Abstract
Background
Testosterone is theorized to play a major role in the pathophysiology of abdominal aortic aneurysms (AAAs) as this disease occurs primarily in males. The role of the androgen receptor (AR) in the formation of AAAs has not been well elucidated and therefore it is hypothesized that androgen blockade will attenuate experimental aortic aneurysm formation.
Methods
Aortas of 8- to 12-week-old male C57Bl/6 wild type (WT) mice or male androgen receptor knockout (AR−/−) mice were perfused with purified porcine pancreatic elastase (0.35 units/ml) to induce AAA formation. Two groups of WT male mice were treated with flutamide or ketoconazole (both androgen receptor blockers, 50mg/kg and 150mg/kg, respectively) twice daily via intraperitoneal injection. Aortas were harvested on day 14 after video micrometry was used to measure AAA diameter. Cytokine arrays and histologic analysis were performed on aortic tissue. Groups were compared using an ANOVA and a Tukey’s post hoc test.
Results
Flutamide and ketoconazole treatment attenuated AAA formation in WT mice (84.2±22.8%, p=0.009, and 91.5±18.2%, p=0.037) compared with WT elastase. 121±5.23% (mean±SEM). In addition, AR−/− mice showed attenuation of AAA growth (64.4±22.7% P< 0.0001) compared to WT elastase. Cytokine arrays of aortic tissue revealed decreased levels of pro-inflammatory cytokines interleukin (IL)-α, IL-6, and IL-17 in both flutamide-treated and AR−/− groups when compared to controls.
Conclusions
Pharmacologic and genetic AR blockade cause attenuation of AAA formation. Therapies for AR blockade utilized in prostate cancer may provide medical treatment to halt progression of AAAs in humans.
Introduction
The incidence of abdominal aortic aneurysms (AAAs) is roughly 1.2 – 1.4% and is most prevalent among elderly, male smokers1–4. Aortic aneurysms are a significant clinical problem as this disorder was primarily responsible for nearly 10,500 deaths in 20095. The pathophysiology of aneurysm degeneration of the aorta involves increased elastin degradation, increased cytokine production, smooth muscle cell apoptosis, and dysregulation of matrix metalloproteinases (MMPs), though the specific pathophysiology is unclear. Androgens, specifically testosterone, have been thought to play a role in aortic aneurysm formation6, 7; however, the role of the androgen receptor in AA development has not been well elucidated.
The androgen pathway has been a therapeutic target for male-specific diseases, such as prostate cancer. Flutamide is an androgen receptor antagonist that historically has been shown to be effective at androgen depletion when combined with gonadotropin releasing hormone agonists, such as leuprolide8–10. Additionally, ketoconazole has been shown to be an effective therapy for prostate cancer given its inhibition of the conversion of cholesterol to pregnenolone, which is a critical step during androgen synthesis11–13.
Androgen receptor blockade has certainly proven beneficial in an androgen-dependent disease states, such as prostate cancer. Given the male preponderance for AAA it is hypothesized that there is a fundamental difference in androgen receptor expression between male and female AAA tissues and pharmacologic blockade and genetic deletion of the androgen receptor will influence the formation of experimental AAA in a murine model.
Materials and Methods
Mice
Wild type (WT) C57Bl/6 mice and Androgen Receptor knockout mice (AR−/−) (strain B6.Cg-Aw-J EdaTa-6J +/+ ArTfm/J, stock number 001809) were ordered from Jackson Laboratories (Bar Harbor, ME) and maintained in house. All mice were fed minimal phytoestrogen diet (2016 Teklad Diet, Harlan Laboratories, Indianapolis, IN) in order to decrease any anti-inflammatory effects of excess dietary estrogen14. Animal experimental protocols were approved by the University of Virginia Institutional Animal Care and Use Committee (IACUC protocol #3648).
Aneurysm Model
All mice underwent elastase perfusion as previously described15–18. Eight to 12-week-old male C57Bl/6 WT mice underwent induction of anesthesia with inhaled isoflurane and maintenance of anesthesia was maintained by inhaled isoflurane. The infrarenal aorta was isolated via midline laparotomy and perfused with 0.35 units/ml of porcine pancreatic elastase (Sigma-Aldrich, St. Louis, MO) for 5 minutes. Lower extremity perfusion was resumed after aortotomy repair and evacuation of residual elastase. Postoperative analgesia was provided with buprenorphine. After 14 days, the mice underwent reoperation where video micrometry was used to estimate the size of maximal aortic dilation relative to an unperfused suprarenal aortic control segment. Subsequently, blood was drawn from the inferior vena cava and left renal vein junction and aortas were harvested from the level of the renal vein to the iliac bifurcation at euthanasia. Negative controls were created by heating elastase at 99°C for 30 minutes creating heat-inactivated elastase (HIE) and using it in place of active elastase or saline for perfusion. Elastase perfusion allows for chemical proteolysis of the aortic media, as well as mechanical disruption of the media, while perfusion with HIE or saline allows for mechanical dilation only. All harvested aortas were either: 1) snap frozen in liquid nitrogen for real-time polymerase chain reaction (PCR) analysis or protein extraction or 2) incubated overnight in 4% paraformaldehyde solution for immunohistochemistry or histology. Blood was centrifuged and the serum was frozen at −80°C. Animal care and use were in accordance with the Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the University of Virginia Institutional Animal Care and Use Committee (#3634) in compliance with the Office of Laboratory Animal Welfare.
