Abstract
Trace amounts of lithium are essential for our physical and mental health, and administration of lithium has improved the quality of life of millions of patients with bipolar disorder for >60 years. However, in a substantial number of patients with bipolar disorder, long–term lithium therapy comes at the cost of severe renal side effects, including nephrogenic diabetes insipidus and rarely, ESRD. Although the mechanisms underlying the lithium–induced renal pathologies are becoming clearer, several recent animal studies revealed that short-term administration of lower amounts of lithium prevents different forms of experimental AKI. In this review, we discuss the knowledge of the pathologic and therapeutic effects of lithium in the kidney. Furthermore, we discuss the underlying mechanisms of these seemingly paradoxical effects of lithium, in which fine-tuned regulation of glycogen synthase kinase type 3, a prime target for lithium, seems to be key. The new discoveries regarding the protective effect of lithium against AKI in rodents call for follow-up studies in humans and suggest that long-term therapy with low lithium concentrations could be beneficial in CKD.
Keywords: acute renal failure, cell and transport physiology, cell signaling, end-stage renal disease, diabetes insipidus, drug nephrotoxicity
The alkali metal lithium is naturally present in the soil, and the uptake of trace amounts of lithium by the drinking water or diet is essential for our mental and physical health.1 Animals fed with a lithium-deficient diet exhibited higher mortality rates, chronic inflammation, and abnormalities in reproduction and behavior.1 In agreement, humans living in areas with relatively high natural lithium levels in their drinking water display reduced all–cause mortality rates and less harmful behavioral problems, like homicide and suicide.2,3 Other than the necessity of trace amounts of lithium, the benefits of lithium in the treatment of bipolar disorder have been known for >60 years, and despite large efforts of pharmaceutical companies to develop alternatives, lithium is even today the most effective medication for this disease.4
However, an important drawback of lithium medication is the development of severe renal side effects (Table 1).5 On the short term (months to years), lithium causes nephrogenic diabetes insipidus (NDI), a urinary concentrating defect, in approximately 20% of patients, resulting in polyuria, dehydration, thirst, and compensatory polydipsia. Dependent on dose and duration of treatment, long–term (decades) lithium therapy increases the chance to develop ESRD 6- to 8-fold.5–7 Recently, two studies also reported an increased incidence of solid renal tumors in chronic lithium users compared with the general population.8,9 However, because of several limitations of these studies as outlined by Licht et al.10 and contradictory findings in the study from Pottegård et al.,11 which included many more patients, we will not regard renal tumor development as a side effect of lithium treatment at this point.10,11
Table 1.
Toxic effects of lithium on the kidney and proposed mechanisms
| Effecta | Segment | Proposed Mechanism | References |
|---|---|---|---|
| NDI | Distal tubule and collecting duct | Reduced water uptake because of downregulation of AQP2 and loss of principal cells | 38,40–42,80,117 |
| Cellular remodeling (increase of intercalated versus principal cells) | Distal tubule and collecting duct | Not known; possibly a consequence of lithium–induced G2 arrest of collecting duct principal cells and/or lithium–induced metabolic acidosis | 42,80,117 |
| Interstitial fibrosis | Throughout all of the kidney | Not known; possibly a result from prolonged activation of the Wnt signaling pathway | 69,71,75,118–120 |
| Tubular atrophy | Proximal tubuleb | Not known but often linked with interstitial fibrosis in various renal diseases (interstitial fibrosis tubular atrophy) | 69,71,74,75,118 |
| Tubular dilation | Collecting ductb | Not known; likely a result from increased cell proliferation | 75,83,86,120,121 |
| Microcysts | Distal tubule and collecting duct | Not known; likely caused by increased cell proliferation | 75,77,83,118,120 |
| Glomerulosclerosis | Glomerulus | Not known; possibly a consequence of progressive renal damage | 75,118 |
The toxic lithium effects are arranged in chronological order of appearance.
Described for these segments, but studies did not exclude occurrence in other segments.
In contrast to these adverse effects of lithium treatment, accumulating evidence suggests that the administration of low lithium amounts (<0.6 mM in blood) improves kidney function in different animal nephropathy models. Single-bolus injections or short-term treatment (<1 week) of lithium reduced adriamycin-, LPS-, cisplatin-, gentamicin-, and ischemia-induced AKI,12–18 whereas prolonged treatment (≥1 month) alleviated kidney damage because of ischemia-reperfusion, hypertension, and the autoimmune disease lupus erythematosus.19–21 To understand these opposing findings, we will first present an overview of renal lithium handling and then, discuss the molecular pathways underlying the toxic and potential therapeutic effects of lithium in the kidney.
