Abstract
Burkitt lymphoma (BL) is the most common paediatric cancer in sub-Saharan Africa (SSA). Anthracyline-based treatment is standard in resource-rich settings, but has not been described in SSA. Children ≤ 18 years of age with newly diagnosed BL were prospectively enrolled from June 2013 to May 2015 in Malawi. Staging and supportive care were standardized, as was treatment with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) for six cycles. Among 73 children with BL, median age was 9.2 years (interquartile range 7.7–11.8), 48 (66%) were male and two were positive for human immunodeficiency virus. Twelve (16%) had stage I/II disease, 36 (49%) stage III and 25 (34%) stage IV. Grade 3/4 neutropenia occurred in 17 (25%), and grade 3/4 anaemia in 29 (42%) of 69 evaluable children. Eighteen-month overall survival was 29% (95% confidence interval [CI] 18–41%) overall. Mortality was associated with age >9 years [hazard ratio [HR] 2.13, 95% CI 1.15–3.94], female gender (HR 2.12, 95% CI 1.12–4.03), stage (HR 1.52 per unit, 95% CI 1.07–2.17), lactate dehydrogenase (HR 1.03 per 100 iu/l, 95% CI 1.01–1.05), albumin (HR 0. 96 per g/l, 95% CI 0.93–0.99) and performance status (HR 0.78 per 10-point increase, 95% CI 0.69–0.89). CHOP did not improve outcomes in paediatric BL compared to less intensive regimens in Malawi.
Keywords: Burkitt lymphoma, Malawi, sub-Saharan Africa, paediatric cancer, Epstein-Barr virus
INTRODUCTION
Burkitt lymphoma (BL) accounts for 50% of paediatric cancer in Malawi, with an annual incidence of 36 cases per 100,000 population, among the highest in sub-Saharan Africa (SSA) [Msyamboza et al (2012); Mwanda (2004); Sinfield et al (2007)]. For BL in resource-rich settings, cure rates of 90% or greater are achievable using intensive chemotherapy regimens incorporating anthracyclines and high-dose methotrexate [Adde et al (1998); Ferry (2006); Magrath (2009); Magrath et al (1996); Patte et al (2001)]. However, in low-resource settings in SSA, there is no accepted standard for BL treatment. Cure rates using low- and medium-intensity chemotherapy in SSA are substantially lower, with event-free survival (EFS) or overall survival (OS) ranging between 25 and 62 % [Depani et al, (2015); Hesseling et al (2009); Hesseling et al (2006); Kazembe et al (2003); Ngoma et al (2012); Nkrumah and Perkins (1976); Olweny et al (1980)]. More intensive protocols incorporating high-dose methotrexate have often resulted in excessive treatment-related mortality given the supportive care limitations in this region [Harif et al (2008); Hesseling et al (2005); Hesseling et al (2003); Hesseling et al (2012); Stefan and Lutchman (2014); Traore et al (2011)]. Toxicity concerns have similarly led to anthracycline-based chemotherapy being infrequently applied in SSA, and the feasibility, safety and efficacy of this approach remain uncertain [Hesseling et al (2009); Hesseling et al (2003)]. However, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) is the regional standard-of-care for adult non-Hodgkin lymphoma in SSA, including demonstrated safety and efficacy for patients with human immunodeficiency virus (HIV) [de Witt et al (2013); Gopal et al (2015)] and anthracycline-based approaches may be similarly applicable in children.
In this paper, we describe the clinical presentation, treatment course and toxicities, and OS for children with BL in Lilongwe treated with CHOP, during a period when this was the institutional standard for BL therapy at our centre. We also examine the risk factors associated with OS in a uniformly treated BL population. To our knowledge, this is the first prospective description of anthracycline-based chemotherapy for paediatric BL from SSA, and provides valuable data to guide treatment strategies for this common childhood cancer in the region.
METHODS
Setting
Kamuzu Central Hospital (KCH) is a national teaching hospital in the Malawian capital, Lilongwe. It receives cancer referrals from the northern and central regions, serving approximately half the total estimated population of nearly 16 million people in Malawi. The paediatric cancer ward consists of 15 beds managed by two full-time clinical officers and two full-time nurses. The treatment protocols described were implemented after consultation and extensive training by paediatric haematologist-oncologists.
Patients and procedures
The KCH Lymphoma Study is an on-going prospective cohort initiated in June 2013. Through active case finding across all hospital departments and referring clinics, adult and paediatric patients with lymphoma were eligible to participate. For these analyses, we focused on children less than 18 years of age with newly diagnosed BL enrolled between 1 June 2013 and 31 May 2015.
