SYNOPSIS
The microcirculation is a series of arterioles, capillaries, and venules that performs essential functions of oxygen and nutrient delivery, customized to the unique physiologic needs of the supplied organ. The homeostatic microcirculatory response to infection, which includes barrier hyperpermeability, leukocyte adhesion, and coagulation activation, can become harmful if overactive and/or dysregulated, contributing to the organ failure characteristic of sepsis. In humans, pathologic microcirculatory dysfunction can be directly visualized by intravital microscopy or indirectly measured via detection of circulating biomarkers, such as endothelial glycocalyx fragments. While several treatments have been shown to protect the microcirculation during sepsis, these therapies have not improved patient outcomes when applied indiscriminately. Future outcomes-oriented studies are needed to test the utility of sepsis therapeutics when applied in a manner “personalized” to a patient’s microcirculatory dysfunction.
Keywords: Sepsis, microcirculation, glycocalyx, intravital microscopy, glycosaminoglycans, heparan sulfate
Anatomy and function of the microvasculature
The microcirculation, comprised of < 100 μm-diameter arterioles, capillary beds, and draining venules, performs essential homeostatic functions including oxygen delivery and solute exchange1. While this simple construct holds true across all human tissues, there is substantial organ specificity of microcirculation structure, reflecting unique functions assigned to different vascular beds. The kidney glomerulus, tasked with plasma ultrafiltration, features afferent and efferent arterioles flanking a capillary network lined with fenestrated endothelium. In contrast, the cerebral and pulmonary vasculature are characterized by tight endothelial barriers (and supporting pericytes), reflecting organ functions that would be threatened by interstitial edema. These organ-specific differences in microvascular function are paralleled by tissue-specific endothelial phenotypes, yielding varied mechanisms of endothelial-leukocyte adhesion (e.g. pulmonary vs. systemic circulations2) and organ-specific endothelial glycocalyces3.
The normal microvascular response to infection
To understand dysfunction of the microcirculation during sepsis, it is necessary to appreciate the appropriate microvascular response to infection. The inflammatory response to infection, as described in the first century AD, consists of calor (heat), rubor (redness), dolor (pain), and tumor (swelling)4. From a microcirculation standpoint, these responses reflect altered regional blood flow, vascular hyperpermeability, leukocyte recruitment, and coagulation1. It is critical to recognize that these physiologic changes are appropriate and effective in the setting of acute infection. The vast majority of viral and bacterial infections are controlled quickly by the host and do not lead to disseminated infection, organ failure, and death. By allowing for the beneficial actions of calor, rubor, dolor, and tumor, the microcirculation facilitates local quarantine of pathogens, targeted delivery of soluble anti-infectious agents (e.g. complement, immunoglobulins), and chemotaxis of activated host immune cells.
Leukocyte adhesion
The recruitment of leukocytes to areas of infection is a highly regulated process, consisting (in systemic venules) of active leukocyte rolling, adhesion, activation, aggregation, and transmigration, demonstrating the importance of these processes to tissue homeostasis5. In the absence of infection, leukocyte-endothelial interactions are limited, occurring primarily in specialized vascular beds (e.g. lymph node high endothelial venules). There is great heterogeneity across different vascular beds regarding processes of leukocyte extravasation, with rolling being essential for diapedesis from systemic venules but dispensable for extravasation from the pulmonary capillaries2,6.
Tissue edema
The intense, multi-process regulation of vascular permeability reflects its critical importance in microvascular function7. Indeed, the targeted extravasation of antibacterial peptides, antibodies, and complement is beneficial to the host response to infection. However, barrier dysfunction can become pathologic if transvascular fluid flux overwhelms lymphatic drainage or other tissue-specific safeguards against interstitial edema8.
Coagulation
Microvascular coagulation is important to the host response to infection. Endothelial damage and inflammatory cytokines lead to a pro-coagulant state in the microvasculature, allowing for the development of microthrombi9,10. This response functions to isolate infection and prevent dissemination. Murine studies have shown that anticoagulants facilitate bacterial spread after peritonitis, leading to worsened sepsis outcomes11. The failures of activated protein C, antithrombin III, and tissue factor antagonists to improve sepsis outcomes perhaps reflect homoeostatic effects of microvascular coagulation12–14.
