Abstract
Aging incurs aortic stiffening and dilation, but these changes are less pronounced in peripheral arteries, resulting in stiffness and geometry gradients influencing progression of the forward and reflected pressure waves. Because premature arterial aging is observed in ESRD, we determined the respective roles of stiffness and aortic geometry gradients in 73 controls and 156 patients on hemodialysis. We measured aortic pulse wave velocity (PWV) and brachial PWV to evaluate the stiffness gradient [(brachial PWV/aortic PWV)0.5] and ascending aortic and aortic bifurcation diameters to assess aortic taper (ascending aortic diameter/aortic bifurcation diameter). The global reflection coefficient was estimated from characteristic impedance and vascular resistance. Cox proportional hazard models were used to determine mortality risk. The age-associated increase in aortic PWV was higher in patients (P<0.001). In controls, aortic ascending and bifurcation diameters increased with age, with an unchanged aortic taper. In patients on hemodialysis, age did not associate with increased ascending aortic diameter but did associate with increased aortic bifurcation diameter and decreased aortic taper, both of which also associated with abdominal aortic calcifications and smaller global reflection coefficient (P<0.001). In patients, multivariate models revealed all-cause and cardiovascular mortality associated with age, aortic PWV, and aortic bifurcation diameter with high specificity and sensitivity. Using stiffness gradient, aortic taper, or global reflection coefficient in the model produced similar results. Thus, whereas aortic stiffness is a known independent predictor of mortality, these results indicate the importance of also evaluating the aortic geometry in patients on hemodialysis.
Keywords: arteries, pulse wave velocity, cardiovascular disease, vascular calcification, chronic kidney failure, mortality
Aging is associated with marked changes in the mechanical and geometric characteristics of the arterial tree.1 It is characterized by markedly increased aortic stiffness (aortic pulse wave velocity [PWV]), stiffening not observed to the same degree in peripheral muscular arteries.2 This change reduces the normal centrifugal impedance gradient between central and peripheral artery systems.2,3 Any change of the impedance gradient generates modifications of partial local wave reflections, which can be considered a continuous phenomenon along the impedance gradient.3,4 The partial reflections summate to form an apparently unique backward–traveling reflected wave.5 In the presence of impedance gradient and low aortic PWV, the backward wave returns to the aorta at end systole and early diastole, thereby enhancing late diastolic pressure and coronary perfusion4 and attenuating the transmission of pulsatile pressure to the microcirculation.3,6 The central effect of aortic stiffness on impedance gradient attenuation and resulting excessive transfer of pressure and pulsatile flow into microvasculature is mainly observed in low-resistance circulations: the kidney or brain.6–9 The associations between increased aortic PWV and decreased renal function10 or extent of brain white matter leukoaraiosis are well described.8,11–13 Impedance gradient is dependent on wall properties together with the structural gradient and taper from thoracic to abdominal aorta.14–16 Those interrelated measurements of arterial function differ considerably in their relationships to the design of the cardiovascular system and arterial lumen radius, PWV = k(Eh/R)0.5 and characteristic impedance (Zc) = k(Eh/R5)0.5, where E is the elastic modulus, h is arterial wall thickness, and R is artery radius.14–16 Consequently, the aorta undergoes marked geometric changes with aging, becoming tortuous and dilatated,17–20 with alterations of aortic taper affecting the pressure wave transmission and the sites, timing, and intensity of wave reflections.20,21
Premature aortic aging is a dominant characteristic of patients with ESRD.22 Increased aortic stiffness has emerged as an independent predictor of cardiovascular events in this population.23,24 However, the role of age-related changes of aortic dimensions and their consequences in patients with ESRD remain to be evaluated precisely and are the aim of this study.
Results
Clinical Characteristics
The characteristics of studied populations are shown in Table 1. The patients with ESRD were on dialysis for 48 months (range =3–304), and follow-up was 60 months (range =6–132). Common carotid artery pulse pressure was elevated in patients with ESRD with higher forward pressure, increased augmentation index, and reflected pressure wave.
Table 1.
Baseline characteristics and data regarding common carotid artery, aortic dimensions, and arterial PWVs
| Variable | Controls(n=73) | ESRD(n=156) | P Value |
|---|---|---|---|
| Age (yr) | 53±1.8 | 54±1.25 | NS |
| Sex (men/women) | 43/30 | 88/48 | NS |
| Body surface area (m2) | 1.87±0.03 | 1.71±0.02 | <0.001 |
| Body height (m) | 1.70±0.02 | 1.65±0.1 | <0.001 |
| Brachial systolic BP (mmHg) | 145±2.6 | 150±2.4 | NS |
| Brachial diastolic BP (mmHg) | 84±1.8 | 81±1.2 | NS |
| Brachial mean BP (mmHg) | 105±1.9 | 104±1.4 | NS |
| Common carotid artery systolic BP (mmHg) | 135±1.8 | 143±2.1 | <0.01 |
| Common carotid artery pulse pressure (mmHg) | 51±2.2 | 62±2.0 | <0.001 |
| Common carotid artery augmentation index (%) | 19±1.4 | 27±1.1 | <0.001 |
| Common carotid artery augmented pressure (mmHg) | 11±1.2 | 18±1.1 | <0.001 |
| Common carotid artery forward pressure (mmHg) | 39±1.2 | 44±1.1 | 0.01 |
| Left ventricular ejection time (ms) | 309±3.0 | 309±3.0 | NS |
| Diastolic time (ms) | 666±17 | 585±10 | <0.001 |
| Aortic PWV (m/s) | 9.6±0.2 | 11.1±0.2 | <0.001 |
| Brachial PWV (m/s) | 11.0±0.2 | 11.4±0.2 | 0.03 |
| Femoral PWV (m/s) | 10.9±0.2 | 11.3±0.1 | 0.03 |
| Brachial/aortic stiffness gradient | 1.08±0.01 | 1.03±0.01 | <0.001 |
| Femoral/aortic stiffness gradient | 1.07±0.01 | 1.03±0.01 | <0.01 |
| Ascending aortic diameter (mm)a | 28.2±0.3 | 27.8±0.3 | NS |
| Aortic bifurcation diameter (mm)b | 16.4±0.2 | 17.7±0.3 | <0.001 |
| Aortic taper (ascending aortic diameter/aortic bifurcation diameter) | 1.72±0.15 | 1.60±0.25 | <0.001 |
Values are means±SEMs.