Renal Lithium Handling
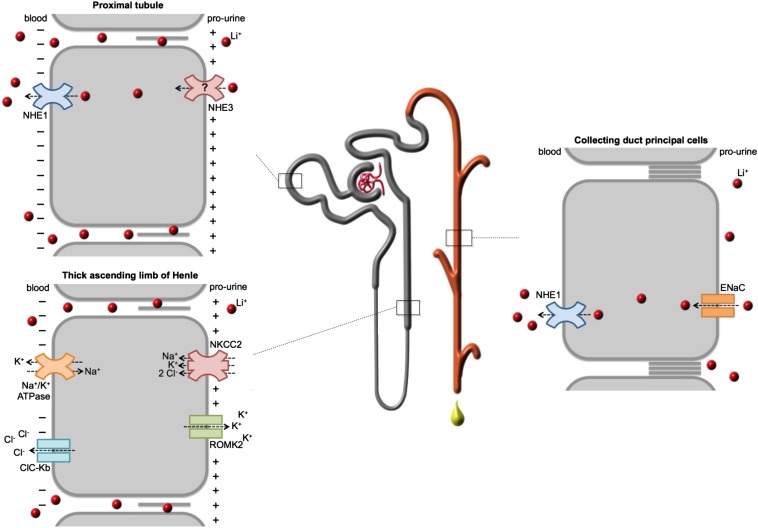
Lithium has a similar charge and size as sodium, and various reports from approximately 1970 to approximately 1985 showed that their renal filtration and reabsorption are similar. Lithium is, thus, freely filtered in the glomerulus and largely reabsorbed in the proximal tubule, which reabsorbs 70% of filtered sodium. Consequently, lithium was proposed as a quantitative marker for sodium reabsorption in the proximal tubule.22,23 Later, however, micropuncture studies showed that the actual delivery of lithium at the end of the proximal convoluted tubules exceeds that of sodium by approximately 14%, showing that lithium is reabsorbed to a lower extent than sodium.24 Proximal sodium reabsorption takes place through transcellular and paracellular pathways, accounting for approximately one third and approximately two thirds of proximal sodium reabsorption, respectively. Although lithium is also reabsorbed through both pathways (Figure 1), the contribution of the transcellular pathway is much less, probably explaining its lower total proximal reabsorption.25–27 The lithium-transporting proteins in the proximal epithelium have not been identified, but ion flux studies give us some insight. The Na+/H+ exchanger (NHE3), responsible for most of the luminal Na+ uptake, is thought to mediate lithium uptake at the apical side of the cell, because its close family member, NHE1, transports lithium very well.28–30 However, this remains to be investigated. Basolateral lithium efflux is likely not mediated by the Na+/K+-ATPase because of its low affinity for lithium but by NHE1, which depending on the electrochemical gradient, can transport lithium in both directions.29,31,32 Because NHE1 is expressed in the basolateral membrane of epithelial cells of all nephron segment, except the macula densa and collecting duct intercalated cells, basolateral lithium transport is attributed to NHE1 in all lithium-transporting segments.31,33
Figure 1.
Lithium is reabsorbed in different segments of the renal tubule and in the collecting duct. Lithium is freely filtered in the glomerulus and subsequently reabsorbed by different nephron segments. In the proximal tubules, lithium is, to a minor extent, reabsorbed through the transcellular pathway, which likely involves NHE3 at the apical plasma membrane and NHE1 at the basolateral plasma membrane. In the distal tubules and collecting duct, transcellular lithium uptake from the prourine occurs through the ENaC, whereas NHE1 likely mediates the cellular efflux to the interstitium. In both the proximal tubule and thick ascending limb of Henle (TAL), lithium is reabsorbed in a paracellular fashion, which is driven by the generated transcellular luminal–positive electrical gradient. In the TAL, this gradient is accomplished by luminal K+ efflux by renal outer medullary K+ 2 (ROMK2); K+, Na+, and Cl− influx b Na+/K+/2Cl– cotransporter 2 (NKCC2); and basolateral extrusion by the Na+/K+-ATPase and ClC-Kb chloride channels (as indicated).
Another 3%–10% of filtered lithium is reabsorbed in the thick ascending limb of Henle (Figure 1).24 This transport is mainly mediated by the paracellular pathway and driven by the transepithelial voltage difference created by potassium efflux by renal outer medullary K+ 2 and activity of the Na+/K+/2Cl– cotransporter 2.28,34,35 Finally, lithium is reabsorbed in late distal tubules and collecting duct, where no paracellular transport takes place because of the impermeability of tight junctions to cations (Figure 1).24,36 Here, lithium is taken up from the prourine by the principal cells of the collecting duct through the epithelial sodium channel (ENaC).37
Toxic Effects of Lithium Treatment
NDI
NDI is characterized by the inability of the kidney to concentrate prourine.38–40 Urine concentration is mediated by the principal cells of the collecting duct that express aquaporin-2 (AQP2) water channels at their apical membrane and thereby, allow transcellular water reabsorption. Lithium treatment causes dysregulation of AQP2 expression and trafficking on the short term and loss of principal cells on the long term.41,42 Lithium–induced AQP2 downregulation is a consequence of ENaC-mediated influx of lithium into principal cells, which is shown by ENaC inhibition studies on cultured collecting duct cells.37 In agreement, ENaC inhibition attenuated and ENaC ablation prevented the development of lithium-induced NDI.43,44 Within the cell, lithium inhibits glycogen synthase kinase type 3 (GSK3), a serine/threonine protein kinase that regulates many processes, including cell cycle progression, cell differentiation, and normal epithelial function and survival.45–49 GSK3 consists of two isoforms (α and β), which are inhibited directly by lithium but also, indirectly, by the increased phosphorylation of serines 9 and 21 on GSK3β and -α, respectively.50 Different studies showed that GSK3 plays an important role in urine concentration and lithium-induced NDI, because the use of GSK3 inhibitors other than lithium also reduced AQP2 abundance in collecting duct cell cultures.37 Furthermore, ablation of GSK3α or -β in mice caused polyuria and a reduced AQP2 abundance, whereas subsequent lithium treatment in the GSK3α knockout mice only slightly reduced their urine concentrating ability.51,52 How the inhibition of GSK3 by lithium ultimately leads to NDI has not been identified but may involve β-catenin, which is increased in expression in lithium-induced NDI and targeted for degradation by GSK3.48,53–55 Although basal β-catenin activity is necessary for AQP2 expression in collecting duct cell cultures, overactivation of the canonical Wnt-β-catenin pathway might contribute to lithium-induced NDI, because it induces transcription of proliferative genes, which is also observed in principal cells of lithium-treated rodents.56,57 Loss of polarization, which occurs with proliferation, reduces AQP2 expression in vitro.58 This, however, may not hold, because treatment of lithium NDI mice with the mammalian target of rapamycin 1 inhibitor rapamycin blocked their principal cell proliferation but not NDI.59 It, thus, remains to be established whether β-catenin has a role in lithium-induced NDI.
