Abstract
Background
Children with brain tumor (BT) are at risk for a number of physical and cognitive problems that may lower their health-related quality of life (HRQoL). Executive functioning (EF) and intellectual ability are hypothesized to associate with HRQoL and deficits in these areas may be amenable to interventions.
Objective
To investigate intellectual function, EF, and HRQoL following conformal radiation therapy (CRT) for pediatric BT.
Method
Forty-five BT survivors (age 12.68±2.56) treated with CRT participated. Thirty-six siblings of BT patients (age 12.36±2.13) and 33 survivors of non-CNS solid tumors (ST; age 12.18±2.88) were comparison groups. IQ estimate (Wechsler Abbreviated Scale of Intelligence; WASI), EF ratings (Behavior Rating Inventory of Executive Function; BRIEF), and HRQoL ratings (KINDL-R) were obtained.
Results
BT survivors reported lower overall HRQoL than ST survivors (p=.012). Parents reported lower overall HRQoL for BT survivors than siblings (p=.014). Parent-report on individual areas of HRQoL was higher than self-report for most subscales. IQ and HRQoL ratings were not related (Parent r=.17, p=.27; Child r=.11, p=.49). EF ratings correlated with Parent (r=-.15 to -.73) but not Child HRQoL ratings.
Conclusion
Children with BT experienced poorer HRQoL than controls. Children's HRQoL was consistently rated higher by parent- than self-report across all domains. HRQoL was associated with EF, but not with IQ. These findings identify interventions targeting EF (e.g., cognitive rehabilitation, medication) as a possible avenue for improving HRQoL in childhood BT survivors.
Background
Children diagnosed with a brain tumor (BT) and treated with cranial radiation therapy are at increased risk for cognitive problems (e.g., reduced IQ, executive dysfunction; Mulhern et al., 1999; Reddick et al., 2000). These children may experience difficulties in vision, hearing, cognition and low growth rate due to tumor location and sequelae of subsequent treatment (Conklin, Li, Xiong, Ogg & Merchant, 2008; Butler & Haser, 2006; Mulhern, Merchant, Gajjar, Reddick & Kun, 2004; Kun, Mulhern & Crisco, 1983). These problems may affect children's educational, social, and overall health-related quality of life (HRQoL). Children with primary central nervous system (CNS) tumors have demonstrated poorer HRQoL in prior studies (Bhat et al., 2005). Although factors contributing to this decline are poorly understood, it has been suggested that radiation therapy is a key risk factor for reduced HRQoL (Reimers, Mortensen, Nysom & Schmiegelow, 2009).
Conformal or intensity-modulated radiation therapy (CRT/IMRT) delivers radiation doses to tightly targeted areas of diseased tissue, while sparing surrounding healthy tissue. This method has the potential to improve functional outcomes, although some cognitive functions may still be vulnerable (Conklin et al., 2008; Netson et al., 2012; Conklin et al. 2012; Netson et al., 2013). While multiple studies have examined HRQoL after conventional radiation therapy, proton beam radiation therapy, chemotherapy, and other treatment modalities (Reimers et al., 2009; Laffond et al., 2012; Benesch et al., 2009), no known studies have examined HRQoL after CRT for pediatric brain tumor.
The literature is inconsistent regarding relationships among IQ, executive functioning (EF) and HRQoL in general, and few studies exist in children with BT. A recent review of the HRQoL literature in children with BT identified only 16 relevant studies (Macartney, Harrison, VanDenKerkhof, et al., 2014). BT survivors were rated lower across a number of HRQoL areas relative to healthy peers and children with other types of cancer. In children with pilocytic astrocytoma, receiving radiation therapy and requiring special education services were associated with lower HRQoL (Aarsen, Paquier, Arts, et al., 2009). In children with cerebellar tumors who underwent surgical resection and craniospinal irradiation, HRQoL scores were below those of healthy peers and lower scores were associated with self-reported cognitive and emotional complaints (Bull, Liossi, Culliford, et al., 2014). Despite these findings, little work has been done to identify specific cognitive complaints that might affect HRQoL or be associated with response patterns on self-report measures of HRQoL.
There are also methodological issues limiting the assessment of HRQoL in children. The most ubiquitous measures are parent-report and self-report questionnaires, which generally ask face-valid questions about various life domains that may or may not be affected by a specific health-related condition. There are marked inconsistencies in the literature regarding the relationship between parent ratings and child self-report. Among healthy children, parents tend to rate their children's HRQoL higher than the children, while among children with chronic, ongoing illnesses (e.g., diabetes, chronic pain), children rate their HRQoL higher than parents (Eiser and Varni, 2013). Childhood BT survivors present a unique population, as there is wide variability regarding the chronic effects of their tumor and treatment; thus, inter-rater agreement between parents and children needs further exploration.
