Synopsis
Sepsis is a common and life-threatening inflammatory response to severe infection treated with antibiotics and fluid resuscitation. Despite the central role of intravenous fluid in sepsis management, fundamental questions regarding “which fluid” and “in what amount” remain unanswered. Recent advances in understanding the physiologic response to fluid administration, as well as large clinical studies examining resuscitation strategies, fluid balance after resuscitation, colloid versus crystalloid solutions, and high- versus low-chloride crystalloids, inform the current approach to sepsis fluid management and suggest areas for future research.
Keywords: fluid resuscitation, sepsis, crystalloids, colloids, albumin, Early Goal Directed Therapy
Introduction
Sepsis is an inflammatory response to severe infection characterized by hypovolemia and vasodilation and treated with early antibiotics and fluid resuscitation1. In the United States, sepsis with organ dysfunction (severe sepsis) or fluid-resistant hypotension (septic shock) account for 2% of hospital admissions and 10% of intensive care unit (ICU) admissions1. In-hospital mortality rates have decreased from 80% in the early years of intensive care to 20-30% in the modern era2–4 through improved surveillance, early treatment of underlying infection, and advances in support for failing organs. Despite the central role intravenous (IV) fluid administration has played in sepsis management for the last 15 years5,6, fundamental questions regarding “which fluid” and “in what amount” remain unanswered. This review addresses the physiologic principles and scientific evidence available to help clinicians address those questions in practice.
Physiology of Fluid Resuscitation in Sepsis
Patients with early sepsis are frequently hypovolemic from decreased intake and increased insensible losses. In addition, inflammation alters vascular resistance, venous capacitance, and vascular leak generating a “relative hypovolemia”. Resultant decreases in stroke volume and cardiac output imbalance oxygen delivery and demand, precipitating tissue hypoxia, anaerobic metabolism, and lactic acidosis.
The classic physiologic rationale for fluid resuscitation in sepsis is to restore intravascular volume, cardiac output, and oxygen delivery. Volume and choice of resuscitation fluids have largely been predicated on this model. Resuscitation endpoints like central venous pressure (CVP), inferior vena cava filling, mixed venous oxygen saturation, and lactate are used to restore preload independence and match oxygen demand and supply. Selection of colloids over crystalloids is intended to optimize volume expansion through colloid retention in the intravascular space.
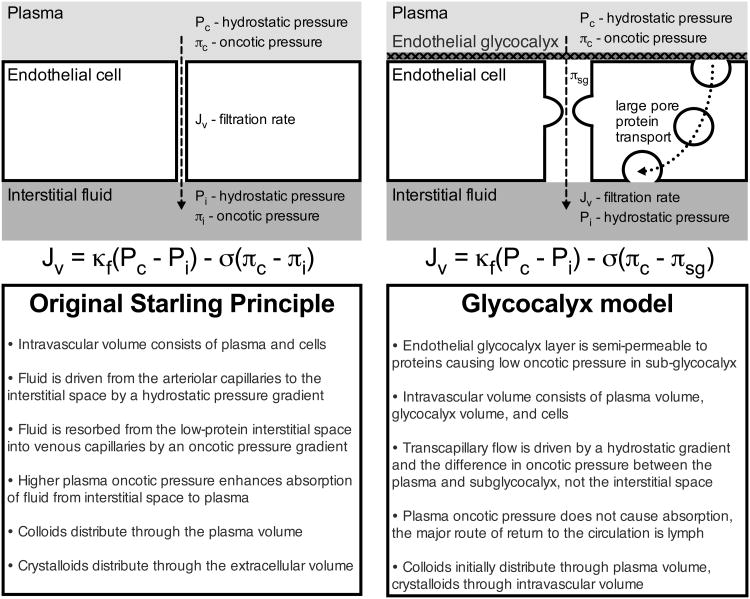
It is increasingly clear, however, that the hemodynamic response to fluid administration is determined by an intricate interaction of mean systemic filling pressure, right atrial pressure, venous resistance, and ventricular compliance, which makes predicting a critically ill patient's response to fluid challenging7. Impaired oxygen utilization and non-hypoxemic causes of lactic acidosis may elevate lactate levels despite adequate perfusion. Perhaps most importantly, the century-old Starling model conceptualizing maintenance of vascular volume as the balance of hydrostatic and oncotic pressure gradients between the vessel lumen and interstitial space has been challenged by the recent recognition of the importance of the endothelial glycocalyx (Figure 1)8. Because it is a primary determinant of membrane permeability, damage to the glycocalyx during sepsis may alter patients' response to fluid resuscitation. While the clinical implications of these findings are not yet fully understood, they argue against an overly-simplified approach to fluid dose (“fill the tank”) and fluid choice (“colloids stay in the vasculature”).
