Abstract
Objective
We examined the association of nutrient intake with microstructural white matter integrity, and the role of white matter integrity in the association between nutrient consumption and cognition.
Methods
This cross-sectional analysis included 239 elderly (≥65 years) participants of a multiethnic cohort. White matter integrity was measured with fractional anisotropy (FA) from diffusion tensor magnetic resonance imaging. Nutrient patterns were derived from principal component analysis based on energy-adjusted intake of 24 selected nutrients. Generalized linear models were used to assess the association between nutrient patterns and mean FA of 26 white matter tracts. Mediation analysis was used to determine if FA mediates the nutrient-cognition relationship. All models were adjusted for age at time of scan, gender, ethnicity, education, caloric intake, and Apolipoprotein genotype.
Results
Among the identified 6 nutrient patterns, one (nutrient pattern-6, characterized by high intakes of Ω-3 and Ω-6 poly-unsaturated fatty acids and vitamin E) was positively associated with FA. Those with the highest tertile of nutrient pattern-6 score had a mean of 0.01 (p=0.01) higher FA value than those with the lowest tertile, similar to the effect of a ten-year decrease in age (b for age=−0.001, p=0.01). FA mediated the relationship between nutrient pattern-6 and memory, language, visuospatial and speed/executive function, and mean cognitive scores.
Interpretation
Our study suggests that older adults consuming more poly-unsaturated fatty acids and vitamin E rich foods had better white matter integrity, and that maintaining white matter microstructural integrity might be a mechanism for the beneficial role of diet on cognition.
Keywords: nutrient, diet, diffusion tensor imaging (DTI), magnetic resonance imaging (MRI), white matter integrity, neuroepidemiology
INTRODUCTION
Mounting evidence suggests that dietary factors are associated with risk for Alzheimer’s disease and cognitive decline among the elderly1. Dietary factors have also been implicated in maintaining brain macrostructures, such as brain volumes assessed by magnetic resonance imaging (MRI)2–7. However, little is known about the association of dietary patterns with microstructural brain imaging markers such as white matter integrity.
Diffusion tensor imaging (DTI) is a sensitive MRI-based neuroimaging technique that assesses the integrity of white matter microstructure in the brain by measuring diffusion properties of water8. Fractional anisotropy (FA), the most widely used metric derived from DTI, ranges from 0 to 1, with higher values indicating greater directionality of diffusion and preserved microstructure. Low FA is associated with subtle white matter abnormalities in prodromal and frank Alzheimer’s disease9, and is associated with cognitive function and decline10. Studies also showed that DTI metrics predicted cognitive decline better than measures of CSF tau and Aβ11, distinguished prodromal Alzheimer’s disease from healthy controls better than gray matter volume12, and correlated more reliably with memory performance in healthy elderly than hippocampal volumes13. Overall, DTI is a promising technique for research in prodromal Alzheimer’s disease14.
Given that there is no effective treatment for dementia to date, an earlier intervention at the prodromal or earlier stage is important in order to delay disease onset. Thus, it is essential to investigate modifiable factors that can contribute to the maintenance of white matter integrity. It is particularly important to examine the role of diet on white matter because certain nutrients such as polyunsaturated fatty acids (PUFAs) are key components of the glial, myelin sheath, and neuronal membrane phospholipid15 factors that comprise the brain’s white matter, thus insufficient consumption of these nutrients may interfere with membrane fluidity and function. Finally, elucidating the relationship between diet and the integrity of white matter tracts may also provide a foundation for further investigation of whether early disruption or preservation in white matter microstructure explain the diet-cognition relationship. However, to date only few studies have addressed these issues. In animal experiments, thiamine-deprived rats had lower FA values16, while calorie restriction in rhesus macaques had increased FA17. Studies in human are also rare with limited evidence suggesting that vitamin B12 supplementation18, higher serum level of 25-hydroxyvitamin D19, plasma PUFA concentration20, and supplementation of Ω-3 PUFA4 or fish oil21 might be associated with favorable DTI metrics like higher FA values. However, except for one study showing adherence to the Mediterranean diet22 was associated with higher FA values, whether and which nutritional or dietary factors directly from foods are related to white matter integrity is largely unknown. In addition, to our knowledge, whether white matter integrity may mediate the relationship between diet and cognition has never been examined.
In the current study, we investigated the association between dietary nutrient intake and white matter integrity among participants of a community-based, multiethnic cohort. We also evaluated the extent to which the association between nutrient intake and cognition is due to the variability in white matter microstructure.
MATERIALS AND METHODS
Study Participants
The study participants were from the Washington Heights/Hamilton Heights Inwood Columbia Aging Project (WHICAP), which were identified (via ethnicity and age stratification processes) from a probability sample of Medicare beneficiaries aged 65 or older, residing in northern Manhattan23. The initial sample for this study included 2,776 participants of the ongoing WHICAP II cohort, with the majority of the participants from a cohort recruited between 1999 and 2001 (n = 2174), and a smaller percent from continuing members of the WHICAP I cohort originally recruited in 1992 (n = 602). Briefly, at entry, a physician elicited each participant’s medical and neurological history, and conducted a standardized physical and neurological examination. Each participant received an assessment of health and function and a neuropsychological battery24. The diagnosis of any type of dementia or its absence25, Alzheimer’s disease26, and mild cognitive impairment27 was based on standard research criteria, using all available information at a consensus conference. Participants were followed every 18 months, repeating the baseline examination and consensus diagnosis. Institutional Review Boards at Columbia University approved this project. All individuals provided written informed consent.
The imaging sub-study was started in 2004 among ongoing dementia-free WHICAP II participants28. In total, 769 WHICAP participants received MRI scans, and they were slightly younger and more likely to be African Americans or male compared with those who were eligible but not undergo MRI28. Approximately 4 years later, we invited the participants to come back for a follow-up MRI scan. A total of 337 subjects received second structural MRI scans, among which 255 subjects also received DTI. We additionally excluded 16 subjects who had no diet assessments. Therefore, a total of 239 (71% of 337) subjects were included in the current analysis. Twenty-eight subjects met diagnostic criteria for dementia at neuroimaging visit and were further excluded in sensitivity analysis.