It has been hypothesized that EF relates to quality of life in adult populations, as EF enables individuals to carry out health-promoting behaviors (Kuo and Lipsitz, 2004); however, this has not been studied in children. Behavioral manifestations of EF, or goal-directed behaviors such as inhibition, organization, task initiation and maintenance, and self-monitoring, do not appear to correlate with IQ in patients with brain disease or healthy participants (Aarsen et al., 2009; Aran-Filipetti V & Richaud de Minzi MC, 2012). In adults with acquired brain injury, coping style may mediate the relationship between executive dysfunction and HRQoL, with more passive coping styles leading to lower HRQoL (Wolters et al., 2015). In children with traumatic brain injuries, injury severity significantly affected both executive dysfunction and HRQoL, although the relationship between the two domains was not investigated (Anderson et al., 2012). In a separate sample of youth with acquired brain injuries (e.g., encephalitis, stroke, traumatic brain injury), executive dysfunction was strongly related to poor occupational/academic performance and poor social relationships, but HRQoL was not explicitly investigated (Soo, Tate, & Brookes, 2014). Understanding the relationship between EF and HRQoL may provide avenues for intervention via cognitive rehabilitation programs specifically targeting EF in pediatric BT survivors.
Accordingly, the current study investigated relationships among IQ, EF, and HRQoL in BT survivors treated with CRT. It was hypothesized that, 1) children with BT would have lower HRQoL relative to the healthy sibling control group and the cancer control group; 2) HRQoL scores would be similarly rated by children and parents; and 3) HRQoL scores would be related to cognitive function in BT survivors, specifically IQ and EF.
Methods
This cross-sectional investigation was conducted at a major pediatric cancer center. This study was approved by the Institutional Review Board of the research institution and written informed consent was required prior to participation. Study enrollment occurred between April 2007 and December 2009.
Participants
Participants were children between 8 and 16 years of age at the time of evaluation and their parents. Forty-five BT survivors, 33 solid tumor (ST) survivors not receiving CNS-directed therapy, and 36 siblings of BT survivors participated. Participant recruitment was stratified based on age (8-12; 13-16) and for BT survivors, on tumor location (infratentorial; supratentorial). Clinical and demographic characteristics of all groups are reported in Table 1, with Table 2 summarizing BT-specific clinical variables.
Table 1. Participant Demographic and Clinical Characteristics.
| Brain Tumor (N=45) |
Siblings (N=36) |
Solid Tumor (N=33) |
p | |
|---|---|---|---|---|
| Gender (% Male) | 46.7 | 47.2 | 48.5 | 0.99 |
| Age at Diagnosis (Y) | 6.11 ± 3.45 | NA | 3.36 ± 2.87 | <0.01* |
| Age at Assessment (Y) | 12.67 ± 2.56 | 12.37 ± 2.13 | 12.18 ± 2.88 | 0.69 |
| Time since Diagnosis (Y) | 6.55 ± 2.52 | NA | 8.82 ± 3.66 | <0.01* |
| SES (BSMSS) | 37.76 ± 12.08 | 42.94 ± 11.19 | 41.24 ± 13.36 | 0.15 |
| Abbreviated IQ (WASI std score) | 98.64 ± 14.24 | 108.69 ± 12.56 | 107.67 ± 13.16 | <0.01* |
P-value indicates whether group is equally distributed across sub-categories using One-Way ANOVA, independent t-test or Chi-square. Y – Years; CRT – conformal radiation therapy; SES – socioeconomic status; BSMSS - Barratt Simplified Measure of Social Status (Scores range from 8 to 66 with higher scores indicative of higher SES); IQ – intelligence quotient; WASI – Wechsler Abbreviated Scale of Intelligence
Table 2. Clinical Characteristics of Brain Tumor Survivors.
| N | % | pa | |
|---|---|---|---|
| Tumor Diagnosis | |||
| Ependymoma | 21 | 47 | 0.09 |
| Low Grade Glioma | 9 | 20 | |
| Craniopharyngioma | 15 | 33 | |
| Tumor Location | |||
| Infratentorial | 20 | 44 | 0.46 |
| Supratentorial | 25 | 56 | |
| Pre-CRT Chemotherapy | |||
| No | 39 | 87 | <0.01 |
| Yes | 6 | 13 | |
| Extent of Surgical Resectionb | |||
| Biopsy/STR | 21 | 47 | 0.66 |
| NTR/GTR | 24 | 53 | |
| Hydrocephalus | |||
| No | 19 | 42 | 0.30 |
| Yes | 26 | 58 | |
| CSF Shunting | |||
| No | 27 | 60 | 0.18 |
| Yes | 18 | 40 |
P-value indicates whether group is equally distributed across sub-categories using Chi-square.
Biopsy= tumor sampling to establish histologic diagnosis without intent to resect; STR= subtotal resection, incomplete tumor resection with gross residual disease present on post-operative neuroimaging; NTR= near total resection, incomplete tumor resection with minimal residual disease present on post-operative neuroimaging; GTR= gross total resection, resection of tumor without apparent gross residual disease observed by the operating neurosurgeon and confirmed on post-operative neuroimaging
BT survivors were treated for a primary CNS tumor (low-grade glioma, ependymoma or craniopharyngioma) on a phase II trial of CRT using photons. Treatment was initiated at least two years prior to current study enrollment with patients having no evidence of recurrent disease. All participants received CRT/IMRT, using conventional fractionation (1.8 Gy per day) with a prescribed dose of 54 Gy (low-grade glioma and craniopharyngioma) or 59.4 Gy (ependymoma). The dose was attenuated to 54.0 Gy for children with ependymoma younger than 18 months of age after gross-total resection. The irradiated clinical target volume included a 10-mm margin surrounding the tumor and/or tumor bed to control microscopic disease, and an additional 3- to 5-mm margin expansion in three dimensions to form the planning target volume and account for uncertainty in patient positioning and image registration.