All diagnoses were pathologically confirmed using biopsies or fine needle aspirates, supported by immunohistochemistry (IHC) and weekly telepathology consultation involving 2–4 pathologists in Malawi and the United States who rendered a consensus opinion [(Gopal et al (2013); Gopal et al (2012)]. Specific IHC stains to support lymphoma diagnosis included CD3, CD20, CD30, CD45, CD138, Ki67, terminal deoxynucleotidyl transferase (TDT) and latency-associated nuclear antigen (LANA). Other stains, including synaptophysin and AE1/AE3, were used to distinguish lymphomas from neuroendocrine or epithelial tumours, respectively, when morphology was equivocal.
At initial diagnosis, a comprehensive baseline evaluation was performed, including chest radiography, abdominal ultrasound, Lansky performance status [Lansky et al (1985)] and cerebrospinal fluid (CSF) cytology to assign Murphy stage [Murphy (1980)]. Based on experience with frequent relapses after less intensive therapy, institutional practice during the study period involved CHOP for all BL patients of any stage. Chemotherapy was initiated with COP prephase [cyclophosphamide 300–400 mg/m2 on day 1, vincristine 1 mg/m2 (maximum 2 mg) on day 1, and prednisone 1.5 mg/kg/day on days 1–5]. CHOP was started seven days later for a total of six cycles administered every 21 days as tolerated.
Chemotherapy toxicities were graded using National Cancer Institute criteria [NCI (2009)].[http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.]. Transportation reimbursement was provided to all paediatric oncology patients to promote retention throughout care. Medicines were freely provided through external funding specifically designed to mitigate treatment interruptions.
Analysis
Baseline characteristics are summarized using numbers with percentages or medians with interquartile ranges (IQRs). OS was estimated using Kaplan-Meier methods, and survival differences between groups were compared using the non-parametric log-rank test. We focused our analyses on OS as the most reliable clinical endpoint in our setting, particularly given that BL is a highly curable cancer for which relapse is uncommon after 12 months. We additionally chose OS as a more valid outcome given non-standardized criteria for response assessment throughout SSA, and a patient population with frequent abdominal disease that was evaluated serially using relatively crude methods incorporating physical examination and ultrasound. However, to provide more detailed data regarding cause of death, all deaths in our study were separately adjudicated through consensus-centralized review involving multiple study investigators. Risk factors for mortality were assessed using unadjusted Cox proportional hazards models. Follow-up time was calculated from enrolment until death, loss to follow-up or censoring, which occurred on31 August 2015. All analyses were performed using STATA SE version 12.1 (Stata Corp., College Station, TX). Statistical significance was considered at a two-sided α-level of 0.05.
Ethical approval
The study was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill (study 12–2255)on 15 August 2013, the Protocol Review Committee of the Lineberger Comprehensive Cancer Centre and the Malawi National Health Sciences Research and Ethics Committee (doccentre@Malawi.net) (NHSRC 1107) on 10 August 2013.
RESULTS
Baseline characteristics
Between 1 June 2013 and 31 May 2015, 73 of 89 eligible children (82%) with newly diagnosed BL were enrolled in the study and planned for CHOP treatment at KCH. Six families refused to participate, six families relocated after diagnosis and could not be reengaged in care, and four children died before they could be enrolled. Baseline characteristics for the final analytic population are shown in Table I. Median age was 9.2 years (IQR 7.7–11.8), 48 (66%) were male and two were HIV-infected. Twelve (16%) presented with stage I/II disease, 36 (49%) stage III, and 25 (34%) stage IV. Thirty-four (47%) children had abdominal disease and 60 (82%) Lansky performance score ≤ 70. Twenty-five children (34%) were underweight, defined as weight-for-age z-score <−2 if less than five years of age or body mass index (BMI) z-score <−2 if older than five years of age, as per World Health Organization (WHO) reference tools [ http://www.who.int/childgrowth/standards/technical_report/en/]. Fifty-five children (75%) were diagnosed by cytology and 18 (25%) by histology or cell-block supported by IHC. Baseline leptomeningeal involvement was present in ten of 57 children (18%) who underwent initial staging lumbar puncture, which was omitted for some children during a five-month period when intrathecal methotrexate could not be purchased for clinical use anywhere in Malawi.
Table I.