Human Aortic Tissues
Abdominal aortic aneurysm tissue was procured from patients undergoing elective open abdominal aortic aneurysm repair at the time of surgery. Control abdominal aortic tissue was taken from transplant donors. Patients who donated aortic samples did not have a known collagen vascular disorder or dissection. Aortic samples were explanted and were immediately flash frozen in liquid nitrogen. Patients gave written consent for collection of aortic aneurysm tissue in compliance with the University of Virginia Human Subjects Review Committee (IRB# 13178).
Quantification of Androgen Receptors in Aortic Tissues
Normal human infrarenal abdominal aortic tissue and AAA tissue underwent analysis for mRNA expression of the androgen receptor (n=10 male and 10 female patients). Eight to 12-week-old C57Bl/6 WT mice were perfused with elastase or saline (n=6 per group). Tissues were harvested on days 0, 3, 7 and 14, and underwent analysis for levels of mRNA specific for androgen receptor and for 18s.
Pharmacologic Blockade of Androgen Receptors
Eight to 12-week-old C57B/Bl6 mice underwent aortic perfusion with elastase (n=7) or saline (n=9). Two experimental treatment arms of perfused mice then underwent subcutaneous implantation with osmotic pumps (Alzet, Cupertino, CA, USA) containing flutamide (50mg/kg) or ketoconazole tablets (150mg/kg) [Figure 1A] at the time of the aortic elastase perfusion in order to give steady states of drug delivery. Additional mice were perfused with HIE for negative controls (n=9). In order to examine an alternate route of drug delivery, two additional groups of mice were given 50 mg/kg of flutamide (n=7) and 150 mg/kg ketoconazole (n=8) via intraperitoneal injection twice daily for 14 days after elastase perfusion [Supplemental Figure I]. It is important to note the variance of the mechanism of action of each pharmacologic agent: flutamide is an androgen receptor blocker, while ketoconazole inhibits the conversion of cholesterol to pregnenolone, an upstream step important in the formation of testosterone.
Figure 1.
Scheme of experimental arms. A) Subcutaneous delivery of drugs for WT mice including aortic perfusion of elastase alone (n=7), perfusion with heat inactivated elastase alone (n=9), subcutaneous osmotic pump placement of flutamide (50 mg/kg) (n=9), and subcutaneous placement of ketoconazole tablet (150mg/kg over 14 days) (n=9). B) Genetic deletion of androgen receptors including aortic perfusion of elastase alone (n=7), perfusion with heat inactivated elastase alone (n=9), and elastase perfusion of aortas from androgen receptor knockout mice (ARK−/−) (n=8).
Genetic Deletion of Androgen Receptors
Eight to 12-week-old C57B/l6 mice underwent aortic perfusion with elastase (n=7) or HIE (n=9). An additional arm of 8 to 12-week old androgen receptor knockout mice (AR−/−) underwent aortic perfusion with elastase (n=8) [Figure 1B].
Cytokine Array
For the purpose of determining the effects of pharmacologic blockade and genetic deletion of androgen receptors and to determine its effect on the pro-inflammatory cytokine mileu of the aortic wall, mouse cytokine arrays (R&D Systems, Minneapolis, MN, USA) were performed using isolated protein from mouse aortas harvested at day 14 after elastase perfusion. Protein samples from each group were pooled for analysis, and all samples were run in duplicate15–18.