Sibling participants were healthy siblings of BT survivors (15 of whom participated in this study) treated at a large cancer center. ST patients received treatment for their tumor (Ewing sarcoma, osteosarcoma, soft tissue/rhabdomyosarcoma, neuroblastoma, or Wilms tumor [no primary brain masses or metastases[) without CNS-directed therapy (i.e., cranial radiation therapy, intrathecal chemotherapy or high dose methotrexate; one patient received whole body irradiation [2 Gy –total dose] in preparation for bone marrow transplant) and were diagnosed at least two years prior to enrollment in the present study.
Individuals with global intellectual impairment (IQ less than 70 for BT patients [obtained during previous protocol-prescribed cognitive assessment] or a history of special education services for siblings and ST survivors) were excluded from participation. Participants were also excluded for a history of CNS injury or disease (predating cancer diagnosis in BT patients), documented ADHD (predating cancer diagnosis for BT patients), treatment with psychotropic or stimulant medication within two weeks of study participation, or major sensory and/or motor impairment that would preclude valid testing (e.g., blindness, hemiparesis, poorly controlled seizures, active psychosis).
Procedures
HRQoL Assessment
All participants completed an age-appropriate version of the KINDL-R (named from the German translation for Children's Quality of Life Questionnaire, KINDer Lebensqualitätsfragebogen) as a measure of their HRQoL. Participants completed either the Kid KINDL-R (age 8-12) or the Kiddo KINDL-R (age 13-16), and parents completed the KINDL-R for Parents (age 8-16). The measure consists of 24 items distributed among six dimensions (Physical Health, Psychological Health, Self-Esteem, Family, Friends, and School). Items are graded on a 5-point Likert scale, with 10 items scored in the reverse direction. Total scores are recorded and transformed to a scale of 0-100 using algorithms provided in the manual. Higher transformed scores indicate better HRQoL. Age- and gender-stratified scores from a healthy reference sample are provided in the manual, with mean and standard deviations provided for each subscale and the total score on the child self-report form; normative data are not available for the parent-report form. In general, mean (standard deviation) subscale scores range from 58.14(19.06) out of 100 for the Self-Esteem subscale to 84.40(12.85) out of 100 for the Family subscale. Mean Total Transformed Scores range from 70.78(10.01) to 76.83(8.63) depending on age and sex in the reference sample (See Figure 1). The KINDL-R has been established as a reliable measure with the ability to discriminate between healthy children and children with medical conditions (Ravens-Sieberer & Bullinger, 2000).
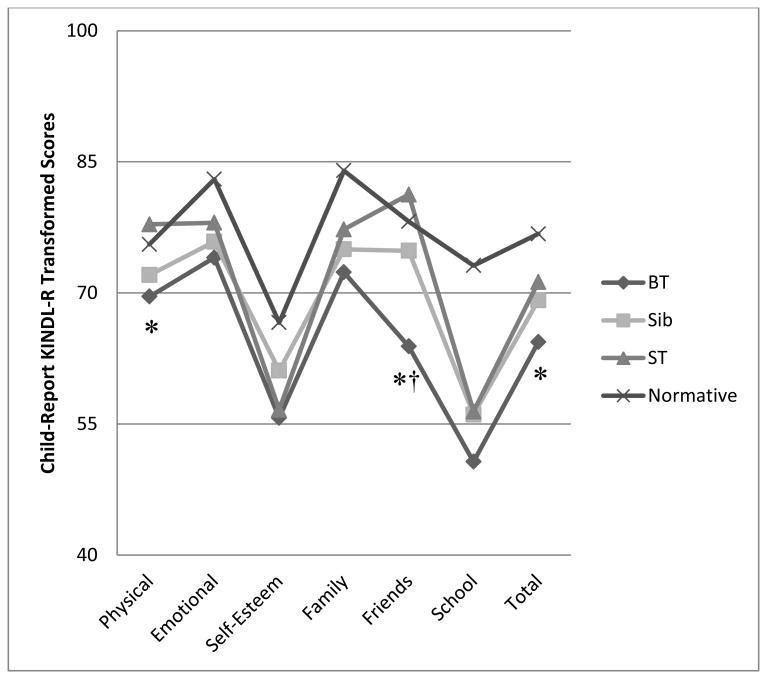
Figure 1. KINDL-R Child ratings by diagnostic group.
Note. BT=Brain Tumor; Sib=Sibling; ST=Solid Tumor;
*BT group significantly lower than ST group at p<.05
†BT group significantly lower than Sib group at p<.05
Executive Function Assessment
Participants' parents also completed the Behavior Rating Inventory of Executive Function (BRIEF) as a measure of participants' EF skills in daily life. The BRIEF has been established as a reliable and valid instrument for assessing EF and takes 10-15 minutes for completion (Gioia, Isquith, Guy & Kenworthy, 2000). It includes 86 items rated as Never, Sometimes, or Often and includes both negativity and consistency indices to address validity concerns. The measure yields eight subscales (Inhibit, Shift, Emotional Control, Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor) as well as two indices (Behavior Regulation Index and Metacognitive Index) and a General Executive Composite. Raw scores are summed onto respective subscale and index scores, then transformed to age- and gender-stratified T-Scores (Mean=50; Standard Deviation=10). T-Scores over 65 indicate clinically significant problems with a specific domain of executive behaviors.