Baseline characteristics of CHOP-treated children with Burkitt lymphoma in Lilongwe, Malawi
| Total, n | 73 |
|---|---|
| Male | 48 (65.8) |
| HIV negative | 71 (97.3) |
| Clinical stage | |
| Stage I/II | 12 (16.4) |
| Stage III | 36 (49.3) |
| Stage IV | 25 (34.3) |
| Age, years, median (IQR) | 9.2 (7.1–11.7) |
| Primary site | |
| Abdominal only | 34 (46.6) |
| Facial only | 20 (27.4) |
| Cervical only | 2 (2.7) |
| Multiple sites | 15 (21.1) |
| Other | 2 (2.7) |
| Diagnostic specimen | |
| Cytology | 55 (75.3) |
| Histology | 18 (24.7) |
| CSF involvement | 10/57 (17.5) |
| B symptoms | 67 (91.8) |
| Weight or BMI-for-age z-score <−2‡ | 25 (34.3) |
| Lansky performance status ≤ 70 | 60 (82.2) |
| White blood cell count, 109/l, median (IQR) | 8.6 (6.8–12.1) |
| Absolute neutrophil count, 109/l, median (IQR) | 4.0 (2.8–6.7) |
| Haemoglobin, g/l, median (IQR) | 102 (87–115) |
| Platelet count, 109/l, median (IQR) | 421 (277–561) |
| Lactate dehydrogenase, iu/l, median (IQR)╪ | 628 (366–1349) |
| Lactate dehydrogenase >2x ULN | 24/66 (36.4) |
| Albumin, g/l, median (IQR) | 34 (31–39) |
Values given as number (%) unless indicated otherwise. CSF, cerebrospinal fluid; ULN, upper limit of normal; IQR, interquartile range.
CSF staging was not done during a five-month period when intrathecal methotrexate could not be purchased anywhere in Malawi.
Weight was used if aged <5 years and BMI if aged 5 years according to World Health Organization (WHO 2007).
Laboratory upper limit of normal for children is 430 iu/l.
Treatment course and toxicities
As of 31 August, 2015, 40 children (55%) had completed six CHOP cycles, of whom 35 (88%) and 5 (12%) achieved complete and partial response respectively. Thirty-three children failed to complete the planned number of CHOP cycles because of death (n=29), treatment abandonment (n=3) or disease progression (n=1). Median number of cycles per patient was 5 (IQR 3–6) and median number of days between cycles was 21 (IQR 20–22). The median cyclophosphamide dose per cycle was 720 mg/m2 (IQR 620–820 mg/m2) and median doxorubicin dose per cycle was 38 mg/m2 (IQR 34–44 mg/m2). CHOP was delayed for more than seven days in 32 (7%) of 432 total cycles, due to delayed absolute neutrophil recovery (ANC) recovery (n=18), social issues (n=12) and pharmacy stock-out (n=2).
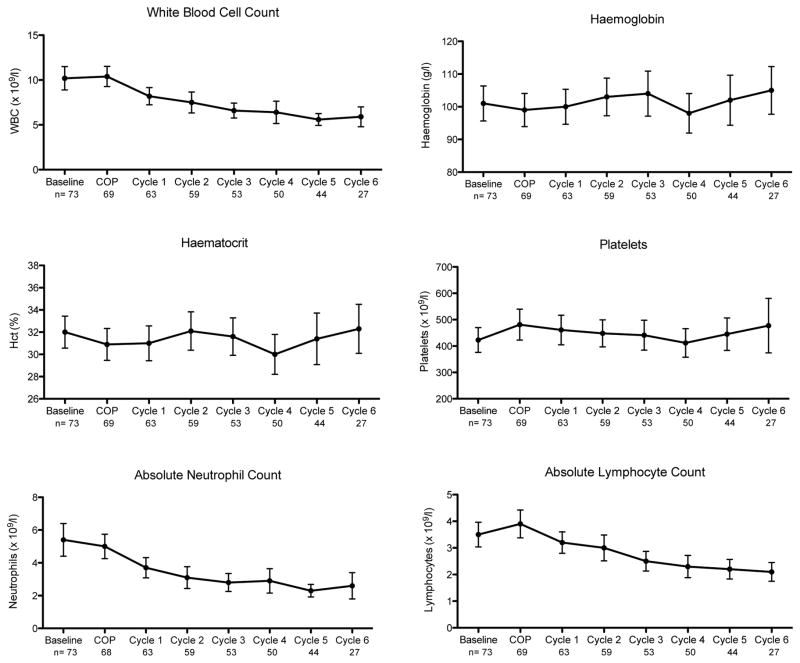
Figure 1 depicts peripheral blood counts over the course of therapy. As expected, the white blood cell count (WBC), ANC and absolute lymphocyte count (ALC) declined over time, but both median ANC and ALC remained ≥ 2 × 109/l throughout treatment. Any grade 3/4 neutropenia occurred in 17 of 69 evaluable patients (25%), including 11 with any grade 3 neutropenia (ANC 0.5–1 × 109/l) and six with any grade 4 neutropenia (ANC <0.5 × 109/l). Any grade 3/4 anaemia occurred in 29 of 69 evaluable patients (42%), including 24 with any grade 3 anaemia [Haemoglobin (Hb) 65–79 g/l] and five with any grade 4 anaemia (Hb <65 g/l). There were no grade 3/4 thrombocytopenia events during treatment.
