Abstract
Background
The purpose of this study was to examine outcomes of a patient cohort undergoing intervention for carotid blowout syndrome (CBS) associated with head and neck cancer.
Methods
Patients with head and neck cancer who presented with carotid-distribution bleeding from 2000-2014 were identified in the medical record. Primary outcomes were short and mid-term mortality and recurrent bleeding. Standard statistical methods and survival analysis were used to analyze study population characteristics and outcomes.
Results
Thirty-seven patients were included in the study. The mean age was 60.1±11.4 (74% male). All malignancies were squamous cell type, stage IV, in a variety of primary locations: 32% oral cavity, 24% larynx, 16% superficial neck, with the remainder in the oro, naso, and hypopharynx. 51% of bleeds were of common carotid, 29% external carotid, and 19% internal carotid origin. 68% presented with acute hemorrhage, 24% with impending bleed, and 8% with threatened bleed. All patients underwent intervention: 38% received endovascular coil embolization, 30% stent grafts, 22% surgical ligation, and 10% primary vessel repair or bypass grafting. 10.8% of patients had perioperative stroke; other major complications were rare. Sixteen recurrent bleeding episodes involving 12 arteries occurred in 11 patients (29.73%). Median rebleeding time was seven days (IQR 6-49). Estimated recurrent bleeding risk at 30-days and 6 months was 24% and 34% respectively. 91.9% of patients survived to hospital discharge. 90-day and 1-year estimated survival was 60.9% and 36.6% respectively.
Conclusions
CBS associated with head and neck cancer carries poor mid- and long-term prognoses; however, mortality may be related more to the advanced stage of disease rather than carotid involvement or associated intervention. Both surgical and endovascular approaches may be efficacious in cases of acute hemorrhage but carry a significant risk of periprocedural stroke and recurrent bleeding.
INTRODUCTION
Carotid blowout syndrome (CBS) is a rare complication associated with head and neck cancer and subsequent surgical and radiation therapy. Carotid blowout traditionally carries average morbidity and mortality rates of up to 40% and 60%, respectively1 although these may have improved with recent advances in diagnosis and endovascular treatment2.
Surgical ligation has previously been the only therapy for CBS; in recent years, however, endovascular approaches involving either parent vessel occlusion or placement of a covered stent have replaced surgery as the treatment of choice2,3. Covered stent usage has been frequently utilized in those patients who have elective failed balloon test occlusion or are at risk for major neurological complications secondary to compromised cerebral perfusion and have been successfully used for the treatment of acute hemorrhage as well4,5. Despite the immediate hemostasis that covered stents provide, patients remain at risk for immediate complications such as stroke as well as long-term morbidities including recurrent bleeding, thrombosis, and infection6,7. The purpose of the current study was to examine short and midterm mortality and recurrent bleeding outcomes for patients with head and neck cancer complicated by CBS in a large tertiary center.
METHODS
Study Design
This retrospective review of medical record data was approved by the institutional review board and exempted from informed consent. The institutional electronic medical record was searched and relevant baseline, operative, and outcome information was extracted for all patients with head and neck cancer who presented with any carotid distribution bleeding from 2000-2014. Patients who did not have a head and neck cancer history or had bleeding from a non-carotid distribution were excluded. Bleeding episodes were classified as “impending” for an initial self-limited or sentinel bleed, “acute hemorrhage” for bleeding requiring urgent surgical or endovascular intervention, or “threatened” if a vessel was exposed but no bleeding had occurred according to previously published standards1. Initial operative or adjunctive interventions for the primary cancer were recorded, including the use of adjuvant or neoadjuvant chemotherapy or radiation, as well as any wound complications and concomitant reconstruction using local or free tissue transfer (“flap coverage”).
Treatment
A multidisciplinary team consisting of an otolaryngologist and a neurointerventionalist or vascular surgeon determined the method of treatment for each patient. Endovascular therapy consisted of covered stent graft placement for bleeding involving the common carotid or proximal internal carotid, or coil embolization for bleeding involving any carotid distribution branches. Dual antiplatelet therapy using aspirin and a P2Y12 inhibitor was initiated after placement of endovascular stent-grafts. Surgical therapy consisted of open exploration and primary resection/reanastamosis of the artery, ligation of the proximal and distal bleeding arterial stump, or exclusion and bypass grafting. Balloon occlusion testing was not routinely performed as our institutional protocol prioritizes covered stent-grafting over embolization or ligation for elective or semi-elective treatment of CBS.