MRI protocol
Scan acquisition was performed on a 1.5T Philips Intera scanner at Columbia University Medical Center29 . T1-weighted images (repetition time = 20ms, echo time = 2.1 ms, field of view 240, 256 x 160 matrix, 1.3 mm slice thickness) were acquired and analyzed with FreeSurfer, version 5.1 (http://surfer.nmr.mgh.harvard.edu/) for cortical thickness and regional and total volume. Whole brain diffusion imaging (field of view=224 x 224, contiguous slices, slice thickness = 2mm, TR=10586, TE=70) were acquired along 15 directions with a maximum b-factor of 800 s/mm2, complemented by two scans with b=0 s/mm2. Fractional anisotropy (FA) maps were constructed for each participant with software implemented in MATLAB® (R2013b, the MathWorks, the FMRIB Software Library (FSL) and Inc., Natick, Massachusetts, USA). Regions-of-interest (ROIs) were derived with the JHU-ICBM-DTI-81 white matter labels atlas30. Each subject’s FA map was first registered to the FA atlas template with the nonlinear transformation tool (FNIRT) in FSL using a linear initialization (default FSL parameters) and the predefined configuration file for FA registration provided by FSL. By applying the inverse nonlinear registration transformation to the atlas, the atlas was warped, allowing for ROI analysis in subject space. Twenty-six ROIs in left and right hemispheres, and midline structures, were considered for analysis. A mean FA value of the 26 tract-specific FAs, measuring global white matter microstructure, was used as the main outcome variable.
Dietary information
Information about average diet over the prior year was obtained using the 61-item version of Willett's semi-quantitative food frequency questionnaire (Channing Laboratory, Cambridge, MA), administered by trained interviewers in English or Spanish. The validity (using two 7-day food records) and reliability (using two 3-month frequency assessments) of various components of the food frequency questionnaire in WHICAP were good and have been previously reported31. The database used for the nutrient content analysis was a specifically designed program32, primarily using United States Department of Agriculture Nutrient Database for Standard Reference, as well as information from McCance and Widdowson's The Composition of Foods, published data, and manufacturers. The daily intake of nutrients was computed by multiplying the consumption frequency of each food item by the nutrient content of the specified portion of the food item which was from this specifically designed nutritional database. The diet information was collected on average 5.1 (SD=2.2) years before the MRI scan.
Cognitive evaluation
Cognition was assessed with a neuropsychological battery 24 which was administered either in English or Spanish at baseline and each follow-up visit. Selected neuropsychological tests scores were combined into four composite scores (memory, language, executive-speed, and visuospatial) based on an exploratory factor analysis using principal axis factoring and oblique rotation24. Memory was assessed with the Selective Reminding Test, including total recall, delayed recall, and delayed recognition, and with recognition from the Benton Visual Retention Test. The language domain was assessed by measuring naming, letter fluency, category fluency, verbal abstract reasoning, and repetition and comprehension. Executive-Speed was assessed with the Color Trails test 1 and 2. Visuospatial function was assessed with the Rosen Drawing Test, the BVRT–Matching, and the Identities and Oddities subtest of the Mattis Dementia Rating Scale.
Means and standard deviations (SD) were calculated from baseline scores for non- demented WHICAP subjects controlling for age, race/ethnicity, and years of education. Z-scores for each of the cognitive tests were calculated and then averaged to create a composite Z-score for each of the four domains33. These four factor domain scores were subsequently averaged to produce a composite “mean cognition” z-score. A higher z-score indicates better cognitive performance.
Other information
Age (years), education (years), caloric intake (kcal), and body mass index (BMI; weight in kilograms divided by height in square meters [kg/m2]) were used as continuous variables. Participants were assigned to one of four groups: African American (Black non-Hispanic), Hispanic, White (non-Hispanic) or Other based on self-report using the format of the 2000 US census. Ethnicity was used as a dummy variable with non-Hispanic White as the reference. Sex was used as a dichotomous variable with male as the reference. Apolipoprotein (APOE) genotype was used dichotomously: absence (as reference) versus presence of either 1 or 2 ε4 alleles. The diagnosis of clinical stroke was based on questioning of the participant or relatives and supplemented by a neurological examination or review of medical records. Heart disease, diabetes mellitus, and hypertension were defined by collective information on measured blood pressure, self-report, and use of disease-specific medications. These four vascular comorbidities were used as dichotomous variables with absence of the condition as the reference. Standard FreeSurfer outputs of brain measures including total brain volume, total white matter volume, or total gray matter volume, all adjusted for intracranial volume using the residual method, were also included in the analysis as a covariate.
Statistical analyses
Principal component analysis (PCA)
To identify underlying latent dietary constructs (dietary patterns) in the population, we used exploratory PCA performed on the correlation matrix of 24 nutrients. The following 24 nutrients were considered in the analysis: protein, carbohydrate, fatty acids (saturated, cholesterol, monounsaturated, Ω-3 and Ω-6 PUFA), B vitamins (B1, B2, B3, B5, B6, B12, folate), A vitamins (lycopene, lutein and zeaxanthin, vitamin A), antioxidants (vitamin C, vitamin E, β-carotenes, β-cryptoxanthin), vitamin D, calcium, and iron, selected based on their biological functions and their relationship with cognition and neurodegenerative conditions reported in the literature6, 34. Nutrient intake was adjusted for total caloric intake using the regression models, and their residuals were used in the analysis.
The number of nutrient patterns to be retained was determined by eigenvalues >1.0, scree plot, parallel analysis, and interpretability of the factors. We considered nutrients with an absolute factor loading value ≥ 0.50 on a nutrient pattern as dominant nutrients contributing to that particular nutrient pattern. Each subject received a factor score (i.e., a linear combination of nutrients weighted by factor loadings) for each identified nutrient pattern, with higher score indicating relatively higher adherence to that nutrient pattern.
Descriptive analysis and association analysis between nutrient patterns and white matter integrity
Characteristics of participants by tertiles of nutrient pattern adherence or mean FA were compared using ANOVA for continuous variables and χ2 test for categorical variables. Generalized linear models were used to assess the association between nutrient patterns and mean FA, initially adjusted for age at time of scan only (Model 1), and then followed by adjustment of age at time of scan, gender, ethnicity, education, caloric intake, and APOE genotype (Model 2). We also additionally included total brain volume, four vascular conditions and BMI as covariates (Model 3). For Model 3, we replaced total brain volume with either total gray matter or white matter volume.
Mediation analysis
We performed mediation analysis to examine the potential mediating role of white matter integrity on the relationship between nutrient intake and cognition. Age, sex, education, race/ethnicity, caloric intake, and APOE status were included as covariates in the models.
Supplementary analyses
We explored the potential effect modification by sex, APOE genotype, or ethnicity on the relationship between nutrient patterns and mean FA. We also examined the association between the individual dominant nutrients of the nutrient pattern that was associated with FA. We excluded participants with dementia and repeated the analyses. To evaluate the robustness of the identified dietary patterns, we performed PCA on an exhaustive set of 34 nutrients that included 10 additional nutrients (zinc, copper, phosphorous, potassium, magnesium, manganese, sodium, alcohol, caffeine, and fiber). We then examined whether these alternative nutrient patterns retained from this PCA were associated with FA. We also additionally adjusted for the supplements intake (including vitamins A, C, E, and B6, and calcium, all as binary variables indicating yes or no of supplementation) in the Model 3. Similar to the PCA method we used for deriving nutrient patterns, we used PCA on the 26 tract-specific FAs as an alternative way to summarize the overall FA value rather than using the mean FA value, and examined the relationship between nutrient patterns and the derived white matter tract FA pattern scores. Finally, we examined whether FA had a mediating role in the associations of all nutrient patterns (not just the ones associated with FA) and cognitive functions.