Figure 1. Peripheral blood counts with interquartile ranges among CHOP-treated children with Burkitt lymphoma in Lilongwe, Malawi.
Chemotherapy was initiated with COP pre-phase [cyclophosphamide 300–400 mg/m2 on day 1, vincristine 1 mg/m2 (maximum 2 mg) on day 1, and prednisone 1.5 mg/kg/day on days 1–5]. CHOP was started seven days later for a total of six cycles administered every 21 days as tolerated. WBC, white blood cells
Survival
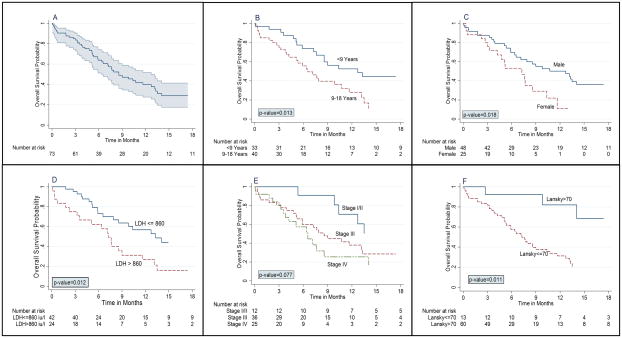
As of 31 August 2015, vital status was known for 70 children (96%) with a median follow-up of 12.3 months (IQR 4.5–19.6) for 28 children not known to have died. This includes a median follow-up of 10.6 months (IQR 4.6–18.5) for 25 children known to be alive and 19.7 months (range 18.8–24.4) for three children who were alive at their last visit but lost to follow-up prior to 31 August 2015. Of three children for whom vital status was not known, two had returned to Mozambique after coming to Malawi for care and could not subsequently be located. OS estimates with 95% confidence intervals (CIs) are shown in Table II, and Kaplan-Meier curves are shown in Figure 2. Estimated 18-month OS was 29% (95% CI 18–41%) for the cohort overall, including 51% (95% CI 19–76%) for stage I/II, 28% (95% CI 13–46%) for stage III, and 17% (95% CI 3–39%) for stage IV. As shown in Figure 2, 18-month OS was worse for children aged more than 9 years, females, children with lactate dehydrogenase (LDH) more than twice the upper limit of normal, children with Lansky performance status <70, and children with weight or BMI for age z-score <−2.
Table II.
Kaplan-Meier overall survival estimates stratified by clinical stage among CHOP-treated children with Burkitt lymphoma in Lilongwe, Malawi
| n | 12-month OS % | 95% CI | 18-month OS % | 95% CI | |
|---|---|---|---|---|---|
| Overall | 73 | 40 | 28 – 52 | 29 | 18 – 41 |
| Stage I/II | 12 | 71 | 34 – 90 | 51 | 19 – 76 |
| Stage III | 36 | 38 | 22 – 54 | 28 | 13 – 46 |
| Stage IV | 25 | 25 | 8 – 47 | 17 | 3 – 39 |
OS, overall survival; CI, confidence interval.
Figure 2. Kaplan-Meier overall survival estimates among CHOP-treated children with Burkitt lymphoma in Lilongwe, Malawi.
(A) Overall cohort with 95% confidence intervals. (B) Stratified by age. (C) Stratified by gender. (D) Stratified by lactate dehydrogenase. (E) Stratified by clinical stage. (F) Stratified by Lansky performance status.
Risk factors for mortality are shown in Table II. Mortality was associated with older age (9–18 years) [hazard ratio (HR) 2.13, 95% CI 1.15–3.94, p=0.016], female gender (HR 2.12, 95% CI 1.12–4.03, p=0.021), clinical stage per Murphy system unit increase (HR 1.52, 95% CI 1.07–2.17, p=0.019), LDH (HR 1.03 per 100 iu/l, 95% CI 1.01–1.05, p=0.003), albumin (HR 0.96 per g/l, 95% CI 0.93–0.99, p=0.033) and weight or BMI for age z-score <−2 (HR 1.90, 95% CI 1.05–3.45, p=0.035). Better Lansky performance status was associated with decreased mortality (HR 0.78 per 10-point increase, 95% CI 0.69–0.89, p<0.001).
Assigning cause of death in Malawi is difficult, as there is no national system for death registration. Although we urged patients to return promptly for interim illnesses to be thoroughly evaluated, deaths often occurred at home or in health facilities where diagnostic resources were limited. However, central adjudication of deaths that occurred during the study period was undertaken to attribute cause of death (Supplemental Table 1). Of 45 deaths, 33 (73%) were attributed to lymphoma-related causes and 12 (27%) to CHOP-related complications. Of note, many CHOP-related deaths occurred among children with severe malnutrition. Estimated treatment-related mortality was 16% (12 of 73 children).