Figure 1. Models of transvascular fluid exchange.
In the original Starling model, the gradient of hydrostatic pressure from the capillary (Pc) to the interstitium (Pi) is opposed by the gradient of oncotic pressure from the capillary (πc) to the interstitium (πi), with filtration (Kf) and reflection (σ) coefficients. Understanding the web of membrane-bound glycoproteins and proteoglycans on the luminal side of endothelial cells (endothelial glycocalyx layer) suggests the low oncotic pressure under this semipermeable membrane (πsg) is a more important regulator of transcapillary flow than the interstitial oncotic pressure.
Fluid Dose
Fluid Administration in Sepsis Resuscitation
Fluid resuscitation is currently considered an essential component of early sepsis management1. Prompt IV fluid administration for patients with sepsis was advanced by a 2001 study of early goal-directed therapy (EGDT)5. In that landmark trial, 263 patients with sepsis and hypoperfusion were randomized to either standard therapy or EGDT. Standard therapy involved arterial and central venous catheterization and a protocol targeting CVP of 8-12 mmHg, mean arterial pressure (MAP) at least 65 mmHg, and urine output at least 0.5 ml/kg/hr. EGDT included all elements of standard therapy in addition to a catheter measuring central venous oxygen saturation (SvO2), six hours of treatment in the emergency department before admission, and protocolized administration of 500 mLs of IV crystalloid every 30 minutes to achieve CVP goals, vasopressors and vasodilators to maintain MAP goals, and blood transfusion or dobutamine to achieve SvO2 at least 70%. During the six hours of intervention, EGDT patients received more IV fluid (5.0 versus 3.5L, p<0.001), red-cell transfusions (64.1% versus 18.5%, p<0.001), and dobutamine (13.7% versus 0.8%, p<0.001). In-hospital mortality was 16% lower with EGDT compared to standard therapy (46.5% versus 30.5%, p=0.009).
The remarkable improvement in mortality propelled early, protocolized fluid resuscitation to the forefront of sepsis management. Based on the 2001 EGDT study, an EGDT trial at eight Chinese centers, and dozens of ‘before-after’ studies of EGDT implementation, the Surviving Sepsis Campaign (SSC) promoted incorporation of goal-directed fluid resuscitation into early sepsis management globally6. The most recent version of the SSC guidelines recommends “protocolized, quantitative resuscitation of patients with sepsis-induced tissue hypoperfusion” beginning with an “initial fluid challenge…to achieve a minimum of 30 mL/kg of crystalloids” targeting CVP, blood pressure, urine output, and venous oxygen saturation goals outlined in the 2001 EGDT trial6.
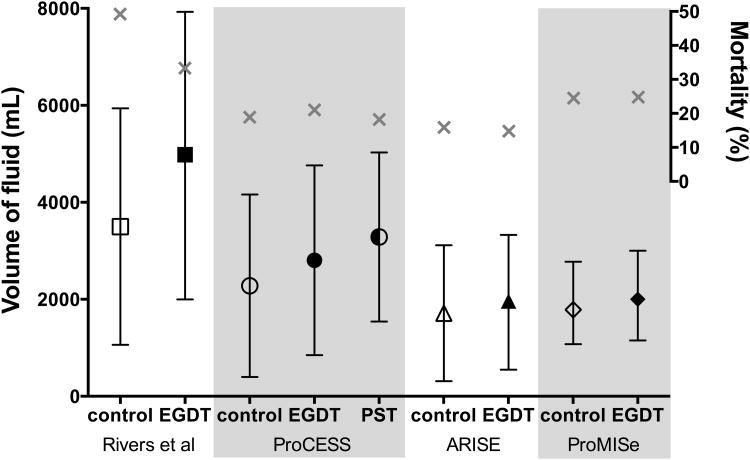
More than a decade after the original EGDT study, three large, multicenter trials attempted to confirm the benefit of EGDT. The ProCESS2, ARISE3, and ProMISe4 trials all compared EGDT to usual care in which invasive management was optional (e.g., central venous access in ProCESS) or forbidden (e.g., SvO2 measurement in ARISE). Fluid resuscitation in the first six hours of each EGDT trial is shown in Figure 2. There were no differences in any clinical outcome between EGDT and usual care among the 4,201 patients in these trials. Understanding the implications of these new EGDT trials for fluid resuscitation presents a number of challenges. First, the largest separation between arms in fluid administration in the first six hours was a 1L difference between modified “protocol-based standard therapy” (3.3L) and usual care (2.2L) – less than the 1.5L difference in the original trial. Advocates of EGDT would suggest that routine sepsis care has shifted to resemble the intervention arm of the original trial, but patients in both arms of the modern trials actually received less IV fluid than either arm of the original trial (Figure 2). Although the modern trials enrolled patients later after presentation, the pre- enrollment fluids were similar to the 20-30ml/kg required before inclusion in the original trial. Patients in the modern trials were less severely ill than patients in the original trial, potentially limiting the impact of early intervention. Ultimately, ancillary aspects of critical care have changed so dramatically in the decade between trials9 that comparing fluid management across EGDT studies may not yield firm conclusions about the optimal approach to early fluid resuscitation.