Ascending aortic diameter (millimeters per meter2): controls: 14.9±1.6 versus ESRD: 16.5±2.10 (P<0.001).
Aortic bifurcation diameter (millimeters per meter2): controls: 8.8±1.4 versus ESRD: 10.5±1.6 (P<0.001).
Arterial Characteristics
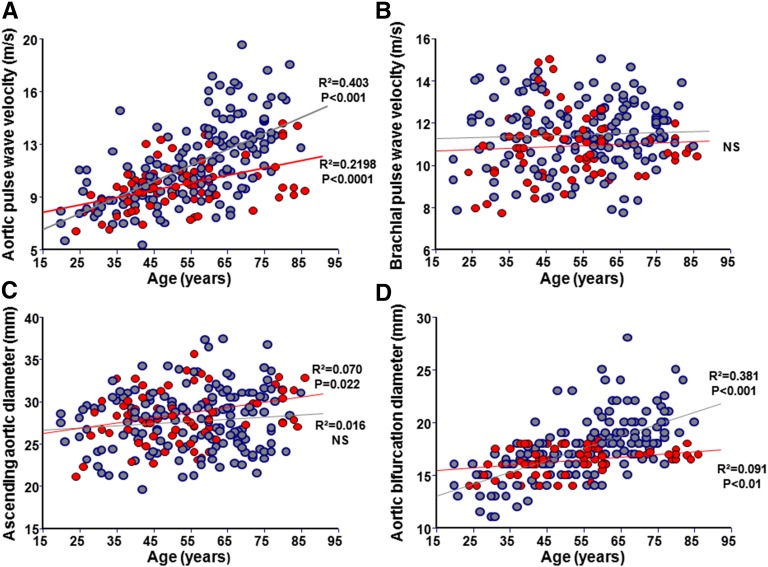
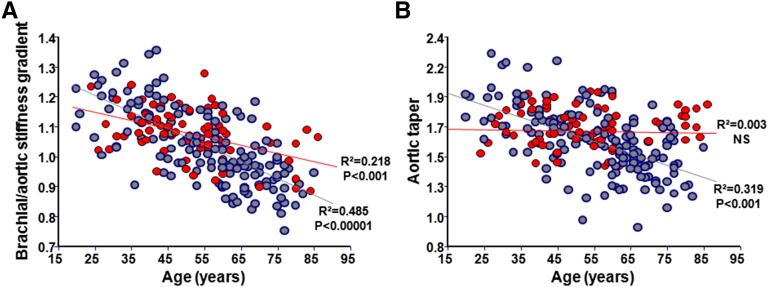
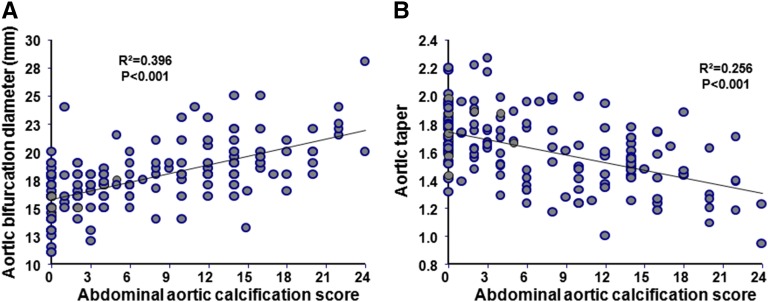
Patients with ESRD had higher aortic PWV (Table 1) associated with significant age–related increases (0.13±0.01 versus 0.08±0.01 m/yr; P=0.001) (Figure 1A, Table 2). Brachial and femoral PWVs (not shown) increased with BP but not with age and were comparable for both groups (Figure 1B, Table 2). In patients with ESRD, the arterial stiffness gradient was lower and declined significantly more with age (β-coefficient: −0.006±0.0005 versus −0.003±0.0006; P<0.001) (Figure 2A). Age, body surface area, and sex are the principal determinants of aortic diameters (Table 2). The ascending aortic diameter increased with age in controls but not in patients with ESRD (Figure 1C). The ascending aortic diameter increased with body surface area in both groups, with significantly higher β-coefficient in ESRD (6.49±1.35 versus 3.49±1.29 mm/m2; P<0.001). Although similar in absolute values, when adjusted for body surface area, the ascending aortic diameter was increased in ESRD (Table 1), but the correlations with age were similar to nonadjusted values (Supplemental Figure 1). The aortic bifurcation diameter was higher in patients with ESRD and increased significantly more with age (β-coefficient: 0.11±0.01 versus 0.03±0.008 mm/yr; P<0.001) (Figure 1D) and body surface area (β-coefficient: 3.06±0.81 versus 1.45±0.51 mm/m2; P<0.001) (Table 2). Patients with ESRD had lower and pronounced age–related aortic taper diminution (β-coefficient: −0.006±0.001 versus −0.003±0.001; P<0.001) (Figure 2B). In patients with ESRD, the abdominal aortic calcification score was positively associated with aortic bifurcation diameter (Figure 3A) and inversely associated with aortic taper (Figure 3B).
Figure 1.
Age-associated aortic and arterial characteristics. Correlations between age and (A) aortic PWV, (B) brachial PWV, (C) ascending aortic diameter, or (D) aortic bifurcation diameter in controls (red) and patients with ESRD (blue).
Table 2.
