Abstract
The sub-renal capsule graft site for in vivo growth and development of developing organs and can be Q2 used to great advantage in the “rescue” of organ rudiments from “embryonic” or “birth” lethal mutant mice, which permits examination of the full impact of gene knockout on all phases of development from morphogenesis to adult functional differentiation. Another use of the sub-renal capsule graft site is the examination of normal and “chemically perturbed” development of human fetal organs. Tissue recombinants composed of various types of epithelium and mesenchyme, when grafted under the renal capsule undergo normal development and in 3–4 weeks achieve full adult functional cytodifferentiation. The investigator can control many of the developmental parameters of the graft such as endocrine status of the host and treatment of the host with a variety of biologically active agents to assess their effects on development and differentiation.
Keywords: Renal capsule, Transplantation
1. Introduction
Transplantation of tissues and organ rudiments under the renal capsule is an extremely useful technique for study of development and differentiation as it allows the investigator to examine organogenesis from its inception through all stages to final functional (secretory) cytodifferentiation under experimental conditions in which the investigator has to control many of the developmental parameters. For example, embryonic urogenital sinuses of both male and female mouse embryos when transplanted under the renal capsule of adult male hosts undergo prostatic development and achieve secretory function following 1 month of growth under the renal capsule (Cunha, 1975). During this process solid prostatic buds emerge from grafts of the ambisexual urogenital sinus a few days post-transplantation. Within a week of post-transplantation, solid prostatic buds canalize to form patent ducts. One month after transplantation the epithelium matures into an adult-like secretory epithelium producing prostate-specific secretory proteins (Donjacour and Cunha, 1993). Of course, in the case of androgen-dependent prostatic development, manipulation of the hormonal status of the male host is an important variable to be controlled by the investigator (intact versus castrated host, treatment with testosterone versus anti-androgens [flutamide], or treatment with environmentally relevant estrogenic chemicals such as bis-phenol A). The ideal conditions of the renal capsule transplantation site are due to the extremely high vascular density of the graft site, which gives the kidney its bright red color. Accordingly, the take rate of grafts under the renal capsule approaches 100%. The method can be learnt in an afternoon and with experience high take rates can be achieved.
2. Rescue of organ development
An important use of renal capsule transplantation is the rescue to organ development in germ-line knockout mice, which may be either “embryonic lethal” or “birth lethal”. In such mutant mice it may be impossible to follow development of organs sufficiently to reveal the full impact of gene knockout due to their early demise. There are two solutions to this problem. A conditional gene knockout mouse line can be constructed and maintained at great expense. Alternatively, the germline mutant organ rudiment can be isolated from “embryonic lethal” or “birth lethal” mice and transplanted under the renal capsule of the appropriate host mouse. This approach was used to study the role of p63 and basal epithelial cells in prostatic biology. Prostatic basal epithelial cells normally express p63, and thus it was suspected that prostates of p63 knockout mice might lack basal cells. This possibility could not be tested directly in p63 knockout mice due to lethality at birth, well before prostatic differentiation. Accordingly, late gestation or newborn p63 knockout prostatic rudiments were grown under the renal capsule of male hosts and formed mature prostatic tissue lacking basal epithelial cells. Such p63 knockout prostates exhibited a variety of abnormalities (Kurita et al., 2004). This important observation was achieved rapidly at reduced expense.
Well before conditional Rb knockout mice were available, we created Rb-KO prostate, also at reduced expense, through a subrenal capsule transplantation strategy. Germ-line Rb-KO mice die around day 12 of gestation, well before the prostatic rudiment exists. The prostate develops from the endoderm of the urogenital sinus, which is derived from the cloaca. Accordingly, we transplanted cloacas from 11-to 12-day embryonic Rb-KO mice under the renal capsule. After 1 month a considerable amount of Rb-KO prostate was present in the grafts from which Rb-KO prostatic epithelium was isolated and studied (Day et al., 2002).
