Case Report
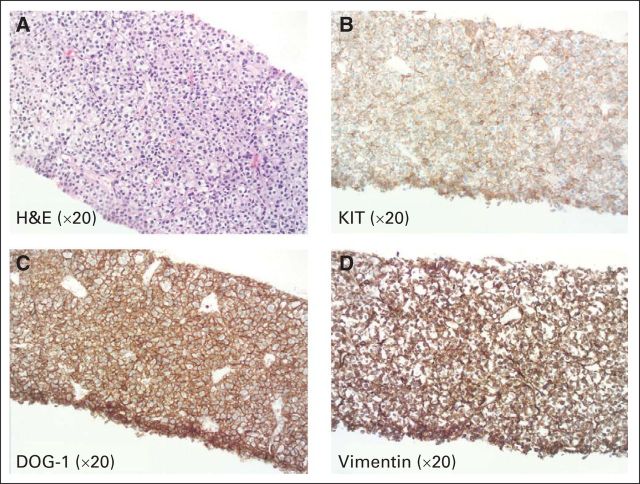
A 76-year-old Asian man presented with symptoms of postprandial epigastric pain and bloating for 3 months. He denied nausea, vomiting, diarrhea, melana, or hematochezia, but complained of chronic constipation. He was previously diagnosed with irritable bowel syndrome for which he was treated with fiber, dicyclomine, and a Chinese herb after a colonoscopy that revealed a tubulovillous adenoma and melanosis coli. Because of progressive upper abdominal discomfort, a computed tomography (CT) scan of his abdomen and pelvis was performed. Imaging revealed a 13.0-cm mass arising from the retrohepatic space and gastrohepatic ligament. The lesion was abutting the porta hepatis, vena cava, liver, duodenum, and right kidney. Metastatic omental involvement was noted. The primary location of the tumor (ie, stomach or duodenum) was indeterminate; however, given the extent of locally advanced and metastatic disease, the tumor was deemed unresectable. Before referral, percutaneous biopsy of the mass revealed a spindle cell neoplasm that was mostly comprised of epithelioid cells with mildly pleomorphic nuclei on hematoxylin and eosin staining (Fig 1A, ×20 magnification). Focal necrosis was present. Immunohistochemical staining demonstrated that the tumor cells were strongly positive for KIT (c-KIT, CD117; Fig 1B, ×20 magnification), DOG-1 (Fig 1C, ×20 magnification), and vimentin (Fig 1D, ×20 magnification). These findings were diagnostic of a GI stromal tumor (GIST). The mitotic index in the tumor was greater than 60 mitoses per 50 high-power fields. In summary, the patient had metastatic, high-grade GIST with epithelioid features.
Fig 1.
On referral to our center, we obtained a CT scan of the chest, abdomen, and pelvis to fully stage the patient's disease. This demonstrated a new, small, left pleural effusion, interval development of ascites, and tumor compression of the inferior vena cava with portal vein abutment (Figs 2A and 2B). Representative coronal (Figs 2A, 2C, and 2E) and axial (Figs 2B, 2D, and 2F) images are shown at the same levels before treatment (Figs 2A and 2B), as well as after 2 months (Figs 2C and 2D) and 9 months (Figs 2E and 2F) of imatinib therapy.
Fig 2.