Multivariate correlation report for arterial PWVs and aortic diameters
| Parameter | β-Coefficient ±SEM | t Value | P Value |
|---|---|---|---|
| Aortic PWV (m/s)a | |||
| Controlsb | |||
| Age (yr) | 0.076±0.012 | 6.100 | <0.001 |
| Mean BP (mmHg) | 0.035±0.010 | 3.114 | <0.01 |
| Sex (1, man; 0, woman) | 0.115±0.301 | 0.301 | NS |
| ESRDc | |||
| Age (yr) | 0.132±0.012 | 11.891 | <0.001 |
| Mean BP (mmHg) | 0.0316±0.010 | 3.110 | <0.01 |
| Sex (1, man; 0, woman) | 0.893±0.363 | 2.456 | 0.01 |
| Brachial PWV (m/s) | |||
| Controld | |||
| Age (yr) | 0.021±0.012 | 1.774 | 0.08 |
| Mean BP (mmHg) | 0.051±0.011 | 4.594 | <0.001 |
| ESRDe | |||
| Age (yr) | 0.015±0.008 | 1.815 | 0.07 |
| Mean BP (mmHg) | 0.040±0.008 | 5.316 | <0.001 |
| Ascending aortic diameter (mm) | |||
| Controlsf | |||
| Age (yr) | 0.068±0.017 | 3.940 | <0.01 |
| Body surface area (m2) | 3.494±1.29 | 2.701 | <0.01 |
| Sex (1, man; 0, woman) | 3.274±0.650 | 5.028 | <0.001 |
| ESRDg | |||
| Age (yr) | 0.0239±0.016 | 1.480 | NS |
| Body surface area (m2) | 6.491±1.350 | 4.780 | <0.001 |
| Sex (1, man; 0, woman) | 1.850±0.577 | 3.204 | <0.01 |
| Aortic bifurcation diameter (mm) | |||
| Controlsh | |||
| Age (yr) | 0.031±0.008 | 4.382 | <0.001 |
| Body surface area (m2) | 1.493±0.512 | 2.917 | <0.01 |
| Sex (1, man; 0, woman) | 1.562±0.303 | 4.495 | <0.001 |
| ESRDi | |||
| Age (yr) | 0.113±0.01 | 11.630 | <0.001 |
| Body surface area (m2) | 3.060±0.809 | 3.767 | <0.01 |
| Sex (1, man; 0, woman) | 1.720±0.356 | 4.908 | <0.001 |
β-Coefficient of aortic PWV versus age of patients with ESRD versus controls: t value, 3.168; P<0.01.
β-Coefficient of aortic PWV versus age of patients with ESRD versus controls: R2=0.402; P<0.001.
β-Coefficient of aortic PWV versus age of patients with ESRD versus controls: R2=0.472; P<0.001.
β-Coefficient of aortic PWV versus age of patients with ESRD versus controls: R2=0.197; P<0.001.
β-Coefficient of aortic PWV versus age of patients with ESRD versus controls: R2=0.265; P<0.001.
β-Coefficient of aortic diameters versus age of patients with ESRD versus controls: R2=0.500; P<0.001.
β-Coefficient of aortic diameters versus age of patients with ESRD versus controls: R2=0.313; P<0.001.
β-Coefficient of aortic diameters versus age of patients with ESRD versus controls: R2=0.489; P<0.001.
β-Coefficient of aortic diameters versus age of patients with ESRD versus controls: R2=0.580; P<0.001.
Figure 2.
Age-associated arterial stiffness gradient and aortic taper. Correlations between age and (A) brachial/aortic stiffness gradient or (B) aortic taper in controls (red) and patients with ESRD (blue).
Figure 3.
Correlations between abdominal aortic calcifications and aortic geometry. Correlations between abdominal aortic calcification score and (A) abdominal bifurcation diameter or (B) aortic taper in patients with ESRD.
Hemodynamics Data
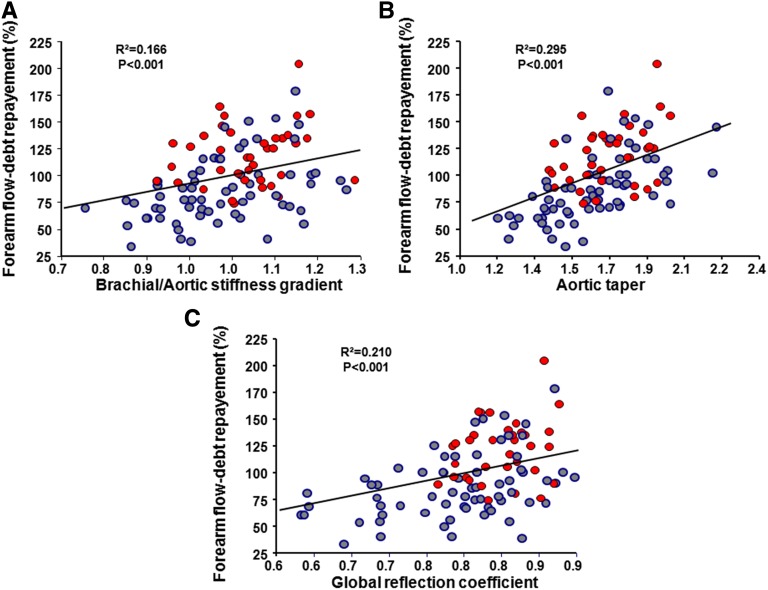
Systemic hemodynamics and forearm parameters are reported in Table 3. Peripheral resistances were comparable for patients and controls, whereas characteristic impedance was higher in patients with ESRD whose global reflection coefficient was lower. Post-ischemic forearm flow debt repayment and duration of post-ischemic vasodilation were lower in patients with ESRD whose minimal flow resistance was higher. Multiple regression analyses retained the global reflection coefficient as negatively associated with ESRD, high aortic PWV, and aortic bifurcation diameter and positively associated with higher brachial PWV, ascending aortic diameter, and the resulting aortic taper and stiffness gradient (Table 4). Forearm flow debt repayment was lower in patients with ESRD, positively associated with ascending aortic diameter and stiffness gradient, and inversely associated with aortic bifurcation diameter (Figure 4, Table 5).
Table 3.