Figure 2. Fluid administration in early goal-directed therapy trials.
Volume of IV fluid during the first six hours in each early goal-directed therapy (EGDT) trial. Volume of fluid (black) is mean and standard deviation for all trials except ProMISe, which is median and interquartile range. Mortality (grey X) is through 60 days in ProCESS and 28 days in all other trials. PST is Protocol-based Standard Therapy.
While broad adoption of EGDT in developed countries complicates the study of sepsis resuscitation, provocative data have emerged elsewhere. The Fluid Expansion as Supportive Therapy (FEAST) study10 randomized 3,170 septic African children to weight-based fluid boluses with 0.9% saline, 5% albumin, or no bolus. The median volume of fluid received by one and eight hours was 20.0 and 40.0 ml/kg for the bolus groups compared to 1.2 and 10.1 ml/kg in the no bolus group. By 48 hours, 10.5% of children in the fluid bolus groups had died compared to 7.3% in the no bolus group (p=0.003). Receipt of fluid was harmful in all subgroups. Although shock resolved more frequently in the bolus groups, excess mortality was evident regardless of blood pressure response11. Similarly, the Simplified Severe Sepsis Protocol (SSSP) trial12 randomized 112 African adults with sepsis and organ dysfunction to usual care or an algorithm of simplified, goal-directed resuscitation. Patients in the intervention arm received 1.3L more fluid in the first six hours (2.9 versus 1.6 L, p<0.001) with no differences in vasopressors, transfusions, or antibiotics. In-hospital mortality was 64.2% with fluid resuscitation compared with 60.7% without when the study was stopped early for high mortality among patients with baseline respiratory failure randomized to the intervention12. The Simplified Severe Sepsis Protocol-2 (SSSP-2) trial currently enrolling patients with septic shock in Zambia (NCT01663701) may provide more definitive data on the impact of fluid compared to little or no resuscitation for early sepsis in this population.
Fluid Management in Sepsis after Resuscitation
In contrast to the intense focus on fluid in the first 6-12 hours of sepsis, little attention has been dedicated to optimal fluid management after resuscitation. There is broad agreement that fluid management may differ between different “phases” of sepsis, but the factors delineating each phase and the optimal fluid strategy for each phase remain largely undefined. The 2012 SSC guidelines recommend a fluid challenge approach for patients requiring hemodynamic support wherein fluid boluses are continued as long as there is hemodynamic improvement6. Frequently in clinical practice this has meant administering IV fluids to patients for changes in heart rate, blood pressure, or urine output. Recognizing the limitations of these traditional indices in assessing intravascular “volume status” and “fluid responsiveness”, researchers and clinicians have sought dynamic predictors of response to fluid administration13,14. Cardiac output monitoring15, pulse pressure and stroke volume variation16, and IVC diameter and stroke volume assessment by echocardiography13 have all been advocated to guide fluid administration. However, many dynamic measures cannot be used for patients who are spontaneously breathing or receiving low tidal-volume ventilation. Moreover, no clear evidence yet correlates improvement in short-term physiologic parameters with improvements in longer-term clinical outcomes.