Outcomes
The primary outcome was in-hospital mortality after the initial bleed intervention. Secondary outcomes included recurrent bleeding, perioperative stroke rate, and graft infection. Technical success of a procedure was defined as cessation of bleeding immediately after the intervention. Patients having more than one documented recurrent bleed were individually qualitatively analyzed. Death was determined by documentation in the medical record and querying the Social Security Death Index through 2014. Patient characteristics and procedural differences were analyzed in those who experienced recurrent bleeding compared to those who did not. Patient characteristics and outcomes were also compared between treatment modalities. Those who presented with acute hemorrhage were also additionally examined as a separate subgroup.
Statistical Analysis
Fisher exact, two-tailed t-test, ANOVA, and Kruskal-Wallis tests were used for between-group comparisons where appropriate. Kaplan-Meier survival methods were used to analyze time-to-event data for death and rebleeding. Multivariate analysis of survival was performed using Cox regression using a mix of clinically significant variables and variables identified using backward selection. Statistical analyses were performed using the Stata SE 13.1 statistical software package (College Station, TX, USA).
RESULTS
Patients and Baseline Characteristics
37 patients met inclusion criteria and were included in the study. The majority of patients were male (26, 74%) with mean age of 60.1±11.4yr . All patients had been previously diagnosed with advanced stage squamous cell carcinoma of the head and neck; the most common locations were in the oral cavity and the larynx. Only one patient did not undergo any radiation therapy (3%), seven patients received primary chemoradiation only without resection (18.9%) and the remainder received adjuvant or neoadjuvant radiation therapy. Of those undergoing an operative resection, 16 had concurrent neck dissection and seven had concomitant flap reconstructions at the time of initial resection. Five (14%) patients had wounds that required packing and four (14%) patients had wounds complicated by persistent salivary exposure (Table 1).
Table I.
Cohort Characteristics
| Total Cohort (N=37) |
No Rebleeding (N=26) |
Rebleeding (N=11) |
P | |
|---|---|---|---|---|
| Age (yr) | 60.11 ± 11.38 | 61.88 ± 8.67 | 55.70 ± 16.08 |
0.15 |
| Male Sex | 26 (74%) | 20 (80%) | 6 (60%) | 0.39 |
| Coronary Artery Disease | 12 (34%) | 9 (36%) | 3 (30%) | 1.00 |
| Diabetes Mellitus | 2 (6%) | 2 (8%) | 0 (0%) | 1.00 |
| Peripheral Vascular Disease | 2 (6%) | 2 (8%) | 0 (0%) | 1.00 |
| Active Smoking | 9 (26%) | 7 (28%) | 2 (20%) | 1.00 |
| Cancer Characteristics | ||||
| Location | 0.54 | |||
| Oral Cavity | 12 (32%) | 9 (35%) | 3 (27%) | |
| Nasopharynx | 2 (5%) | 2 (8%) | 0 (0%) | |
| Oropharynx | 1 (3%) | 0 (0%) | 1 (9%) | |
| Hypopharynx | 4 (11%) | 3 (12%) | 1 (9%) | |
| Larynx | 9 (24%) | 6 (23%) | 3 (27%) | |
| Neck Soft Tissue | 6 (16%) | 5 (19%) | 1 (9%) | |
| Other | 3 (8%) | 1 (4%) | 2 (18%) | |
| Initial Cancer Treatment | ||||
| Therapy Type | 0.33 | |||
| Operative Resection | 30 (81.1%) | 22 (84.6%) | 8 (72.7%) | |