Intellectual Assessment
Global intellect was estimated using the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). The resulting abbreviated IQ score is highly correlated with Full-Scale IQ scores obtained on age-appropriate Wechsler scales (e.g., WISC-IV; WAIS-III).
Clinical and Demographic Characteristics
Parents of each participant completed a questionnaire with relevant demographic and developmental information (e.g., parental education, parental occupation, family income). Socioeconomic status (SES) was then estimated for each family using the Barratt Simplified Measure of Social Status (Barratt, 2006). These scores (ranging from 8 to 66, with higher scores indicating higher SES) are derived using maternal and paternal education and occupation as reported on the questionnaire. Relevant clinical variables (diagnosis, tumor location, number of surgeries, extent of resection, hydrocephalus, shunt placement, chemotherapy, age at CRT) were extracted from the treatment protocol database for the BT group.
Statistical Analyses
Clinical and demographic variables (e.g., age at treatment, presence of complications such as hydrocephalus) were examined using chi-square analyses. The first hypothesis, that BT survivors would have lower HRQoL than healthy siblings and the ST cancer control group, was evaluated using univariate linear models. Cohen's d was calculated to determine the magnitude of the effect, with effect sizes exceeding d=.80 deemed “large” (Cohen, 1988). Follow-up analyses examining clinical and demographic factors that associate with lower HRQoL scores were conducted using Pearson correlations and multivariate linear models. The second hypothesis, that parent and child ratings of HRQoL would agree, was assessed using linear mixed models comparing both rater source and diagnostic group. Again, the magnitude of the effect was assessed with Cohen's d. The third hypothesis, that HRQoL would be related to cognitive function in BT survivors, was evaluated using Pearson correlations and univariate linear modeling. Multiple variable regression was performed to test model fit.
Results
Forty-five childhood BT survivors treated with CRT (22 males; mean age at study=12.67±2.56 years; mean age at irradiation=7.07±3.38 years) participated. Thirty-six siblings of BT patients (16 males; mean age=12.36±2.13 years) served as a healthy control group and 33 survivors of solid tumors (ST) outside of the CNS served as a cancer control group (16 males; mean age=12.18±2.88 years).
HRQoL Ratings for BT Survivors
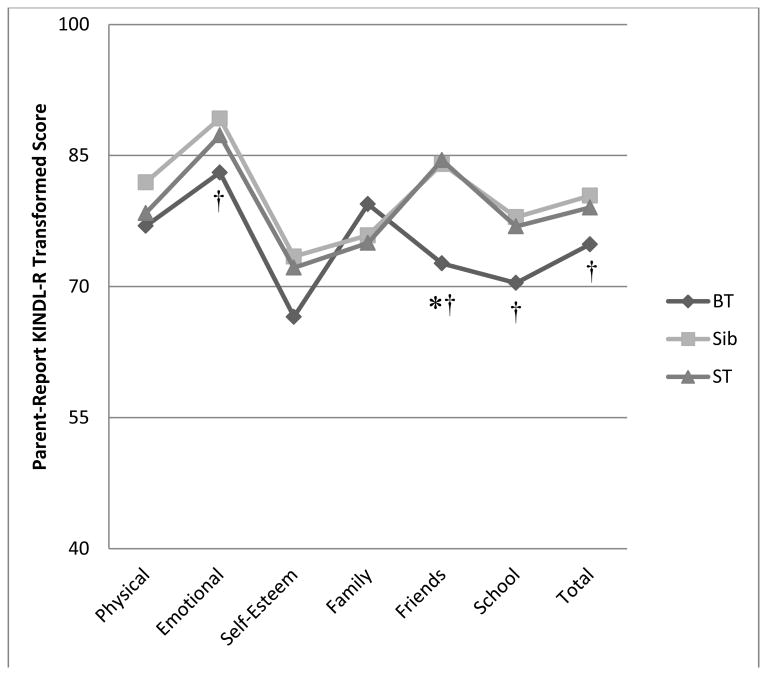
The first hypothesis, that children with BT would have poorer HRQoL than healthy siblings and the ST control group was partially supported. Linear mixed models and calculation of effect sizes (Cohen's d) revealed that BT survivors reported lower KINDL-R Total scores than the ST group (p=.012, d=.59), while scores did not significantly differ from the Sibling group (p=.075, d=.40; See Figure 1). Children with BT also reported significantly lower KINDL-R Physical scores (p=.027, d=.51) and KINDL-R Friendship scores (p<.0001, d=.93) than the ST group. Children with BT reported lower KINDL-R Friendship scores relative to the Sibling group as well (p=.012, d=.57). Parents reported lower KINDL-R Total scores for BT survivors relative to sibling controls (p=.014, d=.56), but not relative to the ST group (p=.091, d=.39; See Figure 2). Parents also rated lower KINDL-R scores for children with BT relative to siblings for Emotional (p=.020, d=.53), Friendship (p=.0002, d=.85), and School (p=.049, d=.44) subscales, with a trend for lower Self-Esteem ratings (p=.053, d=.44). Parents rated children with BT as having lower Friendship ratings (p=.0002, d=.88) than the ST group as well. Children in the Sibling and ST groups did not significantly differ on any KINDL-R subscales for child or parent ratings.
Figure 2. KINDL-R Parent scores by diagnostic group.