In lithium-induced NDI, urinary PGE2 levels are increased and contribute significantly to the disorder, because inhibition of cyclooxygenase-2 in rodents and patients strongly attenuated their lithium-induced polyuria.60–62 PGE2 activates the EP3 receptor in principal cells, leading to a reduced cAMP signaling, AQP2 expression, and plasma membrane targeting.48,63 Importantly, renal PGE2 production is stimulated by flow-stimulated release of ATP and its degradation products by activation of their P2Y receptors.64 Indeed, blocking the P2Y12 receptor attenuated lithium-induced NDI and reduced urinary PGE2 levels.65 A different mechanism was obtained with P2Y2 knockout mice.66 These mice were also protected from the development of lithium-induced polyuria, but because these mice exhibited increased urinary PGE2 levels, this protection was attributed to the observed reduced EP3 receptor abundance. Thus, blocking PGE2 production or modulating EP3 receptor abundance seems effective to attenuate lithium NDI.
ESRD
The most severe clinical side effect of lithium is the development of ESRD. Its prevalence in lithium-using patients was reported as approximately 1.5%, which is six to eight times higher than in the general population.7,67 The duration of lithium treatment is an important factor, because the vast majority of patients with ESRD were treated for >15 years with lithium.7,67 Although a report in 2014 suggested that the less–controlled treatment regime for lithium in the 1960s and 1970s strongly contributed to the development of ESRD, a later report showed that 5% of patients who started lithium treatment after 1980 and continued for 10–29 years also displayed evidence of ESRD.6,68 Whether this treatment regime reduces the occurrence of lithium-induced ESRD compared with the practices in the 1960s and 1970s remains to be addressed.
Kidney damage in humans and rodents chronically treated with lithium is mostly characterized by proximal tubular atrophy and chronic interstitial fibrosis (Table 1).69–72 Some lithium–treated patients also display glomerulosclerosis, but animal studies revealed that this pathology occurs after the onset of interstitial fibrosis and tubular atrophy.7,69,72–75 Although this suggests that glomerulosclerosis results from damage at other renal segments, it probably constitutes an essential step in the final progression to ESRD.76 Other than these pathologic features, histology on biopsies or magnetic resonance imaging scans revealed that a number of lithium-treated patients also displays renal microcysts.75,77 The development of these cysts is likely a direct consequence of lithium uptake by principal cells, because these cysts mainly originate from the distal tubule and collecting duct.75 Renal cyst formation often results from disturbances in the function of the primary cilium.78 Because cilium function is dependent on GSK3 activity, the high levels of inactive GSK3, as observed in renal tubules and cysts from lithium-treated animals, might be the primary cause for renal cyst formation.54,78
It seems evident that the early development of lithium–induced proximal tubular atrophy and interstitial fibrosis is an essential step toward ESRD. How lithium treatment causes these pathologies is still poorly understood. It is not known whether ESRD results from the influx of lithium into principal cells or other renal cells. We recently found that lithium caused a G2 arrest of principal cells, whereas others found a direct link between G2–arrested renal cells and the elevated production of TGF-β1, which plays a crucial role in the development of renal fibrosis.76,79,80 In agreement with these findings, the expression of TGF-β1 is enhanced in collecting ducts of lithium-treated rats.69 Elevated β-catenin levels in principal cells might also contribute to the fibrosis, because Dickkopf-1, an antagonist of canonical Wnt signaling, and ICG-001, which disrupts β-catenin–mediated gene transcription, strongly reduced interstitial fibrosis in mouse nephropathy models.81,82
As pointed out, lithium can easily enter proximal tubule cells, and a recent study showed that, several days after a single lithium dose, which was below or similar to therapeutic amounts given to patients with bipolar disorder, the proximal tubule exhibited an enhanced expression of different proproliferative proteins.18 Importantly, the development of NDI can further aggravate the potential direct pathologic effect of lithium on proximal tubules, because polyuric rats were shown to have an enhanced capacity of transcellular proximal sodium transport and a corresponding increase in NHE3 abundance, likely as a consequence of the hypovolemia-induced activation of the renin-angiotensin system.30,83,84 Considering NHE3 as the main lithium entry site of proximal tubule cells, this likely also elevates the proximal uptake of lithium. Thus, chronic exposure to therapeutic lithium amounts, certainly combined with NDI, will increase lithium fluxes in proximal tubular epithelia, which may directly cause or contribute to the proximal tubular atrophy and interstitial fibrosis. It is clear that, to develop medication to prevent or attenuate lithium–induced ESRD development, a better understanding of the etiology is needed.