Central hemodynamics and forearm circulation data
| Variable | Control Subjects | ESRD Patients | P Value |
|---|---|---|---|
| n | 73 | 156 | |
| Left ventricular outflow tract area (cm2) | 1.91±0.03 | 1.78±0.03 | <0.001 |
| Left ventricular outflow velocity integral (cm) | 21.8±0.55 | 26.8±0.53 | <0.001 |
| Heart period (ms) | 951±16.2 | 890±11.3 | <0.001 |
| Stroke volume (ml) | 62.1±1.9 | 63.8±1.9 | NS |
| Cardiac output (L/min) | 3.92±0.13 | 4.41±0.14 | 0.03 |
| Aortic peak flow velocity (cm/s) | 108±2.4 | 129±2.4 | <0.001 |
| Aortic peak flow (ml/s) | 309±9.5 | 305±8.5 | NS |
| Characteristic impedance (dyne s per cm−5) | 178±6.7 | 218±8.9 | <0.01 |
| Peripheral resistance (dyne s per cm−5) | 2289±65 | 2143±83 | NS |
| Global reflection coefficient | 0.82±0.06 | 0.76±0.10 | <0.001 |
| n | 36 | 64 | |
| Baseline forearm blood flow (ml/100 ml per min) | 3.6±0.25 | 4.0±0.17 | NS |
| Post-ischemic maximal blood flow (% from baseline) | 816±58 | 573±35 | <0.001 |
| Minimal forearm flow resistance (arbitrary units) | 4.0±0.24 | 4.9±0.27 | 0.01 |
| Forearm flow debt repayment (%) | 119±4.6 | 87±3.8 | <0.001 |
| Duration of vasodilation (s) | 127±4.8 | 97±4.2 | <0.001 |
Values are means±SEMs.
Table 4.
Multivariate correlation report for global reflection coefficient
| Parameter | t Value | P Value | Adjusted Partial R2 |
|---|---|---|---|
| Whole modela | |||
| ESRD (1, yes; 0, no) | −2.341 | 0.02 | 0.017 |
| Aortic PWV (m/s) | −7.013 | <0.001 | 0.150 |
| Brachial PWV(m/s) | 3.665 | <0.01 | 0.033 |
| Ascending aortic diameter (cm) | 3.457 | <0.01 | 0.037 |
| Aortic bifurcation diameter (cm) | −3.149 | <0.01 | 0.030 |
| Age (yr) | 0.492 | NS | |
| Stiffness gradient and taperb | |||
| ESRD (0, no; 1, yes) | −2.955 | <0.01 | 0.028 |
| Brachial/aortic stiffness gradient | 7.142 | <0.001 | 0.164 |
| Aortic taper (ratio) | 4.308 | <0.001 | 0.059 |
| Age (yr) | −1.072 | NS |
R2=0.414; P<0.001.
R2=0.378; P<0.001.
Figure 4.
Correlation between forearm flow debt repayement and arteial changes. Correlations between forearm flow debt repayment and (A) brachial/aortic stiffness gradient, (B) aortic taper, or (C) global reflection coefficient in controls (red) and patients with ESRD (blue).
Table 5.
Multivariate correlation report for post-ischemic forearm flow debt repayment
| Parameter | t Value | P Value | Adjusted Partial R2 |
|---|---|---|---|
| Whole modela | |||
| ESRD (1, yes; 0, no) | –4.001 | <0.001 | 0.091 |
| Aortic bifurcation diameter (cm) | –3.970 | <0.001 | 0.090 |
| Ascending aortic diameter (cm) | 2.914 | <0.01 | 0.048 |
| Aortic PWV (m/s) | –1.342 | 0.18 | NS |
| Brachial PWV (m/s) | 0.924 | 0.36 | NS |
| Mean BP (mmHg) | –0.866 | 0.39 | NS |
| Age (yr) | –0.151 | 0.89 | NS |
| Sex (1, man; 0, woman) | 0.280 | 0.78 | NS |
| Stiffness gradient and aortic taperb | |||
| ESRD (1, yes; 0, no) | –4.488 | <0.001 | 0.114 |
| Aortic taper (ratio) | 5.141 | <0.001 | 0.131 |
| Brachial/aortic stiffness gradient | 2.501 | 0.01 | 0.025 |
R2=0.465; P<0.001.
R2=0.467; P<0.001.
Indicators of Outcome
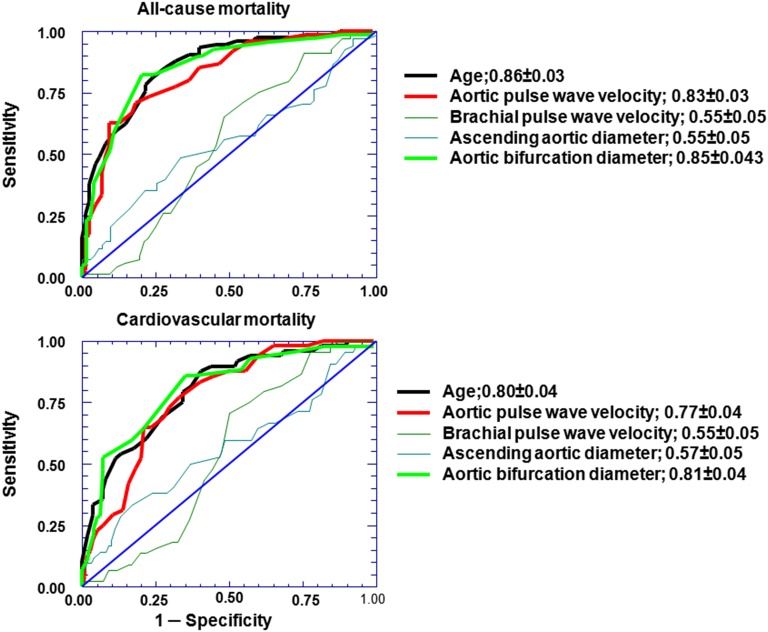
The Cox regression analysis retained age, aortic PWV, and increased aortic bifurcation diameter as being associated with all-cause (n=74) and cardiovascular mortality (n=48) in patients with ESRD (Tables 6 and 7) with high sensitivity and specificity (Figure 5). For all-cause mortality, the area under the receiver operating characteristic curve for age was 0.86±0.03, the area under the receiver operating characteristic curve for aortic bifurcation diameter was 0.85±0.03 (cutoff, 18.0 mm; sensitivity, 0.82; specificity, 0.77), and the area under the receiver operating characteristic curve for aortic PWV was 0.83±0.03 (cutoff, 11.1 m/s; sensitivity, 0.71; specificity, 0.83). The area under the curve was 0.55±0.04 for brachial PWV and 0.55±0.045 for ascending aortic diameter and had low sensitivity and specificity. The area under the curve for stiffness gradient was 0.80±0.04, and the area under the curve for aortic taper was 0.80±0.04 (not shown).