Note. BT=Brain Tumor; Sib=Sibling; ST=Solid Tumor; Normative data not available for KINDL-R Parent version
*BT group significantly lower than ST group at p<.05
†BT group significantly lower than Sib group at p<.05
Clinical and demographic factors influencing ratings of HRQoL were investigated to determine sub-groups with increased risk for problems. Presence of a shunt for management of hydrocephalus (p=.002) and receipt of pre-radiation chemotherapy (p=.018) were both associated with lower child-rated KINDL-R Total scores in the BT group. Parent-rated KINDL-R Total scores were lower in children who were older at the time of the study (p=.0009) and older at the time of diagnosis (p=.015), which are highly correlated clinical variables (r=.552, p<.001).
Parent vs. Child Ratings of HRQoL
The second hypothesis was that parents and children would rate HRQoL similarly. Linear mixed modeling revealed that parents consistently rated age- and gender-adjusted HRQoL higher than children on nearly all KINDL-R subscales across the three diagnostic groups (p<.05; d=.04-1.14). See Table 3 for mean scores and effect sizes. There was a group by form interaction for the Physical subscale (p=.038) such that parent and child ratings for the ST group were nearly equivalent, while parent ratings for the BT (p=.0018) and Sibling (p=.0002) groups were significantly lower than child ratings. No other significant interactions were observed.
Table 3. Linear mixed model comparison of mean Parent vs. Child KINDL-R ratings across tumor groups.
| KINDL-R Subscales | Child KINDL-R Mean (SD) | Parent KINDL-R Mean (SD) | p-valuea | Effect Size db |
|---|---|---|---|---|
| Physical | ||||
| Brain Tumor | 69.58(17.81) | 76.94(18.93) | 0.0018 | 0.45 |
| Sibling | 72.05(13.07) | 81.94(13.26) | 0.0002 | 0.70 |
| Solid Tumor | 77.84(13.54) | 78.41(15.16) | 0.8328 | 0.04 |
| Emotional | ||||
| Brain Tumor | 74.03(16.43) | 83.06(14.82) | 0.0011 | 0.45 |
| Sibling | 75.87(16.10) | 89.24(8.79) | <.0001 | 0.78 |
| Solid Tumor | 78.03(15.24) | 87.31(7.57) | 0.0040 | 0.56 |
| Self-Esteem | ||||
| Brain Tumor | 55.69(25.21) | 66.53(15.49) | 0.0064 | 0.40 |
| Sibling | 61.11(20.65) | 73.44(17.45) | 0.0056 | 0.53 |
| Solid Tumor | 56.63(24.30) | 72.16(12.31) | 0.0009 | 0.56 |
| Family | ||||
| Brain Tumor | 72.36(17.09) | 79.44(15.80) | 0.0195 | 0.33 |
| Sibling | 75.00(15.38) | 75.87(12.78) | 0.7954 | 0.05 |
| Solid Tumor | 77.27(17.80) | 75.00(14.91) | 0.5161 | 0.11 |
| School | ||||
| Brain Tumor | 50.69(21.33) | 70.42(17.29) | <.0001 | 0.89 |
| Sibling | 56.08(17.20) | 77.95(14.68) | <.0001 | 1.13 |
| Solid Tumor | 56.44(19.98) | 76.89(16.35) | <.0001 | 1.14 |
| Friendship | ||||
| Brain Tumor | 63.89(23.15) | 72.64(16.34) | 0.0049 | 0.35 |
| Sibling | 74.83(18.21) | 84.03(11.52) | 0.0081 | 0.53 |
| Solid Tumor | 81.25(13.71) | 84.47(9.52) | 0.3681 | 0.19 |
| Total | ||||
| Brain Tumor | 64.38(13.45) | 74.84(12.15) | <.0001 | 0.66 |
| Sibling | 69.16(10.23) | 80.41(7.35) | <.0001 | 1.05 |
| Solid Tumor | 71.24(11.87) | 79.04(8.00) | 0.0011 | 0.64 |
P-value denotes significant difference between parent and child ratings within each of the three study groups.
Cohen's; ; Cohen (1988) classified effect sizes as small (d>.20), medium (d>.50), and large (d>.80).
HRQoL's Association with Cognitive Function
The final hypothesis, that HRQoL would be correlated with EF in BT survivors, was assessed using Pearson correlations. Mean IQ (98.64±14.23) and mean BRIEF T-scores (Range=49-56) were within the average range. One participant's Negativity Index on the BRIEF was elevated; however, that individual had considerable EF deficits that matched the severity of behavioral ratings. Thus, the data were included in analyses. Pearson correlations revealed no significant relationship between the KINDL-R Total Score and IQ (Parent r=.17, p=.27; Child r=.11, p=.49). All Parent KINDL-R subscales correlated significantly and in the anticipated direction across all BRIEF subscales (Range of significant correlations r=-.304 to -.728) with three exceptions: KINDL-R Emotional subscale did not correlate with BRIEF Inhibition or Emotional Control indices and the KINDL-R Physical subscale did not correlate with BRIEF Organization of Materials (Range of non-significant correlations r=-.149 to -.259). Only four significant correlations in the expected direction between Child KINDL-R and BRIEF subscales were identified: KINDL-R Physical and Self-Esteem correlated significantly with BRIEF Inhibit, and KINDL-R Family and Total Score correlated significantly with BRIEF Shift (Range of significant correlations r=-.304 to -.346; Range of non-significant correlations r=-.011 to -.273). KINDL-R Emotional correlated with BRIEF Emotional Control (r=.295, p=.049), but in an unexpected direction suggesting that better child-reported emotional HRQoL was associated with more parent-reported problems with emotional control. Although statistically significant, the correlations between Child KINDL-R and BRIEF subscales were low at best.
