Abstract
Activation of the EGF receptor (EGFR) or the Hippo signaling pathway can control cell proliferation, apoptosis, and differentiation, and the dysregulation of these pathways can contribute to tumorigenesis. Previous studies showed that activation of EGFR signaling in renal epithelial cells can exacerbate diabetic kidney injury. Moreover, EGFR has been implicated in regulating the Hippo signaling pathway in Drosophila; thus, we examined this potential interaction in mammalian diabetic kidney disease. Yes-associated protein (YAP) is a transcriptional regulator regulated by the Hippo signaling pathway. We found YAP protein expression and phosphorylation were upregulated in diabetic mouse renal proximal tubule epithelial cells, which were inhibited in diabetic proximal tubule EGFR-knockout mice (EGFRptKO) or administration of an EGFR tyrosine kinase inhibitor erlotinib. Furthermore, activation of an EGFR-PI3K-Akt-CREB signaling pathway mediated YAP gene expression and YAP nuclear translocation and interaction with the TEA domain (TEAD) transcription factor complex, which led to upregulated expression of two TEAD-dependent genes, the connective tissue growth factor and amphiregulin genes. In a renal proximal tubule cell line, either pharmacologic or genetic inhibition of EGFR, Akt, or CREB blunted YAP expression in response to high-glucose treatment. Additionally, knocking down YAP expression by specific siRNA inhibited cell proliferation in response to high glucose or exogenous EGF. Therefore, these results link the Hippo pathway to EGFR-mediated renal epithelial injury in diabetes.
Keywords: diabetic nephropathy, cell signaling, epidermal growth factor
Diabetic kidney disease is now the major cause of ESRD, and approximately 30% of all patients with diabetes will develop some form of kidney disease during their lifetime.1 Current evidence suggests that both genetic and environmental factors determine susceptibility to developing diabetic nephropathy (DN) and the risk for progression of DN.2 Apart from genetic susceptibility, long-term exposure of resident and nonresident renal cells to elevated blood glucose levels, with initiation of production of growth factors, humoral mediators, and cytokines, has been suggested to be an essential factor in the development of structural and functional alterations characteristic of DN.3
The EGF receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases. Binding of its ligands, such as EGF, amphiregulin, or HB-EGF, to EGFR leads to activation of the intrinsic kinase domain and subsequent phosphorylation on specific tyrosine residues within the cytoplasmic tail. In addition, EGFR may be transactivated by non–ligand-associated intracellular Src family kinases, indicated by phosphorylation at Y845. EGFR is widely expressed in the mammalian kidney, including the proximal tubule, glomeruli, and cortical and medullary collecting ducts.4,5 Previous studies have demonstrated upregulation of EGFR and its different ligands and activation of EGFR in experimental models of diabetic kidney injury and in cultured renal cells exposed to high glucose.6–9 Renal enlargement is an early feature of both human and experimental diabetes animal models, and inhibition of EGFR activation significantly attenuated diabetes-induced tubular epithelial cell proliferation and increased tubular epithelial cell apoptosis, thereby reducing early diabetic renal enlargement.10 Studies by us and others have found that prolonged EGFR activation in diabetic animals mediated aberrant activation of a TGF-β1-smad signaling pathway, and inhibition of EGFR signaling in diabetic kidney by pharmacologic or genetic strategies markedly preserved renal function and slowed DN progression.11–14
The elucidation of the role of the Hippo pathway, which is conserved from Drosophila to mammals, has provided new insights in understanding the regulation of cell proliferation, cell death, and cell differentiation to control organ size. The core components of the Hippo signaling pathway—Warts, Salvador, Hippo, and Mats—were discovered in genetic screens in Drosophila for tumor suppressor genes.15–20 The mammalian counterparts of these components are Ste20-like serine/threonine kinases 1/2 (MST1/2), the large tumor suppressor 1/2 serine/threonine protein kinases (LATS1/2), and their adaptor proteins SAV (also termed WW45) and Mps-one binder 1 (MOB1).21 The Hippo signaling pathway is a kinase cascade activated in mammals in response to different extracellular cues.22 Activation of the mammalian Hippo signaling pathway causes LATS1-mediated phosphorylation of downstream effectors, Yes-associated protein (YAP), and WW domain–containing protein (TAZ), at specific serine/threonine residues, which results in inactivation of YAP/TAZ by cytoplasmic sequestration and/or proteasome-mediated degradation.22
Reduction in serine phosphorylation by inactivation of the Hippo pathway or increased tyrosine phosphorylation by Src family kinases results in YAP/TAZ nuclear translocation and accumulation, thereby activating downstream target gene expression as a transcriptional co-activator by interacting with transcription factors, such as the TEA domain family members (TEADs).23,24 Genome-wide analyses of YAP and TAZ transcriptional targets have identified many important target genes, such as CTGF, AREG, CYR61, ANKRD1, BIRC5, AXL, InhA, and Col8a1.22,25 Of note, the profibrotic factor CTGF, which is a 38-kD, cysteine-rich, heparin-binding protein and a member of the CCN (CTGF/Fisp 12, Cyr 61/CEF-10, Nov) immediate early gene family of proteins, has been strongly implicated in the development and progression of diabetic kidney injury.26 CTGF expression has been reported to be increased in many fibrotic renal diseases, including diabetic nephropathy.27 Moreover, CTGF expression also correlates with the early enlargement of glomeruli in diabetic rat28 and has been implicated as a mediator of hypertrophy of human proximal tubular cells induced by angiotensin II.29
The current study examined whether regulation of the mammalian Hippo signaling pathway is involved in EGFR signaling in diabetic kidney disease.
RESULTS
YAP Protein Expression and Phosphorylation Increased in Diabetic Mouse Kidney Cortical Tissues and Was Inhibited by Preventing EGFR Expression or Activation
YAP protein expression, as well as YAP phosphorylation at serine127 (S127), was upregulated in mouse kidney cortical tissues of experimental models of both type 1 (streptozotocin [STZ] induced) and type 2 (db/db) diabetes; however, expression of another hippo signaling effector, TAZ, was downregulated (Figure 1, A, C, and D). Immunofluorescence indicated that increased YAP expression was most prominent in lotus tetragonolobus agglutinin (LTA)–expressing (proximal tubule) epithelial cells (Figure 1B). A previous study in Drosophila reported that EGFR signaling could regulate the Hippo signaling pathway30; in the current study we found that increased YAP expression and YAP phosphorylation and decreased TAZ expression were reversed in mice with specific proximal tubule EGFR deletion (EGFRptKO mice)31 (Figure 1C) or with administration of an EGFR tyrosine kinase inhibitor, erlotinib (Figure 1, A and B) without affecting hyperglycemia (Supplemental Figure 1, A and C). In addition, deletion of proximal tubule EGFR or administration of erlotinib to mice significantly but not completely reduced the early diabetic kidney enlargement, as indicated by increased kidney-to-body weight ratio (26.18%±1.905% versus 20.28%±1.391% for EGFRflox/flox versus EGFRptKO mice; 27.75%±1.243% versus 21.25%±1.010% for mice treated with erlotinib versus those not treated without erlotinib (Supplemental Figure 1, B and D), which is consistent with previous findings in diabetic rat kidney.10
Figure 1.
