Abstract
Locally produced 1,25-dihydroxyvitamin D3 may have pleiotropic effects outside of bone. Experimental and observational studies suggest that nutritional vitamin D may enhance erythropoiesis in settings of 25-hydroxy vitamin D (25(OH)D) deficiency. We conducted a double-blind, placebo-controlled, randomized clinical trial to assess the effects of supplementation with ergocalciferol on epoetin utilization and other secondary outcomes in patients on hemodialysis with serum 25(OH)D <30 ng/ml. In all, 276 patients were randomized to 6 months of ergocalciferol or placebo. Mean±SD serum 25(OH)D increased from 16.0±5.9 ng/ml at baseline to 39.2±14.9 ng/ml in the ergocalciferol arm and did not change (16.9±6.4 ng/ml and 17.5±7.4 ng/ml, respectively) in the placebo arm. There was no significant change in epoetin dose over 6 months in the ergocalciferol or placebo arms (geometric mean rate 0.98 [95% confidence interval (95% CI), 0.94 to 1.02] versus 0.99 [95% CI, 0.95 to 1.03], respectively) and no difference across arms (P=0.78). No change occurred in serum calcium, phosphorus, intact parathyroid hormone, or C-reactive protein levels, cinacalcet use, or phosphate binder or calcitriol dose in either study arm. Rates of all-cause, cardiovascular, and infection-related hospitalizations did not differ by study arm, although statistical power was limited for these outcomes. In conclusion, 6 months of supplementation with ergocalciferol increased serum 25(OH)D levels in patients on hemodialysis with vitamin D insufficiency or deficiency, but had no effect on epoetin utilization or secondary biochemical and clinical outcomes.
Keywords: anemia, randomized controlled trials, vitamin D, hyperparathyroidism
Local production of 1,25-dihydroxy vitamin D3 [1,25(OH)2D] via extrarenal 1-α hydroxylases1 is postulated to have paracrine and autocrine functions separate from its classic endocrine function on bone.2 Most patients on dialysis are treated with vitamin D receptor agonists (VDRAs), such as calcitriol, doxercalciferol, or paracalcitol,3 for management of secondary hyperparathyroidism; however, if the hypothesized pleiotropic effects of vitamin D require local production, supplementation with VDRAs without its nutritional vitamin D precursor may not optimize clinical benefits, particularly as more than half of patients on hemodialysis are deficient in total serum 25-hydroxy vitamin D [25(OH)D].4 The 2009 Kidney Disease: Improving Global Outcomes Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) clinical practice guideline suggests supplementing with nutritional vitamin D to achieve 25(OH)D ≥30 ng/ml in patients on hemodialysis.5 A nonrandomized trial of 158 patients on hemodialysis demonstrated a reduction in serum-intact parathyroid hormone (iPTH), VDRA use, calcimemetic use, erythropoietin-stimulating agent (ESA) dose, as well as reductions in BP, left ventricular mass index and infections after 6 months of cholecalciferol supplementation.6
A reduction in ESA dose has been found in nonrandomized trials of nutritional vitamin D supplementation in patients on hemodialysis,6–8 and cross-sectional studies show an inverse relationship between serum 25(OH)D and hemoglobin and ESA dose.9–12 In vitro and in vivo studies under conditions of vitamin D deficiency have demonstrated increased erythropoeisis with addition of vitamin D.13,14 The postulated mechanism for nutritional vitamin D’s effect is via the suppression of cytokines inhibitory to erythroid progenitors.14 Increased availability of iron through suppression of hepcidin may also play a role.15 Given the potential risks16,17 and costs associated with ESAs, reducing ESA dose is desirable, particularly if it can be accomplished safely and inexpensively.
The suppression of parathyroid hormone secretion is another postulated effect of nutritional vitamin D that may be found even in an anuric patient on dialysis, as 1-α hydroxylase is present in parathyroid tissue. Nonrandomized observational studies6,18–20 and small randomized clinical trials (RCTs)19,21–27 have yielded inconsistent results. Accordingly, there is uncertainty about whether patients on dialysis should be treated with nutritional vitamin D in addition to VDRAs.
We conducted a double-blind, placebo-controlled, RCT to assess the effects of 6 months of supplementation with ergocalciferol in 25(OH)D-deficient patients on hemodialysis on epoetin dose, as well as several secondary outcomes including iPTH, treatments targeting CKD-MBD, and hospitalizations.
Results
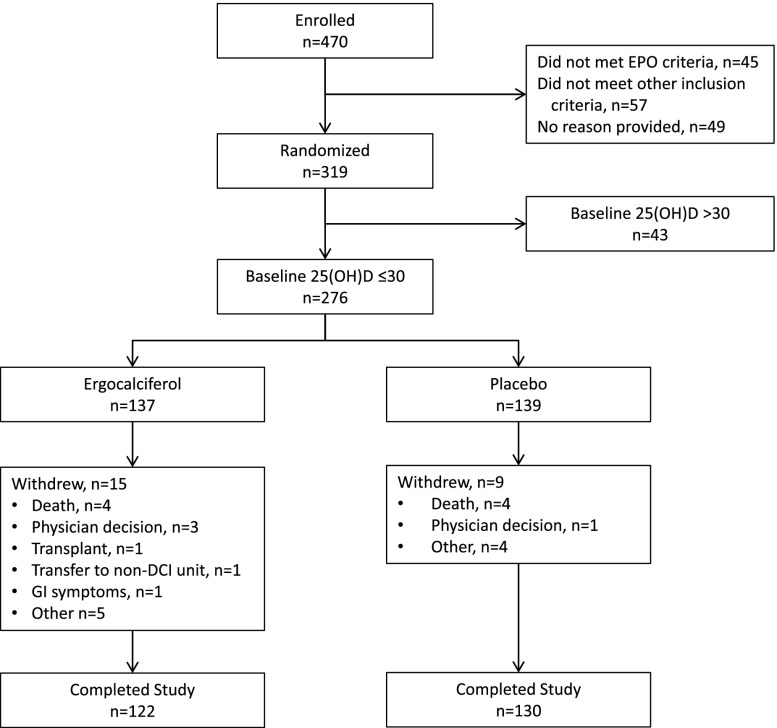
We enrolled 470 patients into baseline between July 2011 and September 2013; 151 were withdrawn prior to randomization and the remaining 319 were randomized (Figure 1). This report describes results in the 276 randomized participants with baseline 25(OH)D levels ≤30 ng/ml. The 6-month follow-up period was completed in April 2014 by 252 (91.3%) of these participants. As shown in Table 1, the mean age of the study population was 61.1 years, median dialysis vintage was 3.5 years, 60% were African American, 46% had diabetes as the cause of ESRD, and 71% were dialyzing via an arteriovenous fistula. The median epoetin dose was 5550 units per week, median iPTH was 427 pg/ml, and mean serum 25(OH)D was 16.4 ng/ml; 47% had 25(OH)D ≤15 ng/ml. At baseline, 82% of patients were treated with a VRDA (approximately half with oral calcitriol and half with intravenous doxercalciferol), and 25% were receiving cinacalcet. Treatment groups were well balanced by baseline characteristics.