For several of our embryonic organ rescue experiments, the mutant tissues were obtained from collaborations who had constructed the mutant mice. Mutant mouse lines are frequently used for very limited purposes, and consequently most of the mutant mouse is discarded at the end of the day. Organs of developing mutant mice are a valuable resource, and typically the investigator with the mutant mouse colony is willing to make available unused mouse parts to others. We have found that embryonic, neonatal or even adult mouse organs/organ rudiments remain viable for up to 3 days (probably longer) if stored at 0–4 °C in medium containing 10% fetal bovine serum. This means that discarded mutant mouse parts from investigators on the east coast can be shipped overnight to the west coast for transplantation the next day. For this purpose, it is best to place mouse parts in 50 ml tubes filled to near the top with medium. This averts the possibility that the tube of medium will freeze solid thus destroying the mouse tissue because ice taken directly from an ice machine is typically maintained well below 0 °C.
A preferred solution to embryonic or birth lethality in germline KO mice is to construct a conditional knockout mouse using, for example, an “epithelial tissue specific” promoter. The problem is that there are very few “epithelial tissue specific” promoters that can be used to delete a gene from all of the cell types within an epithelium. More disturbing is the fact that many investigators fail to distinguish “epithelial tissue-specific” versus “epithelial cell-specific” promoters. This problem is seen frequently in the mammary and prostatic fields, but is not unique to either of these fields. The problem lies in the fact that both mammary and prostatic epithelia contain more than one cell type. For example, mammary and prostatic epithelium contained columnar secretory cells as well as myoepithelial or basal cells. Conditional knockout of a gene in the prostate using the probasin promoter deletes the gene of interest from the secretory luminal cells, but not the basal cells. Likewise, conditional knockout of a gene in the mammary gland using the w promoter or the beta-lactoglobulin milk protein promoter deletes the gene of interest from the secretory luminal cells, but not the myoepithelial cells (Chapman et al., 2000; Walton et al., 2001). Thus, in such mice the epithelium is mosaic with respect to the gene of interest. Accordingly, the strategy of conditional gene knockout has to take into consideration whether the promoter is a tissue-specific or a cell-specific promoter. Of course, the use of germline gene knockout mice and renal capsule transplantation eliminates this problem.
3. Growth of human fetal organs under the renal capsule
With the advent of the athymic nude mouse and other immune-compromised mutant mice, the transplantation of human fetal organs has become possible. In the early 1980s Dr. Stan Robboy and I embarked on a study on the effect of diethylstilbestrol (DES) on development of human female fetal reproductive tract organs as an experimental approach to the human DES episode. Over 1 million young women were exposed in utero to DES from the 1940s to ~ 1970, and later developed upper and lower genital tract anomalies and in rare cases vaginal or cervical clear cell adenocarcinomas (Herbst et al., 1971, 1975; Kaufman et al., 1977, 1980; Jefferies et al., 1984). To directly explore the effects of DES on human reproductive tract development in vivo, human fetal reproductive tracts consisting of Fallopian tubes, uterine corpus, cervix and vagina were transplanted under the renal capsule of female athymic mice. Human fetal female reproductive tracts grown as grafts in intact (untreated) female athymic mice grew in size and developed normally. In this xenograft model the following results were obtained: (a) the paired Mullerian ducts fused to form a single uterovaginal canal, (b) endometrial and tubal mesenchyme differentiated normally into stromal and smooth muscle layers, (c) tubal and endometrial mucosa underwent plication, (d) endometrial glands formed, and (e) the simple columnar Mullerian vaginal epithelium underwent normal squamous differentiation (Robboy et al., 1982; Taguchi et al., 1983; Cunha et al., 1987a, 1987b). In contrast, human fetal female reproductive tract organs grafted into DES-treated female athymic mice developed abnormally exhibiting: (a) partial obliteration of the upper genital tract, (b) inhibition of differentiation of utero-tubal mesenchyme into stromal and smooth muscle layers, (c) inhibition of plical development in the Fallopian (uterine) tube, (d) inhibition of normal transformation of caudal Mullerian epithelium into a stratified squamous vaginal epithelium and (e) induction of vaginal adenosis (Robboy et al., 1982; Taguchi et al., 1983). Subsequent studies showed that certain “fertility drugs” (Clomid) had adverse developmental effects similar to that of DES on developing human female reproductive tracts (Cunha et al., 1987a). Today certain consumer products contain chemicals such as bis-phenol A (BPA, a weak estrogen) alleged to be harmful to human development based upon animal studies and correlational epidemiologic studies (Rochester, 2013; Vandenberg, 2014). Whether BPA actually can elicit an adverse effect on developing human organs has never been directly assessed. Such experiments are possible using transplants of human fetal reproductive tracts into mice treated with BPA. Finally, over many years the Cunha and Baskin labs have used the renal capsule site for the transplantation of tissue recombinants as described in this volume.