EGFR dependence of increased YAP expression and phosphorylation in diabetic mouse proximal tubule epithelial cells. Wild-type balb/c mice 9–10 weeks old were subjected to five consecutive STZ injections followed by administration or no administration of erlotinib by gavage (80 mg/kg per day). The mice were euthanized at 2 weeks after development of hyperglycemia. Renal cortical tissues were collected and analyzed by immunoblotting (A) or immunofluorescence (B, Red:YAP; Green:LTA; Purple: DAPI) Original magnification, upper panel ×400; lower panel, ×1200. (C) EGFRptKO mice 9–10 weeks old and age-matched controls were made diabetic with STZ and euthanized at 2 weeks after development of hyperglycemia. Renal cortical tissue lysates were analyzed as in part A. (D) Renal cortical tissue lysates of diabetic db/db mice (at 24 weeks age) were analyzed by immunoblotting. n=5–7 mice/group. Shown are representative data from three separate experiments. Ctrl, control; Veh, vehicle.
Inhibiting EGFR Expression or Activation Blocked High Glucose–Induced YAP Protein Expression and Phosphorylation in Cultured Renal Epithelial Cells
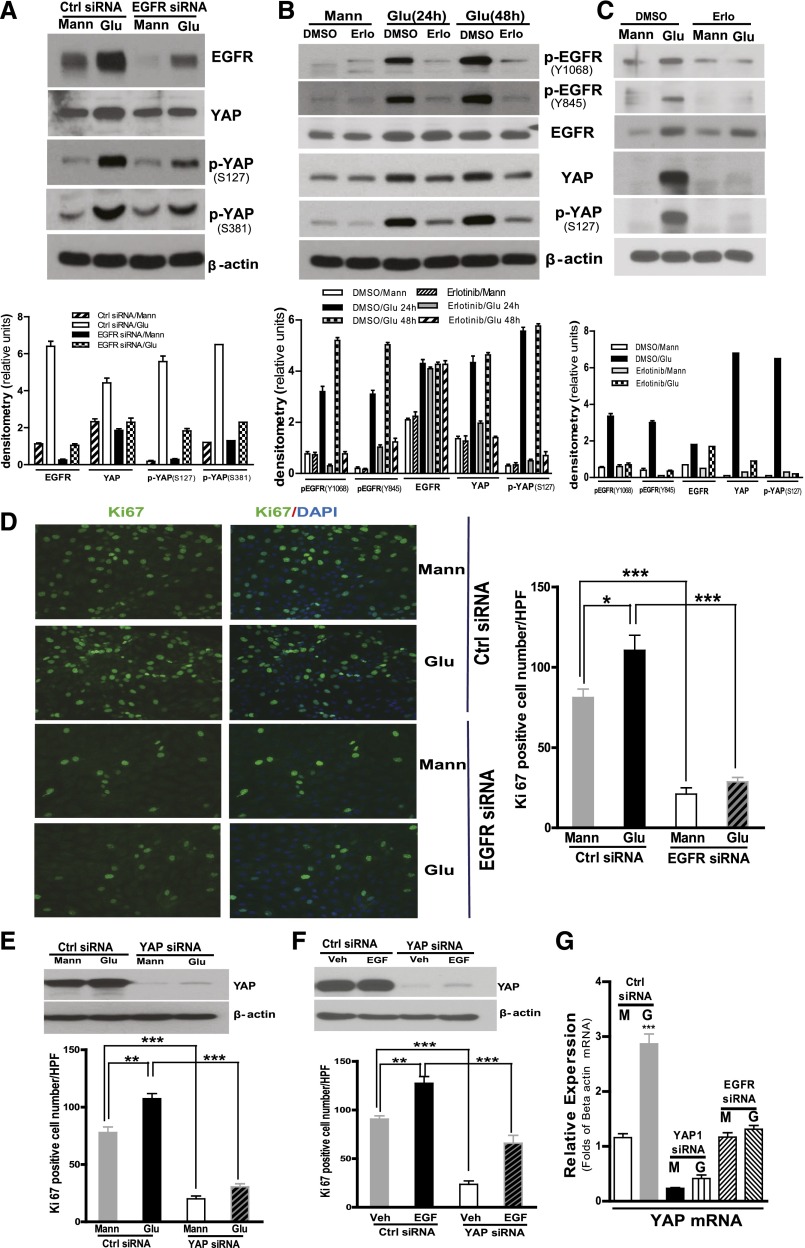
To confirm an interaction of EGFR and YAP, we exposed a well characterized proximal tubule–like epithelial cell, LLCPK-Cl4, to high glucose for 24 hours and found that the high glucose not only increased EGFR expression but also increased expression of both total YAP and YAP phosphorylation at S127, as well as at S381 (Figure 2A). Small interfering RNA (siRNA) knockdown of EGFR expression markedly abrogated this increased expression of YAP and phospho-YAP (Figure 2A). Similarly, erlotinib, which effectively inhibited high glucose–mediated EGFR receptor phosphorylation, also markedly attenuated the increased YAP induced by high glucose (Figure 2B). Exposure of primary cultured mouse renal proximal tubule epithelial cells to high glucose also upregulated EGFR phosphorylation, YAP expression, and phosphorylation, which were all blunted in response to erlotinib treatment (Figure 2C). Silencing EGFR or YAP gene expression significantly inhibited LLCPK-Cl4 cell proliferation in response to high glucose (Figure 2, D and E). Furthermore, silencing YAP expression also significantly inhibited cell proliferation in response to administration of exogenous EGF (Figure 2F). Of note, high glucose increased YAP mRNA expression, which was inhibited when EGFR expression was knocked down (Figure 2G).
Figure 2.
