Abstract
Circulating melanoma cells (CMCs) represent critical mediators of metastatic melanoma progression. However, isolation and characterization of CMCs has been challenging due to the low frequency of these cells and the paucity of melanoma-specific cell surface markers. Herein, we describe a method for the isolation of CMCs that employs two independent markers, displays high sensitivity for CMC enrichment, and can be readily adapted to include additional molecular melanoma markers of interest. CMCs isolated by this method are enriched for ABCB5-positive melanoma stem cells, are tumorigenic in xenotransplantation assays, and can be used for phenotypical, genetic, and functional investigations of CMC biology.
Keywords: Circulating tumor cells, Circulating melanoma cells, Melanoma stem cells, ABCB5, Tumorigenicity, Metastasis, Flow cytometry, Xenotransplantation
1 Introduction
Circulating tumor cells (CTCs) represent an important step in tumor metastasis and have been identified in a number of human malignancies [1]. Detection and quantification of circulating melanoma cells (CMCs) provide diagnostic and prognostic information for melanoma patients, and have potential for the assessment of therapeutic response [2–4]. Different strategies have been explored to detect and isolate CMCs in peripheral blood, including use of melanoma-specific gene transcripts [5], melanoma cell markers such as melanoma-associated chondroitin sulfate proteoglycan andMART-1 [6, 7], the size difference between CMCs and peripheral blood cells [8], and the presence of melanin in pigmented melanoma cells [9]. However, the utility of these approaches are often limited because marker-positive CMCs may represent only subpopulations of phenotypically heterogeneous circulating melanoma cells or because no viable CMCs can be obtained for further characterization and functional testing for tumorigenicity. Here, we describe an experimental method to isolate viable human CMCs from whole blood specimens of subcutaneously xenografted mice using two independent markers, i.e., genetically labeled red fluorescence protein and the human major histocompatibility complex class I antigen HLA-ABC, which maximizes the yield of CMCs and provides a comprehensive representation of the overall CMC population. Additional markers, such as the marker of melanoma initiating cells, ABCB5 [4, 10–14] or CD271 [15] can be readily incorporated so that subsets of CMCs can be further characterized. Moreover, biological functions of viable CMCs can be studied following isolation [12]. We have shown in vivo tumorigenic and metastatic capacity of viable CMCs isolated by this method in serial xenotransplantation studies, using both established human melanoma cell lines or melanoma cells derived from a clinical specimen [12], demonstrating that this method includes selection of malignant melanoma initiating cells. Thus, the method described here represents a sensitive, comprehensive, and flexible assay for the study of CMCs as well as potentially CTCs in other malignancies.
2 Materials
Prepare all reagents under sterile condition.
Lipofectamine™ 2000 transfection reagent (see Note 1).
Red fluorescence protein (RFP) plasmid (see Note 2).
Allophycocyanin (APC)-conjugated murine anti-human HLA-ABC IgG1 antibody (BD Biosciences) (see Note 2).
APC-conjugated isotype control murine IgG1 antibody (Miltenyi Biotec).
Heparin sodium Injection 1000 USP units/mL.
Growth factor-reduced matrigel (BD Biosciences) (see Note 3).
Highly immune-compromised NOD-scid IL2Rγnull (NSG) mice at 6 weeks of age are kept in a defined condition supplied with autoclaved cage and water (see Note 4).
Cytomation MoFlo sorter (Dako) (see Note 2).
Prepare immunostaining buffer for flow cytometry analysis by mixing 490 mL of 1× PBS with 10 mL of fetal bovine serum, followed by filtration sterilization.
3 Methods
3.1 Generation of Fluorescence-Labeled Human Melanoma Cells
Seed 1 × 106 of human melanoma cells in 6-well plate with 2 mL of antibiotics-free culture medium per well and grow at 37 °C over night.
Transfect each well of cells with 4 mg of plasmid DNA coding RFP gene with 10 mL of Lipofectamine™ 2000 transfection reagent.