| Primary Chemoradiation | 7 (18.9%) | 4 (15.4%) | 3 (27.3%) | |
| Neck Dissection | 16 (43%) | 11 (42%) | 5 (45%) | 1.00 |
| Flap Coverage | 7 (19%) | 7 (27%) | 0 (0%) | 0.04 4 |
| Wound Saliva Exposure | 4 (11%) | 2 (8%) | 2 (18%) | 0.57 |
| Wound Packing Required | 5 (14%) | 3 (12%) | 2 (18%) | 0.62 |
| Blowout Characteristics | ||||
| Time from Initial Intervention (d), median (IQR) |
478 (246, 1752) |
862 (260, 1805) |
374 (103, 739) |
0.24 |
| Cancer Recurrence | 21 (60%) | 14 (58%) | 7 (64%) | 1.00 |
| Bleed Type | 1.00 | |||
| Threatened | 3 (8%) | 2 (8%) | 1 (9%) | |
| Impending | 9 (24%) | 6 (23%) | 3 (27%) | |
| Acute Hemorrhage | 25 (68%) | 18 (69%) | 7 (64%) | |
| Blowout Site | 0.40 | |||
| Common Carotid | 19 (51%) | 15 (58%) | 4 (36%) | |
| Internal Carotid | 7 (19%) | 4 (15%) | 3 (27%) | |
| External Carotid | 11 (29%) | 7 (27%) | 4 (36%) | |
| Blowout Treatment | 0.28 | |||
| Surgical Ligation | 8 (22%) | 6 (23%) | 2 (18%) | |
| Covered Stent | 11 (30%) | 8 (31%) | 3 (27%) | |
| Coil Embolization | 14 (38%) | 11 (42%) | 3 (27%) | |
| Bypass Grafting | 2 (5%) | 1 (4%) | 1 (9%) | |
| Primary Repair | 2 (5%) | 0 (0%) | 2 (18%) |
Bleeding and Intervention
The majority of patients presented with acute hemorrhage (25, 68%). Nine (24%) patients presented with impending bleed and three (8%) with threatened bleed. The most common location was the common carotid artery (16, 51%) followed by proximal external artery branches (9, 24%). The median time of bleed from first operation or radiation treatment was 478 days (inter-quartile range 246-1752). All patients identified with CBS underwent intervention. 25 patients received endovascular therapy: 14 patients (38%) received coil embolization while 11 patients (30%) had stent graft placement. The remainder underwent open surgical therapy: 8 patients (22%) had carotid ligation, 2 patients (5%) received bypass grafts with saphenous vein, and 2 patients had primary arterial repair. 11 (30%) also underwent flap coverage of the area for prevention of infection and recurrent blowout. Only 5 patients (15%) received long-term (6 week) antibiotic therapy; the remainder received only a standard perioperative antibiotic course.
Outcomes
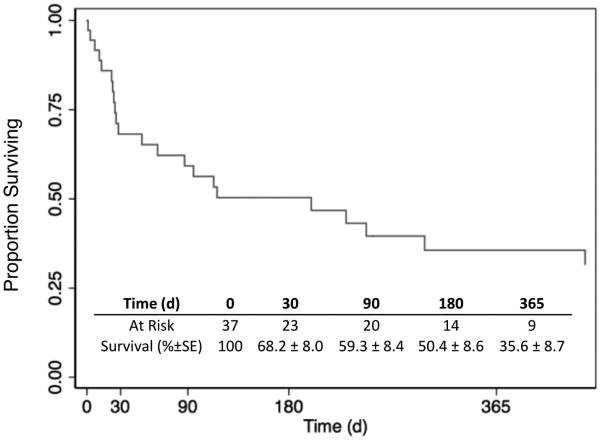
Three patients (8.1%) died perioperatively from bleed related complications and four patients (11%) experienced a perioperative stroke. Two of these patients died after massive stroke during endovascular coiling, and one patient suffered anoxic injury and brain death during the initial bleeding episode prior to primary ligation of the common carotid. All other patients survived to hospital discharge. One patient who received a stent graft was diagnosed with graft infection and sepsis two weeks after implantation; he subsequently underwent uncomplicated explantation of the stent and ligation of the common carotid artery. There were no other infectious complications. The overall estimated 30-day and 1-year survival by Kaplan-Meier analysis was 67.0% and 36.6% respectively [Figure 1]. Univariate and multivariate analysis did not identify any factors significantly associated with mortality risk over time (Supplemental Table I).