All analyses were conducted using PASW Statistics (IBM, Chicago, IL, USA). All p-values were based on two-sided tests. The significance level was set at 0.05 for all tests.
RESULTS
Nutrient Patterns
We retained six major nutrient patterns that had eigenvalues ≥1. Parallel analysis suggested the same number of patterns to be retained. The six nutrient patterns in total explained about 80% of total variance in nutrients (Table 1). Table 1 shows the factor loading matrix for the nutrient patterns. Based on the loading coefficients, the first nutrient pattern (nutrient pattern-1) was characterized by high intake antioxidants and A vitamins, nutrient pattern-2 by B vitamins and iron, nutrient pattern-3 by high vitamin D and calcium and vitamin B2, nutrient pattern-4 by high intakes of saturated fatty acids, monounsaturated fatty acids, cholesterol, and low intake of carbohydrates, nutrient pattern-5 by high protein, cholesterol, vitamin B3 and vitamin B6, and nutrient pattern-6 by high Ω-3 PUFA, Ω-6 PUFA, and vitamin E. Preliminary regression analysis adjusted for age, sex, education, and race/ethnicity showed that nutrient pattern-6 was the only nutrient pattern significantly associated with higher value of FA (Table 1). Therefore, we focused on nutrient pattern-6 and its relationship with FA in the subsequent analyses.
Table 1.
Factor loading matrix and explained variances for the six major dietary patterns identified by principal component analysis.
| Components† | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Communalities | |
|
| |||||||
| Total protein (g) | 0.762 | 0.682 | |||||
| Total carbohydrates (g) | 0.504 | −0.646 | −0.399 | 0.921 | |||
| Saturated fatty acids | −0.471 | 0.707 | 0.838 | ||||
| Cholesterol | 0.571 | 0.593 | 0.730 | ||||
| Monounsaturated fatty acids | −0.437 | 0.719 | 0.881 | ||||
| Ω-3 PUFA | 0.892 | 0.853 | |||||
| Ω-6 PUFA | −0.331 | 0.703 | 0.710 | ||||
| Thiamin (vitamin B1, mg) | 0.876 | 0.910 | |||||
| Riboflavin (vitamin B2, mg) | 0.532 | 0.658 | 0.313 | 0.872 | |||
| Niacin (vitamin B3, mg) | 0.739 | 0.514 | 0.883 | ||||
| Vit B5 (Pantothenic acid ) | 0.757 | 0.450 | 0.844 | ||||
| Vitamin B6 (mg) | 0.334 | 0.798 | 0.820 | ||||
| Vitamin B12 (mg) | 0.646 | 0.482 | |||||
| Total folate (μg) | 0.308 | 0.754 | −0.350 | 0.802 | |||
| Vitamin C (mg) | 0.784 | −0.319 | 0.832 | ||||
| Vitamin E (mg) | 0.742 | 0.413 | 0.856 | ||||
| β Carotene (μg) | 0.903 | 0.887 | |||||
| β Cryptoxanthin | 0.802 | 0.792 | |||||
| Lycopene (μg) | 0.729 | 0.582 | |||||
| Lutein (μg) | 0.715 | −0.342 | 0.670 | ||||
| Vitamin A | 0.919 | 0.910 | |||||
| Vitamin D | 0.826 | 0.763 | |||||
| Calcium (mg) | 0.878 | 0.859 | |||||
| Iron (mg) | 0.844 | 0.786 | |||||
|
| |||||||
| Proportion of variance explained ( % ) | 34.218 | 19.608 | 9.608 | 6.170 | 5.868 | 4.386 | |
| Cumulative Proportion of variance explained ( % ) | 34.218 | 53.826 | 63.434 | 69.604 | 75.471 | 79.857 | |
|
| |||||||
| Association with FA, β(p) ‡ | 0.0001 (0.99) | 0.002 (0.21) | 0.001 (0.39) | −0.003 (0.11) | 0.001 (0.57) | 0.004 (0.006) | |
Principal component analysis with varimax rotation was used to extract all the components, i.e. nutrient patterns. Only loadings with an absolute value ≥0.3 are shown in the table and those <0.30 are suppressed. Each nutrient pattern score is a linear weighted (by loadings) combination of the nutrients. Hence, nutrients with highest absolute loadings (≥0.5, shown in bold) are considered as dominant nutrients contributing to the particular nutrient pattern.
Beta coefficient (β) and p-value for the association with FA was estimated from a regression model with mean FA as the outcome variable, and all 6 nutrient patterns as independent variables, adjusted for age, sex, education, and ethnicity. Acronyms: fractional anisotropy (FA).
Characteristics of the study population
Participants had different language performance, prevalence of diabetes, and mean FA across tertiles of nutrient pattern-6 (Table 2). Post-hoc analyses showed that participants with high tertile nutrient pattern-6 had higher FA than those with low (p=0.04) or middle (p=0.04) tertile, and had higher language z-score than those with low nutrient pattern-6 (p=0.01). Participants with middle tertile of nutrient pattern-6 score had lower total caloric intake than those with low (p=0.02) or high (p=0.05) tertile.
Table 2.
Demographic and cognitive characteristics of the study subjects by tertiles of nutrient pattern-6 score.