Figure 1.
Flow diagram. Few randomized patients failed to complete the study and there was no difference in the rates of dropout across treatment arms.
Table 1.
Participant baseline characteristics
| Characteristica | All (n=276) | Ergocalciferol (n=137) | Placebo (n=139) |
|---|---|---|---|
| 25(OH)D (ng/ml) | 16.4±6.2 | 16.0±5.9 | 16.9±6.4 |
| 25(OH)D level | |||
| ≤15 ng/ml | 130 (47%) | 67 (49%) | 63 (45%) |
| 16–30 ng/ml | 146 (53%) | 70 (51%) | 76 (55%) |
| Age (years) | 61.1±13.6 | 61.4±13.3 | 60.8±13.9 |
| Race | |||
| Caucasian | 92 (33%) | 51 (37%) | 41 (30%) |
| African American | 166 (60%) | 76 (55%) | 90 (65%) |
| Asian | 15 (5%) | 8 (6%) | 7 (5%) |
| Other | 3 (1%) | 2 (1%) | 1 (1%) |
| Cause of ESRD | |||
| Diabetes | 127 (46%) | 66 (48%) | 61 (44%) |
| Hypertension | 83 (30%) | 39 (28%) | 44 (32%) |
| Glomerulonephritis | 33 (12%) | 13 (9%) | 20 (14%) |
| Other | 31 (11%) | 19 (14%) | 12 (9%) |
| Unknown | 2 (1%) | 0 (0%) | 2 (1%) |
| Years on dialysis | 3.5 (1.9, 7.6) | 3.4 (1.8, 8.4) | 3.8 (2.1, 6.6) |
| Vascular access | |||
| AV fistula | 197 (71%) | 99 (72%) | 98 (71%) |
| AV graft | 61 (22%) | 29 (21%) | 32 (23%) |
| Catheter | 18 (7%) | 9 (7%) | 9 (6%) |
| Hospitalizations in prior 90 days | |||
| None | 236 (86%) | 118 (86%) | 118 (85%) |
| 1 | 29 (11%) | 12 (9%) | 17 (12%) |
| 2+ | 11 (4%) | 7 (5%) | 4 (3%) |
| Comorbidity | |||
| Ischemic heart disease | 81 (29%) | 45 (33%) | 36 (26%) |
| Congestive heart failure | 80 (29%) | 45 (33%) | 35 (25%) |
| Peripheral vascular disease | 37 (13%) | 22 (16%) | 15 (11%) |
| Limb amputation | 15 (5%) | 9 (7%) | 6 (4%) |
| Predialysis systolic BP (mmHg) | 149.8±22.5 | 148.1±22.8 | 151.6±22.1 |
| Predialysis diastolic BP (mmHg) | 78.1±14.2 | 77.2±14.9 | 79.0±13.5 |
| VDRA use | |||
| Calcitriol | 115 (42%) | 56 (41%) | 59 (42%) |
| Doxercalciferol | 111 (40%) | 55 (40%) | 56 (40%) |
| None | 50 (18%) | 26 (19%) | 24 (17%) |
| Cinacalcet use (%) | 69 (25%) | 34 (25%) | 35 (25%) |
| Phosphate binder use | 245 (89%) | 117 (85%) | 128 (92%) |
| Epogen dose (units/week) | 5550 (2500, 11,900) | 5800 (2600, 12,200) | 5400 (2400, 11,500) |
| Epogen protocol | |||
| Corporate | 241 (87%) | 121 (88%) | 120 (86%) |
| Local facility | 35 (13%) | 16 (12%) | 19 (14%) |
| Intravenous iron in past month | 77 (28%) | 39 (28%) | 38 (27%) |
| Monthly iron dose (mg/month) | 108.6±27.0 | 104.5±28.9 | 112.8±24.6 |
| Hemoglobin (g/dl) | 11.0±0.7 | 11.0±0.7 | 11.0±0.7 |
| Serum albumin (g/dl) | 3.9±0.3 | 3.9±0.3 | 3.9±0.3 |
| iPTH (pg/ml) | 427 (249, 583) | 425 (251, 558) | 439 (246, 618) |
| hsCRP (mg/l) | 4.3 (1.6, 11.5) | 5.1 (1.8, 10.3) | 3.8 (1.5, 12.0) |
AV, arteriovenous.
Continuous variables expressed as mean±SD or, when not normally distributed, as median (25th to 75th percentile). Categorical variables are n (%).