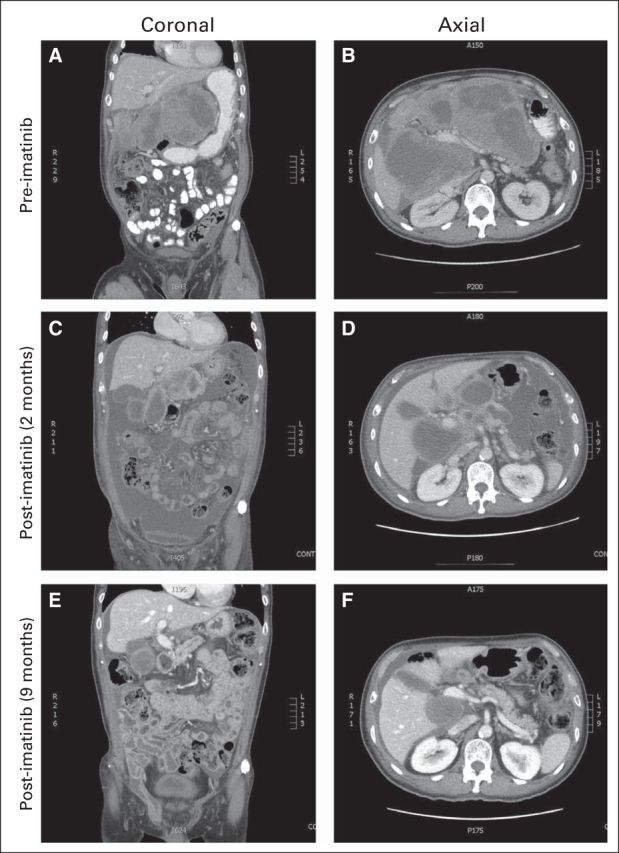
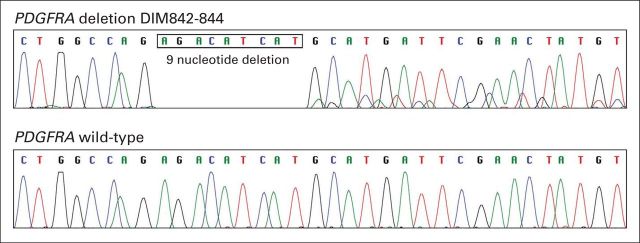
At this time, blood work demonstrated normal renal, hepatic, and thyroid functions with an anemia of chronic inflammation. An echocardiogram revealed normal left ventricular function without evidence of right heart failure or valvular heart disease. To guide optimal tyrosine kinase inhibitor therapy, KIT and platelet-derived growth factor receptor alpha (PDGFRA) gene sequencing analyses were requested. On the basis of the Radiation Therapy Oncology Group (RTOG) 0132/American College of Radiology Imaging Network (ACRIN) 6665 trial, a prospective phase II study evaluating the safety and efficacy of neoadjuvant imatinib for patients with advanced primary and metastatic GIST, the patient was treated with imatinib (Gleevec; Novartis, Basel, Switzerland) 400 mg orally once daily.1 Twenty-three days after initiation of therapy, a CT scan of the abdomen and pelvis was performed because of symptoms of increased abdominal pain. The CT scan demonstrated a decrease in size of the predominately cystic GIST tumor mass. The metastatic omental deposits were stable. At this time, the dominant tumor seemed to demonstrate a radiographic response; however, there was an interval increase in ascites with peritoneal enhancement. The patient's ascites and lower extremity were controlled with intermittent paracenteses, albumin support, diuretics, and compression stockings. Subsequent clinical response was evaluated by imaging 2 months after the initiation of imatinib therapy (Figs 2C and 2D). This demonstrated a decrease in the amount of ascitic fluid with an ongoing interval decrease in the size of the dominant GIST arising from the region of the retrohepatic space and gastrohepatic ligament. At this time, we received the results of the KIT and PDGFRA gene sequencing analyses. These demonstrated wild-type KIT but a PDGFRA deletion, DIM842-844 (Fig 3). Because of the patient's radiographic response, as well as his clinical improvement as gauged by a reduction in the volume and frequency of paracenteses, he continued to receive maintenance therapy with imatinib 400 mg per day. After 9 months of total therapy, there was additional regression of all of the lesions, with a near resolution of the ascites (Figs 2E and 2F). This was consistent with a partial response to imatinib therapy. To our knowledge, this is the first reported case of in vivo sensitivity to imatinib for a GIST with PDGFRA deletion DIM842-844.
Fig 3.
For PDGFRA mutation testing, tumor tissue was dissected from 5-μm unstained sections of formalin-fixed, paraffin-embedded tissue, and DNA was extracted. PDGFRA gene exons 12 and 18 were amplified by polymerase chain reaction, and the products were screened for mutations by direct sequencing. Both forward and reverse sequence chromatograms were reviewed for mutations with software-assisted analysis (Mutation Surveyor; SoftGenetics, State College, PA). A homozygous 9-nucleotide deletion mutation was detected in PDGFRA exon 18 (Fig 3; a sequence trace from a wild-type case [ie, negative control] is shown for reference in the lower panel). This mutation (c.2523_2531del) predicts deletion of three amino acids (p.D842_M844del) within the PDGFRA tyrosine kinase domain.