Blocking EGFR expression or activation inhibited YAP protein expression and phosphorylation in cultured renal proximal tubule epithelial cells. (A) LLCPK-Cl4 cells were exposed to high glucose (Glu) for 24 hours after 3 days of control siRNA or EGFR-specific siRNA transfection. The cell lysates were analyzed with indicated antibodies, and the data were analyzed by densitometry (lower panel). (B) LLCPK-Cl4 cells were exposed to high glucose for 24 or 48 hours with or without erlotinib (50 nM), and the data were analyzed by densitometry (lower panel). (C) Primary cultured mouse renal proximal tubule epithelial cells were exposed to high glucose for 48 hours with or without erlotinib (50 nM), followed by analysis of the cell lysates with indicated antibodies, and the data were analyzed by densitometry (lower panel). (D) LLCPK-Cl4 cells were treated as in part A and then fixed with 4% formalin followed by immunofluorescence analysis with anti-KI67 antibodies. Data represent 10 different fields per group (original magnification, ×400). (E) LLCPK-Cl4 cells were exposed to high glucose for 24 hours after 3 days of control siRNA or YAP-specific siRNA transfection, and Ki67 expression was determined as in part C. (F) LLCPK-Cl4 cells were exposed to 100 nM EGF for 24 hours after 3 days of control siRNA or YAP-specific siRNA transfection, and Ki67 expression was determined as in part C. n=3 per group; *P<0.05; **P<0.001; ***P<0.0001. (G) LLCPK-Cl4 cells were exposed to high glucose for 24 hours after 3 days of control siRNA or EGFR; YAP-specific siRNA transfection and YAP expression were evaluated by real-time PCR. Error bars indicate SEM. Ctrl, control; G, glucose; M, mannitol; Mann, mannitol; Veh, vehicle.
Exposure of Renal Epithelial Cells to High Glucose Increased Nuclear YAP Protein Expression
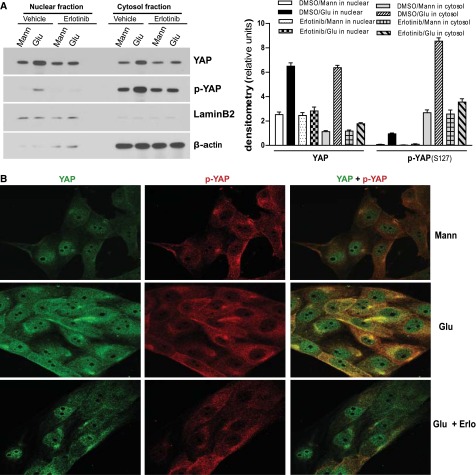
To evaluate the cell distribution of YAP and phospho-YAP in the cells exposed to glucose, we analyzed nuclear and cytoplasm fractions and found that in response to high-glucose treatment, total YAP protein expression was predominantly increased in the nuclear fraction, with a moderate increase in the cytoplasmic fraction. Interestingly, there were also detectable phospho-YAP levels in the nuclear fraction of glucose-treated cells (Figure 3A), although phospho-YAP was predominantly confined to the cytoplasmic fraction, as expected. Immunofluorescence also confirmed that both total YAP protein and phospho-YAP at S127 were augmented (Figure 3B). Moreover, treatment of the cells with erlotinib inhibited the effects of glucose on total and phospho-YAP expression and distribution (Figure 3, A and B).
Figure 3.
EGFR activation–dependent increase of YAP protein expression and phosphorylation in nuclear and cytoplasm fractions in of renal epithelial cells in response to high glucose (Glu). (A) LLCPK-Cl4 cells were exposed to high glucose with or without erlotinib (Erlo; 50 nM), followed by preparation of nuclear and cytoplasm fractions and immunoblotting analysis with indicated antibodies. The data were analyzed by densitometry. (B) LLCPK-Cl4 cells were exposed to high glucose with or without erlotinib (50 nM), followed by immunofluorescence analysis with antibodies against YAP(green) or phospho-YAP(red) (Original magnification, ×400). One representative study from three separate experiments. Error bars indicate SEM. Mann, mannitol.
Exposure of Renal Epithelial Cells to High Glucose Increased Association of YAP with TEAD
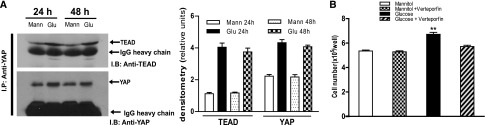
In mammalian cells, interaction with the TEAD transcription factor complex mediates many of the responses observed with translocation of YAP to the nucleus.22 We used a pan-TEAD antibody and found that exposure of LLCPK-Cl4 cells to high glucose increased YAP association with TEAD (Figure 4A). Verteporfin, a well characterized inhibitor of YAP–TEAD interactions,32,33 significantly inhibited the proliferative effect of high glucose in LLCPK-Cl4 cells (Figure 4B).
Figure 4.
Exposure of renal proximal tubule epithelial cells to high glucose (Glu) increased association of YAP with TEAD. (A) LLCPK-Cl4 cells were exposed to high glucose for 24 or 48 hours, and cell lysates were used for immunoprecipitation with antibodies against YAP, followed by immunoblotting with antibodies against TEAD or YAP. The data were analyzed by densitometry. (B) LLCPK-Cl4 cells, 1.5×105/well, were plated in six well plates. The cells were exposed to high glucose for 24 hours with or without verteporfin (10 µM) after 24 hours of quiescence and then trypsinized and counted (n=3; **P<0.001). Error bars indicate SEM. Mann, mannitol.
CTGF and Amphiregulin Were Identified As Downstream Targets of YAP Activation in Response to High Glucose in Renal Epithelial Cells
Many target genes of YAP-mediated transcriptional activation have been identified.22,25,34 We evaluated expression of five of these target genes (CTGF, β1-integrin, smad7, AREG, BIRC5) that are theoretically relevant to progression of DN in response to high-glucose treatment in LLCPK-Cl4 cells and found that expression of two of the genes, CTGF and AREG, was markedly increased (Figure 5A). Immunoblotting further confirmed that CTGF protein expression was markedly upregulated within 24 hours in response to high-glucose treatment, while amphiregulin expression was also markedly upregulated after 48 hours of exposure to high glucose (Figure 5B). To evaluate further YAP-dependent CTGF gene transcription in response to high-glucose treatment, we performed chromatin immunoprecipitation assays (ChIP assays) with a specific antibody against YAP, and we verified increased association of CTGF with YAP in response to high glucose; this association was not detected in the presence of erlotinib (Figure 5C). Knocking down expression of EGFR or YAP by their specific siRNA significantly blocked high glucose treatment–induced expression of CTGF and amphiregulin mRNA and protein (Figure 5, D and E), confirming that EGFR activation is a prerequisite for YAP activation–mediated CTGF and amphiregulin expression in response to high glucose. LATS1 phosphorylation was also moderately stimulated in response to high glucose but was markedly inhibited by knocking down YAP expression, but not by EGFR siRNA (Figure 5E). Treatment of the cells with the YAP-TEAD interaction inhibitor, verteporfin, dose dependently inhibited upregulation of CTGF and amphiregulin expression in response to high glucose (Figure 5F). Furthermore, CTGF and amphiregulin were also upregulated in response to high-glucose treatment in primary cultures of renal proximal tubule epithelial cells, and these effects were attenuated with treatment of the cells with erlotinib (Figure 5G). Moreover, intraperitoneal administration of verteporfin (100 mg/kg every other day for 2 weeks) to STZ-injected mice markedly inhibited CTGF expression upregulation without affecting YAP upregulation (Figure 5H). Interestingly, administration of verteporfin did not affect hyperglycemia but significantly inhibited early diabetic kidney enlargement, as indicated by increased kidney-to-body weight ratio (27.60%±0.8954% versus 14.15%±1.431% for mice treated with verteporfin versus those not treated with verteporfin) (Supplemental Figure 1, E and F).