Figure I.
Estimated survival.
Recurrent Bleeding
Eleven patients (29.73%) experienced at least one recurrent bleeding episode after initial intervention for CBS. 9 patients (82%) had recurrent bleeding from the same site as the initial bleed. Two patients died in-hospital after readmission and intervention for recurrent carotid bleeding; one suffered a stroke with intracranial hemorrhage after coiling of the internal carotid artery, and the other had withdrawal of care after respiratory failure and ventilator dependence post stent-grafting to cover external carotid branch bleeding.
Two patients experienced a third bleeding recurrence and underwent technically successful coil re-embolization of the external carotid artery. One patient required four interventions after presenting initially with tissue necrosis involving both carotids. He underwent primary repair of a bleeding common carotid artery as described previously with immediate success, but subsequently required surgical ligation followed by endovascular coiling due to progression of disease. He eventually underwent a surgical bypass on the contralateral common carotid for a threatened bleed.
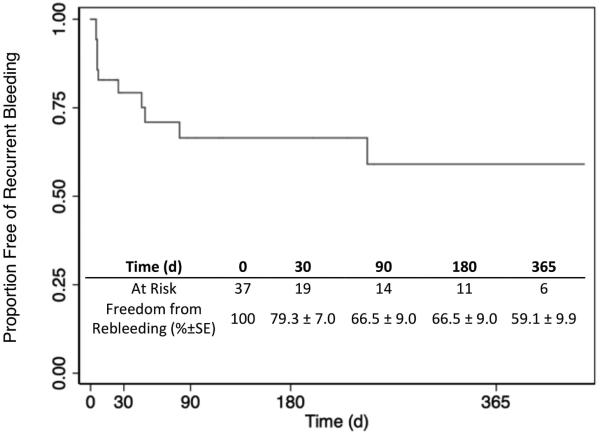
Estimated freedom from the first episode of recurrent bleeding after initial intervention for CBS was 79% at 30 days, 66% at 180 days, and 60% at one year [Figure 2]. The median time to recurrence from the initial blowout was seven days (IQR 6-49). Baseline and previous blowout characteristics did not differ significantly between those with recurrent bleeding and those without [Table 1]. Univariate and multivariate analysis did not identify any factors significantly associated with rebleeding risk over time.
Figure II.
Estimated freedom from rebleeding.
Comparison by Treatment Modality
The cohort was further analyzed by type of intervention performed: surgery, stenting, or embolization. The time from initial cancer intervention to blowout was higher in the stent-graft and embolization groups. Embolization was performed at a higher rate in patients with impending bleed and in the external carotid artery compared to surgical and stent-graft therapy, which were predominantly performed in those with acute hemorrhage and in the common carotid artery. However, perioperative outcomes did not differ significantly between treatment modalities (Table 2).
Table II.
