Abstract
Renal disease variability in autosomal dominant polycystic kidney disease (ADPKD) is strongly influenced by the gene locus (PKD1 versus PKD2). Recent studies identified nontruncating PKD1 mutations in approximately 30% of patients who underwent comprehensive mutation screening, but the clinical significance of these mutations is not well defined. We examined the genotype-renal function correlation in a prospective cohort of 220 unrelated ADPKD families ascertained through probands with serum creatinine ≤1.4 mg/dl at recruitment. We screened these families for PKD1 and PKD2 mutations and reviewed the clinical outcomes of the probands and affected family members. Height–adjusted total kidney volume (htTKV) was obtained in 161 affected subjects. Multivariate Cox proportional hazard modeling for renal and patient survival was performed in 707 affected probands and family members. Overall, we identified pathogenic mutations in 84.5% of our families, in which the prevalence of PKD1 truncating, PKD1 in–frame insertion/deletion, PKD1 nontruncating, and PKD2 mutations was 38.3%, 4.3%, 27.1%, and 30.3%, respectively. Compared with patients with PKD1 truncating mutations, patients with PKD1 in–frame insertion/deletion, PKD1 nontruncating, or PKD2 mutations have smaller htTKV and reduced risks (hazard ratio [95% confidence interval]) of ESRD (0.35 [0.14 to 0.91], 0.10 [0.05 to 0.18], and 0.03 [0.01 to 0.05], respectively) and death (0.31 [0.11 to 0.87], 0.20 [0.11 to 0.38], and 0.18 [0.11 to 0.31], respectively). Refined genotype-renal disease correlation coupled with targeted next generation sequencing of PKD1 and PKD2 may provide useful clinical prognostication for ADPKD.
Keywords: ADPKD, genetic renal disease, genetics and development
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease worldwide, responsible for 5%–10% of ESRD.1,2 Mutations in two genes (PKD1 and PKD2) account for most patients with ADPKD.2,3 Previous studies of European families ascertained through probands enriched with renal failure reported that approximately 85% and approximately 15% of ADPKD families were linked to the PKD1 and PKD2 loci, respectively.3 However, a higher prevalence of PKD2 of 26% has been recently reported in a population-based study.4 Disease progression of ADPKD is highly variable, in part because of a strong gene locus effect.5–8 Adjusted for age, patients with PKD1 have larger kidneys and earlier onset of ESRD than patients with PKD2 (mean age at ESRD, 53.4 versus 72.7 years old, respectively).5,6,8 Additionally, significant intrafamilial renal disease variability in ADPKD suggests a modifier effect.9–11
PKD1 is a large complex gene that shares high DNA sequence identity with six pseudogenes (PKD1P1–PKD1P6), making mutation screening highly challenging.12 Comprehensive screening for PKD1 mutations is now possible using protocols that exploit rare mismatches between its duplicated region and the pseudogenes using PKD1-specific PCR.12 With this advance, the Consortium for Radiologic Imaging Study of PKD (CRISP) reported a comprehensive survey of the spectrum of PKD1 and PKD2 mutations in 180 patients.12 Among the putative deleterious PKD1 variants identified in 153 patients, 70% were protein truncating (PT) and predicted to be definitively pathogenic. By contrast, the clinical significance of the remaining nontruncating (NT) variants (i.e., small in–frame deletion/insertion [IF indel], nonsynonymous missense, or atypical splice site variants) was less certain. More recent studies have shown a significant allelic effect in PKD1, with truncating mutations associated with severe disease and NT mutations associated with milder disease.13–16 Here, we report the results of the Toronto Genetic Epidemiology Study of PKD (TGESP), which examined the prevalence of different mutation classes and the correlation of genotype with renal function in a prospective cohort of 220 families ascertained through probands with near–normal kidney function.
Results
Characteristics of Study Cohort
The study probands comprised 210 affected and 10 at–risk unaffected subjects; the latter allowed us to recruit their affected family members but were not used for the genotype-phenotype analysis (Supplemental Table 1). Their mean (±SD) age and serum creatinine at first presentation to the Hereditary Kidney Disease Clinic were 38.9 (±12.7) years old and 0.90±0.19 mg/dl (or 79.4±16.9 μmol/L), respectively; 55% were men, and 79.5% were of European ancestry. Overall, the multiethnic composition of the probands closely reflects the general population of the greater Toronto area. The complete study cohort comprised 709 affected probands and their affected family members; 48% were men, and 79% were of European ancestry. At the last follow-up, the mean age of our study cohort was 49.6 (±14.8) years old, and 31.6% had developed ESRD.