Histology
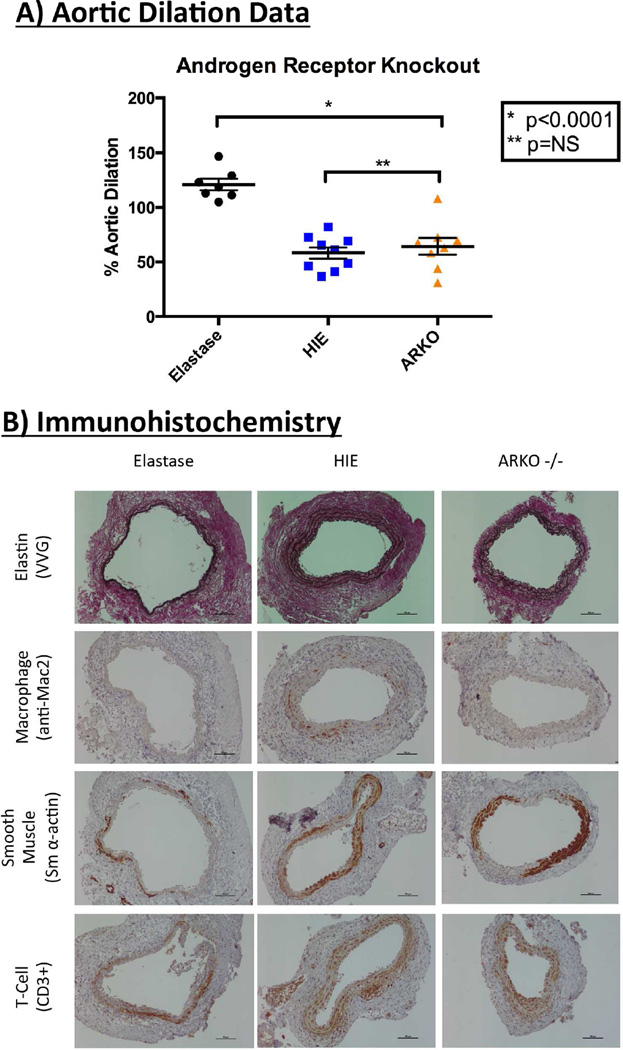
Infrarenal aortas were harvested at euthanasia and irrigated with normal saline. Fixation was achieved with overnight incubation in 4% paraformaldehyde followed by paraffin embedding and sectioning at 5µm. Microwave antigen retrieval was performed and antibodies were bound and detected using Vecta Stain Elite Kit (Vector Laboratories, Burlingame, CA, USA). Verhoeff-Van Gieson stain was used to evaluate aortic elastin content. Antibodies used for immunohistochemistry included: 1) anti-rat Mac2 for macrophages (1:10000; Cedarlane Laboratories, Burlington, Canada), 2) anti-mouse SMαA for SM α-actin (1:1000; Santa Cruz Biotechnology Inc, Santa Cruz, CA), and 3) anti-goat CD3 for CD3+ T-cells (1:500; Santa Cruz Biotechnology Inc, Santa Cruz, CA). Control strategies were employed utilizing controls without the primary antibody and controls with the appropriate IgG control. Visualization color development was completed using diaminobenzidine (Dako Corporation, Carpinteria, CA) for SMαA, Mac2, and CD3ε. Images were obtained using a Nikon eclipse Ti imaging system, and staining densities of photomicrographs were quantified with the NIS-Elements Version 4.2 imaging software.(Melville, NY, USA)
Additional murine aortic cross sections were evaluated with confocal fluorescence immunohistochemistry with staining for nuclei (DAPI), macrophages (Mac2 antibody, 1:1000) smooth muscle cells (Smooth muscle alpha actin, 1:1000), and androgen receptors (androgen receptor antibody, 1:500) to verify the localization of this receptor within the aortic wall. Cells were visualized using an LSM 710 scanning confocal microscope and results are representations of n=5 mice.
Statistical Methods
Statistical analysis was performed using GraphPad Prism Version 6.0e software (Graphpad Software, La Jolla, CA). Maximal aortic dilation (%) was calculated as [(maximal aortic diameter)/(internal control diameter) – 1] × 100. The internal control was an unperfused segment of infrarenal aorta cranial to the proximal ligation site. This section was not perfused with elastase; however, it was susceptible to hemodynamic changes from volume shifts during the operative and postoperative phases, as well as expected animal growth over time. Values are reported as mean ± SE of the mean (SEM), and percent change is referencing a percent change from aortic measurements of the unperfused control segment of native aorta. Aortic dilation between groups was compared using Analysis of Variance (ANOVA), and post hoc analysis was performed using Tukey correction to determine significance of individual comparisons with α=0.05.
Results
Increased levels of Androgen Receptor mRNA in Murine Aneurysm Tissue
Androgen receptors have been shown to be present in a multitude of tissues around the body19–22, though the quantification of ARs in aortic aneurysm tissue has not been performed previously. Wild-type murine AAA tissues revealed significantly increased levels of mRNA coding for ARs over time when comparing murine aortas perfused with elastase versus saline [Figure 2A]. Immunohistochemistry staining for ARs revealed increasing expression of ARs over time [Figure 2B]. Further imaging with confocal microscopy reveals the majority of AR expression is predominately associated with vascular smooth muscle cells [Figure 2C].
Figure 2.
Upregulation of Murine Androgen Receptors in Aortic Aneurysm Tissue. A) mRNA expression of androgen receptors of murine aortas perfused with elastase vs. saline over a 14 day timecourse (n=5 mice per group, *p<0.05). B) Immunohistochemistry staining for ARs in murine AAA tissue revealing increasing expression of ARs over a 14 day timecourse (arrows). C) Confocal microscopy reveals AR staining (purple) concomitantly staining with smooth muscle α-actin (red) as indicated by arrows. (Macrophage (Mac=green), Nuclei (DAPI=blue).