Given the significant number of correlations with BRIEF subscales, the decision was made to utilize BRIEF Index scores (Behavior Regulation Index and Metacognitive Index) in the models to simplify analyses and reduce redundancy. Multiple variable regression revealed that the BRIEF Metacognitive Index (F=20.89; df=1,39) independently accounted for an additional 47% (p<.0001) of the variance in Parent KINDL-R Total score after accounting for contributions by age at diagnosis, sex, and IQ (F=12.13; df=5,39). BRIEF Behavior Regulation Index did not account for a significant amount of variance (p=.42) beyond the Metacognitive Index.
Discussion
HRQoL for BT Survivors
Childhood BT survivors treated with CRT tend to have lower total HRQoL than children with non-CNS tumors and healthy controls, based on both parent- and self-report. This discrepancy persists in spite of average IQ and EF scores, suggesting that HRQoL is a unique construct that should be considered in evaluating treatment outcomes. Results suggest at least some perception, either by parents or children with BT, that areas of physical, emotional, social, academic, and overall well-being are negatively impacted following even conservative treatment for childhood BT.
Some clinical and demographic factors were associated with poorer ratings of HRQoL. Shunts for the management of hydrocephalus and receiving chemotherapy were related to poorer ratings, indicating that children with these medical risk factors may warrant closer monitoring to assess their need for supportive interventions. Shunt placement may reflect increased severity of neurologic sequelae of the tumor itself or complications from surgical or CRT interventions. Pre-treatment chemotherapy may also be an indication of increased tumor burden (i.e., chemotherapy administered to reduce tumor load prior to CRT). Further investigation will be required to determine the functional impact of increased medical complications, such as hydrocephalus and high tumor burden, on treatment outcomes and its relation to HRQoL. This would not be surprising in light of the literature on pediatric traumatic brain injury and the effect of injury severity on HRQoL (Anderson et al., 2012).
Patient age was associated with parent-rated HRQoL. Parents endorsed lower HRQoL in children who were older at the time of diagnosis, suggesting that the impact of a new brain tumor diagnosis on an older child may be more detrimental to HRQoL than adjusting to diagnosis when a child is younger. Thus, age appropriate interventions may need to be considered for recently diagnosed older children in order to mediate potentially damaging effects to their HRQoL. The impact of adolescence on HRQoL may also be unique in children with BT, who often experience hormonal and other endocrine complications that may make their experience of adolescence atypical relative to their siblings and children with non-CNS cancers. Endocrine dysfunction in particular should be examined with regard to its impact on HRQoL.
Parent vs. Child Ratings of HRQoL
It was predicted that parent and child ratings of HRQoL would be similar, but this hypothesis was not supported. Children in all three diagnostic groups rated their HRQoL as significantly lower than did their parents, and this was true for nearly every sub-domain rated on the KINDL-R. This was also in contrast to a recent investigation of HRQoL in craniopharyngioma survivors treated with proton beam radiation therapy, whose parents rated them as having poorer HRQoL than suggested by self-report (Laffond, 2012). Parents' ratings generally suggesting less distress may reflect a general maturity and life experience that parents bring to bear on their judgments. Their perspective on the factors that reduce HRQoL may be more global, while children's own ratings of distress may reflect a developmentally appropriate ego-centrism. Given a lack of normative data for parent ratings on the KINDL-R, and given that this finding was consistent across all tumor groups and nearly all subscales, it is impossible to say with certainty whether this is a developmentally appropriate finding, or whether something about the “cancer experience” within a family has a greater impact on perceived HRQoL for children than parents. Given the discrepancy between parent and child ratings, it is important to obtain independent HRQoL assessments from both patients and their parents.
One notable exception occurred for the ST group, who rated their physical well-being at the same level as their parents. This may reflect the obvious physical effects of some solid tumors (e.g., osteosarcoma), which can result in amputation, repeated orthopedic surgeries, and utilization of crutches, wheelchairs, or prostheses. These outwardly obvious physical manifestations of illness may produce a more cohesive assessment of the physical impact of cancer and treatment than some of the physical symptoms associated with BT (e.g., fatigue, dizziness), which may be harder for parents to assess in their children.
HRQoL's Association with Cognitive Function
Mean IQ and BRIEF scores were within the average range, indicating some preservation of function following CRT, which is consistent with prior findings (Netson, Conklin, Wu, Xiong & Merchant, 2013; Netson, Conklin, Wu, Xiong & Merchant, 2012; Conklin et al., 2008). IQ was not directly correlated with parent- or child-reported HRQoL. In other populations (e.g., children with epilepsy), the relationship between IQ and HRQoL is mediated by the child's mental health status (Fayed, Davis, Streiner, et al., 2015), suggesting that this is a complex relationship that requires further investigation. Moderate to strong correlations were observed between nearly all Parent KINDL-R subscales and BRIEF indices, suggesting a significant relationship between EF and HRQoL in childhood BT survivors. It is interesting to note that these correlations were less pronounced for child-reported HRQoL. EF is presumed to influence HRQoL by affecting a child's ability to independently make decisions, organize behavior, and self-monitor his or her actions. It was hypothesized that the greater a child's capabilities in the EF domain, the more satisfied the child would be with his or her HRQoL. Although trends emerged to suggest some modest associations between parent-rated EF and child-rated HRQoL, these did not rise to the level of significance seen when parents rated both EF and HRQoL. Shared method variance between the BRIEF and the KINDL-R Parent form (i.e., both are parent-report questionnaires) may account for this finding; however, it may also be the case that children and adolescents have limited insight into the impact of their reduced executive skills on their performance of age-appropriate activities, or that they are not bothered by their difficulties.