Historically, patients with sepsis have received significant volumes of fluid throughout their ICU stay. Observational studies report positive fluid balances of five to eleven liters in the week after presentation17,18. After resuscitation, potential benefits of fluid are balanced against risks of pulmonary edema, renal parenchymal edema, and effects of the IV fluid constituents themselves. Observational studies have associated fluid receipt and positive fluid balance with mortality. Among 778 septic shock patients in the Vasopressin in Septic Shock Trial (VASST), odds of mortality doubled for patients with the highest cumulative fluid balance17. For 1,177 sepsis patients in the Sepsis Occurrence in Acutely Ill Patients (SOAP) study, each additional liter of fluid balance at 72 hours was associated with a 10% increase in the odds of death19. These observational studies are inherently limited by the indication bias that patients with higher severity of illness may be more likely to both die and have fluid administered by providers. The Fluid and Catheter Treatment Trial (FACTT) controlled post-resuscitation fluid management for 1,000 acute respiratory distress syndrome (ARDS) patients, of whom 70% had underlying infection. Fluid management emphasizing diuretics and limiting fluid administration increased ventilator-free days and ICU-free days without precipitating cardiovascular or renal dysfunction20. The 2012 SSC recommends conservative fluid management for patients with sepsis and ARDS after the resolution of shock6. Whether a conservative approach to fluid management after resuscitation can improve outcomes for sepsis patients without ARDS is being evaluated in ongoing randomized trials (NCT02079402, NCT02159079, and NCT01309724).
Fluid Choice
Since the advent of IV fluids, there has been debate as to which fluid is best for patients critically ill from infection21. The ideal sepsis resuscitation fluid would increase intravascular volume without accumulating in tissues, contain a chemical composition similar to plasma, and improve patient outcomes in a cost-effective manner. No such fluid exists currently. Available IV fluids are categorized as crystalloid or colloid solutions (Table 1).
Table 1.
Composition of common sepsis resuscitation fluids.
| Plasma | Crystalloid | Colloid | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Hydroxyethyl starch | Gelatin | |||||||||||||
| 0.9% sodium chloride | Ringer's lactate | Hartmann's solution | Plasma-Lyte | 4% albumin | 20% albumin | 10% (200/0.5) [Hemohes] | 6% (450/0.7) [Hextend] | 6% (130/0.4) [Voluven] | 6% (130/0.4) [Volulyte] | 6% (130/0.42) [Venofundin] | 6% (130/0.42) [Tetraspan] | 4% succinylated gelatin [Gelofusine] | 3.5% urea-linked gelatin [Maemaccel] | ||
| Sodium | 135–145 | 154 | 130 | 131 | 140 | 130-160 | 48-100 | 154 | 143 | 154 | 137 | 154 | 140 | 154 | 145 |
| Potassium | 4.5–5.0 | 4.0 | 5.4 | 5.0 | 3.0 | 4.0 | 4.0 | 5.1 | |||||||
| Calcium | 2.2–2.6 | 1.5 | 1.8 | 5.0 | 2.5 | ||||||||||
| Magnesium | 0.8–1.0 | 1.5 | 0.9 | 1.5 | 1.0 | ||||||||||
| Chloride | 94–111 | 154 | 109 | 112 | 98 | 128 | 19 | 154 | 124 | 154 | 110 | 154 | 118 | 120 | 145 |
| Acetate | 27 | 34 | 24 | ||||||||||||
| Lactate | 1–2 | 28 | 28 | 28 | |||||||||||
| Malate | 5.0 | ||||||||||||||
| Gluconate | 23 | ||||||||||||||
| Bicarbonate | 23–27 | ||||||||||||||
| Octanoate | 6.4 | 32.0 | |||||||||||||
| Osmolarity | 291 | 308 | 273 | 277 | 294 | 250 | 210-260 | 308 | 304 | 308 | 286 | 308 | 296 | 274 | 301 |
All values are given in mmol/liter except osmolarity which is in mOsm/liter. Electrolyte concentrations of intravenous fluid preparations may differ by manufacturer -- information is given for Hartmann's Solution (B. Braun Melsungen AG), Plasma-Lyte 148® (Baxter) and Albumex® 20 (CSL Behring). HES solutions are described with regard to their concentration (6-10%), mean molecular weight (70-480 kDa), and degree of molar substitution (range 0-1; tetrastarch 0.4, pentastarch 0.5, hexastrach 0.6).