Figure 5.
YAP-dependent expression of CTGF and amphiregulin in high glucose (Glu)–treated renal epithelial cells. (A) LLCPK-Cl4 cells were exposed to mannitol (Mann) or high glucose for 48 hours, followed by real-time PCR. (B) CTGF and amphiregulin protein expression were evaluated by immunoblotting of LLCPK-Cl4 cell lysates after exposure to high glucose for 24 or 48 h. (C) LLCPK-Cl4 cells were exposed to mannitol or high glucose for 48 hours, with or without erlotinib (Erlo; 50 nM), and ChIP assays were performed using an antibody against YAP to evaluate association with CTGF. (D) LLCPK-Cl4 cells were exposed to mannitol or high glucose for 48 hours after 3 days of control siRNA, YAP, or EGFR-specific siRNA transfection, and expression of CTGF, YAP, and amphiregulin were analyzed by real-time PCR analysis. (E) Protein levels of phospho-LATS1, CTGF, and amphiregulin were evaluated by immunoblotting analysis of the LLCPK-Cl4 cell lysates after exposure to high glucose for 48 hours after 3 days of control siRNA, YAP, or EGFR-specific siRNA transfection. The data were analyzed by densitometry. (F) CTGF and amphiregulin protein expression was evaluated by immunoblotting of LLCPK-Cl4 cell lysates after exposure to high glucose for 48 hours with or without different concentrations of vertoporfin. The data were analyzed by densitometry. (G) Primary cultured mouse renal proximal tubule epithelial cells were exposed to high glucose for 48 hours with or without erlotinib (50 nM), followed by analysis of the cell lysates with indicated antibodies. The data were analyzed by densitometry. (H) Balb/c mice, 9–10 weeks old, were made diabetic with STZ and were administered verteporfin intraperitoneally (100 mg/kg every other day for 2 weeks). This markedly inhibited CTGF expression upregulation without affecting YAP upregulation. Error bars indicate SEM. Veh, vehicle.
PI3K-Akt-CREB Mediated YAP Protein Expression and Phosphorylation in Response to High Glucose in Renal Epithelial Cells
To delineate the underlying mechanism of YAP expression and phosphorylation subsequent to EGFR activation in response to high glucose, we examined the effect of inhibiting signaling of ERK and PI3K-AKT pathways, two well described effector pathways activated by EGFR.35 Inhibition of MEK with PD98059 or AZD6244 did not affect YAP expression and phosphorylation in response to high-glucose treatment (Supplemental Figure 2). In contrast, inhibition of PI3K with LY294002 or wortmannin not only blunted YAP expression and phosphorylation but also inhibited CTGF and amphiregulin expression in response to high-glucose treatment (Figure 6A). Previous studies in other systems had suggested that mitogenic growth factors rapidly inactivate the Hippo signaling pathway in confluent contact-inhibited cells through activation of PI3K-PDK1 but not PI3K-Akt.36 To evaluate the role of Akt in YAP expression and phosphorylation in high glucose–treated LLCPK-Cl4 cells, we silenced Akt expression with siRNA, which reduced YAP expression and phosphorylation and subsequent CTGF and amphiregulin expression (Figure 6B). These results indicated that YAP expression is mediated by a PI3K-Akt–dependent pathway in response to high glucose.
Figure 6.
PI3K-Akt–CREB mediated YAP protein expression and phosphorylation governing CTGF and AREG (amphiregulin) expression in response to high glucose (Glu) in renal epithelial cells. (A) LLCPK-Cl4 cells were exposed to mannitol (Mann) or high glucose for 48 hours with or without the PI3K inhibitors LY294002 (25 µM, LY) or wortmannin (100 nM, WT) after quiescence, followed by analysis of the cell lysates with indicated antibodies. The data were analyzed by densitometry. (B) LLCPK-Cl4 cells were exposed to mannitol or high glucose for 48 hours after 3 days of transfection with control or Akt siRNA transfection, and the cell lysates were subjected to immunoblotting analysis. The data were analyzed by densitometry. (C) LLCPK-Cl4 cells were exposed to mannitol or high glucose for 48 hours after 3 days of transfection with control or CREB siRNA, and the cell lysates were subjected to immunoblotting analysis. The data were analyzed by densitometry. Error bars indicate SEM. Veh, vehicle.
The nuclear factor cAMP response element (CRE)–binding protein, CREB, which was first characterized as a target for PKA and activated after phosphorylation at Ser-133,37 is also identified as a regulatory target of Akt38; moreover, previous studies had suggested that CREB promoted YAP expression to mediate hepatocyte growth and tumorigenesis.39,40 We examined and found that exposing LLCPK-Cl4 cells to high glucose activated CREB, which was blunted by knocking down Akt-specific siRNA (Figure 6B). Furthermore, transfection of CREB-specific siRNA in LLCPK-Cl4 cells not only blunted YAP expression upregulation but also attenuated CTGF and amphiregulin expression in response to high-glucose treatment (Figure 6C), suggesting that CREB mediated the effect of Akt activation in response to high glucose.
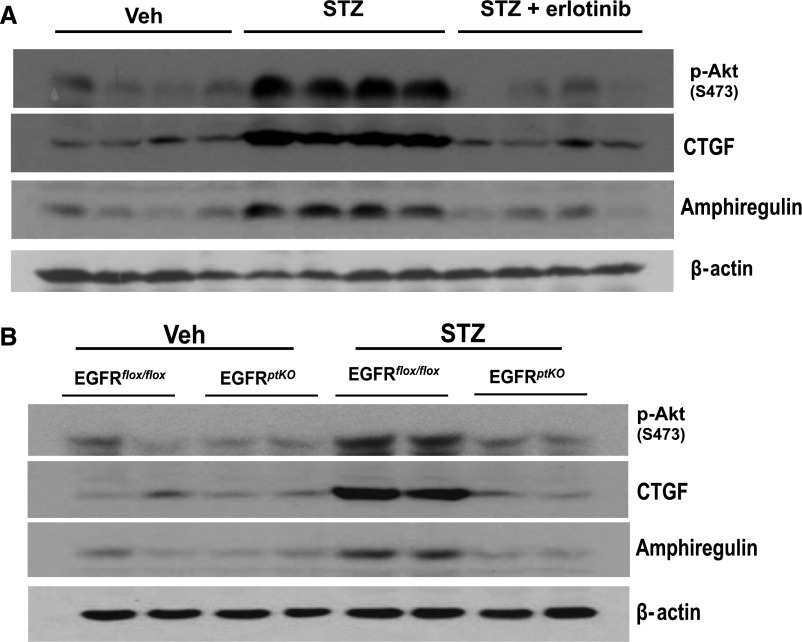
In vivo, STZ-induced diabetes increased Akt phosphorylation, which was markedly decreased in EGFRptKO mice or in response to erlotinib treatment. STZ-induced diabetes also markedly increased renal expression of both CTGF and amphiregulin, and expression was attenuated in EGFRptKO mice or by administration of erlotinib (Figure 7, A and B).