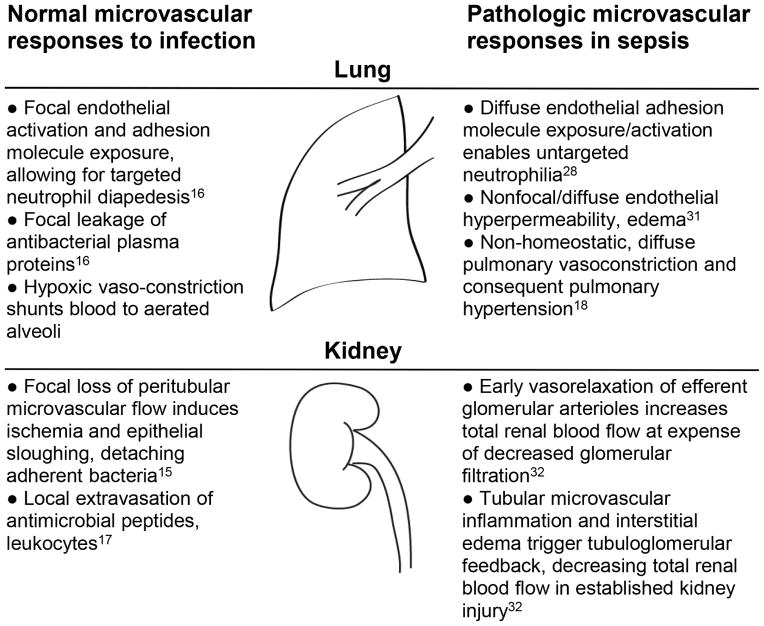
These and other microcirculatory responses are adaptive and often successful in localizing and eliminating infectious insults15–17. However, in extreme cases of overwhelming infection, these processes may contribute to the overall morbidity and mortality of sepsis (Figure 1).
Figure 1.
Homeostatic vs. pathologic (septic) pulmonary, renal microvascular responses to infection
Evidence of microvascular dysfunction during sepsis
Oxygen delivery (DO2) is a function of both cardiac output and blood oxygen content. As early sepsis is characterized by a low systemic vascular resistance/high-cardiac output state, DO2 is typically elevated in sepsis. Indeed, the kidney, brain, and heart all experience augmented blood flow during sepsis19. Despite this increased bulk delivery of oxygen, tissue hypoxia persists in sepsis and contributes to septic organ injury1. This suggests that the defect of sepsis is not a loss of macrovascular blood supply, but rather a loss of microvascular function. Indeed, therapeutic attempts to augment macrocirculatory oxygen delivery by increasing cardiac output or hemoglobin have failed to improve outcomes in sepsis20–24.
This suspected microvascular defect in sepsis has been extensively investigated using animal models25, identifying critical pathogenic roles of endothelial barrier dysfunction26,27, inappropriate leukocyte adhesion28, platelet activation29, activation of microvascular coagulation9, and aberrant control of vascular tone30. These changes broadly mediate injury across numerous organ systems of relevance to sepsis outcomes, including the lung31, kidney32, and brain33. Importantly, there is no discrete, readily-apparent inflection point at which beneficial microvascular responses to infection change to pathologic contributors to sepsis. Sepsis may arise from numerous microcirculatory changes, including activation of anti-infection responses in vascular beds where no pathogens exist, or a magnitude of anti-infection response that outstrips what is necessary for microbial clearance. This complexity warrants a deeper understanding of the precise changes occurring within an individual during sepsis, potentially allowing for personalization of sepsis therapeutics.
Measuring septic microvascular dysfunction in humans
Detecting and characterizing microvascular dysfunction in humans is technically challenging, given difficulties in the direct measurement of clinically-relevant vascular beds. Systemic, circulating biomarkers of tissue ischemia (e.g. central venous oxygenation, lactate) are not sensitive to microcirculatory defects, given the potential for “functional shunting” in which venular PO2 exceeds capillary PO21. Furthermore, the value of therapeutically targeting these markers is uncertain, given recent negative studies of early goal-directed therapy22–24. As recently reviewed elsewhere27,34, numerous promising biomarkers for capillary endothelial dysfunction (e.g. angiopoietins, glycocalyx fragments) have been identified in septic shock. These biomarkers, however, often are not easily measured point-of-care and have yet to be validated as clinically-relevant treatment endpoints.