| Nutrient Pattern-6 tertiles | |||||
|---|---|---|---|---|---|
| Low | Middle | High | Total | p | |
| N | 81 | 82 | 76 | 239 | |
| Age (years) | 83.7 (4.78) | 84.6 (5.3) | 84.1(5.1) | 84.1(5.1) | 0.56 |
| Education (years) | 10.4 (4.9) | 10.7 (4.5) | 12.0 (5.1) | 11.1 (4.9) | 0.08 |
| Female, N(%) | 56 (71) | 57 (71) | 54 (68) | 167 (70) | 0.85 |
| Race/Ethnicity | 0.16 | ||||
| White, N(%) | 24 (30) | 25 (31) | 21 (26) | 70 (29) | |
| African American,N(%) | 26 (33) | 21 (26) | 37 (46) | 84 (35) | |
| Hispanics, N(%) | 28 (35) | 34 (43) | 21 (26) | 83 (35) | |
| Others, N(%) | 1 (1) | 0 (0) | 1 (1) | 2 (1) | |
| APOE ε4, N(%) | 21 (27) | 22 (28) | 19 (24) | 62 (26) | 0.85 |
| Caloric Intake, cal | 1472 (731) | 1260 (408) | 1432 (480) | 1388 (562) | 0.04 |
| Cognitive status | |||||
| Language | 0.12 (0.66) | 0.30 (0.66) | 0.40 (0.62) | 0.27 (0.66) | 0.03 |
| Memory | −0.04 (0.79) | 0.10 (0.8) | 0.09 (0.83) | 0.05 (0.81) | 0.46 |
| speed | 0.10 (1.04) | 0.10 (0.93) | 0.30 (1.02) | 0.17 (0.99) | 0.45 |
| Visuospatial | 0.25 (0.65) | 0.29 (0.58) | 0.37 (0.56) | 0.30 (0.60) | 0.46 |
| Mean cognition | 0.27 (0.6) | 0.26 (0.65) | 0.36 (0.59) | 0.30 (0.61) | 0.56 |
| Dementia, N(%) | 10 (13) | 8 (10) | 10 (13) | 28 (12) | 0.84 |
| Vascular risk/comorbidities | |||||
| BMI, kg/m2 | 28.4 (5.9) | 27.7 (4.5) | 28.0 (5.8) | 28.0 (5.4) | 0.73 |
| Diabetes | 22 (28) | 28 (35) | 13 (16) | 63 (26) | 0.03 |
| hypertension | 64 (81) | 67 (84) | 68 (85) | 199 (83) | 0.79 |
| heart disease | 18 (23) | 27 (34) | 24 (30) | 69 (29) | 0.30 |
| stroke | 6 (8) | 9 (11) | 8 (10) | 23 (10) | 0.73 |
| Total brain volume, mL | 868 (109) | 862 (90) | 884 (106) | 871 (102) | 0.38 |
| Total gray matter volume, mL | 514 (53) | 511 (47) | 523 (53) | 509 (51) | 0.34 |
| Total white matter volume, mL | 375 (56) | 370 (48) | 386 (50) | 377 (52) | 0.16 |
| FA | 0.4460 (0.0251) | 0.4460 (0.0227) | 0.4541 (0.0248) | 0.4487 (0.0244) | 0.05 |
Acronyms: Apolipoprotein (APOE); body mass index (BMI); fractional anisotropy (FA). P values were from ANOVA for continuous variables and χ2 for categorical variables.
Compared with those having the lowest tertile of FA, subjects with the higher tertiles of FA were younger, had better cognition, lower BMI, larger gray matter volume, and larger white matter volume (Table 3).
Table 3.
Demographic and cognitive characteristics of the study subjects by tertiles of FA value.
| Low FA | Middle FA | High FA | Total | p | |
|---|---|---|---|---|---|
| N | 79 | 81 | 79 | 239 | |
| 0.4210 (0.0135; | 0.4505 (0.0057; | 0.4746 (0.0122; | 0.4487 (0.0244; | ||
| FA, mean (SD; range) | 0.38–0.44) | 0.44–0.46) | 0.46–0.51) | 0.38–0.51) | <0.0001 |
| Age (years) | 84.84 (5.38) | 84.54 (5.03) | 82.98 (4.69) | 84.12 (5.09) | 0.046 |
| Education (years) | 10.89 (4.73) | 10.74 (5.13) | 11.53 (4.77) | 11.05 (4.88) | 0.563 |
| Female, N(%) | 54 (68) | 55 (68) | 58 (73) | 167 (70) | 0.702 |
| Race/Ethnicity | 0.053 | ||||
| White, N(%) | 21 (27) | 24 (30) | 25 (32) | 70 (29) | |
| African American, N(%) | 37 (47) | 22 (27) | 25 (32) | 84 (35) | |
| Hispanics, N(%) | 21 (27) | 35 (43) | 27 (34) | 83 (35) | |
| Others, N(%) | 0 | 0 | 2 (2) | 2 (1) | |
| APOE ε4, N(%) | 23 (29) | 20 (25) | 19 (24) | 62 (26) | 0.731 |
| Caloric intake, cal | 1389 (569) | 1390 (662) | 1383 (435) | 1387 (562) | 0.990 |
| Cognitive status | |||||
| Mean cognition | 0.14 (0.65) | 0.27 (0.6) | 0.49 (0.53) | 0.3 (0.61) | 0.001 |
| Memory | −0.18 (0.80) | 0.05 (0.78) | 0.26 (0.79) | 0.05 (0.81) | 0.004 |
| Language | 0.10 (0.68) | 0.28 (0.67) | 0.42 (0.58) | 0.27 (0.66) | 0.010 |
| Speed | −0.17 (1) | 0.12 (1.04) | 0.49 (0.84) | 0.17 (0.99) | 0.001 |
| Visuospatial | 0.13 (0.65) | 0.30 (0.63) | 0.47 (0.45) | 0.30 (0.60) | 0.002 |
| Dementia, N(%) | 18 (23) | 5 (6.2) | 5 (6.3) | 28 (12) | 0.001 |
| Vascular risk/comorbidities | |||||
| BMI (kg/m2) | 29.3 (6.3) | 27.0 (4.6) | 27.8 (5.1) | 28.0 (5.4) | 0.034 |
| Diabetes, N(%) | 24 (30) | 22 (27) | 17 (22) | 63 (26) | 0.441 |
| Heart disease, N(%) | 23 (29) | 22 (27) | 24 (30) | 69 (29) | 0.902 |
| Hypertension , N(%) | 67 (85) | 69 (85) | 63 (70) | 199 (83) | 0.591 |
| Stroke, N(%) | 12 (15) | 4 (5) | 7(9) | 23 (10) | 0.086 |
| Total brain volume, mL | 857 (102) | 885 (110) | 872 (93) | 871 (102) | 0.230 |
| Total gray matter volume, mL | 501 (46) | 520 (55) | 526 (48) | 516 (51) | 0.007 |
| Total white matter volume, mL | 355 (50) | 389 (50) | 387 (49) | 377 (52) | <0.0001 |
| intracranial volume, mL | 1449 (170) | 1462 (217) | 1405 (187) | 1439 (193) | 0.156 |
Acronyms: Apolipoprotein (APOE); body mass index (BMI); fractional anisotropy (FA). P values were from ANOVA for continuous variables and χ2 for categorical variables.
Nutrient Pattern-6 and white matter integrity
Regression analyses showed that higher nutrient pattern-6 score was associated with higher mean FA (Table 4, all models). In the model adjusted for age, sex, education, ethnicity, APOE, and caloric intake (Table 4, Model 2), subjects who had the highest tertile had a mean of 0.01 (p=0.01) higher FA value compared to those with the lowest tertile of nutrient pattern-6 score. Of note, in the same model (Table 4, Model 2), the only other variable associated with FA was age, with one year increase in scan age associated with 0.001 lower FA (b=-0.001, p=0.01). Thus, the estimated effect for FA comparing the highest to the lowest tertile of nutrient pattern-6 was about the same magnitude as a ten-year increase in age.