Prevalence and Spectrum of Mutations in TGESP
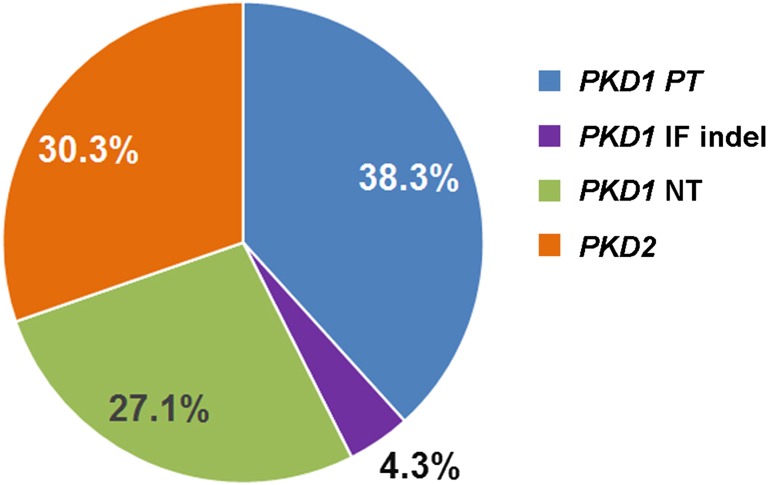
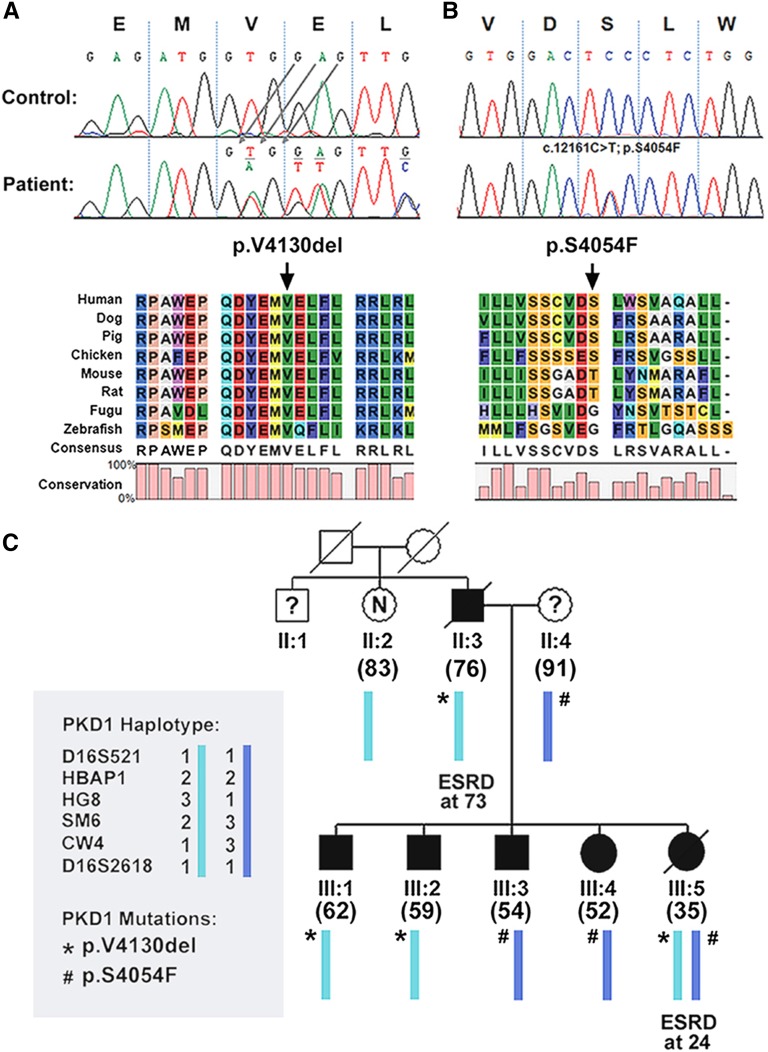
We identified 188 pathogenic mutations in 186 of 220 (84.5%) families, including two families with bilineal ADPKD (Figure 1). PKD1 PT mutations were the most common (72 of 188 or 38.3%) (Supplemental Table 2) followed by PKD2 mutations (57 of 188 or 30.3%) (Supplemental Table 5), PKD1 NT mutations (51 of 188 or 27.1%) (Supplemental Table 3), and PKD1 IF indels (8 of 188 or 4.3%) (Supplemental Table 4). Among the identified mutations, 40% (75 of 188) were novel, and 19 mutations were detected in two or more families not known to be related. Notably, several recurrent mutations (PKD1: c.5014_5015delAG, p.Arg1672fs97X; PKD2: c.1249C>T, p.Arg417X; c.2159dupA, p.Asn720fs4×; and c.2614C>T, p.Arg872X) (Supplemental Tables 2 and 5) have been reported 10 or more times, suggesting that they may represent mutation warm spots and/or a founder effect. TOR179 is a pedigree with clinically apparent bilineal ADPKD from two independently segregating PKD1 NT (p.Arg2767Cys) and PKD2 PT (p.Asn720fs4X) mutations (Supplemental Tables 3 and 5). By contrast, TOR87 is a complex pedigree with cryptic bilineal ADPKD and marked intrafamilial renal disease variability from two independently segregating PKD1 mutations (Supplemental Tables 3 and 4). Figure 2 shows a heterozygous IF deletion of a highly conserved amino acid (c.12389_12391delTGG; p.Val4130del) identified in II:3 and three of his five children (Figure 2A). Follow-up screening of III:4, who was affected but did not carry p.Val4130del, identified a second PKD1 mutation (c.12161C>T; p.Ser4054Phe) inherited from II:4 that was present in III:3 and III:5 (Figure 2B). Repeated imaging of III:3 by renal computed tomography at age 54 years old led to a revision of his disease status to unequivocally affected (Figure 2C). p.Val4130del is a de novo mutation in II:3 that segregated with the disease in III:1, III:2, and III:5, whereas p.Ser4054Phe originated from II:4 and segregated with the disease in III:3, III:4, and III:5. One affected member (III:5) who was transheterozygous for both mutations developed ESRD at age 24 years old. By contrast, all other affected subjects carrying only one hypomorphic PKD1 mutation were mildly affected.