Figure 7.
Upregulation of phospho-Akt, CTGF, and AREG expression in diabetic mice was blunted by administration of erlotinib or in EGFRptKO mice. (A) Balb/c mice, 9–10 weeks old, were made diabetic with STZ and were given or not given erlotinib by gavage (80 mg/kg per day). The mice were euthanized at 2 weeks after development of hyperglycemia, and renal cortical tissues were collected and analyzed by immunoblotting. (B) EGFRptKO mice, 9–10 weeks old, were euthanized after 2 weeks of STZ-induced diabetes and analyzed as in part A. (n=5–7 mice/group.) Shown are representative data from three separate experiments.
DISCUSSION
In the current study, we documented upregulated expression of YAP but downregulated expression of TAZ, two well known downstream effectors of the Hippo pathway, in proximal tubule cells in experimental mouse models of both type 1 and type 2 diabetes. Increased YAP expression and phosphorylation were inhibited in mice with proximal tubule EGFR deletion or by administration of the EGFR tyrosine kinase inhibitor erlotinib. YAP expression also increased in a renal proximal tubule epithelial–like cell line, LLCPK-Cl4 and in primary cultures of mouse proximal tubule cells in response to culturing in high glucose. Although high glucose also increased phospho-YAP, there was increased association of nuclear YAP with the TEAD transcription complex and increased YAP-dependent transcription of known YAP-TEAD–dependent genes, CTGF and AREG, in both high glucose–treated proximal tubule cells and the diabetic kidney.
Diabetes-induced increased expression of EGFR and their ligands and subsequent EGFR activation have been previously reported both in in vivo models and in cultured renal proximal tubule epithelial cells.6–9 Deletion of EGFR expression or inhibition of EGFR tyrosine kinase activity preserved renal function and slowed DN progression, suggesting that continuous aberrant activation of EGFR signaling plays a detrimental role in progression of DN.10–14 We and others have previously shown that EGFR-dependent ERK signaling increased expression of the profibrotic cytokine, TGF-β, in experimental diabetic kidney disease.12,41 However, although previous studies in Drosophila had suggested that activation of EGFR-RAS-MAPK signaling led to YAP activation,30 we could not detect a role for ERK activation in EGFR-mediated increases in YAP expression in response to high glucose. In contrast, we found an essential role for another signaling pathway that is activated by EGFR, the PI-3 kinase-Akt pathway. Knockdown of Akt expression or pharmacologic inhibition of PI3K-Akt activation inhibited high glucose–dependent and EGFR-dependent YAP expression.
In addition to total YAP expression, high glucose and EGFR activation also increased phospho-YAP at S127 and S381. Activation of the Hippo analogue, MST, induces phosphorylation of the serine/threonine kinase, LATS1/2, which phosphorylates YAP at S127,42 creating a binding site for 14–3-3 protein, leading to sequestration of YAP in cytosol.42 Similarly, LATS1/2 phosphorylation of YAP at S381 leads to recruitment of the SCFβ-TRCP E3 ubiquitin ligase, which catalyzes YAP ubiquitination, leading to YAP degradation.43 Therefore, although we observed high glucose–mediated increases in YAP in the nucleus and evidence for YAP-dependent transcriptional activity, we also found increased cytosolic expression of presumably inactivated cytosolic phospho-YAP. In addition, we also detected minimal increases in phospho-YAP in the nuclear fraction. Although some other studies have also detected S127 phosphorylated YAP in the nucleus, its specific function there is still undetermined.22 Our results indicate that activation of EGFR-PI3K-Akt-CREB signaling increases synthesis of YAP, resulting in increases in both cytosolic and nuclear fractions of YAP. Furthermore, high glucose increased phospho-LATS1, an effect that was not EGFR dependent. The mechanism for this glucose-dependent LATS1 activation is still undetermined, but basal expression of YAP appears to be required (Figure 5E).
In addition to an increase in total YAP protein, other mechanisms may be mediating nuclear translocation of YAP in the diabetic milieu. Previous studies by us and others have demonstrated that Src kinase is activated in diabetic or high-glucose milieu.12,14,41,44 Recent studies have suggested that Src family kinases can mediate YAP phosphorylation at tyrosine residue (Y357), which will stabilize YAP and increase its translocation into the nucleus to interact with TEAD transcription factors and regulate downstream gene transcription.24 Furthermore, glucose deprivation activates AMPK-dependent phosphorylation of YAP at S61 or S94, which also leads to cytosolic sequestration of YAP.45,46
Both YAP and TAZ can serve as coactivators by binding with the TEAD transcription complex to control expression of pro-proliferative and antiapoptotic genes. Therefore, YAP and TAZ share some redundant functions, and both YAP and TAZ have been implicated in nephrogenesis47–49 and in development of the urinary tract.50 However, knockout of the YAP gene in mice leads to early embryonic lethality,51 while knockout of TAZ induces development of renal cysts characteristic of polycystic kidney disease and lung abnormalities characteristic of emphysema.47,48,52 In this study, we found upregulation of YAP but downregulation of TAZ expression in the diabetic kidney. Why and how these two paralogues are differentially regulated requires further investigation. However, given that previous studies have suggested that TAZ may play a role in maintaining the integrity of renal cilia to ensure the structural integrity of the kidney47 and our studies indicating a role for YAP in mediating CTGF gene transcription, which has been strongly implicated in the development and progression of diabetic kidney injury,26 our findings suggest that both the upregulation of YAP and downregulation of TAZ may be involved in diabetic kidney injury.