Isolate RFPhigh cells by fluorescence-activated cell sorting 2 weeks after transfection (see Note 5).
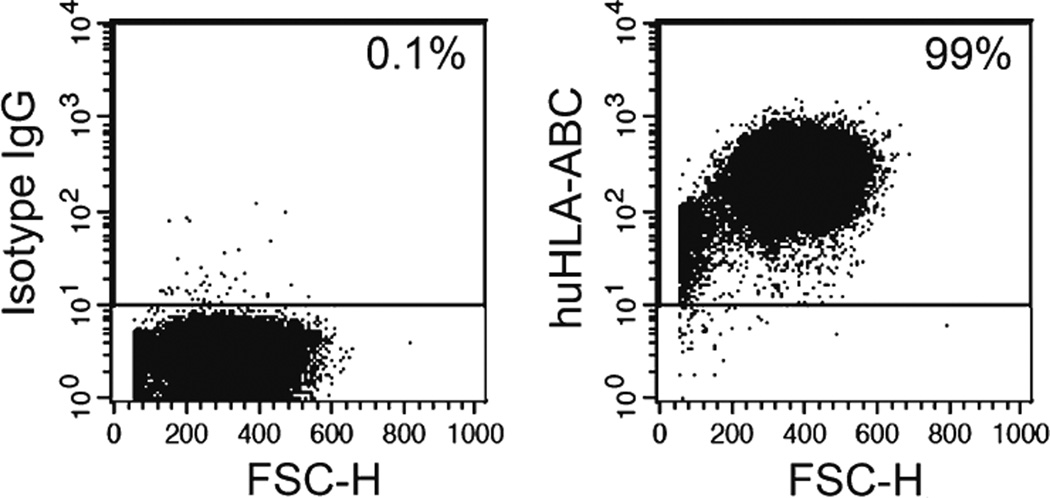
Stain stable RFPpositive cells with APC-conjugated murine anti-human HLA-ABC IgG1 antibody or isotype control antibody. Quantify HLA-ABC expression in human melanoma cells flow cytometry at FL4 spectrum (see Note 6) (Fig. 1).
Fig. 1.
Expression of HLA-ABC antigens in human melanoma cells determined by flow cytometry
3.2 Isolation of Circulating Melanoma Cells
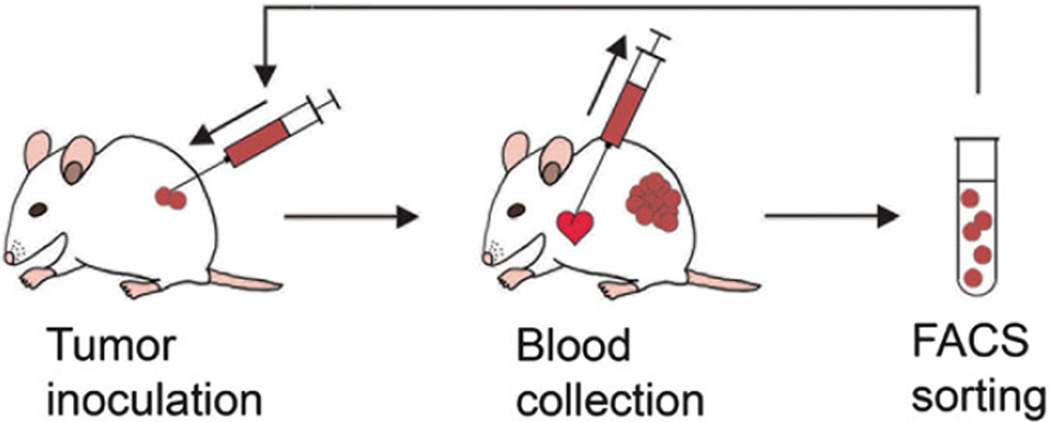
Tag and shave NSG mice. Inoculate RFPpositive human melanoma cells subcutaneously on the right flank of the mice (2 × 104 cells in 100 µL of PBS per inoculum). Tumor size and mouse body weight are measured weekly. Inoculate one tumor-free control mouse inoculated with PBS alone (see Note 7) (Fig. 2).