Figure 1.
Mutation spectrum in TGESP. Percentage distribution of different mutation classes in study families. Overall, we identified 188 pathogenic mutations in 186 of 220 (i.e., 84.5%) families, including 2 families with cryptic bilineal ADPKD. Among 188 mutations identified, 38.3% (72 of 188) were PKD1 PT, 27.1% (51 of 188) were PKD1 NT, 4.3% (8 of 188) were PKD1 IF indel, and 30.3% (57 of 188) were PKD2 mutations (details in Supplemental Table 2).
Figure 2.
Cryptic bilineal ADPKD in TOR87 with marked intrafamilial renal disease variability. A and B show the two putative pathogenic PKD1 mutations (c.12389_12391delTGG, p.Val4130del; and c.12161C>T, p.Ser4054Phe) identified in this pedigree. C shows that p.Val4130del originated from II:3 and was also present in three of his five children. Follow-up screening of III:4, who was affected but did not carry p.Val4130del, identified a second PKD1 mutation (p.Ser4054Phe). p.Val4130del is a de novo mutation in II:3 that segregated with the disease in III:1, III:2, and III:5, whereas p.Ser4054Phe originated from II:4 segregated with the disease in III:3, III:4, and III:5. III:5 was transheterozygous for both mutations and suffered early and severe renal disease. By contrast, all other affected subjects carried only one hypomorphic PKD1 mutation and were mildly affected. The number in parentheses denotes the age at death or last follow-up.
Effects of Mutation Class on Total Kidney Volume
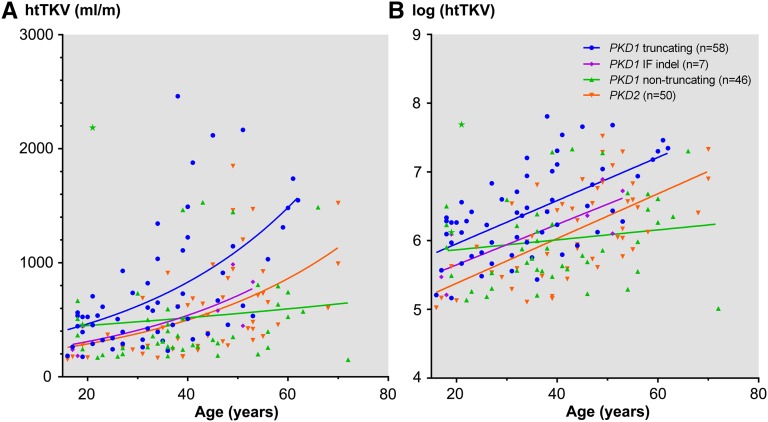
Corrected for age, patients with PT PKD1 mutations generally have larger height–adjusted total kidney volume (htTKV) compared with those from other mutation classes (Figure 3). Assuming exponential growth,17 the rate of log htTKV expansion seems similar between PKD1 PT, PKD1 IF indel, and PKD2 mutations; however, the absolute log htTKV (y intercept) differed between the three mutation classes (Figure 3B). By contrast, the rate of log htTKV expansion associated with NT PKD1 mutations was significantly slower than the other mutation classes. Two affected siblings from TOR264 (Supplemental Table 6) displayed marked discordance of their htTKVs (with 455 and 2184 ml/m at 19 and 21 years of age, respectively), suggesting a modifier effect. Multivariate linear regression showed a significant effect (positive β-values), indicating that men and older age are both associated with larger kidney volumes (Table 1). By contrast, a significant effect (negative β-values) in the same analysis suggests that PKD1 NT and PKD2 mutations (compared with PKD1 PT mutations) were associated with smaller kidney volumes. The number of patients with PKD1 IF indels was small, and therefore, this analysis should be regarded as indeterminate.
Figure 3.