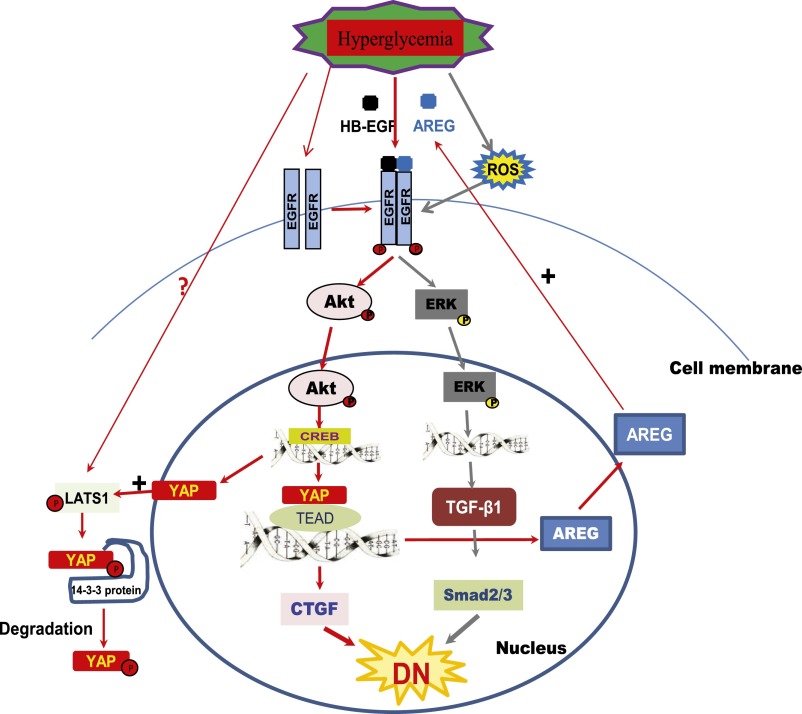
In summary, our study indicates that EGFR-dependent upregulation of YAP occurs in response to the diabetic milieu, and this YAP upregulation is a prerequisite for increased expression of the profibrotic factor CTGF as well as the EGFR ligand amphiregulin. Thus, as indicated in Figure 8, EGFR signaling activation in the diabetic kidney increases expression of two major profibrotic cytokines implicated in diabetic nephropathy: ERK-dependent TGF-β and Akt-dependent CTGF.11–14,26 Therefore, this study demonstrates for the first time cross-talk between the EGFR signaling pathway and Hippo pathway in the diabetic kidney and suggests that EGFR-mediated YAP signaling may be an important underlying mechanism of development and progression of DN. When effective pharmacologic interventions of the Hippo pathway are developed, they may slow progression of diabetic nephropathy.
Figure 8.
Proposed crosstalk between EGFR signaling pathway and Hippo pathway in the diabetic kidney. AREG, amphiregulin; ROS, reactive oxygen species.
CONCISE METHODS
Reagents and Antibodies
Erlotinib was purchased from LC laboratories (Woburn, MA). Antibodies against EGFR, Akt, p-Akt(Ser473), p-EGFR (Y1173, Y845), YAP, p-YAP(Ser 127), Pan-TEAD Lamin B2, CREB, p-CREB(S133), and β-actin were from Cell Signaling Technology (Beverly, MA). LY294002, wortmannin, and antibodies against amphiregulin were from EMD Millipore (Billerica, MA), p-ERK, and all secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Animal Studies
The Vanderbilt University Institutional Animal Use and Care Committee approved all experiments, and all experiments were conducted according to National Institutes of Health guidelines. Specific proximal tubule epithelial cell EGFR deletion mice (EGFRptKO) were generated as we have previously described.41 Ten-week-old, male EGFRptKO and wild-type control mice or wild-type BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were injected daily with STZ (prepared freshly in 0.1 M citrate buffer, pH 4.5, and given intraperitoneally at a dose of 50 mg/kg body weight) or vehicle alone for 5 consecutive days after 4–5 hours of food deprivation to induce diabetes. Blood glucose was measured using the OneTouch Basic Blood Glucose Monitoring System (LifeScan, Milpitas, CA) on blood samples obtained via the saphenous vein after 6 hours of food deprivation. Mice were euthanized 2 weeks after development of hyperglycemia, kidneys were weighed, and renal cortex tissue was harvested and analyzed.
Cell Culture
LLCPK-Cl4 cells, an established adherent proximal tubule-like epithelial cell line derived from pig kidney,53 were maintained as previously described.54 Cells were made quiescent in low glucose DMEM (containing 1 g/L d-glucose) from Gibco (Grand Island, NY) containing 0.5% serum for 24 hours, followed by treatment with 25 mM glucose or 25 mM mannitol for 24 hours or 48 hours with or without other reagent incubation.
Primary Culture of Mouse Proximal Tubule Epithelial Cells
The procedure was performed as previously described,55 with modification. Four wild-type BALB/c mice, age 10 weeks, were euthanized, and kidneys were immediately harvested and washed in sterile ice-cold DMEM medium equilibrated with 95% O2/5% CO2. Each kidney was decapsulated and bisected, and the inner medullary portion was excised. The remaining cortical and outer medullary regions were minced and transferred in a solution of 30 ml of perfusion buffer (105 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 2 mM Na2HPO4, 1 mM MgSO4, 1.5 mM CaCl2, 5 mM d-glucose, 1 mM l-alanine, 10 mM HEPES, pH 7.4,) and 1 mg/ml collagenase (type IV). The solution was bubbled with 95% O2/5% CO2 during incubation for 30 minutes at 37°C. The solution was filtered through a 210-μm mesh sieve and quickly centrifuged; the pellet was resuspended in 5 ml of 45% Percoll solution and slowly added on top of centrifuge tubes containing 20 ml of 45% Percoll solution. The tubes were centrifuged at 30,000 g at 4°C for 30 minutes and three layers were separated. The second layer containing the proximal tubules was aspirated and centrifuged at 405 g at room temperature for 2 minutes to remove the Percoll solution, followed by two washes with serum-free DMEM. The cells were cultured in low glucose (1 g/L) DMEM media supplemented with 2 mM glutamine, 5 μg/ml insulin, 50 nM hydrocortisone, 5 μg/ml transferring, 2 mmol/L butyrate, 2 mmol/L alanine, 2 mmol/L lactate, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% FBS. After 48 hours of the culture, the medium was changed; thereafter it was changed every 2 days. The cells were made quiescent in low-glucose DMEM with 0.5% serum for 24 hours, followed by exposure to 25 mM glucose or 25 mM mannitol with or without erlotinib treatment for 48 hours.