Epstein-Barr virus (EBV) association
In situ hybridization for EBV-encoded RNA (EBER) was available for nine patients for whom tumour blocks were evaluated in the United States, where BL was confirmed using broader immunohistochemistry panels and fluorescence in situ hybridization; six cases were EBER-positive and three EBER-negative. All EBER-negative cases occurred in the older group (aged 9–18 years).
DISCUSSION
We describe the clinical presentation, treatment course and toxicities, and OS for children with BL treated with CHOP in Malawi. To our knowledge, this is the first prospective report of anthracycline-based therapy for this disease from SSA.
The 18-month OS for our cohort with principally advanced disease was 29% overall. While comparison across studies from the region is extremely difficult due to significant heterogeneity in regimens used, patient characteristics, patient selection, staging methods, outcomes analysed, outcome definitions and completeness of follow-up, survival in our cohort does not seem clearly better than published experience using less intensive regimens in SSA. This suggests that uniformly applied intensification with CHOP as per KCH institutional practice did not improve outcomes. Prior studies in Malawi using systemic cyclophosphamide, with or without systemic vincristine and with intrathecal methotrexate/hydrocortisone produced an EFS of 45–50% [Depani et al (2015); Hesseling et al (2009)]. Another regimen developed by the International Network for Cancer Treatment and Research (INCTR) using systemic cyclophosphamide, vincristine and low-dose methotrexate, with intrathecal methotrexate and cytarabine resulted in an OS of 62% [Ngoma et al (2012)]. Based on our results, institutional guidelines at KCH have now been revised, such that stage I/II disease is treated with the less intensive, anthracycline-free COMP regimen (cyclophosphamide, vincristine, low dose methotrexate and prednisone with intrathecal methotrexate and hydrocortisone), as adapted from INCTR studies [Ngoma et al (2012)]. Our site is also now participating in the multicentre Burkitt Lymphoma Trial Network sponsored by the United States National Cancer Institute, which plans to test specific protocols for BL at African and Latin American centres. Experience across SSA overall suggests there may be a ceiling beyond which further intensification of cytotoxic therapy is not feasible, and incorporation of novel non-cytotoxic agents into frontline treatment for BL is needed.
Despite suboptimal outcomes, CHOP in our experience was feasible and well tolerated in many children, with grade 3/4 anaemia in 42% of children, and grade 3/4 neutropenia in 25%. Many deaths appeared to be clearly disease-related on centralized review. CHOP may therefore be another strategy deserving further investigation, particularly for stage III/IV disease in SSA, as these children continue to have poor outcomes using any treatment approach reported to date. Safety and feasibility for CHOP could also be enhanced with relatively modest supportive care refinements, including resource-appropriate incorporation of haematopoietic growth factors, and may be particularly worth studying in environments where paediatric haematologist/oncologist supervision is possible throughout therapy. Intensive nutritional support and/or dose reduction or omission of anthracyclines may also be important among children with poor nutritional status, given that many treatment-related deaths in our cohort occurred among malnourished patients.
Treating BL in SSA is also made difficult by imprecise baseline risk stratification, which relies on physical examination and limited radiographic studies. As a result, any chemotherapy regimen, regardless of intensity, is likely to over treat/undertreat a substantial proportion of children, thereby compromising rates of cure. In a uniformly treated population, we found elevated LDH, older age, female gender, advanced stage and impaired performance status to be associated with mortality. LDH is well known to be a strong prognostic factor for nearly all lymphoma subtypes including BL [Divine et al (2005)]. However, LDH is often not available and typically not reported from BL studies in SSA. In our cohort, LDH demonstrated a continuous association with survival that was stronger than for clinical stage or abdominal disease, suggesting this simple, point-of-care test can be extremely valuable in settings where advanced imaging or bone marrow assessment may not be available. It is imperative that LDH be made more widely available in BL programmes in SSA, and these efforts should not be delayed nor obviated by equally important investigations into novel biomarkers for risk stratification and response assessment.
Worse survival for older children in our study is consistent with other studies from both resource-rich and resource-limited regions, [Burkhardt et al (2005); Divine et al (2005); Patte et al (2001)] and may reflect differences in tumour biology or treatment adherence in the older age group. Intriguingly, in a very small number of cases tested for EBV by EBER in situ hybridization, one third were EBER negative, all of which were among older children. A transition over time from EBV-positive endemic BL to EBV-negative sporadic BL has been described among children in South Africa [Stefan et al (2014)]. Although this requires additional validation in larger numbers of patients, sporadic EBV-negative BL, which is known to principally occur among older children and adolescents, may also occur in Malawi with some frequency. Inferior outcomes for girls with paediatric non-Hodgkin lymphoma have also been observed in other studies, although these results require validation and underlying reasons for this observation remain uncertain [Burkhardt et al (2011); Burkhardt et al (2005)].