Potential Beneficial Effects of Lithium
Lithium Reduces Kidney Damage in Various Nephropathy Models
Although chronic intake of high lithium amounts causes renal damage, accumulating evidence from animal studies indicates that administration of low lithium amounts is beneficial in preventing kidney disease caused by nephrotoxic compounds, inflammation, or oxidative stress. In mice, the administration of a single lithium dose 3 days after the induction of AKI by cisplatin largely prevented acute tubular necrosis and reduced serum creatinine levels.18 The protective effect of lithium was ascribed to its stimulatory effect on tubular cell proliferation and repair, allowing the kidney to repopulate the damaged proximal epithelium.18 Similarly, a 6-hour pretreatment with lithium prevented adriamycin–induced podocyte injury, which was shown by a reduced albuminuria and reduced expression of podocytopathic mediators B7–1 and MCP-1.17 A single lithium bolus 1 hour before or simultaneously with LPS injection strongly reduced inflammation and apoptosis of renal cells in mice.12,17 Importantly, a single dose of 40 mg lithium chloride per 1 kg body wt was effective in all above–mentioned experiments. Although serum lithium levels were not determined in these experiments, the used dose is well below the dose of single lithium chloride injections (200–300 mg/kg body wt) administered to mice to reach the therapeutic range of serum lithium levels in humans.85 Also, long–term lithium treatment of mice with lupus erythematosus, an autoimmune disease, attenuated the progression of tubulointerstitial damage and ESRD.19 Serum lithium levels were not determined, but the mice were injected daily with 4 mg lithium chloride (approximately 120 mg/kg body wt), which likely resulted in lithium levels within the therapeutic range (0.6–1.0 mM).86 It remains to be determined why this dose improved the kidney function of lupus mice, whereas it causes renal disease in other animals and humans.86
Finally, rats that were injected 30 minutes before ischemia-reperfusion with lithium displayed reduced levels of renal oxidative stress, because they exhibited a diminished mitochondrial membrane depolarization and reactive oxygen species (ROS) production.13 In addition, a 30-day pretreatment of rats with lithium reduced renal damage induced by ischemia-reperfusion, which was shown by the reduction in BUN and serum creatinine and the improved preservation of the tubular structure.20 Remarkably, lithium treatment of mice 8 hours after the onset of ischemia-reperfusion was also effective in reducing kidney injury, because these mice exhibited less necrosis and a faster recovery of serum creatinine levels.18 In these experiments, the applied lithium doses were below clinical levels, because they were maximally 50 mg lithium chloride per 1 kg body wt; the 30-day treatment lead to the low serum level of 0.4 mM.
Molecular Mechanisms of the Renoprotective Effects of Lithium
Although lithium also targets inositol monophosphatase and inositol polyphosphate phosphatase,50 there is no evidence that these targets confer renoprotection. Instead, several GSK3–specific inhibitors other than lithium, like TDZD-8 or BIO, mimicked the protective effects of lithium.12,15,16,18,87–89 Consistently, mice carrying an inactive GSK3β mutant were protected from mercuric chloride–induced nephrotoxic injury.90 Therefore, we will focus on GSK3 as the target of lithium contributing to renoprotection with lithium (Figure 2).
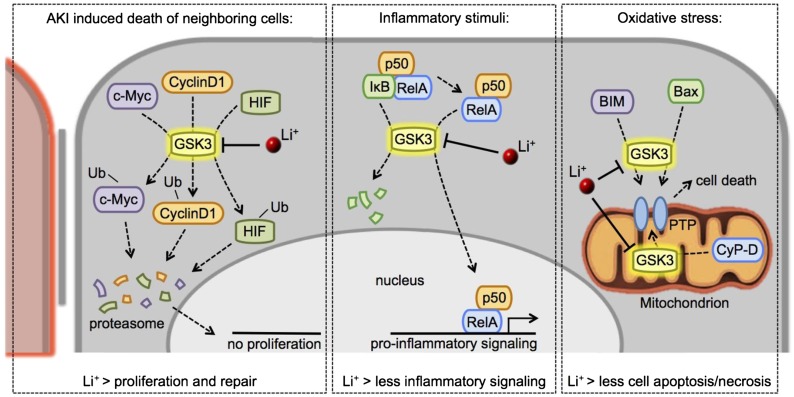
Figure 2.
Lithium-induced GSK3 inhibition confers renoprotection via different mechanisms. Upon AKI–induced cell death, an inflammatory response, or increased oxidative stress of proximal tubule cells, activated GSK3 prevents tubular repair and induces proinflammatory gene transcription and apoptosis/necrosis. By inhibiting GSK3, lithium intervenes and reduces activation of these nephrotoxic pathways. Details are in the text. PTP, permeability transition pore.
GSK3 Inhibition Allows Cell Repair by Activating Proproliferative Pathways
During AKI, the tubular epithelium is damaged and requires repair to prevent chronic damage. Proximal tubule–specific GSK3β knockout or lithium–induced GSK3 inhibition in mice with AKI increased the ability of damaged tubules to undergo repair by enhancing their capacity to proliferate.18,90 This increased capacity might be caused by an enhanced β-catenin–mediated transcription of proliferative genes (canonical Wnt signaling), because this pathway was also activated in cultured mouse proximal tubular cells treated with lithium and the GSK3 inhibitor BIO.89 Although canonical Wnt signaling is essential for self-repair during AKI,91 it is less evident whether pharmacologic repair by lithium also requires canonical Wnt signaling. In fact, the lower lithium amounts used in the AKI mice barely affected canonical Wnt signaling, whereas the expression of proliferative proteins, such as cyclinD1, c-Myc, and HIF-1α, were increased.18 Xu et al.53 suggested that a moderate GSK3 inhibition might activate the repair machinery by preventing GSK3-mediated degradation of these proliferative proteins in a Wnt-independent manner (Figure 2, left panel). Because prolonged canonical Wnt signaling induces kidney damage,81,82 activation of the repair machinery without activating Wnt signaling by moderate GSK3 inhibition might be an exciting potential novel therapeutic strategy in treating kidney disease. Additional research is required to confirm the existence of a Wnt-independent regulation of this repair machinery by GSK3.
GSK3 Inhibition Reduces Inflammatory Responses by Regulating NF-κB
In different forms of human kidney disease, renal NF-κB–mediated transcription is associated with proteinuria and other forms of kidney injury.92 When activated, the NF-κB family members p50, p52, RelA, RelB, and c-Rel form homodimers/heterodimers/trimers, which mediate proinflammatory gene transcription.92 Renal GSK3β deficiency or GSK3 inhibition prevented tubular and glomerular damage induced by proinflammatory NF-κB signaling.17,93 NF-κB function is regulated by both GSK3α and GSK3β.94,95 During kidney injury, active GSK3 promotes the nuclear translocation of NF-κB by phosphorylating RelA at serine 467.17,92 Additionally, GSK3 activates p50 by phosphorylation and induces the nuclear translocation of the NF-κB family members by blocking Inhibitor-κB,95,96 thereby enhancing the inflammatory response (Figure 2, center panel). Direct inhibition of NF-κB in different nephropathy models mostly prevented the proinflammatory signaling92 and reduced damage to both tubular as well as glomerular cells.17,93,97 Importantly, specific NF-κB inhibition was not merely beneficial, because it also induced apoptosis of renal cells.17 Recent studies showed that the adverse effect of NF-κB inhibition might be overcome by regulating GSK3 activity, because GSK3 inhibition prevented nuclear translocation of NF-κB but did not induce cell apoptosis.17 Finally, the anti-inflammatory effect of GSK3 inhibition on the kidney might be partially mediated by the modification of systemic inflammatory responses, because GSK3 also plays an important in this process (an overview is in ref. 98).