Effects of mutation class on htTKV. (A) Corrected for age, patients with PKD1 PT mutations generally have larger htTKV compared with those from other mutation classes. (B) Assuming exponential growth, the rate of htTKV expansion seems similar between patients with PKD1 PT, PKD1 IF indel, and PKD2 mutations; however, the absolute htTKV (y intercept) differed between the three mutation classes. By contrast, the rate of htTKV expansion associated with NT PKD1 mutations seemed to be significantly slower than the rates of the other three mutation classes. Two affected siblings from TOR264 displayed extreme discordance of their htTKV (denoted by stars; 455 and 2184 ml/m at 19 and 21 years of age, respectively), suggesting a modifier effect.
Table 1.
Effects of mutation class on log-transformed htTKV
| Variables | β | SE | 95% CI | P Value |
|---|---|---|---|---|
| Men | 0.315 | 0.093 | 0.12 to 0.49 | 0.001 |
| Age (yr) | 0.026 | 0.0030 | 0.02 to 0.03 | <0.001 |
| PKD1 IF indel versus PKD1 truncating | −0.26 | 0.23 | −0.71 to 0.19 | 0.25 |
| PKD1 NT versus PKD1 truncating | −0.54 | 0.11 | −0.77 to −0.32 | <0.001 |
| PKD2 versus PKD1 truncating | −0.50 | 0.11 | −0.72 to −0.27 | <0.001 |
Data derived from multivariate linear regression analysis.
Effects of Mutation Class on Renal and Patient Survival
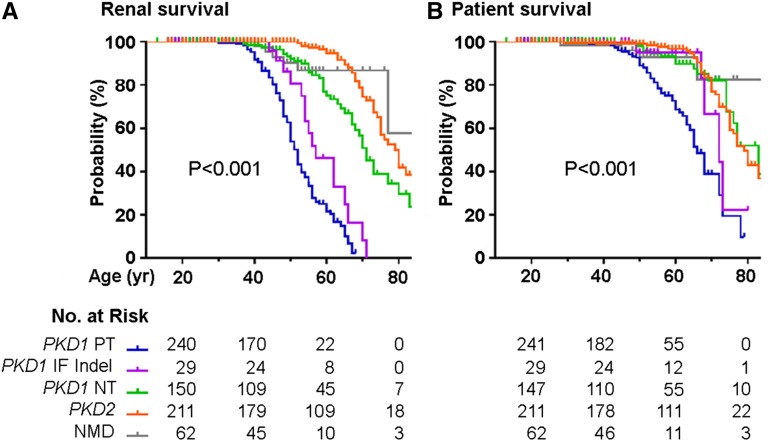
Figure 4 shows the Kaplan–Meier curves for renal and all–cause patient survival in our study cohort. Patients with PKD1 PT mutations have the most severe renal disease followed by those with PKD1 IF indels, PKD1 NT mutations, and PKD2 mutations, with estimated mean ages at ESRD of 52.5, 58.6, 70.8, and 80 years old, respectively (P<0.001) (Table 2). Similarly, the same gene locus and PKD1 allelic effects also affect all–cause patient survival, with the mean ages at death of 65.2, 71.6, 77.5, and 79.1 years old, respectively (P<0.001). Interestingly, patients with no detectable mutation (NMD) had late mean ages at ESRD and death of 77.5 and 80.1 years old, respectively. Multivariate Cox proportional hazard analysis (Table 3) indicates that being a man was a significant risk factor for time to ESRD (hazard ratio [HR], 1.78; 95% confidence interval [95% CI], 1.28 to 2.48 P<0.001). Compared with PKD1 PT mutations (reference group), patients with PKD1 IF indel, PKD1 NT, PKD2 mutations, and NMDs all showed decreased HRs for ESRD (HR, 0.35; 95% CI, 0.14 to 0.91; HR, 0.10; 95% CI, 0.05 to 0.18; HR, 0.03; 95% CI, 0.01 to 0.05; and HR, 0.04; 95% CI, 0.01 to 0.13, respectively) and death (HR, 0.31; 95% CI, 0.11 to 0.87; HR, 0.20; 95% CI, 0.11 to 0.38; HR, 0.18; 95% CI, 0.11 to 0.31; and HR, 0.14; 95% CI, 0.05 to 0.42, respectively).
Figure 4.
Renal and patient survival in TGESP. Kaplan–Meier curves for (A) renal and (B) patient survival by mutation class. P value <0.001 by log-rank test.
Table 2.
Age at ESRD and patient death by mutation class
| Mutation Class | N | Age (yr) at ESRDa | Age (yr) at Patient Deatha |
|---|---|---|---|
| PKD1 truncating | 249 | 52.5 (51.2 to 53.9) | 65.2 (62.7 to 67.7) |
| PKD1 IF indel | 32 | 58.6 (54.9 to 62.4) | 71.6 (67.3 to 75.9) |
| PKD1 NT | 152 | 70.8 (67.5 to 74.2) | 77.5 (74.6 to 80.5) |
| PKD2 | 213 | 80.0 (77.1 to 82.8) | 79.1 (76.6 to 81.6) |
| NMD | 61 | 77.5 (72.1 to 82.9) | 80.1 (75.3 to 84.8) |
Data derived from Kaplan–Meier survival analysis and expressed as means and 95% CIs. P<0.001 by log-rank test.