4. Materials and methods
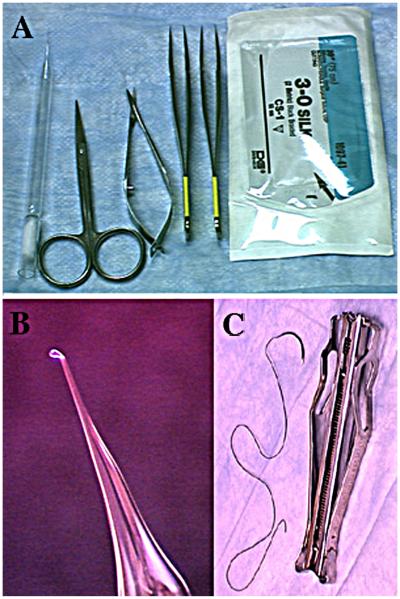
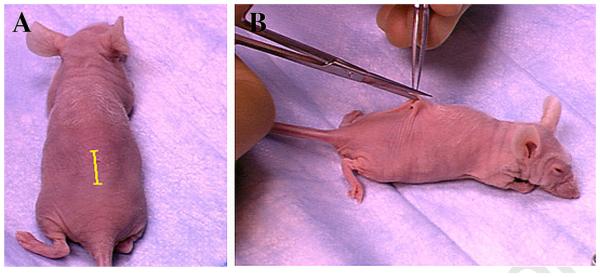
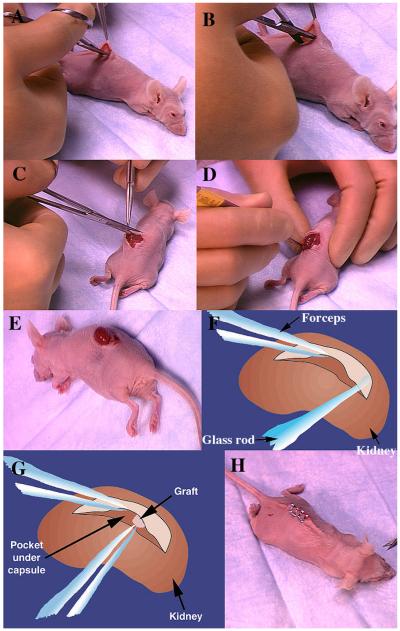
The method of sub-renal capsule transplantation is illustrated in the NIH website on mammary gland biology (http://mammary.nih.gov/tools/mousework/Cunha001/index.html) and will be described below. The following instruments are required: #5 Dumont forceps [Fine Science Tools, Foster City, CA], van Graefe microdissecting knife [Fine Science Tools, Foster City, CA], spring-loaded Vannas scissors [Fine Science Tools, Foster City, CA], a glass Pasteur pipette which has been drawn out thin and fire-polished with a rounded closed end, wound clips or sutures (Fig. 1). Animals are anesthetized with avertin or other agents. Anesthesia is checked by applying pressure to the hind foot. If the animal responds to pressure, more time is allowed for the anesthesia to take effect. Once the animal no longer responds to pressure, the back of the skin is wetted with 70% ethanol. The skin is cut along the dorsal midline with a pair of scissors creating a midline incision approximately 3/4 in. in length (Fig. 2). The dermis is separated from the underlying body wall by blunt dissection on both sides of the incision for bilateral grafting or on one side only (Fig. 3A & B). The animal is placed on its side, and the location of the kidney is identified by viewing it through the muscular body wall. A small incision is made with scissors in the body wall over the kidney just slightly longer than the long axis of the kidney (Fig. 3C). Care is taken to avoid major vessels and nerves. If the anesthetized mouse gulps for air when the body wall is incised, it means that the incision through the body wall was made too far cranially, and thus the pleural cavity was inadvertently opened. In that case, the animal should be euthanized. After successful opening of the body wall, the kidney is “popped out” of the hole in the body wall by applying pressure on either side of the kidney using the forefinger and thumb (Fig. 3D). The exteriorized kidney (Fig. 3E) is allowed to rest on the cut edges of the body wall and skin. In the female mice, exteriorization of the kidney from the body cavity can be facilitated by pulling on the ovarian fat pad with a pair of blunt forceps, gently guiding the kidney through the opening in the body wall and “tucking” the cut edge of the body wall and skin under the exteriorized kidney. Friction with the cut edges of the body wall and skin is usually sufficient