GSK3 Inhibition Protects against Oxidative Stress by Regulating Mitochondrial Permeability
Progression of renal injury is associated with graded increases in oxidative stress.99 ROS mediate or accelerate renal injury by triggering inflammatory responses or promoting cell apoptosis, necrosis, senescence, and fibrosis.99,100 Different GSK3 inhibitors, including lithium, prevent ROS-induced apoptosis of mesangial cells and renal proximal epithelial cells.87,101–103 Although the molecular mechanism by which GSK3 inhibition prevents renal oxidative damage has not been investigated, studies on extrarenal tissues show that ROS–induced GSK3 activation induces the opening of the mitochondrial permeability transition pore, eventually resulting in cell death.104,105 Active GSK3α/β induces permeability transition pore opening by activating Bax and BIM in the cytosol and cyclophilin-D in the mitochondrion (Figure 2, right panel).106 Other than reducing the sensitivity to ROS, lithium and other GSK3 inhibitors also increase the activity of antioxidant proteins, including Bcl-2, in both animal models and humans.107–111 Although the involved mechanisms are poorly studied, they might involve GSK3-mediated degradation of the transcription factor Nrf2, which upregulates the expression of different antioxidant proteins, like Bcl-2, and recently emerged as an important player in protection against AKI.112–116
Concluding Remarks
Although the damaging renal side effects in patients with bipolar disorder treated with lithium are well known, exciting recent data reveal that usage of the same drug on the short term and at a low dose may form a novel and cheap therapy in the prevention of AKI. It will be highly interesting to discover from future studies if this also holds true for humans and whether long-term treatment of low lithium amounts is also beneficial in preventing CKDs.
Disclosures
None.
Acknowledgments
This project received support from Radboud University Medical Center Grant 2012.IGMD (to P.M.T.D.), a grant from the Society of Experimental Laboratory Medicine (to P.M.T.D.), a Niels Stensen Fellowship (to T.d.G.), and Marie Curie Fellowship PIOF-GA-2012-332395 (to T.d.G.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Schrauzer GN: Lithium: Occurrence, dietary intakes, nutritional essentiality. J Am Coll Nutr 21: 14–21, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Blüml V, Regier MD, Hlavin G, Rockett IR, König F, Vyssoki B, Bschor T, Kapusta ND: Lithium in the public water supply and suicide mortality in Texas. J Psychiatr Res 47: 407–411, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Zarse K, Terao T, Tian J, Iwata N, Ishii N, Ristow M: Low-dose lithium uptake promotes longevity in humans and metazoans. Eur J Nutr 50: 387–389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhi GS, Tanious M, Gershon S: The lithiumeter: A measured approach. Bipolar Disord 13: 219–226, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Dols A, Sienaert P, van Gerven H, Schouws S, Stevens A, Kupka R, Stek ML: The prevalence and management of side effects of lithium and anticonvulsants as mood stabilizers in bipolar disorder from a clinical perspective: A review. Int Clin Psychopharmacol 28: 287–296, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Aiff H, Attman PO, Aurell M, Bendz H, Ramsauer B, Schön S, Svedlund J: Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol 29: 608–614, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Bendz H, Schön S, Attman PO, Aurell M: Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int 77: 219–224, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Rookmaaker MB, van Gerven HA, Goldschmeding R, Boer WH: Solid renal tumours of collecting duct origin in patients on chronic lithium therapy. Clin Kidney J 5: 412–415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaidan M, Stucker F, Stengel B, Vasiliu V, Hummel A, Landais P, Boffa JJ, Ronco P, Grünfeld JP, Servais A: Increased risk of solid renal tumors in lithium-treated patients. Kidney Int 86: 184–190, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Licht RW, Grabenhenrich LB, Nielsen RE, Berghöfer A International Group for the Study of Lithium (IGSLi) : Lithium and renal tumors: A critical comment to the report by Zaidan et al. Kidney Int 86: 857, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pottegård A, Hallas J, Jensen BL, Madsen K, Friis S: Long-term lithium use and risk of renal and upper urinary tract cancers [published online ahead of print May 4, 2015]. J Am Soc Nephrol doi:ASN.2015010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Huang WC, Wang CY, Tsai CC, Chen CL, Chang YT, Kai JI, Lin CF: Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. Br J Pharmacol 157: 1004–1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB: The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int 72: 1493–1502, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Plotnikov EY, Grebenchikov OA, Babenko VA, Pevzner IB, Zorova LD, Likhvantsev VV, Zorov DB: Nephroprotective effect of GSK-3β inhibition by lithium ions and δ-opioid receptor agonist dalargin on gentamicin-induced nephrotoxicity. Toxicol Lett 220: 303–308, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Ge Y, Liu Z, Gong R: Glycogen synthase kinase 3β orchestrates microtubule remodeling in compensatory glomerular adaptation to podocyte depletion. J Biol Chem 290: 1348–1363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W, Ge Y, Liu Z, Gong R: Glycogen synthase kinase 3β dictates podocyte motility and focal adhesion turnover by modulating paxillin activity: Implications for the protective effect of low-dose lithium in podocytopathy. Am J Pathol 184: 2742–2756, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao H, Ge Y, Peng A, Gong R: Fine-tuning of NFκB by glycogen synthase kinase 3β directs the fate of glomerular podocytes upon injury. Kidney Int 87: 1176–1190, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao H, Ge Y, Wang Z, Zhuang S, Dworkin L, Peng A, Gong R: Delayed administration of a single dose of lithium promotes recovery from AKI. J Am Soc Nephrol 25: 488–500, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenz SP, Izui S, Benediktsson H, Hart DA: Lithium chloride enhances survival of NZB/W lupus mice: Influence of melatonin and timing of treatment. Int J Immunopharmacol 17: 581–592, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Talab SS, Emami H, Elmi A, Nezami BG, Assa S, Deroee AF, Daneshmand A, Tavangar SM, Dehpour AR: Chronic lithium treatment protects the rat kidney against ischemia/reperfusion injury: The role of nitric oxide and cyclooxygenase pathways. Eur J Pharmacol 647: 171–177, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Scholz A, Rösch N, Blume A, Unger T, Kreutz R, Culman J, Gohlke P: Low-dose lithium combined with captopril prevents stroke and improves survival in salt-loaded, stroke-prone spontaneously hypertensive rats. J Hypertens 23: 2277–2285, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Thomsen K, Schou M, Steiness I, Hansen HE: Lithium as an indicator of proximal sodium reabsorption. Pflugers Arch 308: 180–184, 1969 [DOI] [PubMed] [Google Scholar]