Euthanize mice at the predetermined end point when the establishment of distant metastases has been confirmed. Immediately collect whole blood specimens by heart acupuncture, using a 1 mL syringe/27 G needle prefilled with 100 USP units of heparin (see Note 8).
Mix each volume of blood specimen with 8 volumes of sterile water, gently invert 2–3 times to lyse red blood cells, adding one volume of sterile 10× PBS and gently mixing again (see Note 9).
Centrifuge cells at 2000 rpm for 5 min at 4 °C to isolate peripheral blood mononuclear cells (PBMCs). Resuspend the cell pellet in 3 mL of sterile 1× PBS. Repeat this step once (see Note 10).
Stain PBMCs with the APC-conjugated anti-human HLA-ABC antibody or isotype control antibody for 30 min at 4 °C (see Note 11).
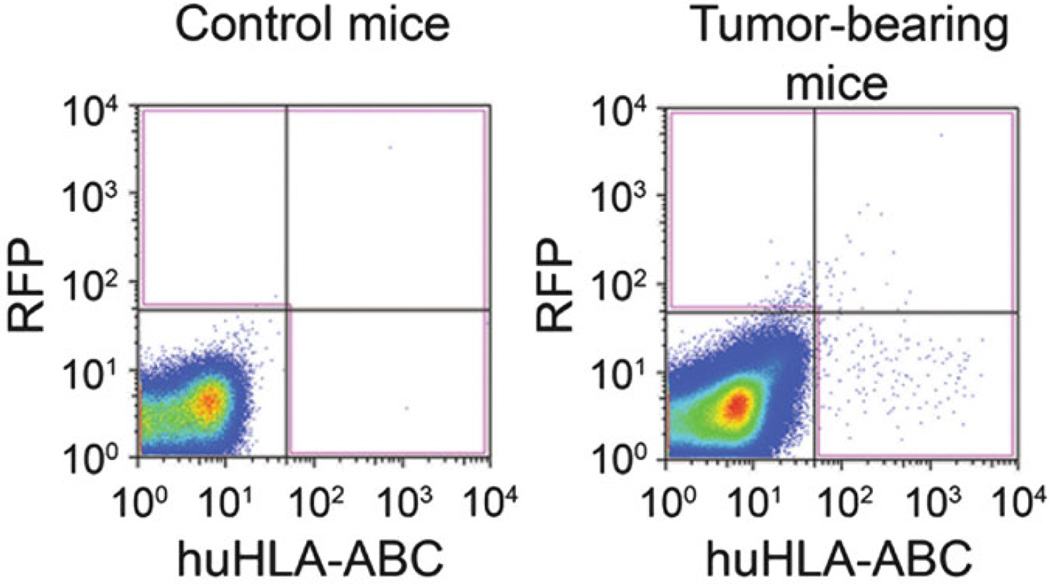
Sort CMCs by flow cytometry. CMCs are defined as RFPpositive and/or human HLA-ABCpositive cells. Cultured RFPpositive human melanoma cells stained with APC-conjugated anti-human HLA-ABC antibody are used as positive controls for RFP (FL2 spectrum) and human HLA-ABC antigens (FL4 spectrum). PBMCs from the tumor-free control mouse stained with APC-conjugated anti-human HLA-ABC antibody are used as negative controls (see Note 12) (Fig. 3).
Fig. 2.
Diagram illustrates the procedure for circulating melanoma cell isolation. Reproduced from Ma et al. 2010 Biochemical and Biophysical Research Communications with permission from Elsevier [12]
Fig. 3.