to maintain the exteriorized kidney in place (Fig. 3E). If the exteriorized kidney slips back into the body cavity, it is because the incisions in the skin and body wall are too long, and thus the technique should be adjusted accordingly. Fine #5 Dumont forceps is used to gently pinch and stretch the capsule upward from the parenchyma of the kidney so that a 2–4 mm incision can be made in the delicate capsule with a van Graefe microdissecting knife. The size of the incision in the capsule is determined by the size of the graft. Care should be taken to insure that the forceps or other instruments do not damage the underlying kidney parenchyma, which will result in bleeding. A glass Pasteur pipette, which has been drawn thin and fire-polished with a rounded closed end (Fig. 1B), is manipulated under the capsule tangential to the surface of the kidney to create a pocket between the capsule and the underlying renal parenchyma (Fig. 3F). Grafts are transferred to the surface of the kidney using forceps or a holding pipette. The cut edge of the kidney capsule is lifted with the fine forceps, and the graft is pushed into the pocket under the renal capsule using the fire-polished glass pipette (Fig. 3G). Several grafts can be placed under the kidney capsule with no apparent ill effect to the mouse. However, each graft should be spaced as far apart as possible to avert their growing together into a common mass. If during the course of grafting the capsule becomes dehydrated, it can be moistened with PBS. When grafting is complete, the kidney is gently eased back into the body cavity. The operation can be repeated using the contralateral kidney. After return of the kidneys to the peritoneal cavity, the edges of the back skin are aligned and closed with wound clips or sutures (Fig. 3H). The animal is allowed to recover from the anesthesia. While grafts of any size can be implanted, thickness of the graft should not exceed 2 mm. Implantation of grafts greater than 2 mm thick will result in central necrosis of the graft prior to establishment of vascularization.
Fig. 1.
(A, C) Instruments and materials used in sub-renal capsule transplantation: small scissors, spring-loaded scissors, Dumont #5 forceps, wound clips and sutures. (B) A “drown out” fire polished Pasteur pipette used to make the pocket between the renal capsule and the underlying renal parenchyma.
Fig. 2.
The mid-dorsal incision in the skin. (A) Depicts the position and length of the skin incision (yellow line), which is carried out in (B).
Fig. 3.