- 23.Thomsen K: Lithium clearance: A new method for determining proximal and distal tubular reabsorption of sodium and water. Nephron 37: 217–223, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Boer WH, Fransen R, Shirley DG, Walter SJ, Boer P, Koomans HA: Evaluation of the lithium clearance method: Direct analysis of tubular lithium handling by micropuncture. Kidney Int 47: 1023–1030, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Hartmann A, Holdaas H, Steen PA, Kiil F: Evidence for bicarbonate-dependent lithium reabsorption in dog kidneys. Acta Physiol Scand 120: 257–264, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Leyssac PP, Holstein-Rathlou NH, Skøtt P, Alfrey AC: A micropuncture study of proximal tubular transport of lithium during osmotic diuresis. Am J Physiol 258: F1090–F1095, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Leyssac PP, Frederiksen O, Holstein-Rathlou NH, Alfrey AC, Christensen P: Active lithium transport by rat renal proximal tubule: A micropuncture study. Am J Physiol 267: F86–F93, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Palmer LG, Schnermann J: Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10: 676–687, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corry DB, Tuck ML, Nicholas S, Weinman EJ: Increased Na/H antiport activity and abundance in uremic red blood cells. Kidney Int 44: 574–578, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Yusufi AN, Christensen S, Dousa TP: Effect of chronic lithium treatment upon the Na(+)-coupled cotransporters in renal brush border membranes. Kidney Int 43: 1074–1080, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Holstein-Rathlou NH: Lithium transport across biological membranes. Kidney Int Suppl 28: S4–S9, 1990 [PubMed] [Google Scholar]
- 32.Thomsen K, Shirley DG: A hypothesis linking sodium and lithium reabsorption in the distal nephron. Nephrol Dial Transplant 21: 869–880, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Bobulescu IA, Moe OW: Na+/H+ exchangers in renal regulation of acid-base balance. Semin Nephrol 26: 334–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unwin RJ, Walter SJ, Shirley DG: Lithium reabsorption in perfused loops of Henle: Effects of perfusion rate and bumetanide. Am J Physiol 266: F806–F812, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Atherton JC, Doyle A, Gee A, Green R, Gingell S, Nicholls F, Pempkowiak L, Plange-Rhule J: Lithium clearance: Modification by the loop of Henle in man. J Physiol 437: 377–391, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J: Regulation of paracellular transport in the distal nephron. Curr Opin Nephrol Hypertens 21: 547–551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM: Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Boton R, Gaviria M, Batlle DC: Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis 10: 329–345, 1987 [DOI] [PubMed] [Google Scholar]
- 39.Stone KA: Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract 12: 43–47, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Walker RJ, Weggery S, Bedford JJ, McDonald FJ, Ellis G, Leader JP: Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int 67: 291–294, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S: Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95: 1838–1845, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen BM, Marples D, Kim YH, Wang W, Frøkiaer J, Nielsen S: Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Christensen BM, Zuber AM, Loffing J, Stehle JC, Deen PM, Rossier BC, Hummler E: alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol 22: 253–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batlle DC, von Riotte AB, Gaviria M, Grupp M: Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med 312: 408–414, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Rao R: Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens 21: 541–546, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doble BW, Woodgett JR: GSK-3: Tricks of the trade for a multi-tasking kinase. J Cell Sci 116: 1175–1186, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Force T, Woodgett JR: Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem 284: 9643–9647, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, Hao CM: Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA: Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: Mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quiroz JA, Gould TD, Manji HK: Molecular effects of lithium. Mol Interv 4: 259–272, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Nørregaard R, Tao S, Nilsson L, Woodgett JR, Kakade V, Yu AS, Howard C, Rao R: Glycogen synthase kinase 3α regulates urine concentrating mechanism in mice. Am J Physiol Renal Physiol 308: F650–F660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao R, Patel S, Hao C, Woodgett J, Harris R: GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol 21: 428–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu C, Kim NG, Gumbiner BM: Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle 8: 4032–4039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kjaersgaard G, Madsen K, Marcussen N, Christensen S, Walter S, Jensen BL: Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol 302: F455–F465, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Kortenoeven ML, Schweer H, Cox R, Wetzels JF, Deen PM: Lithium reduces aquaporin-2 transcription independent of prostaglandins. Am J Physiol Cell Physiol 302: C131–C140, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Paul S, Dey A: Wnt signaling and cancer development: Therapeutic implication. Neoplasma 55: 165–176, 2008 [PubMed] [Google Scholar]