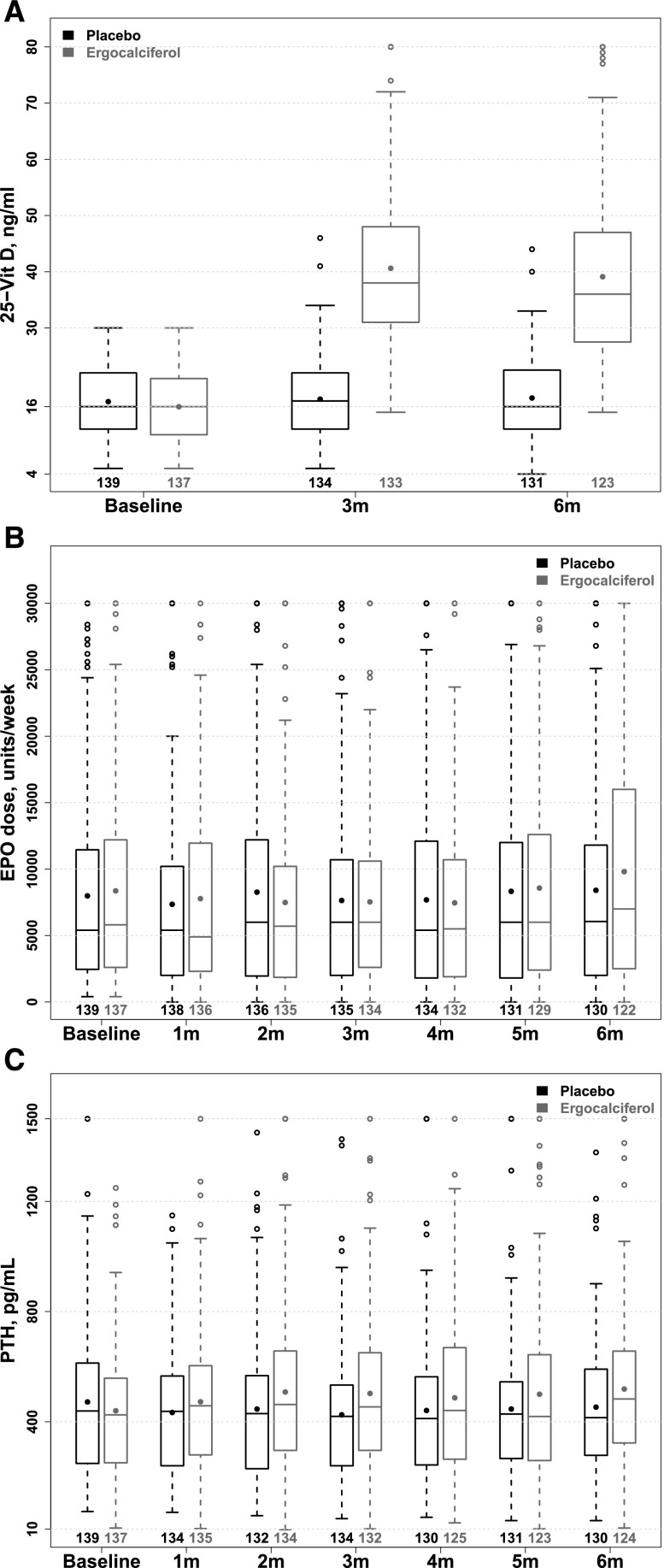
In the ergocalciferol arm, mean±SD serum 25(OH)D increased from 16.0±5.9 ng/ml at baseline to 41.0±15.6 ng/ml at 3 months and 39.2±14.9 ng/ml at 6 months, while corresponding values in the placebo arm were unchanged at 16.9±6.4 ng/ml, 17.3±7.0 ng/ml, and 17.5±7.4 ng/ml, respectively (Figure 2A). The proportion of patients with a serum 25(OH)D ≥30 ng/ml at 0, 3, and 6 months, respectively, were 1.5%, 78.9%, and 67.5% in the ergocalciferol arm and 2.2%, 3.7%, and 6.1% in the placebo arm. Stratified by baseline serum 25(OH)D, serum 25(OH)D increased steadily over 6 months in patients with baseline levels ≤15 ng/ml who received ergocalciferol at 50,000 units weekly, but, in the subgroup with baseline serum 25(OH)D 16–30 ng/ml, levels increased up to 3 months and then declined between months 3 and 6, coincident with the reduction in dose from 50,000 units weekly to monthly (Supplemental Appendix Figure 1).
Figure 2.
Increase in 25(OH)D levels but no effect on epoetin dose or iPTH with nutritional vitamin D supplementation. Boxplots of serum 25(OH)D (Figure 2A), weekly epoetin (EPO) dose (Figure 2B), and iPTH (Figure 2C) over 6 months by treatment allocation. Serum 25(OH)D significantly increased in the ergocalciferol arm at 3 and 6 months and was unchanged in the placebo arm. The results of mixed models revealed no statistically significant change in epoetin dose or iPTH over time in either treatment arm, and no difference across treatment arms.
As shown in Figure 2B and Table 2, weekly epoetin dose did not significantly change in either arm over 6 months (geometric mean rate 0.98 [95% confidence interval (95% CI), 0.94 to 1.02] in the ergocalciferol arm versus 0.99 [95% CI, 0.95 to 1.03] in the placebo arm), and there was no difference in the rate of change by treatment allocation (P=0.78). In a sensitivity analysis, we also found no effect on epoetin dose in subjects who achieved a serum 25(OH)D ≥30 ng/ml at 3 or 6 months (see Supplemental Appendix Table 1). Results were also unchanged when using alternate strategies to handle missing epoetin data (Supplemental Appendix Table 2).
Table 2.