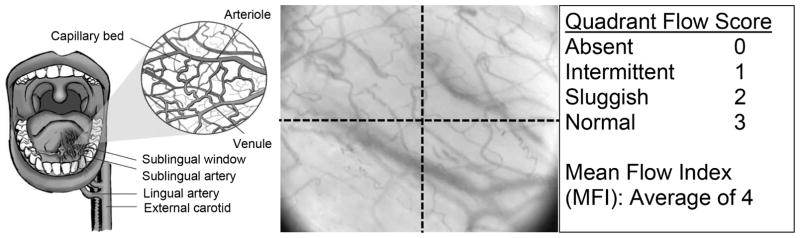
An alternative approach to rapidly measuring microcirculatory function is direct imaging of microvessels using intravital microscopy35. While nail fold or episcleral vessels can be visualized at the bedside35, these vascular beds yield little quantitative data regarding microvascular function without the use of large microscopy systems. However, the development of microscopy techniques such as orthogonal phase spectrometry (OPS) or sidestream darkfield imaging (SDF) has led to increasing enthusiasm for the bedside imaging of the sublingual microvasculature. OPS and SDF imaging can clearly identify RBCs, due to the absorptive effects of hemoglobin. As such, these techniques can identify RBC-perfused vessels (Figure 2); a lack of visualized sublingual vessels serves as evidence of absent or impaired RBC flow36.
Figure 2.
Semiquantitative assessment of sublingual microvascular flow. Intravital microscopy can access the sublingual miscrovasculature (OPS image, middle). Semiquanitative measurements of flow in each quadrant of image yields an average mean flow (MFI); at least 5 images should be measured.
Adapted from Klijn E, Den Uil CA, Bakker J, Ince C. The heterogeneity of the microcirculation in critical illness. Clinics in Chest Medicine. 2008;29(4):643–654; with permission.
This visualized loss of sublingual microvascular RBC perfusion can be quantified via several techniques, either at point-of-care37 or during later review of recorded images. Loss of RBC flow yields a heterogeneous loss of vascular density apparent on OPS and SDF imaging, particularly involving small (< 20 μm) microvessels. This microvascular drop-out can be quantified by using several different validated approaches, including the De Backer score (which employs a stereological-like approach in which vessel density is calculated from intersections with overlying gridlines) or the microvascular flow index (a semiquantitative score determined from the average of qualitative assessments across four visual field quadrants, Figure 2)36,38.
While consensus statements have detailed standardized approaches to the quantification of intravital measures of microvascular function36, there remain several practical challenges to the wide-spread implementation of these approaches. A major concern is the risk of visual artifacts (e.g. capillary dropout) produced from undue pressure of the microscope objective on the sublingual microvessels36,39. Even when excluding video clips that have such artifacts, only 30.8% of SDF recordings were found to be of excellent technical quality39. Despite these concerns, a recently-published international study (“microSOAP”) performed across 56 ICUs performed SDF sublingual microvascular measurements in 501 patients, with low variation in MFI (2%) and De Backer scoring (7%)40.
An additional concern regarding the sublingual microcirculation is the relevance of this vascular bed during sepsis, particularly given divergent responses of the sublingual microvasculature from vascular beds more proximal to the site of a sepsis-inducing infection (e.g. the submucosa of an intestinal ostomy during abdominal sepsis)41. However, convergent findings from multiple groups have linked sublingual microvascular alterations with clinical outcomes in sepsis, providing reassurance for the relevance of these measurements. Using OPS imaging of the sublingual microvasculature, De Backer and colleagues compared 10 healthy volunteers, 16 patients prior to cardiac surgery, 5 non-septic ICU patients, and 50 patients with sepsis/septic shock42. Patients with sepsis had significant loss (or intermittent interruption) of RBC perfusion in small (< 20 μm) sublingual microvessels. Perfusion was highly variable in patients with sepsis, and vessel perfusion was lower in non-survivors. Interestingly, these changes were independent of measures of macrovascular function, including mean arterial pressure and need for vasopressor medications. Further studies demonstrated that septic shock survivors tended to have rapid (albeit incomplete) correction of early microvascular dysfunction, as opposed to persistent abnormalities in patients who ultimately died43. Indeed, an increase in small vessel perfusion of > 7.8% in the first 24 hours of sepsis was 82% specific for survival43. In the microSOAP study, 17% of mixed ICU patients (septic and non-septic) demonstrated abnormal sublingual microvascular function; in the subgroup of patients with tachycardia, this dysfunction predicted hospital mortality40. These studies as well as others44–46 support the feasibility (and reproducibility) of bedside measures of sublingual microvascular function and their relevance to sepsis outcomes.