When considering multiple factors such as gender, age at diagnosis, and cognitive functioning, children's metacognitive skills (e.g., initiation, working memory, organization, self-monitoring) accounted for a significant amount of variance in parent-rated HRQoL. These metacognitive skills are consistent with the cognitive sequelae seen in pediatric cancer survivors who have undergone CNS-directed therapies (Winter et al., 2014; Howarth et al., 2013; Brinkman et al., 2012; Conklin et al., 2012) and may be targeted for pharmaceutical or behavioral interventions. While these metacognitive skills are critical for the age-appropriate performance of day-to-day tasks, they do not often cause overt disruption to a child's behavior or their surrounding environment, and thus may be less likely to be noticed by the child. More externalized behaviors such as those reflected in the BRIEF Behavior Regulation Index (e.g., inhibition, shifting, emotional control) are likely to result in more overtly observable behavioral disruptions and likely behavioral consequences; however, these are not generally the types of executive skills affected by CNS cancer and treatment.
Limitations
Although this study provides important insights into the relation of cognitive performance and HRQoL after CRT, it is not without limitations. The measure selected to assess HRQoL has been validated and found to be reliable; however, it is not the most widely-used measure of HRQoL. The lack of normative data for parent-report forms renders this measure more difficult to use. Given that even our sibling control group had been affected in some way by the “cancer experience” within their family, it would be interesting to compare ratings with those from a healthy, non-cancer-related control group of similar demographic make-up to the BT survivors. It should also be noted that this is a relatively well-functioning group of BT survivors. Participants were intentionally selected with no evidence of premorbid neurologic or psychiatric dysfunction, and did not exhibit impairment in IQ at study entry. As a result, the group showed IQ and executive skills largely within the average range. This may be related to the types of BT diagnoses in this sample, and findings cannot be fully generalized to other types of BT (e.g., medulloblastoma) without further investigation. Likewise, children with impaired IQ either premorbidly or as a result of their BT diagnosis and treatment may also show a different pattern of HRQoL. Finally, this investigation relied on parent-report of executive skills. Utilization of laboratory tests or real-world, third-party observation of EF may limit the effect of shared method variance and provide additional insight into the influence of EF on HRQoL.
Conclusions
These preliminary results show that, while CRT may afford relative sparing of IQ and EF in children with BT, HRQoL continues to be an area of risk. Varying methods of assessment produce variable results, with children self-reporting poorer HRQoL than their parents observe. This discrepancy may reflect maturity or perspective gained from life experience that is absent from children's own ratings, or a belief that well-functioning children are a reflection of parenting skills; however, parents may be unaware of distress about specific areas of functioning such as school performance or self-esteem. Investigating whether EF-based interventions improve HRQoL in BT survivors may provide useful direction in improving the effectiveness of treatment patients receive. Focusing intervention on metacognitive abilities, which can be heavily affected by CNS-directed therapy, may be particularly beneficial.
Compliance with Ethical Standards
In accordance with the Helsinki Declaration, this study was approved by the institutional review board and written, informed consent was obtained prior to participation.
Acknowledgments
Funding: This work was supported in part by the National Cancer Institute (St. Jude Cancer Center Support [CORE] Grant [P30 CA21765]; H.C., R21 CA131616); the International Neuropsychological Society (H.C., Rita G. Rudel Award); and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, Lequin M, Catsman-Berrevoets CE. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. Journal of Clinical Oncology. 2009;27(21):3526–32. doi: 10.1200/JCO.2008.19.6303. [DOI] [PubMed] [Google Scholar]
- Anderson V, Le Brocque R, Iselin G, Eren S, Dob R, Davern TJ. Adaptive ability, behavior and quality of life pre and posttraumatic brain injury in childhood. Disability & Rehabilitation. 2012;34(19):1639–1647. doi: 10.3109/09638288.2012.656789. [DOI] [PubMed] [Google Scholar]
- Aran-Filippetti V, Richaud de Minzi MC. A structural analysis of executive functions and socioeconomic status in school-age children: cognitive factors as effect mediators. Journal of Genetic Psychology. 2012;173(4):393–416. doi: 10.1080/00221325.2011.602374. [DOI] [PubMed] [Google Scholar]
- Barratt WR. The Barratt Simplified Measure of Social Status. 2006 Retrieved from the Indiana State University website: http://wbarratt.indstate.edu/socialclass/Barratt_Simplified_Measure_of_Social_Status.pdf.