Transfection of EGFR, YAP, Akt, and CREB siRNA
A pool of two EGFR siRNAs (sense 1, 5′- CGCUGGAGGAGAAGAAAGUtt -3′, antisense 1, 5′- ACUUUCUUCUCCUCCAGCGtt -3′; sense 2, 5′- CACCGUGGAGAAGAUCCCUtt -3′, antisense 2, 5′- AGGGAUCUUCUCCACGGUGtt-3′), the Silencer Negative Control #1 (catalogue no. AM4611) siRNA (Applied Biosystems/Ambion, Austin, TX), Thermo Scientific Dharmacon ON-TARGETplus YAP1 siRNA SMARTpool L-012200–00–0005, ON-TARGETplus CREB1 siRNA SMARTpool L-003619–00–0005, or ON-TARGETplus Akt1 siRNA SMARTpool L-003000–00–0005 or ON-TARGETplus nontargeting pool (Thermo Fisher Scientific, Lafayette, CO) were transfected into LLCPK-Cl4 cells by using Effectene from Qiagen (Valencia, CA) following the product instructions. For signaling studies, 48 hours after transfection, cells were made quiescent for another 24 hours, followed by exposure to 25 mM glucose or 25 mM mannitol for 48 hours.
Immunoprecipitation and Immunoblotting
These procedures were performed as we previously described.41 Briefly, for in vitro experiments, cells were made quiescent in a low-glucose DMEM medium containing 0.5% serum for 24 hours before treatment of the cells with the indicated reagents for the indicated time, followed by harvesting in RIPA buffer. For in vivo experiments, BALB/c mice or EGFRptKO and wild-type control mice, renal cortices were dissected and homogenized in RIPA buffer at 2 weeks after development of hyperglycemia. After centrifugation at 10,000 g for 5 minutes at 4°C, equal amounts of protein lysate were loaded directly or after immunoprecipitation with indicated antibodies onto 7%–12% SDS-PAGE, transferred onto Immobilon-P transfer membranes (EMD Millipore, Bedford, MA) and probed with the indicated primary antibody. The primary antibodies were detected with peroxidase-labeled goat anti-rabbit IgG or goat anti-mouse IgG and exposed on film by using enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK).
Preparation of Cell Nuclear Protein and Cytosol Protein
Quiescent LLCPK-Cl4 cells were exposed to 25 mM glucose or 25 mM mannitol with or without erlotinib treatment for 24 hours, and the cells were harvested in a 1 ml/100 mm dish of lysis buffer A (0.05% Nonidet P-40, 10 mM KCl, 1.5 mM MgCl2, 10 Mm HEPES, 0.5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, phosphatase inhibitor cocktail 2, pH 7.4) and lysed on ice for 10 minutes. The cells were gently sonicated, followed by centrifugation at 1000 g for 10 minutes at 4°C, then the supernatant was carefully removed and kept (cytosol protein fraction). The pellets were resuspended in 400 μl of buffer B (0.2 mM EDTA, 1.5 mM MgCl2, 5 mM HEPES, 0.5 mM dithiothreitol, 10% glycerol, 300 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, phosphatase inhibitor cocktail 2, pH 7.4) and were sonicated again and kept on ice for 30 minutes for further lysis. Then the lysates were centrifuged at 14,000 g for 10 minutes at 4°C and the supernatant was retained (nuclear protein fraction).
Real-Time PCR
Total RNA was extracted from cells in six well plates using miRNeasy Mini Kit (Qiagen). cDNA was synthesized using SuperScript III First-Strand Synthesis System (Invitrogen), and quantitative PCR was performed using Power SYBR Green PCR Master Mix (Invitrogen) on Bio-Rad CFX96 Touch Real-Time PCR Detection System. β-actin was used as an internal control. The primer sequences for RT-PCR were as follows: β-actin-FW: 5′-CGA GAC CAC CTT CAA CTC GAT CAT-3′, β-actin-RV: 5′-ATC TCC TTC TGC ATC CTG TCG G-3′; CTGF-FW: 5′-TTA CCA ATG ACA ACG CTT CTT GCA G-3′, CTGF-RV: 5′-CAA ACT TGA CGG GCT TGG AGA TTT T-3′; β-integrin-FW: 5′-CGT GAC CAA GAA AAT GGT GAA AAC G-3′, β-integrin-RV: 5′-TGA TCC AAA AAC ACT CTG GAG GAC A-3′; Smad7-FW: 5′-ACC AAG AAG GAT TCG GTC CAT TGT A-3′, Smad7-RV: 5′-GAG CTA AGA GCA GTG TCA ACG TAT C-3′; AREG-FW: 5′-GAA ATG CCT TCT GGT AGC GAC TAT G-3′, AREG-RV: 5′-CTT TCT GTT CTG TTT CTC TTG GGC T–3′; BIRC5-FW: 5′-TGG CTC AAT GTT TCT TCT GCT TCA A-3′, BIRC5-RV: 5′-TGT TCT TGG CTC TTT CTT TGT CCA G-3′ and YAP1-FW: 5′-CTG GAG GGA GAT GGA ATG AA-3′, YAP1-RV: 5′-ATC GCC TTA GCT CCT TCA CA-3′.
ChIP Assay
Quiescent LLCPK-Cl4 cells were exposed to 25 mM glucose or 25 mM mannitol with or without erlotinib treatment for 48 hours and crosslinked by 1% formaldehyde. Cell nuclei were lysed and sonicated to generate DNA fragments with an average size of 0.5 kb. Anti-YAP or mouse IgG control antibodies were added to the sonicated chromatin fragments for immunoprecipitation. PCR was performed with HotStarTaq Master Mix Kit (Qiagen). The primer sequences in CTGF gene promoter amplification were forward primer 5′-TGC TGA GTG TCA AGG GTC AG-3′ reverse primer, 5′- GGG ACA TTC CTC GCA TTC CT-3′, which produces an expected 79–base pair PCR product.
Immunofluorescence Analysis
Immunofluorescence was performed on paraffin-embedded tissues fixed by 4% paraformaldehyde using standard techniques. Briefly, 5-μm kidney sections were deparaffinized, rehydrated, subjected to antigen retrieval, and then incubated with rabbit primary antibodies (EGFR, N-cadherin, Snail, E-cadherin, vimentin or TGF-β) in 5% goat serum in PBS for 1 hour and Cy3-labeled goat anti-rabbit secondary antibodies for 30 minutes, followed by incubation with FITC-conjugated LTA for another hour. Confocal microscopy was performed using Carl Zeiss LSM 510 Laser-Scanning (Vanderbilt Cell Imaging Core). All images were processed using Carl Zeiss LSM Image Browser and Photoshop (Adobe Systems Inc., San Jose, CA).
Statistical Analyses
Data are presented as mean±SEM for at least three separate experiments (each in triplicate). An unpaired t test was used for statistical analyses; for multiple-group comparisons, ANOVA and Bonferroni t tests were used. A P value <0.05 compared with control was considered to indicate a statistically significant difference.
Disclosures
Nothing to disclose.
Supplementary Material
Acknowledgments
This work was supported by funds from National Institutes of Health grants DK51265, DK62794, and DK79341 (to R.C.H.) and the Department of Veterans Affairs (to R.C.H.).