Illustration of the steps in sub-renal capsule transplantation. Blunt dissection by “opening” of the scissors to separate the skin from the underlying body wall (A & B). Incision through the muscular body wall overlying the kidney (C). Exteriorization of the kidney by applying pressure on the animal's body using the forefinger and thumb (D). The exteriorized kidney remains in place as a result of tucking the cut edge of the skin under the kidney (E). After the initial hole is made in the delicate capsule, a pocket is created and enlarged using the fire-polished pipette (F). Insertion of the graft into the pocket by lifting the edge of the renal capsule and pushing the graft into place with forceps or the fire-polished pipette (G). After return of the kidney into the peritoneal cavity, the skin incision is closed with wound clips or with sutures (H). Suturing of the renal capsule and body wall incision is not necessary.
Based upon the design of the experiment we have harvested the grafts after as few as 2 or 3 days, but the value of the technique is achieved by longer periods of growth so that “adult” type differentiation can be achieved (usually in 3–4 weeks). We have maintained some grafts in hosts for up to 1 year. However, note that for secretory organs, copious amounts of secretion accumulate in ductal lumina after 1 month. Since there is no exit for these secretions, cystic distension of the ducts will eventually occur. To avert this problem, secretory organs should be harvested at about 1 month post-grafting. Likewise, for organs such as skin or vagina, continuous epithelial proliferation will generate sloughed epithelial cells into the graft lumen and thus distort tissue architecture. Again the solution is harvest at 3–4 weeks post-grafting.
Finally, it should be noted that sub-renal capsule grafting is also possible in rats using the technique described above. In the case of both rats and mice, graft rejection is averted through use of inbred animals in which that graft and the host are of the same strain. Fisher 344 rats are inbred, while Sprague–Dawley rats are outbred. Of course, immune-compromised rats are now available and should be used as needed to avert graft rejection.
5. Discussion
The renal capsule graft site is perhaps the best site for transplantation of developing organs owing to its high degree of vascularity, which is manifested by the red color of the kidney. In contrast, other graft sites such as the subcutaneous site consists of white connective tissue, which although vascularized does not have the degree of vascularity as that of the kidney. Consequently, we have consistently observed a much higher (nearly 100%) take rate in renal capsule transplants than that in subcutaneous transplants especially when normal tissue is transplanted. In contrast, take rate of highly angiogenic tumor cells is high in both subcutaneous and renal capsule transplants.
The sub-renal capsule graft site is ideal for tissue recombinants. Following combination of epithelium and mesenchyme (see chapter in this issue), the tissue recombinants can be either maintained as short-term organ cultures or alternatively transplanted under the renal capsule. Organ culture provides shortterm growth, limited opportunity for differentiation and no possibility for functional cytodifferentiation and secretion. In contrast, tissue recombinants grown for one month under the renal capsule achieve adult-like differentiation and express tissue-specific secretory proteins (Donjacour and Cunha, 1993; Aboseif et al., 1999). Likewise, grafts of mutant mouse organ rudiments developing under the renal capsule achieve their fullest developmental potential so that the complete impact of gene knockout can be assessed particularly in specimens obtained from embryonic lethal embryos. Human fetal organs also grow and differentiate in a fashion comparable to that seen during normal human development. Lastly, the mouse hosts bearing developing grafts can be manipulated experimentally through alterations of endocrine parameters (male versus female hosts, intact hosts versus gonadectomized hosts, or hormonally treated hosts). Host mice can also be treated with the biologically active agent of choice or with chemicals in the human environment suspected to have adverse developmental effects.
Acknowledgment
This work was supported by NIH Grant RO1 DK0581050.