Characteristics and Outcomes by Treatment
| Surgical (N=12) |
Stent (N=11) |
Embolization (N=14) |
P | |
|---|---|---|---|---|
| Age (y), mean ± SD | 55.63±15.25 | 63.30±10.30 | 61.36±7.74 | 0.3 |
| Male Sex | 8 (73%) | 5 (50%) | 13 (93%) | 0.07 |
| Chemoradiation | 9 (82%) | 4 (36%) | 11 (79%) | 0.05 |
| Prior Neck Dissection | 7 (58%) | 4 (36%) | 5 (36%) | 0.5 |
| Prior Flap Coverage | 4 (34%) | 2 (18%) | 1 (7%) | 0.4 |
| Prior Wound Saliva Exposure | 2 (17%) | 1 (9%) | 1 (7%) | 0.8 |
| Prior Wound Packing | 3 (25%) | 0 (0%) | 2 (14%) | 0.3 |
| Blowout Time (d), median (IQR) |
201.5 (53, 374) |
1402.5 (459, 2387) |
989 (861, 1682) |
0.01 |
| Blowout Characteristics | ||||
| Bleed Type | 0.00 2 |
|||
| Threatened | 1 (8%) | 2 (18%) | 0 (0%) | |
| Impending | 0 (0%) | 1 (9%) | 8 (57%) | |
| Acute Hemorrhage | 11 (92%) | 8 (73%) | 6 (43%) | |
| Blowout Site | 0.00 2 |
|||
| Common Carotid | 8 (67%) | 8 (73%) | 3 (21%) | |
| Internal Carotid | 1 (8%) | 3 (27%) | 3 (21%) | |
| External Carotid | 3 (25%) | 0 (0%) | 8 (57%) | |
| Outcomes | ||||
| Perioperative Stroke | 1 (8%) | 1 (9%) | 2 (14%) | 1.0 |
| Perioperative Death | 1 (8%) | 0 (0%) | 2 (14%) | 0.8 |
| Rebleeding | 5 (41.7%) | 3 (27.3%) | 3 (21.4%) | 0.6 |
Subgroup Analysis of Acute Hemorrhage
Subgroup analysis was performed on twenty-five patients (68%) presenting with acute hemorrhage. Fifteen had bleeding from the common carotid (60%), seven (28%) from the internal carotid artery, and three (12%) from the external carotid trunk. Eleven patients (44%) underwent surgical intervention, eight (32%) had stent-graft placement, and six (24%) coil embolization. The perioperative stroke and death rates were 33% (n=2) and 17% (n=1) respectively. The rate of rebleeding was 28% (n=7), and the mean time to rebleeding was 14.4±15.6d. Small sample size prevented meaningful statistical comparison between those with acute, impending, and threatened bleeding.
DISCUSSION
Carotid blowout syndrome is a morbid condition presenting in patients with head and neck cancer after surgical intervention or radiation therapy. CBS has mainly been described in case reports and series due to the rarity of the condition, precluding practical prospective trials. The incidence has been estimated to be between 2-5%2 in patients receiving intervention for head and neck cancer, and up to 10%6 in those receiving repeated courses of radiation. The morbidity of CBS has been well-described, with a perioperative mortality rate as high as 30% and a perioperative stroke rate as high as 15%, but remain highly variable due to the rarity of the condition1,2,8.
We report a series of 37 patients with CBS, all of whom received intervention for bleeding. The most common locations or tumor location were in the oral cavity and larynx and the most common bleeding origin was the common carotid artery. The location of tumor and bleeding origins vary widely in the literature, potentially due to geographic and chance variations as well as small numbers of cases in each center and series. As a result, the location of the primary tumor and distribution of bleed may affect prognosis, but the magnitude and direction of these effects has not been fully explored. For example, studies of endemic nasopharyngeal carcinoma in Asian populations has shown a tendency for bleeding in distal internal or external carotid distributions9,10. These bleeds tend to be treated with embolization and carry a higher rate of neurologic complications and lower rate of rebleeding11 than the cohort in our study, which had a majority common carotid and oral/laryngeal tumor component. However, the cohort size in our study was too small to further explore these differences.
All interventions were technically successful for immediate bleeding cessation in those with acute hemorrhage, but with a stroke rate of around 11%. Reports have suggested a higher stroke rate with surgical or endovascular ligation/occlusion compared to endovascular graft repair4,7,12, but stroke rates were not different between treatment modalities in our cohort. Endovascular occlusion of the internal and common carotid arteries is not commonly performed in our institution, with stent-grafts being the preferred endovascular solution in these vascular beds. The majority of surgical ligation procedures occurred in patients presenting with acute hemorrhage, necessitating emergency operation and preventing usage of adjuncts such as a balloon occlusion testing. Variable stroke rates have been seen in different cohorts ranging from 0-15%4,7,12.
The in-hospital mortality rate in our study was low at around 8%. Others have also reported relatively high rates of immediate survival after intervention6 but the overall mid-term survival remains poor. In our cohort, survival was 70% at 30 days and 37% at one year, similar to recent reports7. The high post-discharge mortality likely reflects the poor overall condition of these patients who commonly present with either a local recurrence or a second primary tumor. Thus, carotid blowout may serve more as a marker of poor prognosis due to severely advanced disease rather than as a mortal event if bleeding is treated in a timely fashion.