Table 4.
Associations between nutrient pattern-6 and white matter integrity.
| Continuous nutrient pattern-6 | Tertiles of nutrient pattern-6 | ||||
|---|---|---|---|---|---|
| b | p | b | p | ||
| Model 1 | 0.0040 | 0.02 | Middle | 0.001 | 0.87 |
| High | 0.008 | 0.03 | |||
| P-trend | 0.03 | ||||
| Model 2 | 0.0043 | 0.007 | Middle | 0.001 | 0.80 |
| High | 0.010 | 0.01 | |||
| P-trend | 0.01 | ||||
| Model 3 | 0.0045 | 0.006 | Middle | 0.002 | 0.70 |
| High | 0.010 | 0.01 | |||
| P-trend | 0.02 | ||||
Results are from generalized linear models.
Model 1: Adjusted for age only.
Model 2: Adjusted for age, sex, education, ethnicity, APOE, and caloric intake.
Model 3: Adjusted for age, sex, education, ethnicity, APOE, caloric intake, total brain volume, BMI, hypertension, stroke, diabetes, and heart disease.
Additional adjustment for total brain volume, BMI, hypertension, stroke, diabetes, and heart disease did not change the results (Table 4, Model 3). Replacing total brain volume with either total gray matter volume or total white matter volume did not change the results (data not shown).
Mediating effect of white matter integrity for the association between nutrient pattern-6 and cognitive functions
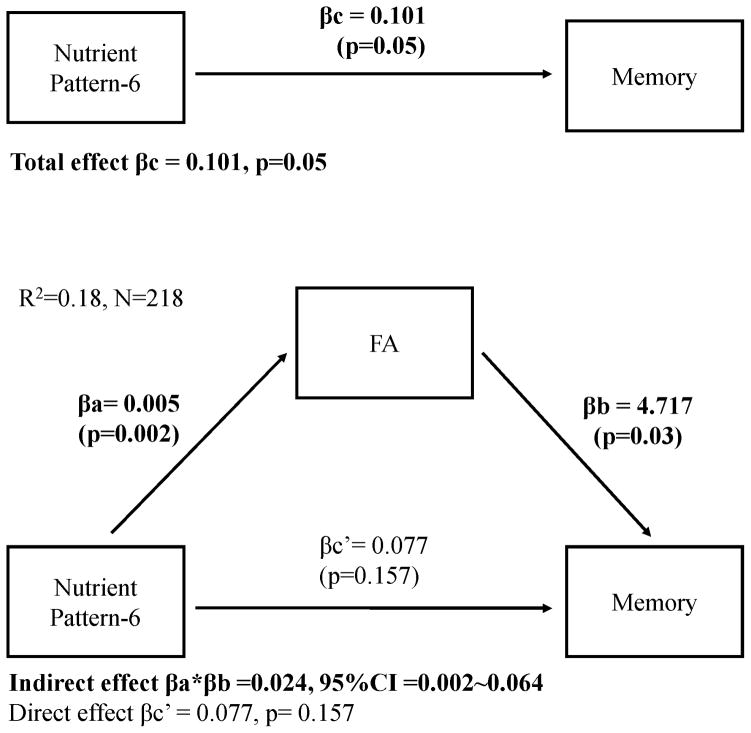
Mediation analysis adjusted for age, sex, education, ethnicity, APOE, and caloric intake showed that mean FA was positively associated with cognitive functions in all domains (positive βb in Table 5). In addition, there was a significant mediation effect (βa*βb in Table 5) of nutrient pattern-6 on cognition through FA, for all cognitive scores, suggesting a path linking diet (nutrient pattern-6) and cognition (cognitive scores) via white matter microstructure (mean FA). Taking memory score as an example (Table 5 and Figure 1), the total effect of nutrient pattern-6 on memory score was significant (βc=0.10, p=0.05), but after taking into consideration the significant path via white matter integrity (the mediation effects βa*βb=0.024, 95%CI 0.002–0.064), the remaining direct effect of nutrient pattern-6 on memory score was reduced and no longer significant (βc'=0.077, p=0.157). When we additionally adjusted for BMI, hypertension, stroke, diabetes, and heart disease in the mediation models, we found the results were similar (data not shown).
Table 5.
Mediation effect of white matter integrity on the association between nutrient pattern and cognitive functions.
| Mean Cognition | Memory | Language | Speed | Visuospatial | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p or 95%CI | β | p or 95%CI | β | p or 95%CI | β | p or 95%CI | β | p or 95%CI | |
| Number of subjects | 233 | 218 | 224 | 187 | 219 | |||||
| R2 (Mediation model) | 0.51 | 0.18 | 0.49 | 0.23 | 0.45 | |||||
| Total effect βc (nutrient pattern-6 on Cog) | −0.009 | 0.760 | 0.101 | 0.050 | 0.057 | 0.090 | 0.099 | 0.150 | 0.002 | 0.940 |
| βa (nutrient pattern-6 on FA) | 0.004 | 0.016 | 0.005 | 0.002 | 0.005 | 0.003 | 0.004 | 0.020 | 0.004 | 0.044 |
| βb (FA on Cog, given nutrient pattern-6) | 3.854 | 0.002 | 4.717 | 0.030 | 3.595 | 0.039 | 11.150 | <0.0001 | 5.078 | 0.001 |
| Mediation effect βa*βb (nutrient pattern-6 on Cog via FA)‡ | 0.017 | (0.003, 0.041) | 0.024 | (0.002, 0.064) | 0.019 | (0.003, 0.046) | 0.049 | (0.012, 0.109) | 0.019 | (0.003, 0.046) |
| Direct effect βc' (nutrient pattern-6 on Cog) | −0.026 | 0.410 | 0.077 | 0.157 | 0.038 | 0.277 | 0.049 | 0.548 | −0.017 | 0.644 |
Mediation analysis was used to examine the potential indirect relationship between nutrient intake (X, independent variable) and cognition (Y, dependent variable) through white matter integrity (M, mediator), in other words, the mediation role of white matter integrity on the relationship between nutrient and cognition. Bold values indicate significance at p<0.05 two-tailed, or a 95%CI that does not include 0 for 95%CI for the indirect effect. P-values were italicized in the table. All models were based on a sub-sample that was limited to subjects with all variables, and thus the sample sizes as well as βas were slightly different due to missing values on each cognitive score. FA from DTI assessment was used as an indicator of white matter integrity, with higher FA value indicating better integrity.