Pharmacologic Blockade of Androgen Receptors and Androgen Synthesis Attenuates Experimental Aortic Aneurysm Formation
Wild type mice were treated with flutamide and ketoconazole by steady state subcutaneous dosing and daily intraperitoneal administration. Aortic dilation of WT mice was significantly increased after exposure of elastase (n=7) when compared to perfusion with HIE (control, n=9) (114%±4.34 vs. 58.3%±5.26, p<0.0001). Mice treated with flutamide revealed significant attenuation when compared with elastase (80.3%±7.2 vs. 114%±4.3, p=0.012), as did mice treated with ketoconazole (65.87%±6.93 vs. 114%±4.34, p=0.0002) [Figure 3A]. Mice treated with intraperitoneal injections of flutamide revealed attenuation of aneurysm formation when compared to elastase (84.24%±8.61 vs. 121%±5.23, p=0.0034), and intraperitoneal ketoconazole injection revealed significant attenuation (65.87%±6.93 vs. 114%±4.34, p=0.018) [Supplemental Figure IB]. No statistical difference existed between mean aortic dilations of flutamide and ketoconazole in either group. Representative, immunohistochemistry reveals decreased macrophage infiltration in both groups treated with flutamide and ketoconazole when compared to the elastase group (p<0.001) [Figure 3B; quantification noted in Supplemental Figure III].
Figure 3.
Pharmacologic androgen receptor blockade and androgen synthesis disruption significantly attenuate experimental aneurysm formation. A) Aortic diameter measured with video micrometry of mice treated with flutamide and ketoconazole revealing significant attenuation of aneurysm development when compared to elastase (p=0.013, p=0.0002, respectively). B) Representative immunohistochemistry of aortas treated with elastase alone, HIE, elastase with flutamide and ketoconazole.
Genetic Deletion of Androgen Receptors Attenuates Experimental Aortic Aneurysm Formation
Androgen receptor knockout mice (AR−/−) (n=8) exhibited significantly less aortic dilation when compared with elastase WT mice perfused (n=7) (64.39%±8.01 vs. 121%±5.23, p<0.0001). There was no difference in dilation between WT mice perfused with HIE (n=9) vs. AR−/− mice (58.25%±5.26 vs. 64.39%±8.01, p=0.76) [Figure 4A]. Representative immunohistochemistry reveals decreased macrophage infiltration [Figure 4B; quantification noted in Supplemental Figure III].
Figure 4.
Genetic androgen receptor deletion significantly attenuates experimental aneurysm formation. A) Aortic diameter measured with video micrometry of androgen receptor knockout mice (ARKO−/−) reveals significant attenuation of aneurysm development when compared to elastase (p<0.0001). B) Representative immunohistochemistry of aortas from ARKO−/− mice.