Change in parameters across treatment arms over the 6-month study duration
| Parameter | Placebo | Ergocalciferol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Value | Month 3 Value | Month 6 Value | Model Slopec (95% CI) | Within-Group P Valued | Baseline Value | Month 3 Value | Month 6 Value | Model Slopec (95%CI) | Within-Group P Valued | Between-Group P Valuee | |
| Serum 25(OH)D (ng/ml) | 16.9±6.4 | 17.3±7.0 | 17.5±7.4 | −0.07 (–0.44 to 0.29) | 0.70 | 16.0±5.9 | 41.0±15.6 | 39.2±14.9 | 4.2 (3.8 to 4.5) | <0.001 | <0.001 |
| Epoetin dose (units/week)a | 5400 (2400, 11,500) | 6000 (2000, 10,800) | 6050 (2000, 11,800) | 0.98 (0.94 to 1.02) | 0.42 | 5800 (2600, 12,200) | 6000 (2600, 10,600) | 7000 (2500, 16,000) | 0.99a (0.95 to 1.03) | 0.68 | 0.78 |
| Serum ferritin (ng/ml)a | 1162 (913, 1421) | 1155 (938, 1515) | 1146 (947, 1470) | 1.01 (1.00 to 1.02) | 0.25 | 1222 (904, 1528) | 1171 (967, 1513) | 1175 (966, 1536) | 1.00 (0.99 to 1.01) | 0.98 | 0.38 |
| TSat (%) | 30.4±10.7 | 30.7±8.7 | 30.4±8.9 | −0.02 (–0.32 to 0.28) | 0.90 | 30.5±10.4 | 30.5±9.1 | 30.1±10.9 | −0.07(–0.37 to 0.23) | 0.65 | 0.79 |
| iPTH (pg/ml)a | 439 (246, 618) | 420 (240, 533) | 415 (278, 591) | 1.01 (0.99 to 1.02) | 0.63 | 425 (251, 558) | 454 (296, 651) | 483 (323, 657) | 1.01a (0.99 to 1.03) | 0.25 | 0.60 |
| Calcium (mg/dl) | 9.0±0.7 | 9.0±0.8 | 9.0±0.8 | 0.00 (–0.02 to 0.02) | 0.87 | 9.1±1.0 | 9.1±0.7 | 9.0±0.7 | −0.01 (–0.03 to 0.01) | 0.41 | 0.61 |
| Phosphorus (mg/dl) | 5.2±1.3 | 5.2±1.4 | 5.3±1.3 | 0.02 (–0.02 to 0.05) | 0.41 | 5.3±1.3 | 5.2±1.4 | 5.2±1.3 | −0.02 (–0.06 to –0.02) | 0.25 | 0.13 |
| Doxercalciferol (μg/tx) | 2.55±1.66 | 2.93±1.82 | 2.86±1.73 | 0.04 (–0.06 to 0.15) | 0.41 | 2.20±1.65 | 3.23±2.29 | 3.80±2.59 | 0.21 (0.10 to 0.31) | <0.001 | 0.02 |
| Calcitriol (μg/tx) | 0.77±0.63 | 0.82±0.64 | 0.82±0.66 | 0.01 (–0.01 to 0.03) | 0.26 | 0.66±0.44 | 0.67±0.46 | 0.64±0.48 | −0.01 (–0.03 to 0.02) | 0.57 | 0.21 |
| Cinacalcet use (%) | 25 | 25 | 25 | 1.00 (0.96 to 1.04) | 0.98 | 25 | 29 | 30 | 1.04 (0.99 to 1.09) | 0.12 | 0.25 |
| Phosphate binder load (pills per day)b | 7.0±3.7 | 6.8±4.0 | 6.7±4.0 | −0.03 (–0.12 to 0.07) | 0.60 | 6.2±4.1 | 6.1±3.7 | 6.4±4.2 | −0.04 (–0.14 to 0.05) | 0.39 | 0.79 |
| hsCRP (mg/L)a | 3.8 (1.5, 12.0) | 4.4 (1.8, 14.1) | 4.4 (1.7, 10.9) | 1.01 (0.98 to 1.04) | 0.51 | 5.1 (1.8, 10.3) | 4.8 (2.2, 12.5) | 5.9 (2.0, 14.5) | 1.03a (1.00 to 1.06) | 0.02 | 0.22 |
| Systolic BP (mmHg) | 151.6±22.1 | 151.4±18.7 | 152.1±19.8 | 0.18 (–0.28 to 0.65) | 0.44 | 148.1±22.8 | 149.7±17.8 | 151.6±18.9 | 0.19 (–0.28 to 0.65) | 0.44 | 0.60 |
TSat iron saturation; tx treatment.
For non-normally distributed parameters, median and interquartile range is shown in the first three columns for each randomized group and the natural log of the variable was modeled. The interpretation is the percent change per month. For example, a β of 0.98 for log epogen is interpreted as a 2% decrease in epoetin dose per month. For the binary variable of cinacalcet, the term is expressed as the percent of participants using the agent, the slope is interpreted as a percent change in the odds of cinacalcet use over time.
The total daily dose of all phosphate binders was converted into the equivalent number of calcium acetate 667 mg pills per day according to Daugirdas et al.48 See the text for details.
The model slope is the change (95% CI) in a given parameter per month. For example a β of –0.07 for serum 25 (OH)D is interpreted as a 0.07 ng/ml decline per month in serum 25(OH)D level.
Within-group P value (the null hypothesis is that there is no change over time) and ebetween-group P value (the null hypothesis is that there is no difference between the slopes of the two arms)
There was also no significant difference in the rate of change in iPTH in either treatment arm, and no difference in the rate of change across arms (P=0.60; Figure 2C) and the results were the same for serum phosphorus, calcium, high-sensitivity C-reactive protein (hsCRP), cinalcalcet use, calcitriol and phosphorous binder dose. There was a statistically significant but clinically negligible difference across study arms in the rate of change in doxercalciferol dose, which increased by 0.21 mg per month (95% CI, 0.10 to 0.31) in the ergocalciferol arm and was unchanged in the placebo arm (0.04 mg per month; 95% CI, –0.06 to 0.15 difference across arms; P=0.02). Neither systolic BP (Table 2) nor the number of antihypertensive drugs used per patient (Supplemental Appendix Table 3) changed in either treatment groups over the study.
Rates of all-cause, cardiovascular (CV) or infectious hospitalization, falls, or fractures did not differ by treatments arms (Table 3). There was a trend toward reduced CV hospitalizations and increased fractures in the ergocalciferol arm, but estimates are imprecise given the small sample size.
Table 3.
Clinical events by treatment arm
| Ergocalciferol (n=137) | Placebo (n=139) | P Value | ||
|---|---|---|---|---|
| All-cause hospitalization | ||||
| Events/total years at risk | 70/61.1 | 88/62.6 | ||
| Event rate (per 100 PY) (95% CI) | 114.6 (90.7 to 144.8) | 140.5 (114.0 to 173.1) | ||
| IRR (95% CI) | 0.82 (0.60 to 1.12) | 1.00 (Reference) | 0.20 | |
| Cardiovascular disease hospitalization | ||||
| Events/total years at risk | 17/61.1 | 29/62.6 | ||
| Event rate (per 100 PY) (95% CI) | 27.8 (17.3 to 44.8) | 46.3 (32.2 to 66.6) | ||
| IRR (95% CI) | 0.60 (0.33 to 1.09) | 1.00 (Reference) | 0.10 | |
| Infection-related hospitalization | ||||
| Events/total years at risk | 15/61.1 | 15/62.6 | ||
| Event rate (per 100 PY) (95% CI) | 24.6 (14.8 to 40.7) | 23.9 (14.4 to 39.7) | ||
| IRR (95% CI) | 1.03 (0.50 to 2.10) | 1.00 (Reference) | 0.95 | |
| Falls | ||||
| Events/total years at risk | 21/61.1 | 21/62.6 | ||
| Event rate (per 100 PY) (95% CI) | 34.4 (22.4 to 52.7) | 33.5 (21.9 to 51.4) | ||
| IRR (95% CI) | 1.03 (0.56 to 1.88) | 1.00 (Reference) | 0.94 | |
| Fractures | ||||
| Events/total years at risk | 5/61.1 | 1/62.6 | ||
| Event rate (per 100 PY) (95% CI) | 8.2 (3.4 to 19.7) | 1.6 (0.2 to 11.3) | ||
| IRR (95% CI) | 5.13 (0.60 to 43.88) | 1.00 (Reference) | 0.14 | |
PY, person-years; IRR, Incidence rate ratio is the ratio of the event rate in the ergocalciferol as compared with the placebo arm.