Some of the data in this article were presented as a poster presentation during at the 2014 American Society of Nephrology Annual Meeting, held November 11-17, 2014, in Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040415/-/DCSupplemental.
References
- 1.Caramori ML, Mauer M: Diabetes and nephropathy. Curr Opin Nephrol Hypertens 12: 273–282, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Susztak K, Böttinger EP: Diabetic nephropathy: A frontier for personalized medicine. J Am Soc Nephrol 17: 361–367, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Schena FP, Gesualdo L: Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16[Suppl 1]: S30–S33, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Harris RC: Response of rat inner medullary collecting duct to epidermal growth factor. Am J Physiol 256: F1117–F1124, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Breyer MD, Redha R, Breyer JA: Segmental distribution of epidermal growth factor binding sites in rabbit nephron. Am J Physiol 259: F553–F558, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Asakawa H, Miyagawa J, Higashiyama S, Goishi K, Hanafusa T, Kuwajima M, Taniguchi N, Matsuzawa Y: High glucose and hyperosmolarity increase heparin-binding epidermal growth factor-like growth factor (HB-EGF) production in cultured human aortic endothelial cells. Cell Biochem Funct 14: 181–186, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Miyazawa T, Zeng F, Wang S, Fan X, Cheng H, Yang H, Bian A, Fogo AB, Harris RC: Low nitric oxide bioavailability upregulates renal heparin binding EGF-like growth factor expression. Kidney Int 84: 1176–1188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayed-Ahmed N, Besbas N, Mundy J, Muchaneta-Kubara E, Cope G, Pearson C, el Nahas M: Upregulation of epidermal growth factor and its receptor in the kidneys of rats with streptozotocin-induced diabetes. Exp Nephrol 4: 330–339, 1996 [PubMed] [Google Scholar]
- 9.Saad S, Stevens VA, Wassef L, Poronnik P, Kelly DJ, Gilbert RE, Pollock CA: High glucose transactivates the EGF receptor and up-regulates serum glucocorticoid kinase in the proximal tubule. Kidney Int 68: 985–997, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Wassef L, Kelly DJ, Gilbert RE: Epidermal growth factor receptor inhibition attenuates early kidney enlargement in experimental diabetes. Kidney Int 66: 1805–1814, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Advani A, Wiggins KJ, Cox AJ, Zhang Y, Gilbert RE, Kelly DJ: Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology (Carlton) 16: 573–581, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi K, Xia L, Goldberg HJ, Lee KW, Shah A, Stavar L, Masson EA, Momen A, Shikatani EA, John R, Husain M, Fantus IG: Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes 62: 3874–3886, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang MZ, Wang Y, Paueksakon P, Harris RC: Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes 63: 2063–2072, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Chen JK, Harris RC:. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol 26: 1115–1125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ: The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 9: 534–546, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Xu T, Wang W, Zhang S, Stewart RA, Yu W: Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK: salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110: 467–478, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Harvey KF, Pfleger CM, Hariharan IK: The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Pantalacci S, Tapon N, Léopold P: The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol 5: 921–927, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Wu S, Huang J, Dong J, Pan D: Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gomez M, Gomez V, Hergovich A: The Hippo pathway in disease and therapy: Cancer and beyond. Clin Transl Med 3: 22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccolo S, Dupont S, Cordenonsi M: The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev 94: 1287–1312, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML: TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev 15: 1229–1241, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamm C, Böwer N, Annerén C: Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J Cell Sci 124: 1136–1144, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA: YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 11: 1444–1450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly SB, Sadlier D, Kieran NE, Doran P, Brady HR: Transcriptome profiling and the pathogenesis of diabetic complications. J Am Soc Nephrol 14[Suppl 3]: S279–S283, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, Goldschmeding R: Expression of connective tissue growth factor in human renal fibrosis. Kidney Int 53: 853–861, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Liu BC, Chen Q, Luo DD, Sun J, Phillips AO, Ruan XZ, Liu NF: Mechanisms of irbesartan in prevention of renal lesion in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 24: 67–73, 2003 [PubMed] [Google Scholar]
- 29.Liu BC, Sun J, Chen Q, Ma KL, Ruan XZ, Phillips AO: Role of connective tissue growth factor in mediating hypertrophy of human proximal tubular cells induced by angiotensin II. Am J Nephrol 23: 429–437, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Reddy BV, Irvine KD: Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell 24: 459–471, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Chen JK, Harris RC: Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D: Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26: 1300–1305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodowska K, Al-Moujahed A, Marmalidou A, Meyer Zu Horste M, Cichy J, Miller JW, Gragoudas E, Vavvas DG: The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res 124: 67–73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL: TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J, Liu N, Zhuang S: Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 83: 804–810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan R, Kim NG, Gumbiner BM: Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A 110: 2569–2574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez GA, Montminy MR: Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Du K, Montminy M: CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273: 32377–32379, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He J, Wang H, Xiao W, Li L, Chu Q, Pan Q, Yu Y, Sun F: Mutual interaction between YAP and CREB promotes tumorigenesis in liver cancer. Hepatology 58: 1011–1020, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Zhang J, You X, Liu Q, Du Y, Gao Y, Shan C, Kong G, Wang Y, Yang X, Ye L, Zhang X: Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology 56: 2051–2059, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC: EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao B, Li L, Lei Q, Guan KL: The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev 24: 862–874, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL: A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 24: 72–85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Q, Sheibani N: High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol 295: C1647–C1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J: AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol 17: 490–499, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL: Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol 17: 500–510, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, Qi Z, Ponniah S, Hong W, Hunziker W: Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A 104: 1631–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H: Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol 294: F542–F553, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H: Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet 9: e1003380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reginensi A, Hoshi M, Boualia SK, Bouchard M, Jain S, McNeill H: Yap and Taz are required for Ret-dependent urinary tract morphogenesis. Development 142: 2696–2703, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O’Neal W, Milgram SL: Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol 26: 77–87, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian Y, Zhou R, Rehg JE, Jackowski S: Role of phosphocholine cytidylyltransferase alpha in lung development. Mol Cell Biol 27: 975–982, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amsler K, Cook JS: Development of Na+-dependent hexose transport in a cultured line of porcine kidney cells. Am J Physiol 242: C94–C101, 1982 [DOI] [PubMed] [Google Scholar]
- 54.Chen J, Chen JK, Falck JR, Guthi JS, Anjaiah S, Capdevila JH, Harris RC: Mitogenic activity and signaling mechanism of 2-(14,15- epoxyeicosatrienoyl)glycerol, a novel cytochrome p450 arachidonate metabolite. Mol Cell Biol 27: 3023–3034, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamiyama M, Garner MK, Farragut KM, Kobori H: The establishment of a primary culture system of proximal tubule segments using specific markers from normal mouse kidneys. Int J Mol Sci 13: 5098–5111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.