Representative flow cytometry results for circulating melanoma cells identified in blood specimens from tumor-free mice (left) or mice xenografted with human melanoma cells (right). Reproduced from Ma et al. 2010 Biochemical and Biophysical Research Communications with permission from Elsevier [12]
3.3 Characterization of Circulating Melanoma Cells
In vivo tumorigenicity and metastatic activity of isolated CMCs can be evaluated in serial xenotransplantation studies. CMCs are directly sorted into growth factor-reduced Matrigel (1:1 diluted with sterile PBS), and injected subcutaneously into NSG mice. Primary tumor formation and growth can be followed as described above. Development of distant metastases can be determined using in vivo fluorescence imaging or pathological examination (see Note 13).
CMCs can be further characterized during or after flow cytometry sorting. The frequency of cancer stem cells in the circulating tumor cell population can be determined by co-staining PBMCs with antibodies against specific cancer stem cell markers, such as ABCB5 or CD271 for melanoma initiating cells. The viability of CMCs can be determined by co-staining PBMCs or the sorted cells with viability reagents. Isolated CMCs can also be analyzed for gene expression profiling (see Note 14).
Acknowledgments
This work was supported by the NIH/NCI (grant numbers 5R01CA113796, 1R01CA158467, 1R01CA138231, and 2P50CA093683 to M.H.F.). Jie Ma was supported by a NIH dermatology training grant (2T32 AR 7098-37).
Footnotes
Alternative transfection reagents or viral vectors can be used to generate stable cell lines [16]. Transfection or transduction protocol should be optimized for each reagent and cell line.
Alternative fluorescence markers and flow cytometers with sorting capabilities can also be used for the identification and isolation of CMCs. The selection of the marker(s) depends on the sorting capabilities of the flow cytometer.
Co-injection of tumor cells with Matrigel increases the tumor-take rate in xenograftedmice [14]. However, caution should be taken as Matrigel provides a microenvironment artificially enriched with the melanoma cell mitogen laminin [11].
Other strains of immune-compromised mice, such as nude mice, scid mice, or NOD-scid mice may also be used as the host in xenotransplantation studies [13]. However, compared to NSG mice, higher residual immunity present in these strains of mice may reduce xenograft tumor-take rates [14, 17].
To ensure high and stable RFP expression, RFPhigh cells can be re-isolated before in vivo inoculation.
Although >95 % of human melanoma cells are expected to be HLA-ABCpositive [12], the expression ofMHCClass-1 antigens is downregulated in malignant melanoma initiating cells [14]. Therefore, an additional genetic marker (RFP) is included to increase the sensitivity of CMC isolation.
Primary tumor growth rate and the time course of metastasis development are to be determined in pilot studies.
The routes of the blood collection have an impact on the CTC enumeration. Heart acupuncture provides the investigator with the highest CTC counts in xenograft models [18]. For each adult NSG mice, 500–1500 µL of blood can be collected through heart acupuncture. Avoid bleeding when euthanizing and dissecting the mice.
This step and the following steps should be done under sterile conditions when CMCs are planned to be grown in vivo or in vitro after sorting. Alternative PBMC isolation method may be used, such as Ficoll-Paque [19]. The viability of human melanoma cells should be determined when alternative red blood cell lysis protocols are used.
After PBS washes, cells can be first incubated with Fc receptor blocking reagents to prevent nonspecific binding of antibody to PBMCs in the immunostaining step.
Most PBMCs will be stained with the human HLA-ABC anti-body [12]. Only a small aliquot of PBMCs (e.g., 5 × 104 cells) needs to be stained with the isotype IgG antibody and serves as a negative control in the sorting procedure.
The number of CMCs isolated may vary significantly, depending on the intrinsic metastatic activities of the tumor cells and on the in vivo growth kinetics of the primary tumors. Cells should be sorted directly to the bottom of collection tubes to facilitate the recovery after sorting.
Sterile condition should be maintained during sorting if the sorted cells will be used for further functional studies. Collection tubes should be placed on ice to maintain the viability of cells.
The presence of circulating melanoma initiating cells (melanoma stem cells) in melanoma patient peripheral blood has been confirmed [4, 7, 12]. Melanoma stem cells, for example marked by ABCB5, have not only demonstrated higher tumorigenic capacity compared to non-stem cell CMC populations in xenotransplantation studies, but have also been shown to be relevant to clinical melanoma progression and patient prognosis [4, 7, 12].