Subgroup Analyses
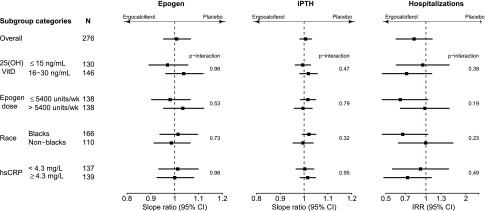
There was no difference in the effects on epoetin dose, iPTH, or all-cause hospitalization across treatment arms by subgroups of baseline serum 25(OH)D level, epoetin dose, race, or hsCRP (Figure 3). The effect of ergocalciferol was in different directions for some subgroups (e.g., baseline 25(OH)D strata and epoetin dose); however, the confidence intervals overlap widely. Tests for interactions were not significant for subgroup comparisons for any outcome.
Figure 3.
Subgroup analysis of the effects of ergocalciferol supplementation on epoetin dose, iPTH, and all-cause hospitalizations. There were no statistically significant differences in the effect of ergocalciferol supplementation on epoetin dose, iPTH level, or all-cause hospitalization by baseline serum 25(OH)D level, baseline weekly epoetin dose, race, or baseline hsCRP level. IRR, incidence rate ratio.
Discussion
Among maintenance patients on hemodialysis with serum 25(OH)D ≤30 ng/ml, ergocalciferol supplementation increased 25(OH)D, but this had no significant effect on epoetin utilization, iPTH, or other biochemical markers or treatments of CKD-MBD. Additionally, there were no significant differences in all-cause, CV or infection-related hospitalizations, falls or fractures, although statistical power was limited for these secondary outcomes. From the present study we can conclude that nutritional vitamin D has no role to play in the management of anemia in dialysis patients.
Prior RCTs of nutritional vitamin D supplementation in patients on hemodialysis have generally been small and do not provide high-level evidence for any outcome. Only one prior RCT reported on ESA dose; it found a reduction in ESA but was a very small (n=22) and unblinded study.24 Reduced expression of proinflammatory cytokines is the proposed mechanism by which nutritional vitamin D is hypothesized to affect erythropoiesis,13 although convincing evidence of a role for nutritional vitamin D in mediating inflammation in a manner that impacts on clinical outcomes remains to be demonstrated.28,29 Prior RCTs that have measured inflammation in patients on hemodialysis after vitamin D supplementation have not found reductions in cytokines or T-cell or monocyte mediators.24,30,31 The observation of an acute decline from baseline in serum 25(OH)D drawn 48 hours after surgery has even led to the suggestion that serum 25(OH)D is a negative acute-phase reactant.32 The current study furthers the skepticism about the therapeutic role for nutritional vitamin D in reducing inflammation by showing no effect of increasing serum 25 (OH)D on epoetin responsiveness or hsCRP levels.
There have been ten previous RCTs with sample size ≥30 patients and follow-up ≥8 weeks that have assessed the effects of nutritional vitamin D or calcifediol supplementation in patients on dialysis on iPTH,19,22–27,31,33–35 and all but one small study33 is consistent with the present study in finding no effect on iPTH. An absence of effect of nutritional vitamin D on iPTH has also been found in stage IV CKD,36,37 while at earlier stages of CKD, there appears to be a reduction in iPTH with nutritional vitamin D. The conclusion from a Cochrane evidence review, published in 2009 before many of the above-mentioned trials were completed, concluded that vitamin D is effective in suppressing iPTH, although (not obvious from the abstract) this statement pertains to VDRAs and not to nutritional vitamin D.38 The results of observational studies lead one to believe that there is a reduction in iPTH in patients on hemodialysis with nutritional vitamin D supplementation,6,8,18,39 although these studies used historical controls and are limited by other biases inherent with a nonrandomized design. Given our result,s as well as those of several smaller clinical trials, we believe there is now strong evidence that nutritional vitamin D has no effect on iPTH in patients on dialysis.
Our study showed lower, albeit nonstatistically significant CV-related hospitalizations among participants randomized to ergocalciferol. Vitamin D receptors are present within the vasculature and heart, and vitamin D deficiency has been implicated in the pathogenesis of left ventricular hypertrophy, cardiac fibrosis, endothelial dysfunction, and renin-angiotensin system activation.40 We found no change in BP in the present study, consistent with a 6-month trial of cholecalciferol at a dose of 3000 IU daily versus placebo in 64 patients on hemodialysis, which also found no effect on arterial stiffness or cardiac structure or function.23 Evidence for reduction in CV events with nutritional vitamin D in the general population is lacking.41 A sequential network meta-analysis of RCTs of nutritional vitamin D supplementation with pooled sample sizes of 48,647 patients from nine trials with myocardial infarction as an outcome and 46,431 patients in eight trials for stroke found no effects of nutritional vitamin D for either outcome and the CIs fell within futility bands, suggesting that any effect of vitamin D would not exceed a 15% relative risk reduction for CV events and a 5% relative risk reduction for mortality.42 RCTs are ongoing in the general population, but even if these do find benefits of nutritional vitamin D, these results may not extend to the dialysis population, where vitamin D receptor activity and 1-α hydroxylase function may be reduced.43
Potential harms with nutritional vitamin D supplementation include hypercalcemia, hyperphosphatemia, and extraskeletal ossification. In our study, in which 80% or more were concomitantly treated with VDRAs, there were no significant changes in calcium or phosphorus, and this is consistent with all of the other RCTs in patients on hemodialysis,19,22–27,31,33–35 including one study that administered 200,000 IU of cholecalciferol weekly for 3 weeks.27 Raising the possibility that there may be harmful outcomes associated with excess nutritional vitamin D administration, an RCT in community dwelling elderly women treated with a one-time dose of 500,000 units of ergocalciferol or placebo found a 15% increase in falls and a 26% increase in fractures in the ergocalciferol arm at 1 year.44 While our present study showed a trend toward more fractures in ergocalciferol-treated patients, event rates were low and these results are inconclusive.