As with any observational human approach, it is difficult to prove that observed changes in microvascular dysfunction during sepsis are causal to, as opposed to a consequence of, organ dysfunction. For example, it is possible that loss of microvascular flow is in fact an appropriate response to decreased tissue metabolic demand. Sepsis-induced suppression of mitochondrial oxidative phosphorylation would be expected to decrease cellular oxygen demand, triggering a reactive decrease in microvascular flow and vascular density. This phenomenon, however, is not supported by available experimental data. During sepsis, extravascular tissue CO2 partial pressures (a measure of cellular respiration quantifiable by sublingual capnometery) increase as microvascular flow decreases, suggesting that tissue metabolic activity outstrips microvascular blood supply47.
Pathogenesis of microvascular dysfunction during sepsis
Given the potential causal importance of microvascular dysfunction during sepsis, the pathogenic mechanisms underlying these changes are attractive therapeutic targets. Likely contributors to these changes include pathophysiologic events typically implicated in septic organ injury, including aberrant vascular tone, inappropriate barrier dysfunction (and consequent tissue edema), inappropriate leukocyte adhesion (and inflammation), and activation of microvascular coagulation1. These pathophysiologic events can yield a signature appearance on intravital microscopy, with extraluminal (tissue edema, vasoconstriction) and intraluminal (coagulation, leukocyte adhesion) events conspiring to produce a loss of visualized RBC flow. Loss of RBC flow has physiologic consequence, leading to tissue hypoxia in the setting of tissue injury-amplified metabolic demands. While loss of microvascular flow can be compensated by increased flow through other vessels, this compensation can produce a “functional shunt”, in which the high velocity of flow through patent collateral microvessels decreases the capillary dwell time of RBCs, diminishing oxygen diffusion and potentially leading to additional hypoxia surrounding perfused microvessels19.
As numerous pathophysiologic events contribute to microvascular dysfunction during sepsis, it is unlikely that targeting a discrete contributor to vascular injury would have broad beneficial effects on patient outcomes in sepsis. As such, there has been great effort invested in identifying, and subsequently targeting, unifying mechanisms upstream of endothelial barrier dysfunction, inflammation, and microthrombosis. Particularly intense attention has been dedicated to the immunopathogenesis of sepsis, a broad topic ranging from the initial infection-associated release of pathogen-associated molecular patterns, consequent induction of pattern receptor (e.g. toll-like) signaling, downstream induction of inflammatory cytokine production (“cytokine storm”), leukocyte recruitment, and tissue damage with release of immune-amplifying damage-associated molecular patterns48. These events coincide with induction/augmentation of coagulation pathway signaling48. These proinflammatory pathways, however, have largely failed to identify clinically-effective immunotherapies for sepsis49–51. While these failures may be largely the consequence of practical challenges in therapeutically interrupting hyperacute events driving sepsis onset, it may also reflect our incomplete understanding of the complex immunologic events surrounding sepsis. Indeed, recent efforts have highlighted the pathologic significance of anti-inflammatory signaling in severe sepsis and septic shock52.