Our recurrent bleeding rate was high at nearly 30%, with a short mean rebleeding time of 44 days. The prognosis for recurrent bleeding was not significantly affected by any factors on multivariate analysis. These included traditionally implicated factors such as open wounds requiring packing, wounds with saliva exposure, or previous radical neck dissection7. Only the rate of muscle flap coverage differed significantly between re-bleeders and non-re-bleeders in a univariate analysis. The clinical significance of this finding is unclear due to the small sample size and dependence on other confounding factors, as significance was lost upon inclusion in a multivariate model. Our rate of rebleeding falls at the higher end of previously reported rates ranging from 13-27%5,6,12,13 and demonstrates the temporary nature of many of the interventions performed for CBS despite initial technical success.
We report in this series one case of late stent-graft infection requiring subsequent explantation and ligation of the common carotid artery. The overall rate of reported late complications in CBS is low4,5,14, likely due in part to the extremely high one-year mortality rates seen in these patients. However, the implantation of prosthetic material into an irradiated field with potential skin exposure and chronic infection may predispose to infection. Chang and colleagues described one patient who underwent an endovascular stent-graft for CBS with stent-graft thrombosis, infection, and brain abscesses in the anterior and middle cerebral artery distributions. This patient was surgically drained, treated with antibiotics, and survived until discharge14,15. Oweis and colleagues likewise describe septic embolization and brain abscesses after covered stent implantation in the common carotid artery for CBS16. Patients should be closely monitored after implantation of stent-grafts in areas with high risk of exposure to a contaminated field.
Limitations of this study are primarily due to the retrospective nature of data collection and analysis, compounded by the rarity of the condition. The selection of patients for open or endovascular repair was not randomized and was at the discretion of the interventionalist. Selection bias may also affect the analysis, as those who may have expired due to carotid bleed at an outside hospital or prior to admission are not captured. For many of those who died after discharge, cause of death was not able to be determined and as such the rate of bleed-related deaths compared to all-cause mortality remains unknown. The loss-to-follow-up rate is also high, as several patients were lost to follow-up immediately after hospital discharge. The heterogeneity of bleeding distributions, tumor locations, and prior operations among our patients also limits analysis as patients with different blowout characteristics or acuity may have a different natural history of disease, and may potentially respond differently to therapy. Due to the limited sample size in our study, factors independently affecting death or rebleeding were unable to be identified by multivariate analysis, and comparative effectiveness of the different interventions was not able to be fully explored.
CONCLUSIONS
Carotid blowout syndrome carries significant mid-term mortality and morbidity. Despite this, there is a high rate of technical success for bleeding cessation after any intervention and a high rate of survival to hospital discharge. Both surgical and endovascular techniques, including endovascular carotid occlusion and stent-grafting, are efficacious and carry a similar risk of perioperative stroke or death. Close follow-up for all patients should be maintained due to the risk of recurrent bleeding regardless of intervention. In many patients, carotid blowout syndrome may be a marker of poor prognosis due to advanced disease rather than a mortal event and should be managed aggressively.
Supplementary Material
Acknowledgments
This work was supported in part by funds from the Department of Veterans Affairs BLSR&D (U.D.) and does not reflect the views of the US Government or the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the plenary session of the 29th Annual Meeting of the Eastern Vascular Society, Baltimore, MD, September 24-26, 2015.