Crystalloids
Crystalloids are solutions of ions which determine fluid tonicity but are freely permeable through capillary membranes. Isotonic crystalloids are the most commonly administered IV fluid internationally22 and the recommended first-line fluid for sepsis resuscitation6. Crystalloid solutions were first prepared in response to the cholera pandemic in 183221. Early solutions comprised of sodium, chloride, and bicarbonate in water21 evolved over the following century into two basic categories of isotonic crystalloid: sodium chloride and ‘physiologically-balanced’ solutions. Normal saline (0.9% sodium chloride) is the most common crystalloid globally, with over 200 million liters administered annually in the United States alone. With 154 mmol/L each of sodium and chloride, normal saline is isotonic to extracellular fluid but contains a chloride concentration significantly higher than plasma. In contrast, so-called balanced crystalloids derived from the original Hartmann's and Ringer's solutions may be slightly hypotonic to extracellular fluid but provide anions that more closely approximate plasma pH (Table 1).
Hyperchloremic metabolic acidosis
The difference in chloride content between saline and balanced crystalloids causes hyperchloremia and metabolic acidosis among critically ill patients23. In the Stewart physicochemical approach24, hydrogen ion concentration is determined by carbon dioxide, weak acids, and the balance of sodium, potassium, magnesium, calcium, chloride, and lactate (strong ion difference). The increased concentration of chloride with saline infusion decreases the strong ion difference, increases dissociation of water into hydrogen ions, and induces a non-anion gap metabolic acidosis23. Whether metabolic acidosis associated with saline infusion influences patient outcomes remains unclear.
Acute Kidney Injury
Crystalloid chloride content also regulates renal blood flow and may contribute to AKI. Delivery of chloride to the macula densa drives mesangial contraction and decreases glomerular filtration. Denervated dog kidneys infused with chloride-rich solutions demonstrate renal vasoconstriction25. Human volunteers experience decreased renal blood flow with high-chloride fluids26, and surgery patients have decreased urine output after saline administration27. A ‘before-after’ study of 1400 patients in an ICU transitioning from higher to lower chloride solutions found an association between higher chloride fluid and development of AKI28. However, subsequent analyses suggested unidentified confounders beyond fluid choice may have contributed to the difference in AKI29. A meta-analysis of high- versus low-chloride IV fluid in critically ill patients found increased AKI but not mortality30.
Isotonic crystalloids in sepsis
Animal models of sepsis link saline administration to acidosis, inflammation, and mortality. An observational study of adults with septic shock associated higher chloride and increased mortality31, with a dose-response curve for chloride that appears independent of volume of fluid received32. A recent meta-analysis linked balanced crystalloids to reduced mortality in sepsis33, although another suggested no relationship between chloride content and renal replacement therapy34. Ongoing randomized trials (ACTRN12613001370796, NCT02444988) comparing saline to balanced crystalloids in critically ill populations may definitively establish the impact of crystalloid choice on AKI and mortality among patients with sepsis.
Colloids
Colloids are suspensions of molecules in a carrier fluid with high enough molecular weight to prevent crossing of healthy capillary membranes. Available colloids include derivatives of human plasma (albumin solutions) and semisynthetic colloids (gelatins, dextrans, and hydroxyethyl starches). The physiologic rationale favoring colloids over crystalloids is that colloids may more effectively expand intravascular volume by remaining in the intravascular space and maintaining colloid oncotic pressure.
Albumin
Human serum albumin is a small protein synthesized by the liver and maintained in the vasculature through a dynamic equilibrium of leak into the interstitium matched by lymphatic return. Beyond providing 75% of plasma colloid oncotic pressure, albumin binds nitric oxide, protects against lipid peroxidation, and regulates inflammation – leading to the enticing proposition that albumin solutions might both expand intravascular volume and directly mediate sepsis pathogenesis.
Administration of human albumin was introduced in World War II for victims of traumatic and thermal injury. Commercial preparations of isotonic 4-5% albumin solution for fluid replacement and hyperoncotic 20-25% albumin solution to support colloidal pressure led to expanded use in civilian operating rooms, emergency departments, and ICUs. Fifty years after albumin's introduction into clinical practice, the first systematic evaluation of albumin's effect on clinical outcomes reported an alarming 6% increase in the risk of death with albumin use35 and calls were made for large, rigorously-conducted trials of albumin administration in critical illness.