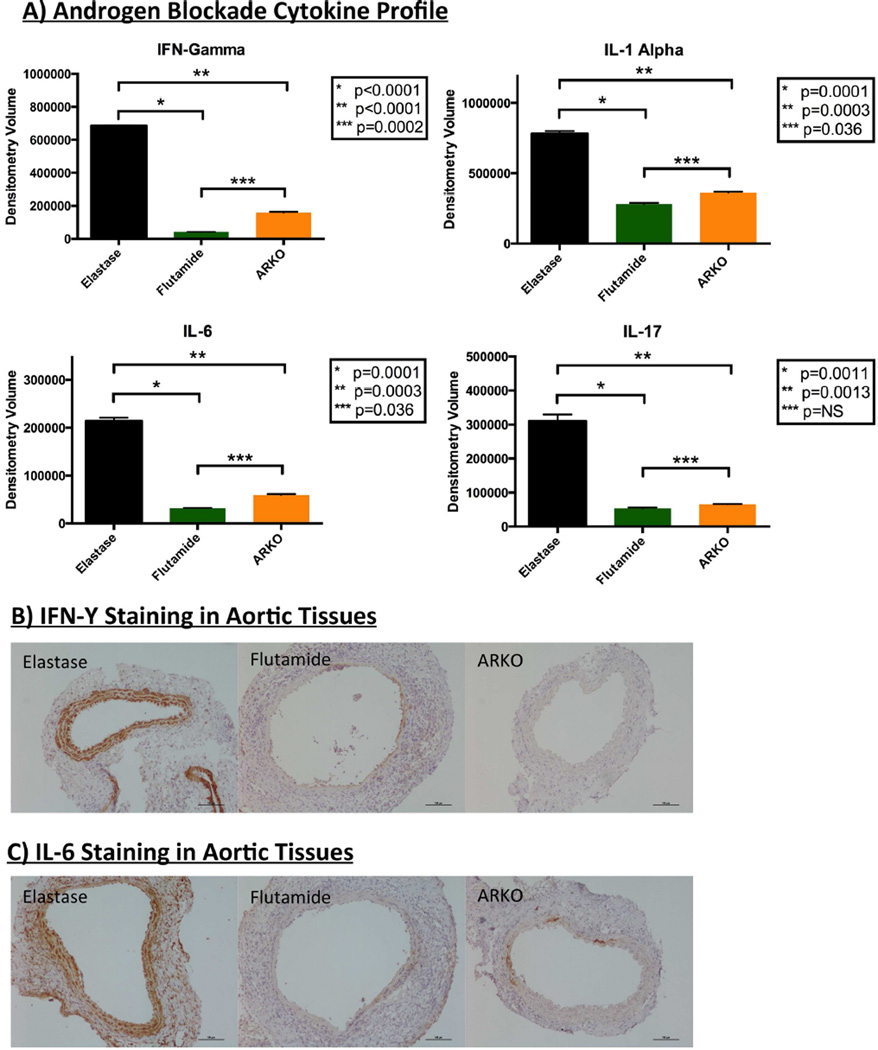
Pro-Inflammatory Cytokines are Decreased After Pharmacologic Blockade and Genetic Deletion of Androgen Receptors
Murine aortic tissues that underwent pharmacologic androgen receptor blockade with flutamide exhibited a significant decrease in pro-inflammatory cytokines interferon (IFN)-γ (p<0.0001), interleukin (IL)-1α (p=0.0001), IL-6 (p=0.0001), and IL-17 (p=0.0011) when compared with with the elastase group. Decreased levels of IFN-γ (p<0.0001), IL-1α (p=0.0003), IL-6 (p=0.0003), and IL-17 (p=0.0013) were also noted in aortic tissue from androgen receptor −/− mice when compared to elastase controls [Figure 5A]. Each of these pro-inflammatory mediators has been shown to play a role in aneurysm disease of the aorta via vascular inflammation23, 24. Representative histologic analysis revealed a trend toward decreased expression of IFN-γ and IL-6 in aneurysm tissues of mice treated with flutamide and from AR−/− mice when compared to elastase controls [Figures 5B and 5C; formal quantification noted in Supplemental Figure III].
Figure 5.
Pharmacologic androgen receptor blockade and genetic androgen receptor deletion decrease pro-inflammatory cytokines. A) Aortic aneurysm tissues from mice treated with flutamide and androgen receptor knockout mice (AR−/−) reveal significantly decreased expression of proinflammatory cytokines IFN-γ, IL-1α, IL-6, and IL-17. B) Representative immunohistochemistry revealing a trend toward decreased expression of IFN- in aneurysm tissues of mice treated with flutamide and aneurysm tissues AR−/− mice when compared to elastase controls. C) Representative immunohistochemistry revealing a trend toward decreased expression of IL-6 in aneurysm tissues of mice treated with flutamide and aneurysm tissues AR−/− mice when compared to elastase controls.
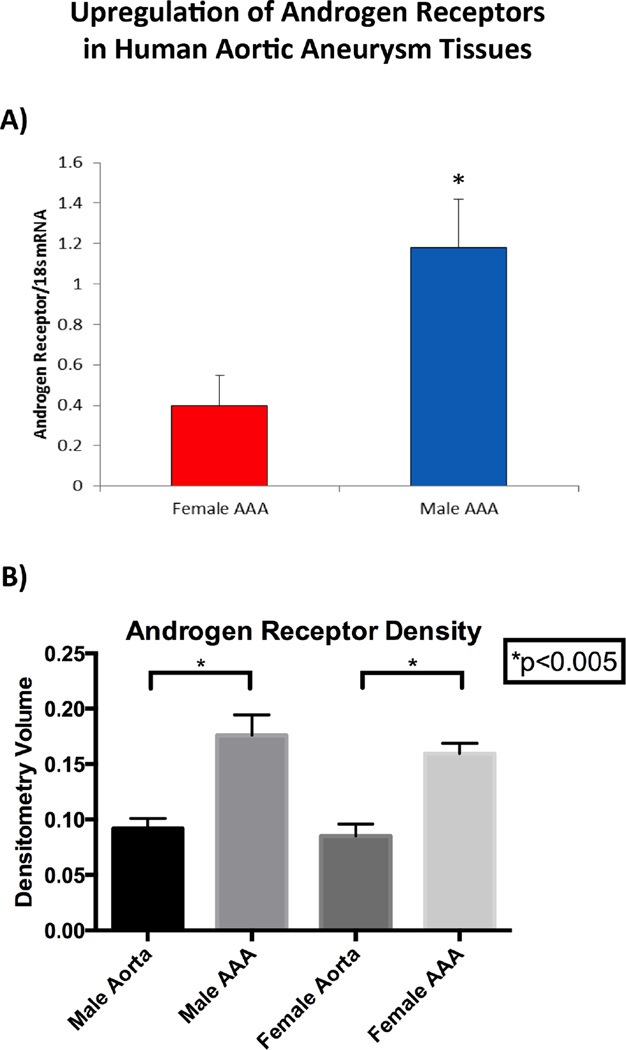
Human Aortic Aneurysms Express Elevated Levels of Androgen Receptors
Human aortic tissue samples were obtained from patients undergoing elective open AAA repair from both genders (n= 7 male and female patients). Significantly increased levels of mRNA for AR were noted in male human AAA tissue compared to female (p=<0.05) [Figure 6A]. Separately, protein was isolated from male and female AAA samples and control aortic tissue. Protein expression of AR in human aortic tissues reveal elevated levels of AR in both male and female aneurysm tissues when compared to control aortic tissues (p=<0.005). [Figure 6B], but with no differences between male and female AAAs. Additionally, there is increased expression of AR in the media and adventitia of human AAA tissues compared to normal aortic tissue [Supplemental Figure II].