We acknowledge that this trial is not able to determine whether ergocalciferol is efficacious in the absence of VDRAs, as the majority of trial participants were treated with VDRAs. However, given the wide use and efficacy of VDRAs for the treatment of mineral and bone disorder (the latter unproven with nutritional vitamin D), we felt that potential benefits associated with nutritional vitamin D would need to be seen in the setting of concomitant VDRA use. The results of this trial are applicable to the treatment of most American patients on dialysis, 80% of whom are treated with VDRAs, and to the majority of European patients on dialysis, 60% of whom are treated with VDRAs per the latest Dialysis Outcomes and Practice Patterns Study.3 A trial of nutritional vitamin D supplementation in the absence of VDRA use may be warranted in settings where VDRA use is not as widespread.
This study has many strengths: it is the largest placebo-controlled, doubled-blinded RCT assessing nutritional vitamin D supplementation in hemodialysis to date; the double-blinding was successful; the study groups were well balanced at baseline; drug therapy was administered in the unit and therefore witnessed; epoetin- and iron-dosing protocols were kept constant throughout the trial; and the outcomes were carefully ascertained through prospective data collection.
The major limitation is that the study is underpowered for death and other major clinical outcomes. Effect sizes estimated from the general population (no more than a 15% relative risk reduction for CV events and 5% for mortality42,45) may be even less in patients on dialysis, considering that interventions that have proven beneficial in the general population, for example statins,46 have not extended to dialysis. Accordingly, in designing a trial to assess death or CV events in dialysis, sample size calculations should probably assume smaller effects than those estimated in the general population, the latter of which are already quite small. The feasibility of such a trial or even the need for it (in the case of mortality) is questionable. A second limitation of the present study is that serum 25(OH)D started to decline in the subgroup with baseline 25(OH)D 16–30 ng/ml after 3 months, coinciding with a protocol-driven reduction in ergocalciferol dose. The decline in 25(OH)D levels when the dose was reduced from 50,000 units weekly to monthly after 3 months would suggest that at least 50,000 IU of ergocalciferol every 2 weeks is needed to maintain serum 25(OH)D levels >30 ng/ml during the first 6 months in most patients on hemodialysis with 25(OH)D levels starting at <30 ng/ml. It is unclear whether such doses will be needed beyond the first 6 months, and levels should be monitored in practice because not all patients will respond uniformly. Third, we lack data on other potential effects of ergocalciferol, including whether there is a change in fibroblast growth factor 23 levels or changes in other nontraditional CV disease risk factors in response to ergocalciferol supplementation. Future post hoc analyses may be able to evaluate these potential effects. Fourth, the 25(OH)D level that defines deficiency is uncertain,4 and it is possible that the effects of supplementation are only seen in severely deficient patients; hence, our inclusion of patients with 25(OH)D levels up to 30 mg/dl may have diluted results. However, there was no suggestion of a differential effect by 25(OH)D level on the outcomes studied in subgroup analyses. Finally, there was a large proportion of African Americans in this study population. A recent publication suggests that African Americans have higher free 25(OH)D than Caucasians at any given total 25(OH)D level.47 Although this finding raises the possibility that the lack of effect in the present study is because some patients were not deficient in 25(OH)D, we did not find a differential effect by race or baseline 25(OH)D for any outcome (Figure 3).
In conclusion, administration of ergocalciferol to 25(OH)D-deficient patients on hemodialysis increased 25(OH)D levels without significant increases in serum calcium or phosphorus; however, treatment had no effect on the primary outcome of epoetin dose. While the study was not designed to examine all postulated actions that nutritional vitamin D might have, and thus, we cannot conclude that nutritional vitamin D is of no value in patients on dialysis, the study design was sufficient for us to conclude that there is no role for nutritional vitamin D in the management of anemia in patients on hemodialysis.
Concise Methods
The study was a multicenter, parallel-arm, randomized, double-blind, placebo-controlled trial. The study was approved by the Western Institutional Review Board and registered with Clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT01395823).
Study Population
Patients were recruited from 12 geographic sites at 20 dialysis units operated by Dialysis Clinic Inc. (DCI), the largest not-for-profit dialysis provider in the United States. Eligibility criteria were: receipt of thrice weekly in-center hemodialysis for ≥90 days; age ≥18 years; life expectancy >6 months; absence of gastrointestinal illness or surgery that could affect vitamin D absorption; epoetin and IV iron treated per the same protocol for ≥90 days; not currently treated with ergocalciferol ≥50,000 IU or cholecalciferol ≥30,000 IU per month; and ability to provide informed consent. Exclusion criteria were: serum calcium ≥10.5 mg/dl or phosphorous >8.0 mg/dl within the past 30 days; infection within the past 30 days; current use of immunosuppressants other than low-dose corticosteroids or equivalent; transfusion within the past 30 days; hematologic malignancy or nonmalignant condition requiring transfusion in the past 90 days; Kt/V <1.3 on past three monthly labs; or pregnancy.
Baseline Period
Following informed consent, participants entered a 4–8-week baseline period. To proceed to randomization, weekly epoetin needed to be stable, defined as not having been held or missed during the past 4 weeks. If weekly epoetin was not administered, most often because hemoglobin level exceeded the threshold for epoetin discontinuation, the participant could re-enter a new 4-week baseline period, one more time, after epoetin was restarted.
Intervention
Participants with baseline serum 25(OH)D ≤15 ng/ml received 50,000 IU weekly for 6 months, and those with 25(OH)D 16–30 ng/ml received 50,000 IU every week for the first 3 months followed by 50,000 IU monthly for 3 months. The placebo was encapsulated by the DCI pharmacy and looked, felt, smelt, and tasted the same as ergocalciferol. The study drug was administered by the dialysis unit staff in the dialysis unit per electronic orders in DCI’s Medical Information System (MIS).
Follow-Up
Study visits were conducted monthly during dialysis treatments to ascertain adverse events and review medications. Data were entered into a web-based electronic data-capturing system designed by DCI’s Information Services.