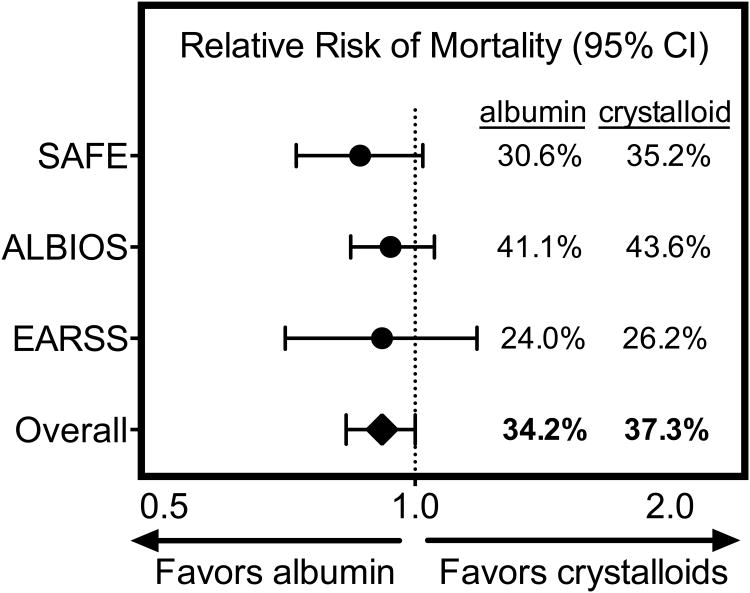
Three large trials now inform the utility of albumin administration for patients with sepsis36–38. The Saline versus Albumin Fluid Evaluation (SAFE) Study randomized nearly 7,000 critically ill adults to 4% albumin versus 0.9% sodium chloride for fluid resuscitation throughout the ICU stay36. The albumin group received slightly less fluid input but demonstrated similar heart rate and MAP. Overall there was no difference in 28-day mortality between albumin and saline. However, analysis of a pre-specified subgroup of patients with severe sepsis (n=1,218) suggested reduced in-hospital mortality with albumin (RR 0.87; 95% CI 0.74 – 1.02)36. In contrast to the SAFE study of 4% albumin for fluid resuscitation, the Albumin Italian Outcome Sepsis (ALBIOS) study examined daily administration of 20% albumin targeting a serum albumin level of 3 g/L37. Among 1,818 septic ICU patients, albumin administration resulted in higher serum albumin levels, lower net fluid balance, lower heart rate, higher MAP, and more rapid freedom from vasopressors. The 28-day mortality was identical in the two groups but a post hoc subgroup analysis suggested fewer deaths with albumin among patients in shock (RR 0.87; 95% CI 0.77 – 0.99; p interaction = 0.03). The third trial, Early Albumin Resuscitation during Septic Shock (EARSS) (available only in abstract form), randomized septic shock patients within 6 hours of vasopressor initiation to receive 100 mL of 20% albumin or 100 mL of 0.9% saline every 8 hours for three days. Among 798 patients, vasopressor-free days were higher with albumin without improvement in 28-day mortality (24.1% versus 26.3%)38. (Although the Colloids Versus Crystalloids for the Resuscitation of the Critically Ill (CRISTAL) trial allowed use of 4% or 20% albumin, albumin administration was too similar between the colloid and crystalloid arms (20.4% versus 16.5%) to allow inferences about the relative effects of albumin39).
Despite no overall benefit in each of the individual trials, multiple meta-analyses33,40–42 have suggested improved mortality with albumin administration in sepsis (Figure 3). The SCC in 2012 continued to recommend crystalloids as the initial sepsis resuscitation fluid, but advised consideration of albumin “when patients require substantial amounts of crystalloids”6. Given albumin's cost and a more recent meta-analysis showing no impact on sepsis mortality43, ongoing trials evaluating earlier albumin administration (NCT01337934, NCT00819416) will need to demonstrate clear mortality benefit for albumin to replace crystalloids as the gold-standard fluid for sepsis resuscitation.
Figure 3. Mortality of sepsis patients in trials of albumin administration.
Relative risks of death by 28 days with albumin (n=603) versus saline (n=615) for patients with severe sepsis in the SAFE study36, death by 90 days with albumin (n=888) versus crystalloid (n=893) in the ALBIOS study37, and death by 28 days with albumin (n=399) versus saline (n=393) in the EARSS study38 are displayed with accompanying 95% confidence intervals.
Adapted from Wiedermann, C. J. & Joannidis, M. Albumin replacement in severe sepsis or septic shock. N. Engl. J. Med. 371, 83 (2014); with permission.
Semisynthetic Colloids
The expense and limited availability of human albumin has prompted the development of semisynthetic colloid solutions (gelatins, dextrans, and hydroxyethyl starches (HES)) (Table 1). Gelatins are prepared by hydrolysis of bovine collagen, dextrans biosynthesized from sucrose by bacteria, and HES synthesized from the maize-derived D-glucose polymer amylopectin. Each colloid's duration of volume expansion is governed by rate of loss from the circulation (determined by molecular weight) and metabolism (determined by chemical properties like molar substitution). Each colloid has been linked to a unique profile of adverse events: increased risk of AKI (HES, gelatin), allergic reactions (gelatins, dextrans), and bleeding (dextrans, HES).