Table 6.
Univariate Cox regression for mortality in patients with ESRD
| Parameter | Wald Z Value | P Value | RR (95% CI) |
|---|---|---|---|
| All cause | |||
| Age (yr) | 5.726 | <0.001 | 1.06 (1.04 to 1.09) |
| Aortic PWV (m/s) | 7.053 | <0.001 | 1.33 (1.23 to 1.44) |
| Aortic bifurcation diameter (mm) | 6.648 | <0.001 | 1.31 (1.21 to 1.41) |
| Brachial PWV (m/s) | 1.761 | NS (0.07) | 1.19 (0.98 to 1.43) |
| Ascending aortic diameter (mm) | 1.575 | NS | 1.74 (0.86 to 3.53) |
| Sex (1, man; 0, woman) | 0.425 | NS | 0.88 (0.53 to 1.50) |
| Mean BP (mmHg) | 0.100 | NS | 1.00 (0.98 to 1.02) |
| Cardiovascular | |||
| Age (yr) | 5.018 | <0.001 | 1.07 (1.04 to 1.10) |
| Aortic PWV (m/s) | 6.154 | <0.001 | 1.36 (1.23 to 1.49) |
| Aortic bifurcation diameter (mm) | 6.124 | <0.001 | 1.35 (1.23 to 1.48) |
| Brachial PWV (m/s) | 1.905 | NS (0.06) | 1.22 (0.99 to 1.50) |
| Ascending aortic diameter (mm) | 1.903 | NS (0.06) | 2.41 (0.96 to 6.05) |
| Sex (1, man; 0, woman) | 0.325 | NS | 0.96 (0.48 to 1.88) |
| Mean BP (mmHg) | 0.309 | NS | 1.00 (0.98 to 1.02) |
RR, relative risk; 95% CI, 95% confidence interval.
Table 7.
Multivariate Cox proportional hazard for mortality
| Parameter | Wald Z Value | P Value | RR (95% CI) | Pseudo-R2 |
|---|---|---|---|---|
| All-causea | ||||
| Age (yr) | 1.448 | 0.14 | 1.02 (0.99 to 1.05) | 0.036 |
| Aortic PWV (m/s) | 2.917 | <0.01 | 1.20 (1.06 to 1.36) | 0.133 |
| Aortic bifurcation diameter (mm) | 2.061 | 0.04 | 1.15 (1.01 to 1.31) | 0.071 |
| Ascending aortic diameter (mm) | 0.383 | 0.70 | 1.02 (0.94 to 1.10) | 0.003 |
| Brachial PWV (m/s) | −0.212 | 0.83 | 0.98 (0.79 to 1.21) | 0.001 |
| Sex (1, man; 0, woman) | 1.343 | 0.18 | 0.64 (0.34 to 1.23) | 0.032 |
| Mean BP (mmHg) | 0.484 | 0.63 | 1.01 (0.98 to 1.02) | 0.004 |
| Cardiovascularb | ||||
| Age (yr) | 0.815 | 0.42 | 1.02 (0.98 to 1.05) | 0.021 |
| Aortic PWV (m/s) | 2.391 | 0.02 | 1.21 (1.03 to 1.41) | 0.153 |
| Aortic bifurcation diameter (mm) | 2.471 | 0.01 | 1.23 (1.04 to 1.45) | 0.162 |
| Ascending aortic diameter (mm) | 0.831 | 0.41 | 1.04 (0.94 to 1.16) | 0.021 |
| Brachial PWV (m/s) | −0.12 | 0.90 | 0.98 (0.74 to 1.30) | 0.001 |
| Sex (1, man; 0, woman) | 1.795 | 0.07 | 0.47 (0.20 to 1.07) | 0.092 |
| Mean BP (mmHg) | −0.091 | 0.93 | 0.99 (0.97 to 1.02) | 0.001 |
RR, relative risk; 95% CI, 95% confidence interval.
R2=0.369; P<0.001.
R2=0.304; P<0.001.
Figure 5.
Receiver operating characteristic curves and mortality. Receiver operating characteristic curves for all-cause and cardiovascular mortality in patients with ESRD.
Discussion
The marked aging–associated changes of the mechanical and geometric characteristics of the arterial tree1 are characterized by notably increased aortic stiffness (aortic PWV) only mildly affecting peripheral muscular arteries.2,3 This degradation reduces the normal centrifugal stiffness/impedance gradient between central and peripheral arterial systems.2,3 In the general populations, aortic and brachial PWVs became nearly equal around the eighth decade, and reversal of the stiffness gradient (aortic PWV > brachial PWV) is rarely observed.2 The situation is different in patients with ESRD, whose aortic and peripheral arteries PWVs become equal around the age of 60–70 years old, with possible inversion of the stiffness gradient.22 Confirming previous publications,22,23,25–28 our results indicate that the age-related changes of the stiffness gradient are largely caused by premature age–related increased aortic stiffness, which is accelerated in ESRD with a significantly steeper age-aortic PWV correlation (Figure 1A, Table 2). In contrast, brachial (or femoral) (Table 1) stiffness remains almost stable throughout aging (Figure 1B, Table 2) and cannot negate for the effect of premature aortic aging on pressure transmission.2,28,29 Fortier et al.30 proposed arterial stiffness gradient as an independent index of ESRD survival. In our study, the predictive value of the arterial stiffness gradient depends entirely on aortic PWV changes. In the paper by Fortier et al.,30 the stiffness gradient was expressed as aortic PWV/brachial PWV and not in the usual way as (brachial PWV/aortic PWV)0.5.2 The population in the work by Fortier et al.30 was older and included patients with diabetes, and the population was characterized principally by unusually low brachial PWV, which could account for the stiffness gradient as a better predictor. However, the most important finding to consider in such studies is that, with aging, the aorta undergoes marked structural and geometric changes and becomes stiff and tortuous, with increased length, diameters, and wall thickness.1,2,17–19
It is now widely accepted that the aorta shows major regional heterogeneity between thoracic and abdominal segments.14,17,18,31,32 This heterogeneity mainly reflects embryologic origin: neural crest for aortic arch and mesoderm for abdominal aorta. Structural and mechanical degradation with severe and progressive atherosclerosis in the abdominal aorta is observed.31–36 Indeed, this heterogeneity is associated with differences in age–related segmental changes of aortic diameters. In general populations, the aging is associated with gradually decreasing slopes (β- coefficients) of diameter-age relationships along the aorta. They are more marked in the ascending aorta and less pronounced in the terminal abdominal aorta, with less or maintained aortic taper.17,18 Tortuosity and dilation of ascending aorta are attributable to a lower elastin-to-collagen ratio and fragmentation of elastic lamellae. In this study, these usual age-diameter relationships were observed in the controls: positive association between age and ascending aortic diameter and to a lesser degree, age-diameter–related changes in aortic bifurcation diameter with stable aortic taper (Figures 1 C and D and 2, Table 2). Those relationships are very different in patients with ESRD. Indeed, no significant relationships were found between age and ascending aortic diameter (Figure 1C, Table 2), but rather, a significant age–associated aortic bifurcation diameter increase leading to decreased aortic taper was found (Figure 2B, Tables 1 and 2). The abnormal age-diameter relationship was associated with extensive abdominal aortic calcifications (Figure 3), characteristic in patients with ESRD and strongly associated with aortic stiffness.33–36