Abbreviations
- DES
diethylstilbestrol
- BPA
bis-phenol A
References
- Aboseif S, El-Sakka A, Young P, Cunha G. Mesenchymal reprogramming of adult human epithelial differentiation. Differ. Res. Biol. Divers. 1999;65:113–118. doi: 10.1046/j.1432-0436.1999.6520113.x. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Lourenco P, Tonner E, Flint D, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. The role of Stat3 in apoptosis and mammary gland involution. Conditional deletion of Stat3. Adv. Exp. Med. Biol. 2000;480:129–138. doi: 10.1007/0-306-46832-8_16. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Age-dependent loss of sensitivity of female urogenital sinus to androgenic conditions as a function of the epithelial–stromal interaction. En-docrinology. 1975;95:665–673. doi: 10.1210/endo-97-3-665. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Namikawa R, Nishizuka Y, Robboy SJ. Teratogenic effects of clomid, tamoxifen, and diethylstilbestrol on the developing human female genital tract. Hum. Pathol. 1987a;18:1132–1143. doi: 10.1016/s0046-8177(87)80381-7. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Sugimura Y, Lawrence DW, Mahmood F, Robboy SJ. Absence of teratogenic effects of progesterone on the developing gential tract of the human female fetus. Hum. Pathol. 1987b;19:777–783. doi: 10.1016/s0046-8177(88)80260-0. [DOI] [PubMed] [Google Scholar]
- Day KC, McCabe MT, Zhao X, Wang Y, Davis JN, Phillips J, Von Geldern M, Ried T, KuKuruga MA, Cunha GR, Hayward SW, Day ML. Rescue of embryonic epithelium reveals that the homozygous deletion of the retinoblastoma gene confers growth factor independence and immortality but does not in?uence epithelial differentiation or tissue morphogenesis. J. Biol. Chem. 2002;277:44475–44484. doi: 10.1074/jbc.M205361200. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;131:2342–2350. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Scully RE, Robboy SJ. Vaginal adenosis and other diethylstilbestrol-related abnormalities. Clin. Obstet. Gynecol. 1975;18:185–194. doi: 10.1097/00003081-197509000-00021. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina: association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Jefferies JA, Robboy SJ, O’Brien PC, Bergstralh EJ, Labarthe DR, Barnes AB, Noller KL, Hatab PA, Kaufman RH, Townsend DE. Structural anomalies of the cervix and vagina in women enrolled in the Diethylstilbestrol Adenosis (DESAD) Project. Am. J. Obstet. Gynecol. 1984;148:59–66. doi: 10.1016/s0002-9378(84)80033-2. [DOI] [PubMed] [Google Scholar]
- Kaufman RH, Adam E, Binder GL, Gerthoffer EA. Upper genital tract changes and pregnancy outcome in offspring exposed in utero to diethylstilbestrol. Am. J. Obstet. Gynecol. 1980;154:330–332. doi: 10.1016/0002-9378(80)90913-8. [DOI] [PubMed] [Google Scholar]
- Kaufman RH, Binder GL, Gray Jr. PM, Adam E. Upper genital tract Q changes associated with exposure in utero to diethylstilbestrol. Am. J. Obstet. Gynecol. 1977;128:51–59. doi: 10.1016/0002-9378(77)90294-0. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Taguchi O, Cunha GR. Normal development of the human female reproductive tract and alterations resulting from experimental exposure to diethylstilbestrol. Hum. Pathol. 1982;13:190–198. doi: 10.1016/s0046-8177(82)80177-9. [DOI] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Robboy SJ. Experimental study of the effect of diethylstilbestrol on the development of the human female reproductive tract. Int. J. Biol. Res. Pregnancy. 1983;4:56–70. [PubMed] [Google Scholar]
- Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitam. Horm. 2014;94:129–165. doi: 10.1016/B978-0-12-800095-3.00005-5. [DOI] [PubMed] [Google Scholar]
- Walton KD, Wagner KU, Rucker 3rd EB, Shillingford JM, Miyoshi K, Hennighausen L. Conditional deletion of the bcl-x gene from mouse mam-mary epithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech. Dev. 2001;109:281–293. doi: 10.1016/s0925-4773(01)00549-4. [DOI] [PubMed] [Google Scholar]