Table 3.
Multivariate Cox proportional hazard model for ESRD and patient death by mutation class
| Clinical Outcome | β | SE | HR | 95% CI | P Value |
|---|---|---|---|---|---|
| ESRD | |||||
| Men versus women | 0.58 | 0.17 | 1.78 | 1.28 to 2.48 | <0.001 |
| PKD1 IF indel versus PKD1 truncating | −1.04 | 0.48 | 0.35 | 0.14 to 0.91 | 0.03 |
| PKD1 NT versus PKD1 truncating | −2.40 | 0.33 | 0.10 | 0.05 to 0.18 | <0.001 |
| PKD2 versus PKD1 truncating | −3.60 | 0.36 | 0.03 | 0.01 to 0.05 | <0.001 |
| NMD versus PKD1 truncating | −3.21 | 0.58 | 0.04 | 0.01 to 0.13 | <0.001 |
| Patient death | |||||
| Men versus women | 0.30 | 0.20 | 1.35 | 0.92 to 1.99 | 0.13 |
| PKD1 IF indel versus PKD1 truncating | −1.17 | 0.53 | 0.31 | 0.11 to 0.87 | 0.03 |
| PKD1 NT versus PKD1 truncating | −1.59 | 0.31 | 0.20 | 0.11 to 0.38 | <0.001 |
| PKD2 versus PKD1 truncating | −1.69 | 0.27 | 0.18 | 0.11 to 0.31 | <0.001 |
| NMD versus PKD1 truncating | −1.97 | 0.57 | 0.14 | 0.05 to 0.42 | <0.001 |
With frailty model to account for random cluster effect within family.
Refining Genotype-Phenotype Correlation for PKD1 NT Mutations
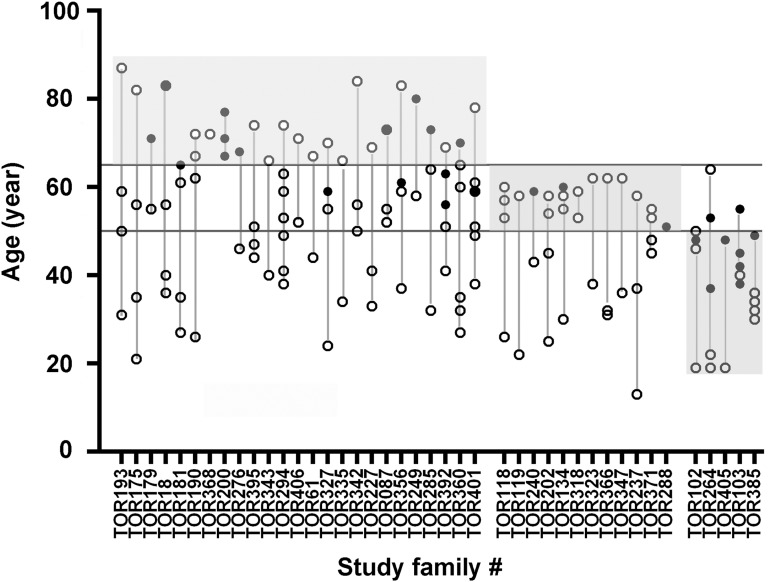
The family-based design of our study provides us an opportunity to estimate the mean genotypic effect to refine the renal disease correlation for this class of mutations. Figure 5 shows the age of the affected subjects at ESRD or last follow-up plotted within each family. Nine probands without any affected family members were also not shown in Figure 5. Of 42 of 51 families with PKD1 NT mutations thus analyzed, 25 have at least one affected member who remained renal sufficient (n=18; 5 with CKD stage 2, 7 with CKD stage 3, and 6 with CKD stage 4) or developed ESRD (n=11) at or after age 65 years old. Thus, the mutations in these families with mild renal disease likely function as hypomorphic alleles.13–15 By contrast, five families have at least one affected member who developed ESRD before age 50 years old. The mutations in these families with severe renal disease are likely completely inactivating. Twelve noninformative families were considered indeterminate. Although PKD1 NT mutations, on average, are associated with milder renal disease than PKD1 PT mutations, our study shows that patients with the former class of mutations comprised a heterogeneous group with both mild and severe renal disease.
Figure 5.
Renal disease severity in families with PKD1 nontruncating mutations. The age of affected relatives (denoted by circles) at ESRD or last follow-up is plotted for each family. White circles denote subjects who remained renal sufficient at their last follow-up, whereas black circles denote those who had reached ESRD. Families with one or more affected relatives with ESRD or renal sufficiency after age of 65 years old (top shaded area) are considered to have mild disease. By contrast, families with one or more affected relative who developed ESRD before age 50 years old are considered to have severe disease (bottom shaded area). Noninformative families are considered as indeterminate (middle shaded area). Nine families with probands but no other affected relatives were not included here.