The strengths of our study include a detailed, prospective cohort with longitudinal follow-up including active tracing. Tracing of patients who missed appointments almost eliminated loss to follow-up, and allowed us to identify many deaths occurring at home. Short median follow-up times and incomplete follow-up undermine many BL studies from the region, and these shortcomings often lead to significantly overestimated survival in SSA [Brinkhof et al (2010); Egger et al (2011)]. Secondly, enrolment involved active case finding from all hospital departments and referral clinics. Thirdly, all diagnoses were confirmed through weekly telepathology consensus review involving pathologists in Malawi and the United States. Finally, all patients received standardized and uniform treatment according to institutional guidelines during the study period. Limitations include referral bias at a national teaching hospital, and assigning cause of death based on inference through centralized review of all available data, as deaths are not certified in Malawi and autopsies are not commonly done.
In addition to more effective chemotherapy and better baseline risk stratification, several additional elements can further improve outcomes. Children with BL in Lilongwe presented overwhelmingly with advanced disease and impaired performance status. It is critical to implement community education programmes to promote earlier referral and diagnosis. Secondly, many families left the hospital and/or refused treatment for a period of time, eliminating or compromising opportunities for cure. Detailed qualitative studies to assess reasons for treatment abandonment in SSA must be conducted, to inform optimal strategies for retaining families in care throughout curative treatment. Finally, improved supportive care, including better availability of blood products, haematopoietic growth factors, antimicrobial agents and nutritional support, will also lead to better outcomes.
In conclusion, CHOP treatment among children with advanced BL in Malawi did not improve survival compared to less intensive protocols from SSA. We do not advocate this approach for all children, although it may be worth investigating further for those with advanced disease. Many baseline characteristics in addition to clinical stage were associated with survival, highlighting a need to develop better risk stratification tools that are specific to SSA to guide BL treatment, using locally available data, including LDH. Parallel efforts to promote earlier diagnosis, improve supportive care and retain families throughout the care continuum are also essential. Through these efforts, the care continuum will include ever more children experiencing long-term cure and survivorship, even in SSA.
Supplementary Material
Table III.
Unadjusted hazard ratios for mortality among CHOP-treated children with Burkitt lymphoma in Lilongwe, Malawi
| Variable | Hazard ratio | 95% CI | p |
|---|---|---|---|
| Female gender | 2.12 | 1.12 – 4.03 | 0.021 |
| Age ≥ 9 years | 2.13 | 1.15 – 3.94 | 0.016 |
| Clinical stage, per Murphy system unit increase | 1.52 | 1.07 – 2.17 | 0.019 |
| Lansky performance status, per 10-point increase | 0.78 | 0.69 – 0.89 | <0.001 |
| Haemoglobin, per g/l | 0.99 | 0.98 – 1.01 | 0.352 |
| Lactate dehydrogenase, per 100 iu/l | 1.03 | 1.01 – 1.05 | 0.003 |
| Albumin, per g/l | 0.96 | 0.93 – 0.99 | 0.033 |
| Weight or BMI for age z-score <−2 | 1.90 | 1.05 – 3.45 | 0.035 |
| Abdominal involvement | 1.63 | 0.91 – 2.93 | 0.101 |
BMI, body mass index; CI, confidence interval.
Acknowledgments
The authors would like to thank children and their families for agreeing to participate in the study; Wiza Kumwenda for developing the study database; Kamuzu Central Hospital, Malawi Ministry of Health, UNC Project-Malawi, Lineberger Comprehensive Cancer Centre for providing leadership and Baylor College of Medicine Children’s Foundation Malawi for support of this study. This work is supported by grants from the National Institutes of Health (K01TW009488, R21CA180815 and U54CA190152) (S.G.), the Medical Education Partnership Initiative (U2GPS001965), the Lineberger Comprehensive Cancer Centre (P30CA016086) and AIDS Malignancy Consortium (U01CA121947).
Footnotes
Author contributions: S.G., Y.F. and G.N.L. designed the study; C.C.S. collected and analysed data, and wrote the first draft of the manuscript; S.G., recruited patients, provided clinical care, collected data; P.W., M.B, I.M., M.M., M.C., B.K., E.K. V.M., R.N., M.C., V.M. and S.I. assisted with patient recruitment, clinical care and data collection; Y.F., N.D.M., B.M.D., F.C., C.K., R.K. and G.N.L. processed and interpreted all pathological specimens; N.E.R. assisted in data analysis. All authors reviewed and commented on the manuscript and approved the final submitted version.
Conflict of interest: The authors declare no competing financial interests.