These limitations of the classic “cytokine storm” theory as a unifying mechanism of microcirculatory dysfunction have raised the need to identify novel pathophysiologic pathways of organ dysfunction (and microcirculatory failure) in sepsis. De Backer and colleagues demonstrated that septic sublingual microcirculatory heterogeneity can be completely corrected by the topical administration of vasodilators (e.g. acetylcholine)42,53. This rapid reversibility suggests that septic microvascular failure may arise largely from pathologic involvement of processes associated with the dynamic regulation of vascular tone. Accordingly, nitric oxide (NO) has been the intense focus of research as a mediator of sepsis and septic organ injury. Unfortunately, NO signaling is highly complex, with context-specific functions that can be both homeostatic and pathologic54–56. Human studies of NO-targeted microvascular therapeutics have accordingly been disappointing57,58, potentially reflecting broad, nonspecific effects of NO manipulation59. These challenges (and opportunities) of NO-based therapies have been reviewed in detail elsewhere60.
The limitations of systemic, NO-targeted therapeutic approaches in sepsis have raised interest in other, more specific manipulations of vascular tone. While many pathways are currently the focus of intense investigation, this review will focus upon one particularly-promising therapeutic target—the endothelial glycocalyx.
Endothelial glycocalyx and the septic microcirculation
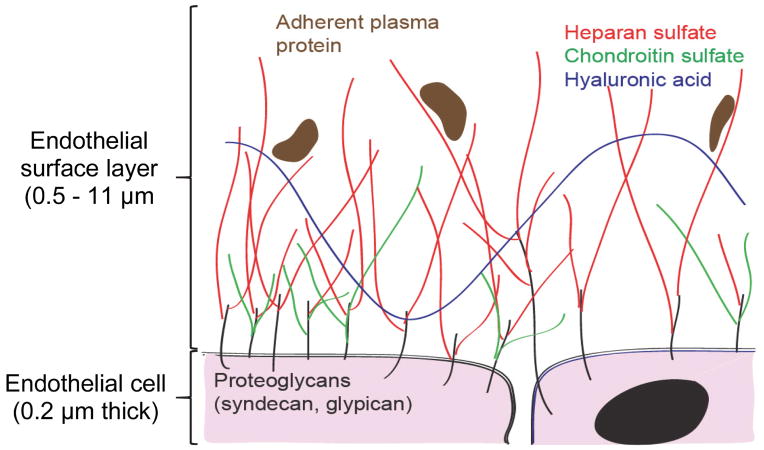
The endothelial glycocalyx is a layer of glycosaminoglycans (GAGs) and associated proteoglycans lining the vascular lumen (Figure 3)61. First described by as a 20 nm-thick “endocapillary layer” in 1966, the glycocalyx was long thought to be a structure of trifling significance62. This underappreciation of glycocalyx structure/significance likely reflected glycocalyx aberrance in vitro63 as well as its frequent degradation during tissue fixation64. With the advent and optimization of intravital microscopy, it is now apparent that in vivo, negatively-charged glycocalyx GAGs sequester water, forming a massive (0.5 – 11 μm) endothelial surface layer (ESL) with measurable rigidity61,65,66. The ESL has several homeostatic functions, including maintenance of the endothelial barrier to fluid and protein67 as well as regulation of leukocyte-endothelial adhesion5. The ESL also serves as a mechanotransducer of shear stress: in the presence of sufficient shear, the ESL-replete endothelium activates endothelial NO synthase, leading to vasodilation and accommodation of increased flow68. Experimental ESL degradation induces edema67, inappropriate leukocyte adhesion69, and loss of microvascular autoregulation68. Accordingly, degradation of the ESL in animal models increased microvascular heterogeneity, with some vessels becoming occluded to RBC flow and others becoming hyperemic70,71.
Figure 3.
The Endothelial Glycocalyx
Endothelial glycocalyx/ESL integrity is therefore highly relevant to septic organ injury and microvascular dysfunction. In experimental models of polymicrobial sepsis, GAG degradation occurred within the pulmonary and renal vascular beds, contributing to both lung edema/inflammation8,28 as well as loss of glomerular filtration72. In a rat model of endotoxemia, loss of intestinal capillary density occurred in association with mesenteric ESL degradation73. In humans, several techniques exist for the detection and quantification of glycocalyx degradation in critically-ill patients (Table 1). Loss of glycocalyx/ESL integrity was apparent within the sublingual microcirculation after endotoxin administration to healthy volunteers, coincident with loss of capillary density74. Patients with sepsis demonstrate elevated circulating ESL degradation products, including proteoglycans75–77 as well as GAGs heparan sulfate, hyaluronic acid, and chondroitin sulfate75,78–83. Accordingly, glycocalyx/ESL degradation is predictive of clinical outcomes in critical illness78,79. The development of rapid, point-of-care assays for glycocalyx breakdown products (Table 1) may allow for microvascular “personalization” of sepsis treatment, identifying patients who may benefit the most from vascular-protective therapies.