Discussion
In a large cohort of families ascertained through probands with near–normal kidney function, we found PKD1 PT and IF indel mutations, PKD1 NT mutations, and PKD2 mutations in 42.6%, 27.1%, and 30.3%, respectively, in the families with identifiable mutations. By contrast, the reported prevalence of PKD1 PT and IF indel mutations, PKD1 NT mutations, and PKD2 mutations was 59.5%, 25.5%, and 15% in the CRISP12 and 58.6%, 22.9%, and 18.5% in the Genkyst Study.15 Compared with the two latter studies, we found a lower prevalence of PKD1 PT and IF indel mutations, which are associated with severe renal disease, and a higher prevalence of PKD2 mutations, which are associated with mild renal disease. However, the CRISP was designed to enrich patients at high risk for renal disease progression, whereas Genkyst was enriched with probands with advanced CKD.12,15 Thus, the latter studies displayed a spectrum of mutations associated with more severe renal disease, whereas our study provides estimates that are likely more representative of the general population.
Independent of the mutation effect, men are associated with larger kidneys and increased risk for ESRD, raising the possibility that environmental factors (e.g., cigarette smoking and lifestyle) and/or sex hormones may modify disease severity in ADPKD. We confirmed that both the gene locus (PKD1 versus PKD2) and PKD1 allele effects provide useful clinical prognostic information.12,15 Specifically, patients with PKD1 PT mutations have the most severe renal disease followed by those with PKD1 IF indels, PKD1 NT mutations, and PKD2 mutations; the same mutation classes also affect patient survival. Women with PKD2 mutations have the best renal prognosis, and many will die of old age without ESRD.7
PKD1 NT mutations are increasingly recognized as a distinct prognostic group with milder renal disease compared with PKD1 PT mutations.13–16 However, our study shows that the renal disease severity in this class of mutations is heterogeneous (Figure 3). Although a majority of PKD1 NT mutations displayed mild renal disease and likely functions as hypomorphic alleles, several associated with severe renal disease are likely completely inactivating (Figure 5). Thus, the apparently low total kidney volume (TKV) expansion rate reflects mostly patients with mild disease but also, a few with severe disease in our cohort (Figure 3B) and should be confirmed with a larger sample size. In the absence of a well validated bioinformatic or functional assay, assessing the putative pathogenicity of PKD1 NT mutations is not always reliable,18–20 and predicting their clinical effects is even more challenging. How then can one use this class of mutations for clinical prognostication? In addition to in silico bioinformatics prediction,18–20 family–based segregation studies can provide additional support for the pathogenicity of a putative PKD1 NT mutation. Moreover, the presence of other affected family members with sufficient renal function or ESRD after 65 years of age can be taken as evidence that the NT mutation of interest likely functions as a hypomorphic allele. Conversely, the presence of other affected family members with ESRD before 50 years of age suggests that the NT mutation is likely completely inactivating. In the absence of an informative family history, TKV may be used as another indicator for renal disease severity.17 The presence of a modifier effect in ADPKD has the potential to complicate the use of mutation-based prognostication,9–11 particularly in PKD1 NT mutations as discussed above. Among the three mutation classes with sizable patient numbers (i.e., PKD1 truncating, n=249; PKD1 NT, n=152; and PKD2, n=213), the spans of the 95% CIs were 2.7, 6.7, and 5.7 years, respectively, suggesting that PKD1 truncating mutations were associated with less (although not necessarily all because of intrafamilial) variability than the other two mutation classes. The importance of a modifier effect is well illustrated by the affected sibling pairs from TOR264 with markedly discordant TKV (Figure 1). However, the availability of multiple affected members in an informative family will provide more confidence for the mutation–based clinical prognostication. In rare instances, complex genetic inheritance may also be confounding as exemplified by TOR87, which harbored cryptic bilineal ADPKD caused by two different mutations in PKD1 (Figure 2). Although rare, the identification of pedigrees with bilineal ADPKD has important implications for genetic counseling. Specifically, the risk of disease in the offspring of an affected spousal pair is increased from 50% to 75%, with one in four children expected to be more severely affected from carrying two mutant alleles.21 Although bilineal disease may be obvious from the family history, this was not the case in TOR87. When a family history of ADPKD is apparent, the spouse of an affected subject, who may be affected with a mild form of the disease, is rarely screened. When then should one suspect a bilineal pedigree? An important clinical clue is an increased disease segregation ratio of approximately 75%, especially in larger pedigrees, caused by two independently segregating mutations. Additionally, marked discordance in renal disease severity within family can signify the presence of individuals carrying two PKD mutations, which was seen in III:5 from TOR87. Families suspicious of a complex genetic underpinning should be referred to specialized centers for further testing.
Interestingly, our patients with NMD have very mild renal disease with low risk for ESRD. Although the reasons for this favorable prognosis are unclear, some of them may have atypical PKD with unilateral, segmental, or asymmetric cystic involvement, which has been reported to be associated with stable kidney function.22 Other patients with de novo disease and markedly asymmetric cystic kidney involvement may have somatic mosaicism. The hallmark of mosaicism is the presence of more than one genetically distinct cell line in the affected subject whose disease is invariably mild or focal because of dilution of the mutant gene dosage at the tissue level.23 Because of the low signal to noise ratio in conventional Sanger sequencing, the pathogenic mutations in mosaic patients are typically missed.