Outcomes
Participants were followed up on for 6 months. The primary outcome was the change in epoetin (epogen alfa) dose over 6 months. Epoetin dose was ascertained through drug administration records within the MIS. If a participant was absent from the dialysis unit for ≤2 weeks in a month, the median dose during the nonabsent period was extrapolated to represent the monthly dose. If absent for >2 weeks, which occurred for 2.3% of epoetin data (174 of 7629 patient-weeks), the epoetin dose was set to missing. In the sensitivity analyses, we tested two alternate approaches: (1) using the last value carried forward, regardless of the duration of missing epoetin values; and (2) leaving all values as missing. Cinacalcet and phosphate binder doses were reconciled at monthly study visits. VDRAs were administered in the dialysis unit and doses were captured from drug administration records in the MIS. Serum calcium and phosphorus were measured every other week for 8 weeks and monthly thereafter. iPTH was measured monthly, while serum 25(OH)D and hsCRP were measured at baseline, and 3 and 6 months. Serum ferritin and iron saturation (serum iron/total iron binding capacity) was measured every 3 months and so we chose the value closest to but not exceeding 3 and 6 months after randomization in defining these parameters. Laboratory samples were assayed by DCI Laboratory, a Clinical Laboratory Improvement Amendments-certified laboratory. Serum total 25(OH)D and iPTH were measured via chemiluminescence using the Diasorin Liason assay and Diasorin Liason XL assay, respectively.
The median of the weekly epoetin dose was calculated for each month from baseline through to month 6. Different formulations of phosphate binders were converted into an equivalent number of 667 mg calcium acetate pills using a published algorithm.48 There is no well accepted method for converting different VDRAs into a uniform dose metric; accordingly, results were analyzed by VDRA preparation. The VDRA preparation at the start and end of the study was the same for all but three patients who were excluded from the VDRA analyses. No outcomes were changed after study commencement.
Randomization and Blinding
Randomization was stratified by dialysis unit, baseline serum 25(OH)D level (≤15 versus 16–30 ng/ml) and baseline epoetin dose (above versus below 5500 units/week, the 75th percentile in January 2011). Randomization schedules were generated by ordering blocks of eight according to a random number generator, which were then programmed into the electronic data capturing system. Upon randomization, the MIS was autopopulated with medication orders. Only the DCI Pharmacy was aware of which medication (A versus B) was ergocalciferol versus placebo.
Sample Size
The power calculation was based on a nonparametric Wilcoxon–Mann–Whitney test, assuming that the SD of the differences in epoetin dose over 6 months would be 4000 units and that the distribution of differences was heavy-tailed (double exponential). The estimate of the SD of the differences was calculated using existing data within DCI. With a two-sided α of 0.05 to reject the null hypothesis and assuming a 20% dropout rate for death, transplant, or loss to follow-up, 276 patients would be needed to provide 80% power to detect a ≥1200 unit per week or greater decline in epoetin administration at 6 months.
Statistical Analyses
Change in epoetin dose and other continuous outcome variables were modeled using linear mixed models of monthly values with a linear function of time. The natural log of epoetin dose and iPTH was modeled because distributions were skewed. The mixed models allowed different random intercepts and slopes for each subject. Slopes were compared across treatment arms. Event rates for hospitalizations and other binary outcomes were compared using Poisson regression. Subgroup analyses were conducted for three outcomes, epoetin dose, iPTH, and all-cause hospitalization rate, by subgroups of baseline 25(OH)D, race, baseline epoetin dose (≤ versus > median), and hsCRP values (≤ versus > median). Analyses were performed using R software (version 3.1.0) and SAS software (version 9.4, Cary, NC).
Disclosures
All authors are either employed by Dialysis Clinic Inc. (DCI), receive salary support from DCI, or are DCI medical directors. No authors have additional competing financial interests.
Supplementary Material
Acknowledgments
We are indebted to the study participants for taking part in the study, the Corporate Project Managers (Alice Martin and Mary Ann McCain) and the dialysis unit staff at the participating units, who made this research possible. We acknowledge the Dialysis Clinic Inc. (DCI) Pharmacy for encapsulating and distributing study drugs and DCI’s Information Technology Group for developing the electronic research application for data collection and randomization of subjects.
This study was supported by DCI. The funder had no role in the design, conduct, interpretation, or writing up of results.
Previously presented at the Annual Meeting of the American Society of Nephrology Late Breaking Clinical Trials Session, November 15, 2014 in Philadelphia, PA. Published in the meeting abstract book: Miskulin D et al., J Am Soc Nephrol 25: B2, 2014.
D.C.M. and H.T. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
We thank the principal investigators and study coordinators at the participating dialysis units, who were as follows: Toros Kapoian, Neeta O’Mara (DCI, North Brunswick), Jeffrey Bell, Brittany Stalvey (DCI, Albany), Garry Reams, Kay Dye, Marci Wilson, Harold Moore, (DCI, Columbia), Richard Muther, Martha Jo Dunkelberg (DCI, Belton), Khalid Bashir, Lisa Moffatt, Tiffany Floro (DCI, Omaha), Jean Lee, David Conway (DCI, Philadelphia), Daniel Weiner, Lilly Chan (DCI, Boston), Kenneth Abreo, Joellen Ford (DCI, Shreveport), Andrew Covit, Alicia Ibanez (DCI, New Brunswick), Paul Serrell, Becky Spurgeon (DCI, Maryville), Jeffrey Krahling, Suki Whitaker (DCI, Redding), Denise Rivers, April Warren (DCI, Knoxville).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Mushroom Clouds for Vitamin D?,” on pages 1581–1584.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040468/-/DCSupplemental.