- Benesch M, Spiegl K, Winter A, Passini A, Lackner H, Moser A, Urban C. A scoring system to quantify late effects in children after treatment for medulloblastoma / ependymoma and its correlation with quality of life and neurocognitive functioning. Child's Nervous System. 2009;25(2):173–81. doi: 10.1007/s00381-008-0742-1. [DOI] [PubMed] [Google Scholar]
- Bhat SR, Goodwin TL, Burwinkle TM, Lansdale MF, Dahl GV, Huhn SL, Fisher PG. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. Journal of Clinical Oncology. 2005;23(24):5493–500. doi: 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- Brinkman TM, Reddick WE, Luxton J, Glass JO, Sabin ND, Srivastava DK, Krull KR. Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro-Oncology. 2012;14(Suppl 4):iv25–36. doi: 10.1093/neuonc/nos214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull KS, Liossi C, Culliford D, Peacock JL, Kennedy CR, Children's Cancer and Leukaemia Group Child-related characteristics predicting subsequent health-related quality of life in 8- to 14-year-old children with and without cerebellar tumors: a prospective longitudinal study. Neuro-oncology Practice. 2014;1:114–122. doi: 10.1093/nop/npu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Mental Retardation and Developmental Disabilities Research Reviews. 2006;12(3):184–91. doi: 10.1002/mrdd.20110. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Conklin HM, Ashford JA, Howarth RA, Merchant TE, Ogg RJ, Santana V, Xiong X. Working memory performance among childhood brain tumor survivors. Journal of the International Neuropsychological Society. 2012;18:996–1005. doi: 10.1017/S1355617712000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. Journal of Clinical Oncology. 2008;26(24):3965–70. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiser C, Varni JW. Health-related quality of life and symptom reporting: similarities and differences between children and their parents. European Journal of Pediatrics. 2013;172:1299–1304. doi: 10.1007/s00431-013-2049-9. [DOI] [PubMed] [Google Scholar]
- Fayed N, Davis AM, Streiner DL, Rosenbaum PL, Cunningham CE, Lach LM, Boyle MH, Ronen GM, QUALITÉ Study Group Children's perspective of quality of life in epilepsy. Neurology. 2015;84:1830–7. doi: 10.1212/WNL.0000000000001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Odessa, FL: Psychological Assesssment Resources, Inc; 2000. [Google Scholar]
- Howarth RA, Ashford JM, Merchant TE, Ogg RJ, Santana V, Wu S, Conklin HM. The utility of parent report in the assessment of working memory among childhood brain tumor survivors. Journal of the International Neuropsychological Society. 2013 doi: 10.1017/S1355617712001567. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun LE, Mulhern RK, Crisco JJ. Quality of life in children treated for brain tumors. Intellectual, emotional, and academic function. Journal of Neurosurgery. 1983;58(1):1–6. doi: 10.3171/jns.1983.58.1.0001. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? The Journals of Gerontology, Series A, Biological Sciences and Medical Sciences. 2004;59:818–26. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- Laffond C, Dellatolas G, Alapetite C, Puget S, Grill J, Habrand JL. Quality-of-life, mood and executive functioning after childhood craniopharyngioma treated with surgery and proton beam therapy. Brain Injury. 2012;26:270–281. doi: 10.3109/02699052.2011.648709. [DOI] [PubMed] [Google Scholar]
- Macartney G, Harrison MB, VanDenKerkhof E, Stacey D, McCarthy P. Quality of life and symptoms in pediatric brain tumor survivors: a systematic review. Journal of Pediatric Oncology Nursing. 2014;31:65–77. doi: 10.1177/1043454213520191. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Reddick WE, Palmer SL, Glass JO, Elkin TD, Kun LE, Gajjar A. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Annuals of Neurology. 1999;46:834–841. doi: 10.1002/1531-8249(199912)46:6<834::aid-ana5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncology. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children's adaptive functioning following conformal radiation therapy for localized ependymoma. International Journal of Radiation Oncology Biology Physics. 2012;84(1):217–223. doi: 10.1016/j.ijrobp.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. International Journal of Radiation Oncology Biology Physics. 2013;85(5):1301–6. doi: 10.1016/j.ijrobp.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens-Sieberer U, Bullinger M. KINDL-R Manual (English) 2000 Retrieved from: http://kindl.org/english/manual/
- Reddick WE, Russell JM, Glass JO, Xiong X, Mulhern RK, Langston JW, Gajjar A. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magnetic Resonance Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. [DOI] [PubMed] [Google Scholar]
- Reimers TS, Mortensen EL, Nysom K, Schmiegelow K. Health-related quality of life in long-term survivors of childhood brain tumors. Pediatric Blood and Cancer. 2009;53(6):1086–91. doi: 10.1002/pbc.22122. [DOI] [PubMed] [Google Scholar]
- Soo C, Tate R, Brookes N. Psychosocial adjustment following acquired brain injury in childhood and adolescence: Executive, behavioural and emotional contributions. Brain Injury. 2014;28:906–914. doi: 10.3109/02699052.2014.888762. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Winter AL, Conklin HM, Tyc VL, Stancel H, Hinds PS, Hudson MM, Kahalley LS. Executive function late effects in survivors of pediatric brain tumors and acute lymphoblastic leukemia. Journal of Clinical and Experimental Neuropsychology. 2014;36(8):818–830. doi: 10.1080/13803395.2014.943695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters Gregorio G, Ponds RW, Smeets SM, Jonker F, Pouwels CG, Verhey FR, van Heugten CM. Associations between executive functioning, coping, and psychosocial functioning after acquired brain injury. British Journal of Clinical Psychology. 2015 doi: 10.1111/bjc.12074. Advance online publication. [DOI] [PubMed] [Google Scholar]