- 57.Jung HJ, Kim SY, Choi HJ, Park EJ, Lim JS, Frøkiaer J, Nielsen S, Kwon TH: Tankyrase-mediated β-catenin activity regulates vasopressin-induced AQP2 expression in kidney collecting duct mpkCCDc14 cells. Am J Physiol Renal Physiol 308: F473–F486, 2015 [DOI] [PubMed] [Google Scholar]
- 58.van Beest M, Robben JH, Savelkoul PJ, Hendriks G, Devonald MA, Konings IB, Lagendijk AK, Karet F, Deen PM: Polarisation, key to good localisation. Biochim Biophys Acta 1758: 1126–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Gao Y, Romero-Aleshire MJ, Cai Q, Price TJ, Brooks HL: Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol 305: F1201–F1208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia Z, Wang H, Yang T: Mice lacking mPGES-1 are resistant to lithium-induced polyuria. Am J Physiol Renal Physiol 297: F1689–F1696, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim GH, Choi NW, Jung JY, Song JH, Lee CH, Kang CM, Knepper MA: Treating lithium-induced nephrogenic diabetes insipidus with a COX-2 inhibitor improves polyuria via upregulation of AQP2 and NKCC2. Am J Physiol Renal Physiol 294: F702–F709, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Allen HM, Jackson RL, Winchester MD, Deck LV, Allon M: Indomethacin in the treatment of lithium-induced nephrogenic diabetes insipidus. Arch Intern Med 149: 1123–1126, 1989 [PubMed] [Google Scholar]
- 63.Olesen ET, Fenton RA: Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol 24: 169–178, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Kishore BK, Carlson NG, Ecelbarger CM, Kohan DE, Müller CE, Nelson RD, Peti-Peterdi J, Zhang Y: Targeting renal purinergic signalling for the treatment of lithium-induced nephrogenic diabetes insipidus. Acta Physiol (Oxf) 214: 176–188, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Peti-Peterdi J, Heiney KM, Riquier-Brison A, Carlson NG, Müller CE, Ecelbarger CM, Kishore BK: Clopidogrel attenuates lithium-induced alterations in renal water and sodium channels/transporters in mice [published online ahead of print September 19, 2015]. Purinergic Signal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Pop IL, Carlson NG, Kishore BK: Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol 302: F70–F77, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Aiff H, Attman PO, Aurell M, Bendz H, Schön S, Svedlund J: End-stage renal disease associated with prophylactic lithium treatment. Eur Neuropsychopharmacol 24: 540–544, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Aiff H, Attman PO, Aurell M, Bendz H, Schön S, Svedlund J: The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol 28: 151–154, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Walker RJ, Leader JP, Bedford JJ, Gobe G, Davis G, Vos FE, deJong S, Schollum JB: Chronic interstitial fibrosis in the rat kidney induced by long-term (6-mo) exposure to lithium. Am J Physiol Renal Physiol 304: F300–F307, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Hansen HE, Hestbech J, Sørensen JL, Nørgaard K, Heilskov J, Amdisen A: Chronic interstitial nephropathy in patients on long-term lithium treatment. Q J Med 48: 577–591, 1979 [PubMed] [Google Scholar]
- 71.Presne C, Fakhouri F, Noël LH, Stengel B, Even C, Kreis H, Mignon F, Grünfeld JP: Lithium-induced nephropathy: Rate of progression and prognostic factors. Kidney Int 64: 585–592, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Ottosen PD, Sigh B, Kristensen J, Olsen S, Christensen S: Lithium induced interstitial nephropathy associated with chronic renal failure. Reversibility and correlation between functional and structural changes. Acta Pathol Microbiol Immunol Scand A 92: 447–454, 1984 [DOI] [PubMed] [Google Scholar]
- 73.Walker RG, Escott M, Birchall I, Dowling JP, Kincaid-Smith P: Chronic progressive renal lesions induced by lithium. Kidney Int 29: 875–881, 1986 [DOI] [PubMed] [Google Scholar]
- 74.Ottosen PD, Nyengård JR, Olsen TS, Christensen S: Interstitial focal fibrosis and reduction in proximal tubular length in adult rats after lithium treatment. Acta Pathol Microbiol Immunol Scand A 94: 401–403, 1986 [DOI] [PubMed] [Google Scholar]
- 75.Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D’Agati VD: Lithium nephrotoxicity: A progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11: 1439–1448, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Liu Y: Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Farres MT, Ronco P, Saadoun D, Remy P, Vincent F, Khalil A, Le Blanche AF: Chronic lithium nephropathy: MR imaging for diagnosis. Radiology 229: 570–574, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W: pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol 9: 588–595, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Groot T, Alsady M, Jaklofsky M, Otte-Höller I, Baumgarten R, Giles RH, Deen PM: Lithium causes G2 arrest of renal principal cells. J Am Soc Nephrol 25: 501–510, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kjaersgaard G, Madsen K, Marcussen N, Jensen BL: Lithium induces microcysts and polyuria in adolescent rat kidney independent of cyclooxygenase-2. Physiol Rep 2: e00202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwon TH, Laursen UH, Marples D, Maunsbach AB, Knepper MA, Frokiaer J, Nielsen S: Altered expression of renal AQPs and Na(+) transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 279: F552–F564, 2000 [DOI] [PubMed] [Google Scholar]
- 85.Can A, Blackwell RA, Piantadosi SC, Dao DT, O’Donnell KC, Gould TD: Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes Brain Behav 10: 434–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plenge P, Mellerup ET, Nørgaard T: Functional and structural rat kidney changes caused by peroral or parenteral lithium treatment. Acta Psychiatr Scand 63: 303–313, 1981 [DOI] [PubMed] [Google Scholar]