To analyze the effect of age–associated arterial properties on pressure-wave propagations, we estimated the global reflection coefficient—the percentage of incident pressure waves reflected in controls and patients with ESRD. The controls global reflection coefficient was higher (0.82±0.06) (Table 1), relatively close to that observed for the whole systemic circulation in an experimental setting.4,15 The global reflection coefficient was significantly lower in patients with ESRD (0.76±0.10) (Table 1). Other than the presence of ESRD, reflection coefficient declines with increased aortic stiffness and increased aortic bifurcation diameter (Table 4). It is positively associated with ascending aortic diameter and peripheral arteries PWVs (or positively associated with stiffness gradient and aortic taper). This change reduces the normal centrifugal impedance between central and peripheral artery systems.2,3
In the presence of an impedance gradient and low aortic PWV, the reflected wave returns to the aorta at end systole and early diastole, thereby enhancing late diastolic BP and coronary perfusion4 and by generating partial reflections, attenuating the transmission of pulsatile pressure to the microcirculation.3,6 In this study, the decreased impedance gradient (decreased stiffness gradient and increased aortic taper) and global reflection coefficient were associated with deteriorated peripheral microcirculation (Tables 3 and 5), involving low post-ischemic forearm flow debt repayment (Figure 5) and increased minimal forearm flow resistance. In the general population and subjects with hypertension, the role of impedance gradient attenuation and the resulting microvascular changes were mainly observed in low-resistance circulations, such as kidney or brain.6–13 In patients with ESRD, the microvascular disorders are generalized, involving muscular, cutaneous, renal, and myocardial circulations.37–40 Decreased postocclusion or thermal muscular and cutaneous hyperemia was observed in patients with ESRD and associated with cardiovascular risk and increased mortality.36,37,40 These abnormalities reflect reduced microvascular and capillary density, abnormal vessel recruitment, structural changes of the microvasculature, and endothelial dysfunction.36–40 In the investigation, the effect of aortic and arterial changes on outcomes of patients with ESRD was studied using Cox models and receiver operating characteristic curves (Figure 5, Tables 6 and 7). The only arterial parameters associated with all-cause and cardiovascular mortality are increased aortic PWV and aortic bifurcation diameter, which are both significantly associated with premature arterial aging in ESRD. One of the principal limitations is the observational nature of the study in a population of incident and prevalent patients. Duration of hemodialysis treatment was not associated with age-related changes, indicating that these occurred already before therapy initiation during progression of CKD. We are unable to analyze the history and progression of arterial changes and determine at what stage of CKD these changes develop, but previous studies in carotid and peripheral muscular arteries indicated that arterial remodeling was already observed in CKD stages 3 and 4 and associated with stage progression.27,41 Finally, in this study, we analyze ESRD–associated premature aging–associated arterial changes, but we cannot discount the role and nature of specific uremic risk factors.
In conclusion, the premature arterial aging in patients with ESRD was associated with increased aortic stiffness and increased terminal abdominal aortic diameters. These changes are responsible for decreased stiffness gradient and less aortic taper, resulting in significantly lower global reflection coefficient and deteriorated peripheral microcirculation. Aortic PWV is an independent predictor of mortality in ESRD. Aortic stiffening is associated with early return of wave reflections, high aortic systolic and pulse pressures, decreased coronary perfusion pressure, decreased arterial stiffness gradient, decreased reflective properties of arterial tree, and abnormal microcirculation. The aortic stiffness attenuation with angiotensin–converting enzyme inhibitors has a favorable effect on survival in patients with ESRD.42 In CKD stages 2–5, the prescription of antihypertensive drugs, including angiotensin–converting enzyme inhibitors and angiotensin receptor blockers, slows down the progression of aortic stiffness but did not prevent the carotid artery remodeling and dilation that is associated with cardiovascular events.41,43 Although the aortic geometry is frequently neglected, the multivariate Cox model indicates that the predictive values of aortic geometry changes are almost similar to aortic PWV. This study shows the importance of evaluating not only aortic stiffness but also, the alterations in aortic geometry, because these changes are associated with microvascular dysfunctions and predicted all–cause and cardiovascular mortality. These results indicate that studies are needed to better explore the effect of pharmacologic treatments on hemodynamic and structural properties of the arterial system.
This investigation did not exclusively address patients with ESRD but examined the important relationships between arterial stiffness and aortic taper in physiologic conditions as analyzed by Taylor14 in mammals. Our findings herein showed the role of stiffness gradient and aortic taper in patients’ outcomes and their implications for the microcirculation. These results indicate that studies are needed to better explore structural and hemodynamic mechanisms in cardiovascular diseases.