βc: Indicates the total effect of nutrient pattern-6 on cognition. The beta coefficient for nutrient pattern-6 on cognition from an adjusted model with age, sex, education, race/ethnicity, APOE, and caloric intake, and nutrient pattern-6 as predicting variables, and cognitive z-score as outcome variable. βa: Indicates the association between nutrient pattern-6 and FA. Beta coefficient for nutrient pattern-6 on FA from an adjusted model with age, sex, education, race/ethnicity, APOE, and caloric intake, and nutrient pattern-6 as predicting variables, and FA as outcome variable.
βb: Indicates the association between FA and cognition. Beta coefficient for FA on cognition from a mediation model with age, sex, education, race/ethnicity, APOE, and caloric intake, nutrient pattern-6, and FA as predicting variables, and cognitive z-score as outcome variable.
βa*βb: Indicates the indirect effect of nutrient pattern-6 on cognition via FA. Bias-corrected bootstrap 95%CI confidence intervals (95%CI) were estimated from 10000 bootstrap samples using Preacher and Hayes’s49 PROCESS SPSS macro. A 95%CI that does not include 0 is considered as statistically significant, and suggesting that a significant mediating role of FA on the relationship between nutrient pattern-6 on cognition.
βc': Indicates the remaining direct effect of nutrient pattern-6 on cognition after controlling for the mediating factor as well as other covariates, thus it is approximately equivalent to βc-βa*βb. The beta coefficient for nutrient pattern-6 from a mediation model with age, sex, education, race/ethnicity, APOE, and caloric intake, nutrient pattern-6, and FA as predicting variables, and cognitive z-score as outcome variable.
Figure 1.
Mediation model.
Supplementary analyses
Interaction analyses indicated that the relationships of nutrient pattern-6 with FA was not modified by gender (p for interaction=0.52), education (p=0.44), ethnicity (p=0.99 Whites and Blacks, p=0.35 Hispanic and Whites), or APOE _4 status (p=0.66). Post-hoc stratified analysis showed similar results in Whites and African-American (b=0.008, p=0.005, and b=0.007, p=0.025, respectively) while no association was observed in Hispanics (b=-0.00005, p=0.84) with Model 3 covariates except for race/ethnicity.
Among the three dominant nutrients contributing to the nutrient pattern-6, we found subjects with high and middle tertile of Ω-3 PUFA both had higher FA than those with low tertile (b=0.009, p=0.02, and b=0.011, p=0.005, respectively; p-trend=0.005), and those with high tertile of vitamin E had higher FA (b=0.009, p=0.02) than those with low vitamin E tertile (p-trend=0.02), while Ω-6 PUFA by itself was not associated with FA. When all individual 24 nutrients were included in the model simultaneously, Ω-3 PUFA remained independently associated with FA (b=0.018 for one SD increase, p=0.04).
When the analysis was limited to 211 non-demented subjects, nutrient pattern-6 and Ω-3 PUFA (both as continuous variables) remained associated with FA, with b=0.006, p =0.001 and b=0.018, p=0.001 in Model 3, respectively.
When we included 10 additional nutrients, we retained 8 alternative nutrient patterns. Among them, alternative nutrient pattern-6 was the only one significantly associated with FA (b=0.004, p=0.009, Model 3), and the nutrients with highest loadings for this alternative nutrient pattern-6 were again Ω-3 PUFA, Ω-6 PUFA, and vitamin E (loadings=0.88, 0.74, and 0.45, respectively). Interestingly, the original and alternative nutrient patterns-6 had a correlation coefficient of 0.94 (p<0.0001), suggesting the robustness of extracting the unobservable pattern in this population using PCA.
When additionally adjusted for the supplements intake in the Model 3, the results did not change much: compared to the low tertile of nutrient pattern-6, those with middle and high tertiels had 0.001 (p=0.073) and 0.01 (p=0.006) higher FA values, respectively.
We used PCA to derive 5 white matter tract FA pattern scores which had eigenvalues larger than 1. As the first FA pattern score explained a large proportion of variance (41%), and all 26 tract FAs had positive loadings larger than 0.3, we focused on the first pattern score as an alternative summary of the global white matter integrity. We found nutrient pattern-6 was the only pattern significantly associated with this FA pattern (b=0.20, p=0.005).
Mean FA did not mediate the relationship between other derived nutrient patterns (i.e. nutrient patterns 1 to 5 in Table 1) and cognitive functions (data not shown).
DISCUSSION
We identified a ‘PUFAs and vitamin E’ nutrient pattern that was associated with better microstructural white matter integrity indicated by higher FA. The strength of the association was about the same magnitude as a ten-year increase in age, which is a well-established factor contributing to white matter integrity deterioration35. In addition, white matter integrity was associated with cognitive function, and it mediated the relationship between this ‘PUFAs and vitamin E’ nutrient pattern and cognitive function.
Few prior epidemiological studies have examined the role of diet in microstructural white matter integrity. In young male adults with psychosis, plasma total PUFA concentration was positively correlated with FA20. In a double-blind randomized interventional study, Ω-3 PUFA supplementation led to significant increases in FA during the 26-week follow-up4. A recent study on 146 non-demented elderly people found that higher adherence to a Mediterranean diet was associated with preserved white matter microstructure in extensive brain areas22. Interestingly, the main food sources for Ω-3 PUFA, Ω-6 PUFA and vitamin E are fish, nuts, cereals, and vegetable, all of which are considered as the beneficial food components in the Mediterranean diet. Thus, our study is in line with the previous reports in supporting beneficial roles of PUFAs and other healthy elements of diet on brain white matter. In addition, our study used data-driven method to find dietary patterns naturally existing in this multiethnic study population, who might not consume a typical Mediterranean diet. Rather than giving equal weights to the food components in Mediterranean diet, the PCA-derived nutrient pattern identified the most relevant nutrients and allowing different weights for the nutrients in the pattern.
The nutrient pattern-6 only explains a small percentage of total variation of nutrients intake in this study population. However, this nutrient pattern can be viewed similar to those risk factors that are rare but strongly associated with certain diseases. In addition to its strong association with white matter integrity, there is also strong evidence supporting the potential biological feasibility for PUFAs and vitamin E to play a role in maintaining white matter microstructural integrity. PUFAs and vitamin E are well documented to have beneficial effects on vascular factors36. Therefore it is possible that following a diet diverging from nutrient pattern-6 may lead to accumulated vascular risk factors such as hypertension and diabetes which in turn have been linked with damaged white matter integrity37. However, as adjusting vascular factors did not change the association between nutrient pattern-6 and FA in our study, other mechanisms might be involved. For example, PUFAs may be related to white matter integrity through inflammation or oxidative stress, both of which have been implicated in the pathochemistry of myelin membrane disruptions38, 39. In the cuprizone mouse model of multiple sclerosis, Ω-3 PUFAs in the brain inhibited inflammation by reducing the release of nitric oxide and tumor necrosis factor-α from primary microglia while at the same time enhancing beneficial immune responses such as microglial phagocytosis40. Furthermore, the mechanisms underlying the association between nutrient pattern-6 and FA can be explained by the biophysiological roles of the PUFAs and vitamin E on pathways directly involving axonal loss or demyelization41. An in vivo study showed that a Ω-3 PUFA injection in the rat brain increased myelinogenesis42. Another mouse study also demonstrated that Ω-3 PUFAs prevented the loss of myelin basic protein, preserved the integrity of the myelin sheath, maintained nerve fiber conductivity, and protected oligodendrocytes from excitotoxicity and death43. Similarly, in vivo and in vitro studies suggest that vitamin E deficiency induces axonal degeneration and loss of myelination44, while vitamin E treatment attenuates demyelination and promotes remyelination45.