References
- Adde M, Shad A, Venzon D, Arndt C, Gootenberg J, Neely J, Nieder M, Owen W, Seibel N, Wilson W, Horak ID, Magrath I. Additional chemotherapy agents improve treatment outcome for children and adults with advanced B-cell lymphomas. Semin Oncol. 1998;25:33–39. discussion 45–38. [PubMed] [Google Scholar]
- Brinkhof MW, Spycher BD, Yiannoutsos C, Weigel R, Wood R, Messou E, Boulle A, Egger M, Sterne JA. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS One. 2010;5:e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt B, Zimmermann M, Oschlies I, Niggli F, Mann G, Parwaresch R, Riehm H, Schrappe M, Reiter A. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39–49. doi: 10.1111/j.1365-2141.2005.05735.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt B, Oschlies I, Klapper W, Zimmermann M, Woessmann W, Meinhardt A, Landmann E, Attarbaschi A, Niggli F, Schrappe M, Reiter A. Non-Hodgkin’s lymphoma in adolescents: experiences in 378 adolescent NHL patients treated according to pediatric NHL-BFM protocols. Leukemia. 2011;25:153–160. doi: 10.1038/leu.2010.245. [DOI] [PubMed] [Google Scholar]
- de Witt P, Maartens DJ, Uldrick TS, Sissolak G. Treatment outcomes in AIDS-related diffuse large B-cell lymphoma in the setting roll out of combination antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2013;64:66–73. doi: 10.1097/QAI.0b013e3182a03e9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depani S, Banda K, Bailey S, Israels T, Chagaluka G, Molyneux E. Outcome is unchanged by adding vincristine upfront to the Malawi 28-day protocol for endemic Burkitt lymphoma. Pediatr Blood Cancer. 2015;62:1929–1934. doi: 10.1002/pbc.25612. [DOI] [PubMed] [Google Scholar]
- Divine M, Casassus P, Koscielny S, Bosq J, Sebban C, Le Maignan C, Stamattoulas A, Dupriez B, Raphael M, Pico JL, Ribrag V. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16:1928–1935. doi: 10.1093/annonc/mdi403. [DOI] [PubMed] [Google Scholar]
- Egger M, Spycher BD, Sidle J, Weigel R, Geng EH, Fox MP, MacPhail P, van Cutsem G, Messou E, Wood R, Nash D, Pascoe M, Dickinson D, Etard JF, McIntyre JA, Brinkhof MW. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8:e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry JA. Burkitt’s lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006;11:375–383. doi: 10.1634/theoncologist.11-4-375. [DOI] [PubMed] [Google Scholar]
- Gopal S, Wood WA, Lee SJ, Shea TC, Naresh KN, Kazembe PN, Casper C, Hesseling PB, Mitsuyasu RT. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119:5078–5087. doi: 10.1182/blood-2012-02-387092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Krysiak R, Liomba G. Building a pathology laboratory in Malawi. Lancet Oncol. 2013;14:291–292. doi: 10.1016/S1470-2045(13)70109-8. [DOI] [PubMed] [Google Scholar]
- Gopal S, Fedoriw Y, Moses A, Montgomery N, Kaimila W, Kampani C, Krysiak R, Richards K, Shea T, Liomba G. CHOP Is Feasible for HIV-Associated Lymphoma in the ART Era in Malawi. Conference on Retroviruses and Opportunistic Infections; 2015; 2015. p. Abstract 719. http://www.croiconference.org/sites/default/files/posters-2015/719.pdf. [Google Scholar]
- Harif M, Barsaoui S, Benchekroun S, Bouhas R, Doumbe P, Khattab M, Ladjaj Y, Moreira C, Msefer-Alaoui F, Patte C, Rakotonirina G, Raphael M, Raquin MA, Lemerle J. Treatment of B-cell lymphoma with LMB modified protocols in Africa--report of the French-African Pediatric Oncology Group (GFAOP) Pediatr Blood Cancer. 2008;50:1138–1142. doi: 10.1002/pbc.21452. [DOI] [PubMed] [Google Scholar]
- Hesseling PB, Broadhead R, Mansvelt E, Louw M, Wessels G, Borgstein E, Schneider J, Molyneux E. The 2000 Burkitt lymphoma trial in Malawi. Pediatr Blood Cancer. 2005;44:245–250. doi: 10.1002/pbc.20254. [DOI] [PubMed] [Google Scholar]
- Hesseling PB, Broadhead R, Molyneux E, Borgstein E, Schneider JW, Louw M, Mansvelt EP, Wessels G. Malawi pilot study of Burkitt lymphoma treatment. Med Pediatr Oncol. 2003;41:532–540. doi: 10.1002/mpo.10322. [DOI] [PubMed] [Google Scholar]
