ: A revised mechanism for the activation of complement C3 to C3b: a molecular explanation of a disease-associated polymorphism. J Biol Chem 290: 2334–2350, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winters MS, Spellman DS, Lambris JD: Solvent accessibility of native and hydrolyzed human complement protein 3 analyzed by hydrogen/deuterium exchange and mass spectrometry. J Immunol 174: 3469–3474, 2005 [DOI] [PubMed] [Google Scholar]