REFERENCES
- 1.Chaloupka JC, Putman CM, Citardi MJ, Ross DA, Sasaki CT. Endovascular therapy for the carotid blowout syndrome in head and neck surgical patients: Diagnostic and managerial considerations. Am J Neuroradiol. 1996;17(5):843–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J, Rad I. Contemporary management of carotid blowout. Curr Opin Otolaryngol Head Neck Surg. 2004 Apr;12(2):110–5. doi: 10.1097/00020840-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Wan WS, Lai V, Lau HY, Wong YC, Poon WL, Tan CB. Endovascular treatment paradigm of carotid blowout syndrome: review of 8-years experience. Eur J Radiol. 2013 Jan;82(1):95–9. doi: 10.1016/j.ejrad.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor BG, Haussen DC, Ambekar S, Peterson EC, Yavagal DR, Elhammady MS. Covered Stents for the Prevention and Treatment of Carotid Blowout Syndrome. Neurosurgery. 2015;77(2):1. doi: 10.1227/NEU.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 5.Zussman B, Gonzalez LF, Dumont A, Tjoumakaris S, Rosenwasser R, Hasan D, et al. Endovascular management of carotid blowout. World Neurosurg. 2012 Jul;78(1-2):109–14. doi: 10.1016/j.wneu.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Chaloupka JC, Roth TC, Putman CM, Mitra S, Ross DA, Lowlicht RA, et al. Recurrent carotid blowout syndrome: diagnostic and therapeutic challenges in a newly recognized subgroup of patients. AJNR Am J Neuroradiol. 1999;20(6):1069–77. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y-J, Wang C-P, Wang C-C, Jiang R-S, Lin J-C, Liu S-A. Carotid blowout in patients with head and neck cancer: associated factors and treatment outcomes. Head Neck. 2015 Feb;37(2):265–72. doi: 10.1002/hed.23590. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey DD, Andersen PE, Nesbit GM, Barnwell SL, Everts EC, Cohen JI. Endovascular management of hemorrhage in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1997;123(1):15–9. doi: 10.1001/archotol.1997.01900010017002. [DOI] [PubMed] [Google Scholar]
- 9.Wong GKC, Chan KK, Yu SCH, Tsang RKY, Poon WS. Treatment of Profuse Epistaxis in Patients Irradiated for Nasopharyngeal Carcinoma. ANZ J Surg. 2007;77(4):270–4. doi: 10.1111/j.1445-2197.2007.04032.x. [DOI] [PubMed] [Google Scholar]
- 10.Lam JW-K, Chan JY-W, Lui W-M, Ho W-K, Lee R, Tsang RK-Y. Management of pseudoaneurysms of the internal carotid artery in postirradiated nasopharyngeal carcinoma patients. Laryngoscope. 2014 Apr;16:1–5. doi: 10.1002/lary.24721. [DOI] [PubMed] [Google Scholar]
- 11.Chen K-C, Yen T, Hsieh Y, Chen H-C, Jiang R, Chen W, et al. Postirradiated carotid blowout syndrome in patients with nasopharyngeal carcinoma: A case-control study. Head Neck. 2014 Mar;7:1–6. doi: 10.1002/hed.23671. [DOI] [PubMed] [Google Scholar]
- 12.Lu H-J, Chen K-W, Chen M-H, Chu P-Y, Tai S-K, Wang L-W, et al. Predisposing factors, management, and prognostic evaluation of acute carotid blowout syndrome. J Vasc Surg. 2013 Nov;58(5):1226–35. doi: 10.1016/j.jvs.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Chang F, Luo C, Lirng J, Lin C, Wu H. Evaluation of the outcomes of endovascular management for patients with head and neck cancers and associated carotid blowout syndrome of the external carotid artery. Clin Radiol. 2013 doi: 10.1016/j.crad.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Chang FC, Luo CB, Lirng JF, Guo WY, Wu HM, Teng MMH, et al. Complications of carotid blowout syndrome in patients with head and neck cancers treated by covered stents. Interv Neuroradiol. 2008 Nov 11;14(Suppl 2):29–33. doi: 10.1177/15910199080140S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang F-C, Lirng J-F, Tai S-K, Luo C-B, Teng MMH, Chang C-Y. Brain abscess formation: a delayed complication of carotid blowout syndrome treated by self-expandable stent-graft. AJNR Am J Neuroradiol. 2006 Aug;27(7):1543–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Oweis Y, Gemmete JJ, Chaudhary N, Pandey A, Ansari S. Delayed development of brain abscesses following stent-graft placement in a head and neck cancer patient presenting with carotid blowout syndrome. Cardiovasc Intervent Radiol. 2011 Feb;34(Suppl 2):S31–5. doi: 10.1007/s00270-009-9778-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.