Figure 6.
Upregulation of Androgen Receptors in Human Aortic Aneurysm Tissue. A) Androgen receptor levels are increased in AAA tissue from male compared to female patients (n=10 per group; *p<0.05 vs. female AAA). B) ELISA of human aortic tissue reveal increased levels of androgen receptors in aneurysm tissues when compared to control aortic tissues in both males and females (p<0.005, n=10 samples per group).
Discussion
The present study documents the significant role of ARs in AAA development, and most importantly reveals the efficacy of anti-androgen therapies commonly utilized in the treatment of prostate cancer. This is the first report of up-regulation of AR expression in aneurysm tissues in both an experimental murine AAA model and human aortic aneurysm tissue. We also demonstrate that pharmacologic blockade and genetic deletion of the androgen receptor significantly attenuated AAA development in a murine animal model, and subsequently that blockade of ARs inhibits the expression of pro-inflammatory cytokines within aortic aneurysm tissue.
A number of studies have implicated testosterone and other circulating androgens play a role in the inflammation and pathogenesis of AAA6, 25. In previous studies, exogenous administration of dihydrotestosterone (DHT) was associated with an increased formation of AAA in apolipoprotein E (apoE) knockout mice infused with angiotensin26. Furthermore, Ailawadi and colleagues previously demonstrated gender differences in experimental AAA formation in rats and illustrated a higher incidence and larger diameter aortic aneurysm formation associated with increased aortic inflammation in male rats27. Despite multiple reports implicating androgens as an important factor in cardiovascular disease, the precise role of androgens, and more specifically the androgen receptor, in AAA has not been well defined.
Androgen receptors have been shown to be present in all human vascular tissues, and multiple studies have revealed increased expression of ARs in multiple male tissues and cells when compared to females19–22. With regards to the mechanism of ARs in vascular tissues, androgens have been shown to stimulate endothelial cell proliferation by increasing the expression of ARs, vascular endothelial growth factor (VEGF), cyclin A, and cyclin D1. Exposing cells to DHT exhibits a dose-dependent increase expression of these ARs, VEGF, cyclin A, and cyclin D1. Interestingly, the introduction of an AR blocking agent, bicalutamide, inhibited further cell proliferation28. Further ex vivo studies have linked testosterone to the production of nitric oxide (NO) in aortic smooth muscle cells and endothelial cells in rats29. Similarly, blockade of the androgen receptor with flutamide significantly attenuated the production of NO, as well as proliferation of vascular smooth muscle cells29. These studies strengthen the association of androgen receptors in cardiovascular pathology and importance of targeting ARs for further therapy for AAAs.
Androgen deprivation therapy has proven to be an effective treatment strategy for prostate cancer. Flutamide and ketoconazole are two of the pharmacologic agents commonly utilized to achieve androgen deprivation. Flutamide is an androgen receptor antagonist and directly interferes with interactions of circulating androgens and their target tissues. Ketoconazole is more commonly known for its antifungal properties, but it is an inhibitor of CYP-17, a catalyst for both 17α-hydroxylase and C17,20-lyase30. Both of these enzymes are critical for androgen synthesis as 17α-hydroxylase converts pregnenolone into 17OH-pregnenolone, while C17,20-lyase works directly downstream by converting 17OH-pregnenolone into dihydroepiandrostenedione (DHEA), a precursor to testosterone synthesis30, 31. These agents are commonly utilized in prostate cancer, but have not been reported in applications for treatment of AAAs. Despite these androgen biochemical pathway implications for AR blockade and enzymatic inhibition, there is potential for each of these agents to have a number of alternative mechanisms of action.