In conclusion, our study suggests that mild forms of ADPKD may be more common than generally appreciated because of selection bias for more severe patients in the published studies. In the era when disease–modifying drug therapy is already becoming a reality for ADPKD,24 there is an urgent need for biomarkers to identify high-risk patients who are most likely to benefit from treatment. In this regard, both mutation classes and TKV provide useful prognostication for disease severity, although neither of them is perfect. The former test can be affected by a modifier effect resulting in intrafamilial renal disease variability. By contrast, baseline htTKV explains only approximately 42% of the variance (r=0.65; r2 is approximately 0.42) in GFR at the eighth year of follow-up in the CRISP Study.25 Thus, both tests have limitations and need to be recognized. To select patients for novel or experimental drug treatment on the basis of mutation class, those with PKD1 PT mutations or IF indels may be considered as at high risk for early renal disease progression. By contrast, low-risk patients with PKD2 mutations may not need the treatment until a later age, perhaps further risk stratified on the basis of their TKV. Additional studies are needed to evaluate the clinical utility of PKD1 NT mutations, which can be improved with an informative family history or combined with TKV measurement. High–throughput targeted exome resequencing protocols have recently been successfully used in a research setting for PKD1 and PKD2 mutation screening at reduced costs,26 can be adapted to screen additional cystic disease genes,27 and can be used to unravel patients with complex genetics, including bilineal disease and somatic mosaicism. These innovations, in turn, have the potential to transform mutation-based diagnostics for clinical prognostication in ADPKD in the near future.
Concise Methods
Study Protocol and Population
Between December 1, 2006 and August 30, 2012, 288 subjects at risk or affected with ADPKD were seen as new patients at the Hereditary Kidney Disease Clinic, University Health Network in Toronto, Canada; 33% of them were referred to us by a family doctor, 29% were referred by nephrologists, 27% were referred by internists, and 11% were referred by medical geneticists. Two hundred twenty probands with serum creatinine ≤1.4 mg/dl at presentation were recruited into our study. Of 68 patients not recruited, 35 had a serum creatinine >1.4 mg/dl at presentation, 11 refused genetic testing, and 22 were lost to follow-up. Among 35 patients excluded with a serum creatinine >1.4 mg/dl, 12 had atypical PKD, including 9 presenting with CKD stages 3–4 without significant kidney enlargement (i.e., <13 cm) and 3 presenting with features suggestive of syndromic PKD (with two subsequently found to harbor HNF1B mutations); 12 presented with CKD stages 3–4, and 13 present with CKD stage 2 before age 50 years old. Among 33 patients who refused genetic testing or were lost to follow-up, 23 presented with a serum creatinine ≤1.4 mg/dl at age 50 years old or older, and 10 presented with a serum creatinine ≤1.2 mg/dl before age 50 years old, suggesting that most of them had mild PKD. All study subjects underwent standardized evaluation, including a detailed family history of ADPKD, renal function tests, and PKD1 and PKD2 mutation screening. Kidney disease severity, including ESRD and survival status, was also collected from all available affected family members. The diagnosis of ADPKD in the study subjects was confirmed by ultrasound-based criteria28 and/or mutation screening. For patients without a positive family history, the diagnosis of ADPKD required the presence of ≥10 cysts in each kidney, with both kidneys >15 cm in length. The research teams (N.H. and K.W. from Toronto and J.L.S. and C.M.H. from Mayo Clinic) that performed the mutation screen were blinded to the study subject identity. TKV was measured in 161 study patients by magnetic resonance imaging or computed tomography scan and scored by J.C. and M.H. without any knowledge of the identity of the study patient. The study protocol was approved by the Research Ethics Board at the University Health Network.
Genetic Testing
DNA samples were collected from all probands and one or more of their affected family members. We screened one affected member from each family by bidirectional sequencing of the coding regions and splice junctions of both PKD1 and PKD2. For PKD1 screening, we used a validated PCR protocol to generate locus-specific amplicons.12 Nonsense, frameshift, and canonical splice site mutations are grouped as PT mutations, whereas nonsynonymous missense or atypical splice site mutations are grouped as NT mutations. Small IF indels affecting less than five amino acids are grouped as a separate mutation class. All NT mutations and IF indels were evaluated for their potential pathogenicity using prediction algorithms (PolyPhen-2,18 SIFT,19 Align GVGD,12 Human Splicing Finder,29 BDGP splice site predictor, and PROVEAN20), by review of the PKD mutation database (http://pkdb.mayo.edu), and by segregation analysis with additional affected family members whenever possible. All mutation-negative patients were rescreened by multiplex ligation–dependent probe amplification for detection of large gene rearrangements.30 P.C.H. and Y.P. reviewed and approved the results of genetic testing.