- Hesseling PB, Molyneux E, Tchintseme F, McCormick P, Achu P, Kamiza S, Wessels G, Hesseling A, Broadhead R. Oral cyclophosphamide and intrathecal methotrexate in 140 children with Burkitt lymphoma. Pediatric Blood and Cancer. 2006;47:381. [Google Scholar]
- Hesseling PB, Molyneux E, Kamiza S, Israels T, Broadhead R. Endemic Burkitt lymphoma: a 28-day treatment schedule with cyclophosphamide and intrathecal methotrexate. Ann Trop Paediatr. 2009;29:29–34. doi: 10.1179/146532809X402006. [DOI] [PubMed] [Google Scholar]
- Hesseling PB, Njume E, Kouya F, Katayi T, Wharin P, Tamannai M, Achu P, Kidd M, McCormick P. The Cameroon 2008 Burkitt lymphoma protocol: improved event-free survival with treatment adapted to disease stage and the response to induction therapy. Pediatr Hematol Oncol. 2012;29:119–129. doi: 10.3109/08880018.2011.644881. [DOI] [PubMed] [Google Scholar]
- Kazembe P, Hesseling PB, Griffin BE, Lampert I, Wessels G. Long term survival of children with Burkitt lymphoma in Malawi after cyclophosphamide monotherapy. Med Pediatr Oncol. 2003;40:23–25. doi: 10.1002/mpo.10190. [DOI] [PubMed] [Google Scholar]
- Lansky LL, List MA, Lansky SB, Cohen ME, Sinks LF. Toward the development of a play performance scale for children (PPSC) Cancer. 1985;56:1837–1840. doi: 10.1002/1097-0142(19851001)56:7+<1837::aid-cncr2820561324>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Magrath I. Lessons from clinical trials in African Burkitt lymphoma. Curr Opin Oncol. 2009;21:462–468. doi: 10.1097/CCO.0b013e32832f3dcd. [DOI] [PubMed] [Google Scholar]
- Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, Neely J, Arndt C, Nieder M, Jaffe E, Wittes RA, Horak ID. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- Msyamboza KP, Dzamalala C, Mdokwe C, Kamiza S, Lemerani M, Dzowela T, Kathyola D. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- Mwanda OW, Rochford R, Rainey J, Wilson ML. Challenges in the epidemiological and clinical aspects of Burkitt’s lymphoma in Kenya: linking evidence and experience. East Afr Med J. 2004:S111–116. doi: 10.4314/eamj.v81i8.9215. [DOI] [PubMed] [Google Scholar]
- Ngoma T, Adde M, Durosinmi M, Githang’a J, Aken’Ova Y, Kaijage J, Adeodou O, Rajab J, Brown BJ, Leoncini L, Naresh K, Raphael M, Hurwitz N, Scanlan P, Rohatiner A, Venzon D, Magrath I. Treatment of Burkitt lymphoma in equatorial Africa using a simple three-drug combination followed by a salvage regimen for patients with persistent or recurrent disease. Br J Haematol. 2012;158:749–762. doi: 10.1111/j.1365-2141.2012.09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkrumah FK, Perkins IV. Burkitt’s lymphoma: a clinical study of 110 patients. Cancer. 1976;37:671–676. doi: 10.1002/1097-0142(197602)37:2<671::aid-cncr2820370210>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Olweny CL, Katongole-Mbidde E, Otim D, Lwanga SK, Magrath IT, Ziegler JL. Long-term experience with Burkitt’s lymphoma in Uganda. Int J Cancer. 1980;26:261–266. doi: 10.1002/ijc.2910260302. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D, Lutz P, Coze C, Perel Y, Raphael M, Terrier-Lacombe MJ. The Societe Francaise d’Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- Sinfield RL, Molyneux EM, Banda K, Borgstein E, Broadhead R, Hesseling P, Newton R, Casabonne D, Mkandawire N, Nkume H, Hodgson T, Liomba G. Spectrum and presentation of pediatric malignancies in the HIV era: experience from Blantyre, Malawi, 1998–2003. Pediatr Blood Cancer. 2007;48:515–520. doi: 10.1002/pbc.20917. [DOI] [PubMed] [Google Scholar]
- Stefan DC, Lutchman R. Burkitt lymphoma: epidemiological features and survival in a South African centre. Infect Agent Cancer. 2014;9:19. doi: 10.1186/1750-9378-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore F, Coze C, Atteby JJ, Andre N, Moreira C, Doumbe P, Ravelomanana N, Ye D, Patte C, Raquin MA, Raphael M, Lemerle J. Cyclophosphamide monotherapy in children with Burkitt lymphoma: a study from the French-African Pediatric Oncology Group (GFAOP) Pediatr Blood Cancer. 2011;56:70–76. doi: 10.1002/pbc.22746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.