Concise Methods
Study Subjects
The study included 156 Caucasians patients with ESRD and without diabetes and 73 age–, sex–, and mean BP–matched control subjects who agreed to participate. Patients were on hemodialysis for 48 (3–304) months and enrolled when they had (1) been on hemodialysis for ≥3 months, (2) no cardiovascular complication during the previous 6 months, and (3) no hemodynamically significant aortic stenosis. The follow-up was 53 (6–110) months. The protocol was approved by our Institutional Review Board.
Hemodynamics Study
Noninvasive hemodynamic data were obtained with previously described techniques.2,23,25,26,33–35,40,41,44–51 The measurements were done 24 hours after the midweek hemodialysis session. After 10 minutes in a supine position, the brachial artery systolic BP (Korotkoff phase I) and diastolic BP (phase V) were recorded by mercury sphygmomanometer at the closest 2 mmHg. Mean BP was integrated from the radial artery pressure waveform recorded by applanation tonometry during common carotid artery pressure wave measurement (Sphygmocor, Sydney, Australia). Diastolic and mean BPs were used to calibrate the common carotid artery wave. The difference between BPs at the foot and the initial flow peak of the common carotid artery pressure curve was taken as the forward pressure. The difference between common carotid artery systolic BP and forward pressure is expressed as the percentage of common carotid artery pulse pressure (i.e., the augmentation index or in millimeters of mercury as the augmented [reflected] pressure).4,25,41,44,45 Arterial PWV was measured with simultaneous pressure wave tracings from common carotid artery to the femoral arteries in the groin (aortic PWV), from common carotid artery to the radial artery (brachial PWV), and from the femoral artery in the groin to the arteria tibialis posterior (femoral PWV; Complior; Colson, Les Lilas, France) as previously described.2,23,26,28,41,44,45,50,52 Stiffness gradients were estimated from (brachial PWV/aortic PWV)0.5 and (femoral PWV/aortic PWV)0.5 ratios.2 The diameters of the ascending aorta 2 cm above the root (ascending aortic diameter) and at the aortic bifurcation (aortic bifurcation diameter) were measured with two–dimensionally directed M–mode echocardiograms (HP Sonos 100; Hewlett-Packard, Evry, France) as previously described.52 Aortic taper was estimated as the ascending aortic/aortic bifurcation diameters ratio.52 The Kauppila score was used to quantify abdominal aortic calcification on lateral abdominal native x-rays as previously described.46,49
Two–dimensionally directed M–mode echocardiography was performed simultaneously with common carotid artery tonometry (2.5- and 3.5-MHz transducers; HP Sonos 100; Hewlett-Packard). Aortic flow velocity was evaluated with a pulsed Doppler26,48,50,51: the sample volume was positioned at the tip of the aortic leaflets, and flow velocity was determined by spectral analysis of the digitized broadband audio signal. The leading edge of the spectral envelope provided a signal–averaged flow-velocity waveform. Peak flow (Qmax) was calculated from the aortic annular cross–sectional area and maximal aortic flow velocity. Stroke volume was calculated as the aortic annular cross–sectional area multiplied by the velocity integral of left ventricular outflow, and cardiac output was calculated as stroke volume multiplied by heart rate. Total peripheral resistance (Zr) was estimated from cardiac output and mean BP. Characteristic impedance (Zc) was estimated in the time domain as described previously8,50,51: the point at which flow reaches its Qmax was located on the flow tracing. The carotid pressure change from the foot to the time of Qmax was determined (PZ). Characteristic impedance was computed from those two values: Zc = PZ/Qmax. The global reflection coefficient (Γ) was computed as Γ=(1− Zc/Zr)/(1+ Zc/Zr).15,16
Forearm Reactive Hyperemia
Forearm blood flow (milliliters per 100 ml per minute) was determined by venous occlusion plethysmography (Perivein, Janssens, Paris) as previously described.40 After control flow measurements, the arterial cuff was inflated to 300 mmHg for 5 minutes. At time 0, the arterial cuff was deflated, and the forearm blood flow was immediately measured, with repetition of measurements every 15 seconds. The following parameters were determined (Supplemental Figure 2): the flow debt (A; area under the curve between the start and end of the ischemia period), the post-ischemic peak flow (also expressed as flow maximum as a percentage of baseline), the excess hyperemic flow (B; area under the curve of ischemia release versus hyperemia duration), and the flow debt repayment (B/A).
Statistical Analyses
The D’Agostino Omnibus test was used to assess the distribution shape, with data expressed as medians (ranges) or means±SEMs according to normality. Continuous variables were compared using t test or Mann–Whitney test. Sex (1, man; 0, woman) and ESRD (yes, 1; no, 0) were used as dummy categorical variables. Pearson or Spearman regression was used to evaluate associations between hemodynamic parameters. The predictor with probability P<0.15 in that analysis was subsequently tested in a multivariable linear correlation model. The outcome events studied were all-cause and cardiovascular mortality. Factors prognostic of survival were identified using the Cox proportional hazards model and specificity and sensitivity with receiver operating characteristics curves. All prognostic factors were also adjusted to dialysis vintage, sex, and history of cardiovascular disease. P<0.05 defined significance. All comparisons were computed with NCSS 2001 software (J. Hintze, Kaysville, UT).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Janet Jacobson for editorial assistance.
The study was supported by the Groupe d’Études de la Pathophysiologie de l’Insuffisance rénale and Groupe de Pharmacologie et d’Hémodynamique Cardiovasculaire Paris.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060617/-/DCSupplemental.