Our study revealed that microstructural white matter integrity was positively associated with cognitive performance. Consistent with our results, several longitudinal studies showed that deficits in white matter microstructure predicted cognitive decline11. Combined with the observation that DTI can detect diffuse abnormalities in white matter that appears to be normal on conventional MRI images46, DTI-derived white matter indices may be a sensitive method to detect the underpinnings of subtle cognitive changes. It is therefore not surprising to find that beneficial dietary factors might be related with better cognition via maintaining better white matter integrity. To our knowledge, there are very few studies that have examined whether brain structural abnormalities mediated the effect of dietary factors on cognition47, 48. These studies found that white matter hyperintensity volume48 or gray matter volume47 might explain the relationship between Ω-3 PUFAs and executive function. In our study, we expanded the findings to several cognitive domains, and to nutrient patterns, and for the first time established a mediating role of white matter integrity in the relationship between a PUFA-and-vitamin E dietary pattern and cognition.
Our study has many strengths. The study included a relatively large sample of multi-ethnic community-dwelling elderly population with extensive data on demographic, clinical, and lifestyle factors including nutritional data. In sensitivity analysis we excluded participants with dementia, thus limiting our analysis to a preclinical stage, and the results remained the same. In addition, we used mediation analysis to estimate whether there was a potential mediating role of white matter integrity in the relationship of diet and cognition, and the results shed light on our understanding of the mechanisms for dietary factor to be related with cognition. Our study has some limitations that need to note. The study is a cross-sectional study, and therefore no causal relationship between diet and DTI, or the relationship between DTI and cognition, can be established. However, dietary assessment was performed a few years before MRI scan, which helped to reduce the possibility of reverse causality to some extent. While the food frequency questionnaire has been validated for some nutrients (including vitamin A, C, and E)31, the accuracy for the measurement of other selected nutrients was unknown, and there is a possibility of information bias. There might be a concern for selection bias as our MRI study requested subjects to be dementia-free. It is possible that the proportion of subjects who had developed dementia and excluded from the MRI study might be higher among those with worse diet than among those with healthier diet, leaving people with healthier brain over-selected among people with worse diet (such as those with low nutrient pattern-6 scores). However, such selection process might have biased the results toward null. Although we adjusted for many potential confounders, we cannot completely rule out residual confounding. For example, despite that we have used as much information as possible, including self-report of the diseases, actual measure of blood pressure, and medication use, to best judge whether a subject had a vascular comorbidity or not, we could not rule out the residual confounding from unmeasured vascular factors. Another limitation of our study is that we only used nutrient intake from food in the analysis, due to the less detailed and reliable information we collected on supplements intakes. However, foods contain a variety of nutrients that may work synergistically to provide the best biological benefits. In addition, the Dietary Guidelines for Americans (http://health.gov/dietaryguidelines/2015/guidelines/) recommend that nutritional needs should be met primarily from foods. Thus, our results may help to provide scientific evidence on the benefits of food sources of nutrients for such guidelines.
Overall, the current study found that a diet high in PUFAs and vitamin E was associated with less white matter damage in the elderly people. Our study also found that white matter integrity was associated with cognitive performances and it might explain, at least partially, certain dietary factors’ association with cognition. Future studies, especially longitudinal ones, are warranted to confirm our findings and to test causality in these observed relationships.
Acknowledgments
This work is supported by NIH grants AG042483, AG037212, and AG034189.
Non-standard abbreviations
- APOE
Apolipoprotein
- BMI
body mass index
- DTI
Diffusion tensor imaging
- FA
fractional anisotropy
- MRI
magnetic resonance imaging
- PCA
Principal component analysis
- Ω-3 PUFA
omega-3 polyunsaturated fatty acids
- ROI
region of interest
- WHICAP
Washington Heights/Hamilton Heights Inwood Columbia Aging Project
Footnotes
Author Contributions: YG (Gu) contributed to study concept and design; YG (Gu), RSV, YG (Gazes), CGH, YS, JAL, JJM, NS, RM, and AMB contributed to data acquisition and analysis; YG (Gu) and AMB contributed to drafting the manuscript and figures.
Potential Conflicts of Interest
Dr. Gu reports grants from National Institute of Health R00 AG042483, during the conduct of the study. Other authors have nothing to disclose; Dr. Manly reports grants from NIH/NIA R01 AG037212, during the conduct of the study; Dr. Brickman reports grants from National Institutes of Health, personal fees from Keystone Heart, personal fees from ProPhase LLC, outside the submitted work.