Interestingly, pure pharmacologic blockade and genetic deletion of the androgen receptor seem to decrease the overall inflammatory state of aneurysm aortic tissue. Both methods of androgen receptor alteration reveal decreased levels of pro-inflammatory cytokines IFN-γ, IL-1α, IL-6, and IL-17. Interferon- γ, IL-1α, and IL-6 have been shown to be elevated in human aortic tissue samples23, while previous work by our laboratory has identified IL-17 as an important regulator for development of experimental AAA32. Aortic tissues from mice treated with flutamide and ketoconazole revealed decreased levels of IFN- γ and IL-17. Xiong and colleagues have identified the association of T-cells and IFN- γ with formation of experimental AAA33, and inhibition of T-cells and IFN- γ after administration of these antiandrogen agents suggest an interplay between androgens and T-cells and IFN- γ, Sharma and colleagues also suggest T-cells play a critical role in AAA formation through production of IL-1732 However, our results were not congruent with prior experiments as there was no significant inhibition of T-cell activity on quantitative measure of AAA immunohistochemistry. Interestingly, pure pharmacologic blockade and genetic deletion of the androgen receptor had a significant effect on macrophage presence in aneurysm tissue, which suggests a potential interplay between the androgen receptor and macrophage signaling with smooth muscle cells. Recent data by Owens et al suggests that there is phenotype switching between macrophages and SMCS and androgens may impact this process34.Ketoconazole has been shown to decrease local and systemic inflammation35, 36; however, flutamide has not been reported to possess anti-inflammatory properties. Our results demonstrate the effectiveness of both flutamide and ketoconazole with respect to inflammation associated with AAA formation.
This study is not without limitations. Our model utilizes mice aged 8–12 weeks, which are relatively young with respect to the elderly male human population that is affected by AA. Rates of experimental aneurysm formation with aortic elastase perfusion in mice at different age points are not available in the literature at this point in time. However, our laboratory has performed studies evaluating the role of aging on aortic aneurysm formation, and we have found there exists no difference in the cytokine expression or dilation data between mice at 8–10 weeks of age and older mice at 6 months or 1 year (Lu and Upchurch, unpublished observation). Furthermore, our model induces an acute inflammatory process to mimic a long-term inflammatory process in the human aorta. Drug delivery was also altered with regards to how human patients take the drug. While drug delivery was performed by two vehicles, namely IP and subcutaneous pump, we recognize these drugs will most likely be delivered by oral administration. However, our results suggest depot dosing is an effective method of drug delivery. Finally, there are issues related to feminization associated with many of these androgen receptor blocking drugs that may make chronic and sustained treatment in patients with AAA not practical.
In summary, the present study is the first to explore the role of ARs in experimental AA formation and identify a potential target for future medical therapy for AAs. We have demonstrated that pharmacologic blockade and genetic deletion of the androgen receptor attenuates experimental AAA formation and decreases the inflammatory milieu of aortic aneurysm tissue. Aside from risk factor modification and overall risk reduction for cardiovascular disease, there is no medical therapy available for AAA, and operative repair is the definitive cure. Therapies for AR blockade utilized in prostate cancer may provide insight into medical treatment to halt progression of AAAs in humans.
Supplementary Material
Clinical Relevance.
Abdominal aortic aneurysms (AAAs) carry a significant mortality and morbidity. Currently, a medical cure is not available for AAAs. This work presents a potential target for medical treatment of AAAs: the androgen receptor (AR). Pharmacologic agents approved for prostate cancer reveal attenuation of experimental aneurysm formation and decreased proinflammatory cytokine profile in aortic tissues. Furthermore, genetic deletion of AR reveals similar results. Therefore, AR appears to play a critical role in aneurysm formation, and pharmacologic agents currently utilized for prostate cancer treatment may play a role in inhibition of aneurysm progression and may prolong time to definitive repair.
Acknowledgments
We would like to thank Sheila Hammond, Cindy Dodson, and Tony Herring for their knowledge and technical expertise.
Funding
This project was supported by Award Number 2T32 HL007849 from the National Heart, Lung, and Blood Institute (NHLBI) (J. Davis, PI: Irving L. Kron, MD) and Award Number 2R01 HL081629 from the NHLBI (PI Gilbert R. Upchurch, Jr., MD) and AHA Scientist Development Grant 14SDG18730000 (M.S.). The content is solely the responsibility of the authors and does not necessarily represent the views of the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented as a poster at the American Heart Association Scientific Session 2014: Late Breaking Science in Chicago, IL on November 15–19, 2014.