References
- 1.O’Rourke MF, Hashimoto J: Mechanical factors in arterial aging: A clinical perspective. J Am Coll Cardiol 50: 1–13, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF: Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 68: 50–58, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF: Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols WW, O’Rourke MF: Wave reflection. In: McDonald’s Blood Flow in the Arteries, 5th Ed., London, Hodder Arnold Publishers, 2005, pp 193–213 [Google Scholar]
- 5.Burattini R, Knowlen GG, Campbell KB: Two arterial effective reflecting sites may appear as one to the heart. Circ Res 68: 85–99, 1991 [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke MF, Safar ME: Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46: 200–204, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Safar ME, London GM, Plante GE: Arterial stiffness and kidney function. Hypertension 43: 163–168, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ: Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility--Reykjavik study. Brain 134: 3398–3407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF: Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol 26: 1181–1187, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ML, Tomlinson LA, Chapman TPE, Rajkumar C, Holt SG: Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 55: 1110–1115, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Poels MMF, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, Witteman JC, Breteler MM, Mattace-Raso FU, Ikram MA: Arterial stiffness and cerebral small vessel disease: The Rotterdam Scan Study. Stroke 43: 2637–2642, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Webb AJS, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM: Increased cerebral arterial pulsatility in patients with leukoaraiosis: Arterial stiffness enhances transmission of aortic pulsatility. Stroke 43: 2631–2636, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Henskens LHG, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW: Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 52: 1120–1126, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Taylor MG: Wave travel in the arteries and the design of the cardiovascular system. In: Pulsatile Blood Flow, edited by Attinger EO, New York, McGraw, 1964, pp 343–367 [Google Scholar]
- 15.Nichols WW, O’Rourke MF: Vascular impedance. In: McDonald’s Blood Flow in the Arteries, 5th Ed., London, Hodder Arnold Publishers, 2005, pp 233–267 [Google Scholar]
- 16.Milnor WR: Vascular impedance. In: Hemodynamics, 2nd Ed., Baltimore, MD, Williams & Wilkins, 1989, pp 167–203 [Google Scholar]
- 17.Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, Guo S-Y, Liu T-H, Ou D-Y, O’Rourke M: Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol 139: 1119–1129, 1991 [PMC free article] [PubMed] [Google Scholar]
- 18.O’Rourke M, Farnsworth A, O’Rourke J: Aortic dimensions and stiffness in normal adults. JACC Cardiovasc Imaging 1: 749–751, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Pearce WH, Slaughter MS, LeMaire S, Salyapongse AN, Feinglass J, McCarthy WJ, Yao JS: Aortic diameter as a function of age, gender, and body surface area. Surgery 114: 691–697, 1993 [PubMed] [Google Scholar]
- 20.Latham RD, Westerhof N, Sipkema P, Rubal BJ, Reuderink P, Murgo JP: Regional wave travel and reflections along the human aorta: A study with six simultaneous micromanometric pressures. Circulation 72: 1257–1269, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Segers P, Verdonck P: Role of tapering in aortic wave reflection: Hydraulic and mathematical model study. J Biomech 33: 299–306, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Briet M, Boutouyrie P, Laurent S, London GM: Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int 82: 388–400, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Blacher J, Guérin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T, Nishizawa Y: Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 12: 2117–2124, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D: Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart Study. Hypertension 43: 1239–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 26.London GM, Guérin AP, Marchais SJ, Pannier B, Safar ME, Day M, Métivier F: Cardiac and arterial interactions in end-stage renal disease. Kidney Int 50: 600–608, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P: Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 69: 350–357, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM: Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension 45: 592–596, 2005 [DOI] [PubMed] [Google Scholar]
- 29.van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM: Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: A population study. Hypertension 35: 637–642, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Fortier C, Mac-Way F, Desmeules S, Marquis K, De Serres SA, Lebel M, Boutouyrie P, Agharazii M: Aortic-brachial stiffness mismatch and mortality in dialysis population. Hypertension 65: 378–384, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Majesky MW: Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248–1258, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS: Regional heterogeneity within the aorta: Relevance to aneurysm disease. J Thorac Cardiovasc Surg 136: 1123–1130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guérin AP, London GM, Marchais SJ, Métivier F: Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 15: 1014–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Blacher J, Guérin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 35.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS: Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: Correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Bejan-Angoulvant T, Bergerot C, Juillard L, Mezergues A, Morelon E, Pouteil-Noble C, André-Fouët X, Angoulvant D: Myocardial microvascular disease and major adverse cardiovascular events in patients with end-stage renal disease: Rationale and design of the MICROCARD study. Nephrol Dial Transplant 27: 2886–2891, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Aalkjaer C, Pedersen EB, Danielsen H, Fjeldborg O, Jespersen B, Kjaer T, Sørensen SS, Mulvany MJ: Morphological and functional characteristics of isolated resistance vessels in advanced uraemia. Clin Sci (Lond) 71: 657–663, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Arismendi-Morillo G, Fernández-Abreu M: Ultrastructural cutaneous microvascular pathology of young adults aged up to 50 years with chronic kidney disease and vascular cognitive impairment. Ultrastruct Pathol 34: 214–218, 2010 [DOI] [PubMed] [Google Scholar]
- 40.London GM, Pannier B, Agharazii M, Guérin AP, Verbeke FH, Marchais SJ: Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int 65: 700–704, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Guérin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P Nephrotest Study Group : Arterial remodeling associates with CKD progression. J Am Soc Nephrol 22: 967–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karras A, Haymann J-P, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M Nephro Test Study Group : Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension 60: 1451–1457, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF: Components of hemodynamic load and cardiovascular events: The Framingham Heart Study. Circulation 131: 354–361, discussion 361, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.London G, Guérin A, Pannier B, Marchais S, Benetos A, Safar M: Increased systolic pressure in chronic uremia. Role of arterial wave reflections. Hypertension 20: 10–19, 1992 [DOI] [PubMed] [Google Scholar]
- 46.London GM, Marchais SJ, Guérin AP, Boutouyrie P, Métivier F, de Vernejoul MC: Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 19: 1827–1835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murgo JP, Westerhof N, Giolma JP, Altobelli SA: Aortic input impedance in normal man: Relationship to pressure wave forms. Circulation 62: 105–116, 1980 [DOI] [PubMed] [Google Scholar]
- 48.London GM, Pannier B, Guérin AP, Marchais SJ, Safar ME, Cuche JL: Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation 90: 2786–2796, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Wilson PWF, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA: Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 103: 1529–1534, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM: Central artery pulse pressure in end-stage renal disease: The roles of aortic diameter, aortic stiffness and wave reflection. Blood Purif 31: 107–112, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS: Hemodynamic correlates of blood pressure across the adult age spectrum: Noninvasive evaluation in the Framingham Heart Study. Circulation 122: 1379–1386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.London GM, Guérin AP, Pannier B, Marchais SJ, Stimpel M: Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension 26: 514–519, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.