References
- 1.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol. 2004 Nov;3(10):579–87. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 2.Douaud G, Refsum H, de Jager CA, et al. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jun 4;110(23):9523–8. doi: 10.1073/pnas.1301816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titova OE, Sjogren P, Brooks SJ, et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordrecht, Netherlands) 2013 Aug;35(4):1495–505. doi: 10.1007/s11357-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witte AV, Kerti L, Hermannstadter HM, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cerebral cortex. 2014 Nov;24(11):3059–68. doi: 10.1093/cercor/bht163. [DOI] [PubMed] [Google Scholar]
- 5.Mosconi L, Murray J, Tsui WH, et al. Mediterranean Diet and Magnetic Resonance Imaging-Assessed Brain Atrophy in Cognitively Normal Individuals at Risk for Alzheimer's Disease. The journal of prevention of Alzheimer's disease. 2014 Jun;1(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson KI, Suever BL, Prakash RS, Colcombe SJ, McAuley E, Kramer AF. Greater intake of vitamins B6 and B12 spares gray matter in healthy elderly: a voxel-based morphometry study. Brain research. 2008 Mar 14;1199:20–6. doi: 10.1016/j.brainres.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Y, Brickman AM, Stern Y, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015 Nov 17;85(20):1744–51. doi: 10.1212/WNL.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006 Sep 7;51(5):527–39. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Kantarci K, Senjem ML, Avula R, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011 Jul 5;77(1):26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosano C, Aizenstein HJ, Newman AB, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. NeuroImage. 2012 Aug 1;62(1):307–13. doi: 10.1016/j.neuroimage.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selnes P, Aarsland D, Bjornerud A, et al. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. Journal of Alzheimer's disease : JAD. 2013;33(3):723–36. doi: 10.3233/JAD-2012-121603. [DOI] [PubMed] [Google Scholar]
- 12.Dyrba M, Barkhof F, Fellgiebel A, et al. Predicting Prodromal Alzheimer's Disease in Subjects with Mild Cognitive Impairment Using Machine Learning Classification of Multimodal Multicenter Diffusion-Tensor and Magnetic Resonance Imaging Data. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2015 Jan 28; doi: 10.1111/jon.12214. [DOI] [PubMed] [Google Scholar]
- 13.Carlesimo GA, Cherubini A, Caltagirone C, Spalletta G. Hippocampal mean diffusivity and memory in healthy elderly individuals: a cross-sectional study. Neurology. 2010 Jan 19;74(3):194–200. doi: 10.1212/WNL.0b013e3181cb3e39. [DOI] [PubMed] [Google Scholar]
- 14.Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer's disease and mild cognitive impairment. Neuroscience. 2014 Sep 12;276:206–15. doi: 10.1016/j.neuroscience.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt S, Castelvetri LC, Simons M. Metabolism and functions of lipids in myelin. Biochimica et biophysica acta. 2015 Aug;1851(8):999–1005. doi: 10.1016/j.bbalip.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Dror V, Rehavi M, Biton IE, Eliash S. Rasagiline prevents neurodegeneration in thiamine deficient rats-a longitudinal MRI study. Brain research. 2014 Apr 4;1557:43–54. doi: 10.1016/j.brainres.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Bendlin BB, Canu E, Willette A, et al. Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiology of aging. 2011 Dec;32(12):2319, e1–11. doi: 10.1016/j.neurobiolaging.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta PK, Garg RK, Gupta RK, et al. Diffusion tensor tractography and neuropsychological assessment in patients with vitamin B12 deficiency. Neuroradiology. 2014 Feb;56(2):97–106. doi: 10.1007/s00234-013-1306-y. [DOI] [PubMed] [Google Scholar]
- 19.Moon Y, Moon WJ, Kwon H, Lee JM, Han SH. Vitamin d deficiency disrupts neuronal integrity in cognitively impaired patients. Journal of Alzheimer's disease : JAD. 2015 Jan 1;45(4):1089–96. doi: 10.3233/JAD-143063. [DOI] [PubMed] [Google Scholar]
- 20.Peters BD, Machielsen MW, Hoen WP, et al. Polyunsaturated fatty acid concentration predicts myelin integrity in early-phase psychosis. Schizophrenia bulletin. 2013 Jul;39(4):830–8. doi: 10.1093/schbul/sbs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chhetry BT, Hezghia A, Miller JM, et al. Omega-3 polyunsaturated fatty acid supplementation and white matter changes in major depression. Journal of psychiatric research. 2016 Jan 11;75:65–74. doi: 10.1016/j.jpsychires.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier A, Barul C, Feart C, et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015 Sep;11(9):1023–31. doi: 10.1016/j.jalz.2015.06.1888. [DOI] [PubMed] [Google Scholar]
- 23.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001 Jan 9;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of neurology. 1992;49(5):453–60. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Archives of neurology. 2001;58(12):1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 28.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Archives of neurology. 2008 Aug;65(8):1053–61. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brickman AM, Zahodne LB, Guzman VA, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiology of aging. 2015 Jan;36(1):27–32. doi: 10.1016/j.neurobiolaging.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008 Apr 1;40(2):570–82. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Archives of neurology. 2003 Mar;60(2):203–8. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 32.Holmes MD, Powell IJ, Campos H, Stampfer MJ, Giovannucci EL, Willett WC. Validation of a food frequency questionnaire measurement of selected nutrients using biological markers in African-American men. European journal of clinical nutrition. 2007 Nov;61(11):1328–36. doi: 10.1038/sj.ejcn.1602641. [DOI] [PubMed] [Google Scholar]
- 33.Siedlecki KL, Honig LS, Stern Y. Exploring the structure of a neuropsychological battery across healthy elders and those with questionable dementia and Alzheimer's disease. Neuropsychology. 2008 May;22(3):400–11. doi: 10.1037/0894-4105.22.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer’s disease risk: a protective diet. Archives of neurology. 2010;67(6):699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salat DH, Tuch DS, Hevelone ND, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005 Dec;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- 36.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascular pharmacology. 2015 Apr 11; doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Wang R, Fratiglioni L, Laukka EJ, et al. Effects of vascular risk factors and APOE epsilon4 on white matter integrity and cognitive decline. Neurology. 2015 Mar 17;84(11):1128–35. doi: 10.1212/WNL.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bongarzone ER, Pasquini JM, Soto EF. Oxidative damage to proteins and lipids of CNS myelin produced by in vitro generated reactive oxygen species. Journal of neuroscience research. 1995 Jun 1;41(2):213–21. doi: 10.1002/jnr.490410209. [DOI] [PubMed] [Google Scholar]
- 39.Linker RA, Maurer M, Gaupp S, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nature medicine. 2002 Jun;8(6):620–4. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Zhang H, Pu H, et al. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Scientific reports. 2014;4:7458. doi: 10.1038/srep07458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Progress in lipid research. 2014 Jan;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Salvati S, Natali F, Attorri L, et al. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. Journal of neuroscience research. 2008 Mar;86(4):776–84. doi: 10.1002/jnr.21537. [DOI] [PubMed] [Google Scholar]
- 43.Pu H, Guo Y, Zhang W, et al. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013 Sep;33(9):1474–84. doi: 10.1038/jcbfm.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukui K, Kawakami H, Honjo T, et al. Vitamin E deficiency induces axonal degeneration in mouse hippocampal neurons. Journal of nutritional science and vitaminology. 2012;58(6):377–83. doi: 10.3177/jnsv.58.377. [DOI] [PubMed] [Google Scholar]
- 45.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cellular and molecular neurobiology. 2010 Mar;30(2):289–99. doi: 10.1007/s10571-009-9451-x. [DOI] [PubMed] [Google Scholar]
- 46.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999 May 12;52(8):1626–32. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 47.Zamroziewicz MK, Paul EJ, Rubin RD, Barbey AK. Anterior cingulate cortex mediates the relationship between O3PUFAs and executive functions in APOE e4 carriers. Frontiers in aging neuroscience. 2015;7:87. doi: 10.3389/fnagi.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowman GL, Dodge HH, Mattek N, et al. Plasma omega-3 PUFA and white matter mediated executive decline in older adults. Frontiers in aging neuroscience. 2013;5:92. doi: 10.3389/fnagi.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008 Aug;40(3):879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]