References
- 1.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. Journal of Vascular Surgery. 2010;52(3):539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Archives of Internal Medicine. 2000;160(10):1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 3.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Annals of Internal Medicine. 1997;126(6):441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ramanath VS, Oh JK, Sundt TM, 3rd, Eagle KA. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clinic Proceedings. 2009;84(5):465–481. doi: 10.1016/S0025-6196(11)60566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: final data for 2009. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;60(3):1–116. [PubMed] [Google Scholar]
- 6.Yeap BB, Hyde Z, Norman PE, Chubb SA, Golledge J. Associations of total testosterone, sex hormone-binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. The Journal of Clinical Endocrinology and Metabolism. 2010;95(3):1123–1130. doi: 10.1210/jc.2009-1696. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, et al. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circulation Research. 2012;110(11):e73–e85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. The New England Journal of Medicine. 1989;321(7):419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 9.Denis LJ, Carnelro de Moura JL, Bono A, Sylvester R, Whelan P, Newling D, et al. Goserelin acetate and flutamide versus bilateral orchiectomy: a phase III EORTC trial (30853). EORTC GU Group and EORTC Data Center. Urology. 1993;42(2):119–129. doi: 10.1016/0090-4295(93)90634-m. discussion 29–30. [DOI] [PubMed] [Google Scholar]
- 10.Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, et al. Phase III radiation therapy oncology group (RTOG) trial 86–10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics. 2001;50(5):1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Current Opinion in Pharmacology. 2008;8(4):440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. Journal of Clinical Oncology. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 13.Yap TA, Carden CP, Attard G, de Bono JS. Targeting CYP17: established and novel approaches in prostate cancer. Current Opinion in Pharmacology. 2008;8(4):449–457. doi: 10.1016/j.coph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Lu G, Su G, Zhao Y, Johnston WF, Sherman NE, Rissman EF, et al. Dietary phytoestrogens inhibit experimental aneurysm formation in male mice. The Journal of Surgical Research. 2014;188(1):326–338. doi: 10.1016/j.jss.2013.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, et al. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 2014;130(11 Suppl 1):S51–S59. doi: 10.1161/CIRCULATIONAHA.113.006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston WF, Salmon M, Su G, Lu G, Ailawadi G, Upchurch GR., Jr Aromatase is required for female abdominal aortic aneurysm protection. Journal of Vascular Surgery. 2014 doi: 10.1016/j.jvs.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, et al. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(2):294–304. doi: 10.1161/ATVBAHA.112.300432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmon M, Johnston WF, Woo A, Pope NH, Su G, Upchurch GR, Jr, et al. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation. 2013;128(11 Suppl 1):S163–S174. doi: 10.1161/CIRCULATIONAHA.112.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Death AK, McGrath KC, Sader MA, Nakhla S, Jessup W, Handelsman DJ, et al. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology. 2004;145(4):1889–1897. doi: 10.1210/en.2003-0789. [DOI] [PubMed] [Google Scholar]
- 20.Higashiura K, Mathur RS, Halushka PV. Gender-related differences in androgen regulation of thromboxane A2 receptors in rat aortic smooth-muscle cells. Journal of Cardiovascular Pharmacology. 1997;29(3):311–315. doi: 10.1097/00005344-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocrine Reviews. 2003;24(3):313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 22.McCrohon JA, Death AK, Nakhla S, Jessup W, Handelsman DJ, Stanley KK, et al. Androgen receptor expression is greater in macrophages from male than from female donors. A sex difference with implications for atherogenesis. Circulation. 2000;101(3):224–226. doi: 10.1161/01.cir.101.3.224. [DOI] [PubMed] [Google Scholar]
- 23.Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The proinflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. Journal of Vascular Surgery. 2007;45(3):574–580. doi: 10.1016/j.jvs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents and Actions. 1994;42(3–4):159–162. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 25.Makrygiannis G, Courtois A, Drion P, Defraigne JO, Kuivaniemi H, Sakalihasan N. Sex Differences in Abdominal Aortic Aneurysm: The Role of Sex Hormones. Annals of Vascular Surgery. 2014;28(8):1946–1958. doi: 10.1016/j.avsg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen increases AT1a receptor expression in abdominal aortas to promote angiotensin II-induced AAAs in apolipoprotein E-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(7):1251–1256. doi: 10.1161/ATVBAHA.107.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ailawadi G, Eliason JL, Roelofs KJ, Sinha I, Hannawa KK, Kaldjian EP, et al. Gender differences in experimental aortic aneurysm formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(11):2116–2122. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 28.Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. American Journal of Physiology: Heart and Circulatory Physiology. 2011;300(4):H1210–H1221. doi: 10.1152/ajpheart.01210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campelo AE, Cutini PH, Massheimer VL. Cellular actions of testosterone in vascular cells: mechanism independent of aromatization to estradiol. Steroids. 2012;77(11):1033–1040. doi: 10.1016/j.steroids.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids. 1997;62(1):133–142. doi: 10.1016/s0039-128x(96)00172-9. [DOI] [PubMed] [Google Scholar]
- 31.Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Seminars in Reproductive Medicine. 2004;22(4):281–288. doi: 10.1055/s-2004-861545. [DOI] [PubMed] [Google Scholar]
- 32.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126(11 Suppl 1):S38–S45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. Journal of Immunology. 2004;172(4):2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 34.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nature Medicine. 2015;21(6):628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeschke MG, Williams FN, Finnerty CC, Rodriguez NA, Kulp GA, Ferrando A, et al. The effect of ketoconazole on post-burn inflammation, hypermetabolism and clinical outcomes. PloS one. 2012;7(5):e35465. doi: 10.1371/journal.pone.0035465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Cutsem J, Van Gerven F, Cauwenbergh G, Odds F, Janssen PA. The antiinflammatory effects of ketoconazole. A comparative study with hydrocortisone acetate in a model using living and killed Staphylococcus aureus on the skin of guinea-pigs. Journal of the American Academy of Dermatology. 1991;25(2 Pt 1):257–261. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.