HES is the only semisynthetic colloid for which large trials enrolling septic patients have been conducted. The 2004 Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial comparing Ringer's lactate to 10% HES 200/0.5 among 537 severe sepsis patients was stopped early for increased AKI (34.9% versus 22.8%, p=0.002) and a trend toward increased 90-day mortality (41.0% versus 33.9%, p=0.09) with HES44. Based on a reportedly improved safety profile for starches with lower molecular weight and molar substitution, 6% HES 130/0.4 was compared to 0.9% sodium chloride among 196 septic patients in the CRYSTMAS study45. Differences between HES and 0.9% sodium chloride in mortality (31.0% versus 25.3%) and AKI (24.5% versus 20.0%) failed to reach statistical significance. However, in the larger Scandinavian Starch for Severe Sepsis/Septic Shock (6S) trial in which 804 patients with severe sepsis were resuscitated with 6% HES 130/4.2 or Ringer's acetate, both renal replacement therapy (22% versus 16%, p=0.04) and 90-day mortality (51% versus 43%, p=0.03) were significantly higher with HES46. Among 7,000 critically ill adults (1,937 with sepsis) in the Crystalloid versus Hydroxyethyl Starch Trial (CHEST) trial, those randomized to 6% HES 130/0.4 received more renal replacement therapy (7.0% versus 5.8%, p=0.04) with similar 90-day mortality (18.0% versus 17.0%). A subsequent meta-analysis confirmed an association between HES and both AKI and mortality47. In contrast, the Colloids Versus Crystalloids for the Resuscitation of the Critically Ill (CRISTAL) trial found similar short-term mortality and improved ventilator-free days and long-term mortality with colloids compared to crystalloids39. The CRISTAL trial randomized 2,857 adult ICU patients (55% with sepsis) to resuscitation with colloids or crystalloids. Patients in the colloid arm received less 0.9% saline and Ringer's lactate, more gelatins and HES, and a similar amount of albumin to patients in the crystalloid arm. The 28-day mortality was 25.4% with colloids compared to 27.0% with crystalloids (p=0.26), a difference which increased to favor colloids at 90 days (30.7% versus 34.2%, p=0.03). Given the preponderance of data linking HES to AKI and the relatively high use of albumin in both arms of the CRISTAL trial, unless the improvement in long-term mortality seen in CRISTAL is replicated, the cost and potential risks prevent colloids from replacing crystalloids as first-line fluid therapy in sepsis6.
Key points.
Fluid resuscitation to correct hypovolemia and support organ perfusion is central to current management of severe sepsis and septic shock.
Recent randomized trials have not confirmed a benefit for targeting invasive physiologic parameters; the ideal fluid volume and endpoints in sepsis resuscitation remain unknown.
Increased fluid balance is associated with increased mortality in early and late sepsis; whether conservative fluid management can improve sepsis outcomes requires further study.
Hydroxyethyl starch increases risk of acute kidney injury and may increase mortality in patients with sepsis.
Whether albumin or ‘physiologically-balanced’ crystalloids improve clinical outcomes in sepsis remains the focus of ongoing study.
Recommendation for clinical practice.
For patients with severe sepsis and septic shock, early administration of IV fluids to correct hypovolemia and potentially improve blood pressure and tissue perfusion remains standard of care. The optimal amount, rate, and endpoint for fluid administration in early sepsis are unknown. Fluid resuscitation beyond euvolemia may be detrimental.
Recommendation for clinical practice.
For patients beyond the early phase of sepsis, the risks and benefits of further IV fluid administration should be weighed. Hypervolemia should be avoided and consideration should be given to targeting a net even-to-negative fluid balance.
Recommendation for clinical practice.
For patients with sepsis, administration of normal saline contributes to metabolic acidosis and may increase the risk of AKI. Whether use of balanced crystalloids can prevent AKI and decrease mortality remains unknown.
Recommendation for clinical practice.
Colloid solutions should not be used as first-line fluid therapy for patients with sepsis. Hydroxyethyl starch appears to increase AKI and potentially mortality; the safety of other semisynthetic colloids is not established. Unless the potential beneficial effects of albumin infusion are confirmed by further trials, cost precludes its routine use.