Contributor Information
Jie Ma, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Transplantation Research Program, Division of Nephrology, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, Boston, MA 02115, USA.
Markus H. Frank, Email: markus.frank@childrens.harvard.edu, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Transplantation Research Program, Division of Nephrology, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, Boston, MA 02115, USA.
References
- 1.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 2.Koyanagi K, O’Day SJ, Boasberg P, Atkins MB, Wang HJ, Gonzalez R, Lewis K, Thompson JA, Anderson CM, Lutzky J, et al. Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clin Cancer Res. 2010;16:2402–2408. doi: 10.1158/1078-0432.CCR-10-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–4613. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 4.Reid AL, Millward M, Pearce R, Lee M, Frank MH, Ireland A, Monshizadeh L, Rai T, Heenan P, Medic S, et al. Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br J Dermatol. 2012;168:85–92. doi: 10.1111/bjd.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medic S, Pearce RL, Heenan PJ, Ziman M. Molecular markers of circulating melanoma cells. Pigment Cell Res. 2007;20:80–91. doi: 10.1111/j.1600-0749.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 6.Ulmer A, Beutel J, Susskind D, Hilgers RD, Ziemssen F, Luke M, Rocken M, Rohrbach M, Fierlbeck G, Bartz-Schmidt KU, et al. Visualization of circulating melanoma cells in peripheral blood of patients with primary uveal melanoma. Clin Cancer Res. 2008;14:4469–4474. doi: 10.1158/1078-0432.CCR-08-0012. [DOI] [PubMed] [Google Scholar]
- 7.Kupas V, Weishaupt C, Siepmann D, Kaserer ML, Eickelmann M, Metze D, Luger TA, Beissert S, Loser K. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J Invest Dermatol. 2011;131:944–955. doi: 10.1038/jid.2010.377. [DOI] [PubMed] [Google Scholar]
- 8.De Giorgi V, Pinzani P, Salvianti F, Panelos J, Paglierani M, Janowska A, Grazzini M, Wechsler J, Orlando C, Santucci M, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol. 2010;130:2440–2447. doi: 10.1038/jid.2010.141. [DOI] [PubMed] [Google Scholar]
- 9.Galanzha EI, Shashkov EV, Spring PM, Suen JY, Zharov VP. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009;69:7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 11.Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, Saab KR, Osherov V, Widlund HR, Gasser M, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71:1474–1485. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma J, Lin JY, Alloo A, Wilson BJ, Schatton T, Zhan Q, Murphy GF, Waaga-Gasser AM, Gasser M, Stephen Hodi F, et al. Isolation of tumorigenic circulating melanoma cells. Biochem Biophys Res Commun. 2010;402:711–717. doi: 10.1016/j.bbrc.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedosekin DA, Sarimollaoglu M, Galanzha EI, Sawant R, Torchilin VP, Verkhusha VV, Ma J, Frank MH, Biris AS, Zharov VP. Synergy of photoacoustic and fluorescence flow cytometry of circulating cells with negative and positive contrasts. J Biophotonics. 2013;6(5):425–434. doi: 10.1002/jbio.201200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schatton T, Frank MH. Antitumor immunity and cancer stem cells. Ann N Y Acad Sci. 2009;1176:154–169. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eliane JP, Repollet M, Luker KE, Brown M, Rae JM, Dontu G, Schott AF, Wicha M, Doyle GV, Hayes DF, et al. Monitoring serial changes in circulating human breast cancer cells in murine xenograft models. Cancer Res. 2008;68:5529–5532. doi: 10.1158/0008-5472.CAN-08-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank MH, Denton MD, Alexander SI, Khoury SJ, Sayegh MH, Briscoe DM. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J Immunol. 2001;166:2451–2459. doi: 10.4049/jimmunol.166.4.2451. [DOI] [PubMed] [Google Scholar]