References
- 1.Lambert PW, Stern PH, Avioli RC, Brackett NC, Turner RT, Greene A, Fu IY, Bell NH: Evidence for extrarenal production of 1 alpha, 25-dihydroxyvitamin D in man. J Clin Invest 69: 722–725, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JS, Hewison M: Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys 523: 95–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, Andreucci VE, Fukagawa M, Frimat L, Mendelssohn DC, Port FK, Pisoni RL, Robinson BM: Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 10: 98–109, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer RF: Vitamin D in dialysis: defining deficiency and rationale for supplementation. Semin Dial 26: 40–46, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes CKDMBDWG: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A: Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 5: 905–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar VA, Kujubu DA, Sim JJ, Rasgon SA, Yang PS: Vitamin D supplementation and recombinant human erythropoietin utilization in vitamin D-deficient hemodialysis patients. J Nephrol 24: 98–105, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW: Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract 105: c132–c138, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Andress DL: Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int 69: 33–43, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kendrick J, Targher G, Smits G, Chonchol M: 25-Hydroxyvitamin D deficiency and inflammation and their association with hemoglobin levels in chronic kidney disease. Am J Nephrol 30: 64–72, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Kiss Z, Ambrus C, Almasi C, Berta K, Deak G, Horonyi P, Kiss I, Lakatos P, Marton A, Molnar MZ, Nemeth Z, Szabo A, Mucsi I: Serum 25(OH)-cholecalciferol concentration is associated with hemoglobin level and erythropoietin resistance in patients on maintenance hemodialysis. Nephron Clin Pract 117: c373–c378, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Patel NM, Gutiérrez OM, Andress DL, Coyne DW, Levin A, Wolf M: Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int 77: 715–720, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Aucella F, Scalzulli RP, Gatta G, Vigilante M, Carella AM, Stallone C: Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure. A synergistic effect with r-HuEpo. Nephron Clin Pract 95: c121–c127, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Icardi A, Paoletti E, De Nicola L, Mazzaferro S, Russo R, Cozzolino M: Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transplant 28: 1672–1679, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Zughaier SM, Alvarez JA, Sloan JH, Konrad RJ, Tangpricha V: The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J Clin Transl Endocrinol 1: 19–25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A: Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 373: 1532–1542, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, Graziano G, McGee R, Nicolucci A, Tognoni G, Strippoli GF: Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 153: 23–33, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Jean G, Souberbielle JC, Chazot C: Monthly cholecalciferol administration in haemodialysis patients: a simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant 24: 3799–3805, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Armas LA, Andukuri R, Barger-Lux J, Heaney RP, Lund R: 25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol 7: 1428–1434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González EA, Sachdeva A, Oliver DA, Martin KJ: Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol 24: 503–510, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ: Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol 8: 1143–1149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massart A, Debelle FD, Racapé J, Gervy C, Husson C, Dhaene M, Wissing KM, Nortier JL: Biochemical parameters after cholecalciferol repletion in hemodialysis: results From the VitaDial randomized trial. Am J Kidney Dis 64: 696–705, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Mose FH, Vase H, Larsen T, Kancir AS, Kosierkiewic R, Jonczy B, Hansen AB, Oczachowska-Kulik AE, Thomsen IM, Bech JN, Pedersen EB: Cardiovascular effects of cholecalciferol treatment in dialysis patients--a randomized controlled trial. BMC Nephrol 15: 50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seibert E, Heine GH, Ulrich C, Seiler S, Köhler H, Girndt M: Influence of cholecalciferol supplementation in hemodialysis patients on monocyte subsets: a randomized, double-blind, placebo-controlled clinical trial. Nephron Clin Pract 123: 209–219, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Shirazian S, Schanler M, Shastry S, Dwivedi S, Kumar M, Rice K, Miyawaki N, Ghosh S, Fishbane S: The effect of ergocalciferol on uremic pruritus severity: a randomized controlled trial. J Ren Nutr 23: 308–314, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Piñera-Haces C, Izquierdo-Ortiz MJ, Martín-de Francisco AL, García-Unzueta MT, López-Hoyos M, Toyos C, Allende N, Quintela E, Arias M: Double treatment with paricalcitol-associated calcifediol and cardiovascular risk biomarkers in haemodialysis. Nefrologia 33: 77–84, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Wasse H, Huang R, Long Q, Singapuri S, Raggi P, Tangpricha V: Efficacy and safety of a short course of very-high-dose cholecalciferol in hemodialysis. Am J Clin Nutr 95: 522–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Autier P, Boniol M, Pizot C, Mullie P: Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2: 76–89, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Mangin M, Sinha R, Fincher K: Inflammation and vitamin D: the infection connection. Inflamm Res 63: 803–819, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Marckmann P, Agerskov H, Thineshkumar S, Bladbjerg EM, Sidelmann JJ, Jespersen J, Nybo M, Rasmussen LM, Hansen D, Scholze A: Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant 27: 3523–3531, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Waldron JL, Ashby HL, Cornes MP, Bechervaise J, Razavi C, Thomas OL, Chugh S, Deshpande S, Ford C, Gama R: Vitamin D: a negative acute phase reactant. J Clin Pathol 66: 620–622, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Delanaye P, Weekers L, Warling X, Moonen M, Smelten N, Médart L, Krzesinski JM, Cavalier E: Cholecalciferol in haemodialysis patients: a randomized, double-blind, proof-of-concept and safety study. Nephrol Dial Transplant 28: 1779–1786, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Rianthavorn P, Boonyapapong P: Ergocalciferol decreases erythropoietin resistance in children with chronic kidney disease stage 5. Pediatr Nephrol 28: 1261–1266, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Bhan I, Dobens D, Tamez H, Deferio JJ, Li YC, Warren HS, Ankers E, Wenger J, Tucker JK, Trottier C, Pathan F, Kalim S, Nigwekar SU, Thadhani R: Nutritional vitamin D supplementation in dialysis: a randomized trial. Clin J Am Soc Nephrol 10: 611–619, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V: Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract 14: 10–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovesdy CP, Lu JL, Malakauskas SM, Andress DL, Kalantar-Zadeh K, Ahmadzadeh S: Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: a randomized controlled trial. Am J Kidney Dis 59: 58–66, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF: Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev (4): CD005633, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Pipili C, Dimitriadis C, Sekercioglu N, Bargman JM, Oreopoulos DD: Effect of nutritional vitamin D preparations on parathyroid hormone in patients with chronic kidney disease. Int Urol Nephrol 44: 167–171, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Norman PE, Powell JT: Vitamin D and cardiovascular disease. Circ Res 114: 379–393, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Mao PJ, Zhang C, Tang L, Xian YQ, Li YS, Wang WD, Zhu XH, Qiu HL, He J, Zhou YH: Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 169: 106–111, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Bolland MJ, Grey A, Gamble GD, Reid IR: The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol 2: 307–320, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Nigwekar SU, Bhan I, Thadhani R: Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis 60: 139–156, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC: Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303: 1815–1822, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Bolland MJ, Grey A: Are trials of vitamin D with mortality as an endpoint really needed? BMJ 349: g4452, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, Hegbrant J, Strippoli GF: HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev 9: CD004289, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R: Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 369: 1991–2000, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daugirdas JT, Finn WF, Emmett M, Chertow GM Frequent Hemodialysis Network Trial Group : The phosphate binder equivalent dose. Semin Dial 24: 41–49, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.