Summary.
Sepsis remains a common and lethal illness with few effective therapies.
Since the 2001 EGDT trial, fluid resuscitation targeting hemodynamic parameters in sepsis has been disseminated globally.
- Recent trials have not confirmed the benefits of EGDT and question reliance on resuscitation targets, but leave unanswered how fluid should be ‘dosed’ in sepsis.
- Trials in the Third World examining outcomes of early fluid therapy compared to limited sepsis resuscitation are ongoing.
- Conservative fluid management after sepsis resuscitation is being studied in the United States and Europe.
- Pending further evidence, an initial 20cc/kg IV fluid bolus for patients with severe sepsis or septic shock will remain common practice; the optimal volume and endpoints of additional fluid administration are unclear.
- Crystalloids remain the first-line sepsis resuscitation fluid because they are widely available, inexpensive, and have not been shown to result in worse outcomes.
- Whether balanced crystalloids result in better organ function or outcomes is the focus of ongoing trials.
- Despite extensive study, the effect of albumin solutions on sepsis outcomes remains unclear.
- Hydroxyethyl starch is the only semisynthetic colloid robustly studied in sepsis and increases the incidence of AKI and potentially mortality.
Ongoing research on the endothelial glycocalyx, balanced crystalloids, and early albumin administration hold the potential to further improve sepsis survival.
Acknowledgments
Funding: When this review was prepared, Dr. Semler was supported by a National Heart, Lung, and Blood Institute (NHLBI) T32 award (HL087738 09).
Footnotes
Disclosure Statement: Conflicts of Interest: Dr. Semler and Dr. Rice have no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.ProCESS Investigators et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ARISE Investigators et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 4.Mouncey PR, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 5.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 7.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 8.Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108:384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 9.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 10.Maitland K, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 11.Maitland K, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews B, et al. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med. 2014;42:2315–2324. doi: 10.1097/CCM.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1:1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhashemi JA, Cecconi M, Hofer CK. Cardiac output monitoring: an integrative perspective. Crit Care Lond Engl. 2011;15:214. doi: 10.1186/cc9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care Lond Engl. 2014;18:650. doi: 10.1186/s13054-014-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 18.Micek ST, et al. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care Lond Engl. 2013;17:R246. doi: 10.1186/cc13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 21.Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr Edinb Scotl. 2008;27:179–188. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Finfer S, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care Lond Engl. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yunos NM, et al. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–2424. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 24.Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 27.Wilkes NJ, et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg. 2001;93:811–816. doi: 10.1097/00000539-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Yunos NM, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA J Am Med Assoc. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 29.Yunos NM, et al. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–264. doi: 10.1007/s00134-014-3593-0. [DOI] [PubMed] [Google Scholar]
- 30.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102:24–36. doi: 10.1002/bjs.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghunathan K, et al. Association Between the Choice of IV Crystalloid and In-Hospital Mortality Among Critically Ill Adults With Sepsis*. Crit Care Med. 2014;42:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 32.Shaw AD, et al. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40:1897–1905. doi: 10.1007/s00134-014-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochwerg B, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–355. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 34.Rochwerg B, et al. Fluid type and the use of renal replacement therapy in sepsis: a systematic review and network meta-analysis. Intensive Care Med. 2015 doi: 10.1007/s00134-015-3794-1. [DOI] [PubMed] [Google Scholar]
- 35.Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finfer S, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 37.Caironi P, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 38.Charpentier J, Mira JP. Efficacy and tolerance of hyperoncotic albumin administration in septic shock patients: the EARSS study. abstract. [Google Scholar]
- 39.Annane D, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310:1809–1817. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 40.Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39:386–391. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 41.Bansal M, Farrugia A, Balboni S, Martin G. Relative survival benefit and morbidity with fluids in severe sepsis - a network meta-analysis of alternative therapies. Curr Drug Saf. 2013;8:236–245. doi: 10.2174/15748863113089990046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiedermann CJ, Joannidis M. Albumin replacement in severe sepsis or septic shock. N Engl J Med. 2014;371:83. doi: 10.1056/NEJMc1405675. [DOI] [PubMed] [Google Scholar]
- 43.Patel A, Laffan MA, Waheed U, Brett SJ. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ. 2014;349:g4561. doi: 10.1136/bmj.g4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunkhorst FM, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 45.Guidet B, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care Lond Engl. 2012;16:R94. doi: 10.1186/cc11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perner A, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 47.Zarychanski R, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]