- 87.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS: Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol 17: 2812–2820, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL: Sustained Wnt/β-catenin signaling rescues high glucose induction of transforming growth factor-β1-mediated renal fibrosis. Am J Med Sci 344: 374–382, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Sinha D, Wang Z, Ruchalski KL, Levine JS, Krishnan S, Lieberthal W, Schwartz JH, Borkan SC: Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am J Physiol Renal Physiol 288: F703–F713, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Howard C, Tao S, Yang HC, Fogo AB, Woodgett JR, Harris RC, Rao R: Specific deletion of glycogen synthase kinase-3β in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney Int 82: 1000–1009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y: Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int 82: 537–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: NF-kappaB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Gong R, Rifai A, Ge Y, Chen S, Dworkin LD: Hepatocyte growth factor suppresses proinflammatory NFkappaB activation through GSK3beta inactivation in renal tubular epithelial cells. J Biol Chem 283: 7401–7410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pak C, Miyamoto S: A new alpha in line between KRAS and NF-κB activation? Cancer Discov 3: 613–615, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR: Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406: 86–90, 2000 [DOI] [PubMed] [Google Scholar]
- 96.Cortés-Vieyra R, Bravo-Patiño A, Valdez-Alarcón JJ, Juárez MC, Finlay BB, Baizabal-Aguirre VM: Role of glycogen synthase kinase-3 beta in the inflammatory response caused by bacterial pathogens. J Inflamm (Lond) 9: 23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guijarro C, Egido J: Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 59: 415–424, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Maddu N, Raghavendra PB: Review of lithium effects on immune cells. Immunopharmacol Immunotoxicol 37: 111–125, 2015 [DOI] [PubMed] [Google Scholar]
- 99.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J: Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 111: S4–S9, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC: Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 17: 311–321, 2012 [DOI] [PubMed] [Google Scholar]
- 101.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, Borkan SC: GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 21: 284–294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z, Ge Y, Bao H, Dworkin L, Peng A, Gong R: Redox-sensitive glycogen synthase kinase 3β-directed control of mitochondrial permeability transition: Rheostatic regulation of acute kidney injury. Free Radic Biol Med 65: 849–858, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang SH, Shih YL, Kuo TC, Ko WC, Shih CM: Cadmium toxicity toward autophagy through ROS-activated GSK-3beta in mesangial cells. Toxicol Sci 108: 124–131, 2009 [DOI] [PubMed] [Google Scholar]
- 104.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ: Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chiara F, Gambalunga A, Sciacovelli M, Nicolli A, Ronconi L, Fregona D, Bernardi P, Rasola A, Trevisan A: Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3α/β and Bax, leading to permeability transition pore opening and tumor cell death. Cell Death Dis 3: e444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Obligado SH, Ibraghimov-Beskrovnaya O, Zuk A, Meijer L, Nelson PJ: CDK/GSK-3 inhibitors as therapeutic agents for parenchymal renal diseases. Kidney Int 73: 684–690, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V Jr., da Silva Vargas R, Kapczinski F, Portela LV, Souza DO, Salvador M, Gentil V: Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. Neurosci Lett 421: 33–36, 2007 [DOI] [PubMed] [Google Scholar]
- 108.Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LM, Nardin P, Cunha AB, Ceresér KM, Santin A, Gottfried C, Salvador M, Kapczinski F, Gonçalves CA: Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res 41: 523–529, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J: Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31: 326–332, 2006 [PMC free article] [PubMed] [Google Scholar]
- 110.Khairova R, Pawar R, Salvadore G, Juruena MF, de Sousa RT, Soeiro-de-Souza MG, Salvador M, Zarate CA, Gattaz WF, Machado-Vieira R: Effects of lithium on oxidative stress parameters in healthy subjects. Mol Med Rep 5: 680–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manji HK, Moore GJ, Chen G: Lithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: A role for neurotrophic and neuroprotective effects in manic depressive illness. J Clin Psychiatry 61[Suppl 9]: 82–96, 2000 [PubMed] [Google Scholar]
- 112.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A: SCF/beta-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31: 1121–1133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rojo AI, Sagarra MR, Cuadrado A: GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: Relevance to exposure of neuronal cells to oxidative stress. J Neurochem 105: 192–202, 2008 [DOI] [PubMed] [Google Scholar]
- 114.Jiang Y, Bao H, Ge Y, Tang W, Cheng D, Luo K, Gong G, Gong R: Therapeutic targeting of GSK3β enhances the Nrf2 antioxidant response and confers hepatic cytoprotection in hepatitis C. Gut 64: 168–179, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A: Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem 281: 14841–14851, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Shelton LM, Park BK, Copple IM: Role of Nrf2 in protection against acute kidney injury. Kidney Int 84: 1090–1095, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Hestbech J, Hansen HE, Amdisen A, Olsen S: Chronic renal lesions following long-term treatment with lithium. Kidney Int 12: 205–213, 1977 [DOI] [PubMed] [Google Scholar]
- 119.Hestbech J, Olesen OV, Thomsen K: Lithium-induced focal interstitial fibrosis in the rat kidney. Acta Pathol Microbiol Scand A 86: 195–197, 1978 [DOI] [PubMed] [Google Scholar]
- 120.Aurell M, Svalander C, Wallin L, Alling C: Renal function and biopsy findings in patients on long-term lithium treatment. Kidney Int 20: 663–670, 1981 [DOI] [PubMed] [Google Scholar]
- 121.Kling MA, Fox JG, Johnston SM, Tolkoff-Rubin NE, Rubin RH, Colvin RB: Effects of long-term lithium administration on renal structure and function in rats. A distinctive tubular lesion. Lab Invest 50: 526–535, 1984 [PubMed] [Google Scholar]