Discussion
KIT and PDGFRA mutations are mutually exclusive events in GIST, with the genetic changes resulting in the expression of a mutant kinase isoform with constitutive tyrosine kinase activity. Approximately 5% to 7% of GISTs have a mutation in the gene encoding PDGFRA; D842V is the most common mutation and is associated with imatinib resistance. In 2005, Corless et al2 reported 289 cases of PDGFRA-mutant GISTs in which they identified mutations associated with varying sensitivity to imatinib. Other groups have reported similar findings and added to a growing body of knowledge about PDGFRA-driven GISTs. Although most of our understanding is based on knowledge of imatinib resistance in tumors with missense mutations, less is known about imatinib sensitivity in GISTs with PDGFRA exon 18 deletions or insertions. Table 1 summarizes the reported in vitro and in vivo sensitivity of imatinib inhibition in GIST cell lines and tumors with various PDGFRA deletions.2–6 In tumors with PDGFRA exon 18 deletions, in vivo responses to imatinib include the following: one complete response (7.1%), four partial responses (28.6%), eight stable diseases (57.1%), and one progressive disease (7.1%). It is noteworthy that our patient's tumor deletion, DIM842-844, is a rare mutation, with only six reported cases in the literature.3–6 Dewaele et al7 reported that deletion DIM842-844 has in vitro sensitivity to four tyrosine kinase inhibitors, including imatinib (inhibitory concentration 50% [IC50] = 20 nmol/L), dasatinib (IC50 = 10 nmol/L), sorafenib (IC50 = 17 nmol/L), and nilotinib (IC50 = 56 nmol/L). Despite this data, in vivo sensitivity remained unknown until recently. In 2012, Cassier et al6 reported two cases in which imatinib therapy resulted in stable disease in GISTs with deletion DIM842-844. Our patient's partial response according to RECIST criteria represents the first reported case, to our knowledge, of an in vivo response to imatinib in a GIST bearing PDGFRA deletion DIM842-844. This is in alignment with the previously reported in vitro sensitivity.
Table 1.
PDGFRA Deletions: Published In Vitro Imatinib Sensitivity and In Vivo Tumor Responses to Imatinib Therapy
| PDGFRA Deletion/Insertion | Imatinib Sensitivity In Vitro | Tumor Responses In Vivo |
|---|---|---|
| Del D842 | Unknown | 1 PR, 1 PD |
| Del I843 | Yes | Unknown |
| Del RD841-842 | Unknown | Unknown |
| Del DIM842-844 | Yes | 2 SD |
| Del DIMH842-845 | Yes | 1 PR, 2 SD |
| Del IMHD843-846 | Yes | 1 CR, 2 SD |
| Del DIMHDS842-847; Ins VL | Unknown | 1 SD |
| Del IMHD843-845 | Unknown | 1 PR |
| Del IMHDS843-847 | Unknown | 1 PR |
| Del IMHDS843-847; Ins T | Unknown | 1 SD |
Abbreviations: CR, complete response; Del, deletion; Ins, insertion; PD, progressive disease; PR, partial response; SD, stable disease.
In summary, this case underscores the need for mutational testing of GISTs. Furthermore, it provides the first evidence that GISTs with PDGFRA deletion DIM842-844 should be considered for treatment with imatinib in the neoadjuvant, adjuvant, or metastatic settings as appropriate. Finally, this highlights the ongoing need to report and update our knowledge about the genetic data and responses of GISTs to tyrosine kinase-based therapies to help shape future treatment algorithms.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jason K. Sicklick, Novartis (C) Stock Ownership: None Honoraria: Jason K. Sicklick, Novartis Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
REFERENCES
- 1.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): Early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–5364. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 3.Debiec-Rychter M, Wasag B, Stul M, et al. Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol. 2004;202:430–438. doi: 10.1002/path.1546. [DOI] [PubMed] [Google Scholar]
- 4.Lasota J, Dansonka-Mieszkowska A, Sobin LH, et al. A great majority of GISTs with PDGFRA mutations represent gastric tumors of low or no malignant potential. Lab Invest. 2004;84:874–883. doi: 10.1038/labinvest.3700122. [DOI] [PubMed] [Google Scholar]
- 5.Wasag B, Debiec-Rychter M, Pauwels P, et al. Differential expression of KIT/PDGFRA mutant isoforms in epithelioid and mixed variants of gastrointestinal stromal tumors depends predominantly on the tumor site. Mod Pathol. 2004;17:889–894. doi: 10.1038/modpathol.3800136. [DOI] [PubMed] [Google Scholar]
- 6.Cassier PA, Fumagalli E, Rutkowski P, et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res. 2012;18:4458–4464. doi: 10.1158/1078-0432.CCR-11-3025. [DOI] [PubMed] [Google Scholar]
- 7.Dewaele B, Wasag B, Cools J, et al. Activity of dasatinib, a dual SRC/ABL kinase inhibitor, and IPI-504, a heat shock protein 90 inhibitor, against gastrointestinal stromal tumor-associated PDGFRAD842V mutation. Clin Cancer Res. 2008;14:5749–5758. doi: 10.1158/1078-0432.CCR-08-0533. [DOI] [PubMed] [Google Scholar]