Statistical Analyses
Continuous variables are expressed as means and 95% CIs or medians and interquartile ranges, and discrete variables are expressed as percentages. We performed multivariate linear regression analysis to examine the effects of age, sex, and mutation class on log-transformed htTKV. We also examined ESRD and all–cause patient death by Kaplan–Meier analysis according to different mutation classes. Two patients with bilineal disease were excluded. Cox proportional hazard analysis was performed with frailty model (Supplemental Tables 1–6) to account for the effect of related members within family.31 Both sex and mutation classes satisfied the proportional hazard assumption when it was tested by log − log plots. R version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria), SPSS 15.0 (IBM Software, Somers, NY), and GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) were used for the statistical analyses.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank all of the study subjects for their participation.
This work was supported by Canadian Institutes for Health Research Grant MOP 123429 (to M.H., A.D.P., and Y.P.) and National Institutes of Health for the Molecular Genetics and Proteomics Core of the Mayo Translational Polycystic Kidney Disease Center Grant DK090728 (to P.C.H.).
Part of this study was presented at the American Society of Nephrology Kidney Week November 15, 2014 at Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060648/-/DCSupplemental.
References
- 1.Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Abad JM, Aresté N, de la Torre RA, Caskey F, Couchoud C, Finne P, Heaf J, Hoitsma A, de Meester J, Pascual J, Postorino M, Ravani P, Zurriaga O, Jager KJ, Gansevoort RT ERA-EDTA Registry EuroCYST Consortium WGIKD : Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: Prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant 29[Suppl 4]: iv15–iv25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris PC, Rossetti S: Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 197–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters DJ, Sandkuijl LA: Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol 97: 128–139, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Barua M, Cil O, Paterson AD, Wang K, He N, Dicks E, Parfrey P, Pei Y: Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 20: 1833–1838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Dicks E, Ravani P, Langman D, Davidson WS, Pei Y, Parfrey PS: Incident renal events and risk factors in autosomal dominant polycystic kidney disease: A population and family-based cohort followed for 22 years. Clin J Am Soc Nephrol 1: 710–717, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Magistroni R, He N, Wang K, Andrew R, Johnson A, Gabow P, Dicks E, Parfrey P, Torra R, San-Millan JL, Coto E, Van Dijk M, Breuning M, Peters D, Bogdanova N, Ligabue G, Albertazzi A, Hateboer N, Demetriou K, Pierides A, Deltas C, St George-Hyslop P, Ravine D, Pei Y: Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 1164–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Persu A, Duyme M, Pirson Y, Lens XM, Messiaen T, Breuning MH, Chauveau D, Levy M, Grünfeld JP, Devuyst O: Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int 66: 2132–2136, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Paterson AD, Magistroni R, He N, Wang K, Johnson A, Fain PR, Dicks E, Parfrey P, St George-Hyslop P, Pei Y: Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 16: 755–762, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Fain PR, McFann KK, Taylor MR, Tison M, Johnson AM, Reed B, Schrier RW: Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int 67: 1256–1267, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang QJ, Thompson PA, Miller JP, Harris PC CRISP Consortium : Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van’t Hoff WG, Niaudet P, Torres VE, Harris PC: Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int 75: 848–855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei Y, Lan Z, Wang KR, Garcia-Gonzalez M, He N, Dicks E, Parfrey P, Germino G, Watnick T: A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int 81: 412–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornec-Le Gall E, Audrézet M-P, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC: Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr., Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng PC, Henikoff S: Predicting deleterious amino acid substitutions. Genome Res 11: 863–874, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP: Predicting the functional effect of amino acid substitutions and indels. PLoS One 7: e46688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei Y, Paterson AD, Wang KR, He N, Hefferton D, Watnick T, Germino GG, Parfrey P, Somlo S, St George-Hyslop P: Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet 68: 355–363, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youssoufian H, Pyeritz RE: Mechanisms and consequences of somatic mosaicism in humans. Nat Rev Genet 3: 748–758, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 25.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossetti S, Hopp K, Sikkink RA, Sundsbak JL, Lee YK, Kubly V, Eckloff BW, Ward CJ, Winearls CG, Torres VE, Harris PC: Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol 23: 915–933, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergmann C, von Bothmer J, Ortiz Brüchle N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Santos A, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K: Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol 22: 2047–2056, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C: Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res 37: e67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Consugar MB, Wong WC, Lundquist PA, Rossetti S, Kubly VJ, Walker DL, Rangel LJ, Aspinwall R, Niaudet WP, Ozen S, David A, Velinov M, Bergstralh EJ, Bae KT, Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Sampson JR, Dawson BD, Harris PC CRISP Consortium : Characterization of large rearrangements in autosomal dominant polycystic kidney disease and the PKD1/TSC2 contiguous gene syndrome. Kidney Int 74: 1468–1479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rondeau V, Mazroui Y, Gonzalez J: Frailtypack: An R package for analysis of correlated survival data with Frailty models using penalized likelihood estimation or parametrical estimation. J Stat Softw 47: 1–28, 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.