Table 1.
Measurement of endothelial glycocalyx/ESL degradation in humans.
| Assay | Human studies | Advantages | Disadvantages |
|---|---|---|---|
| Detection of circulating glycocalyx fragments | |||
| DMMB/Alcian Blue | Rapid, inexpensive colorimetric assay | Only detects sulfated glycosaminoglycans (cannot detect hyaluronic acid) | |
| ELISA/Latex Agglutination Assay | Quantitative measure of proteoglycans, glycosaminoglycans | Insufficient rapidity to date for bedside use; uncertain specificity of antibody binding to glycosaminoglycans | |
| Mass spectrometry | High sensitivity; allows detection of sulfation signatures, potentially identifying tissue source | Expensive, impractical for rapid bedside use | |
| Thromboelastography (TEG) |
|
Rapid, inexpensive measurement of circulating heparan sulfate fragments with anticoagulant ability | Detection limited to highly-sulfated heparan pentasaccharides (or larger) |
| Measurement of whole-body glycocalyx volume | |||
| Tracer dilution technique86 |
|
Can measure whole-body endothelial surface layer volume based upon differences in tracer volumes of distribution | Technical assumptions controversial87 |
| Intravital microscopy | |||
| Sublingual sidestream dark field (SDF) imaging | Rapid, point-of-care assay. Allows for simultaneous measurements of microvascular function. | Concerns regarding relevance of imaged vascular bed and interobserver variability; need for specialized training | |
Therapeutic targeting of the microcirculation in sepsis
The ability to directly visualize the sublingual microvasculature in human subjects has allowed for hypothesis-generating human studies identifying treatments that, by virtue of rescuing the dysfunctional microvasculature, could serve as clinically-effective treatments for sepsis89. Such microcirculation-protective therapies, however, have largely failed to improve patient outcomes when broadly applied across large, multicenter trials (Table 2).
Table 2.
Microcirculation-protective therapies and outcomes in clinical trials of sepsis.
| Intervention | Microcirculation benefit | Benefit as sepsis therapeutic |
|---|---|---|
| Anticoagulants | ||
| Activated Protein C |
|
Initial benefit91 unable to be reproduced in confirmatory studies12,92. |
| Vasoactive medications | ||
| Vasopressors | Targeting MAP of 80 – 85 mm Hg equivalent to MAP of 65 mm Hg to 70 mm Hg in septic shock95. | |
| Inotropic agents |
|
No benefit from early goal directed therapy studies which included dobutamine therapy22–24. No benefit and potential harm from supranormal oxygen delivery in established sepsis96. |
| Anti-inflammatory therapy | ||
| Corticosteroids |
|
High dose steroids without benefit98,99 and potential harm100. Stress dose steroids without benefit101. |
| Hemofiltration (endotoxin removal) | Potential early mortality benefit of two hemofiltration sessions after surgery for intra-abdominal infection; benefit lost at 30 days104. | |
| Fluid therapy | ||
| Early goal-directed therapy |
|
No benefit from early goal-directed therapy in sepsis22–24. This may reflect efficacy/implementation of very early fluid resuscitation (i.e. prior to study enrollment). |
| Colloids | While albumin may be beneficial in septic shock (based on post-hoc subgroup analyses107), hydroxyethyl starch is associated with harm108–110. | |
The failure of activated protein C as a treatment for sepsis is particularly disappointing, not only due to the promising microcirculation-protective effects observed in animal models and preliminary human studies90, but also due to the initial success of drotrecogin alfa as reported in the seminal PROWESS91 study. Ultimately, the futility of drotrecogin alfa was demonstrated in the ADDRESS92 and PROWESS-SHOCK12 studies, paralleling negative studies of other anticoagulants such as antithrombin III14 and tissue factor antagonists13. However, a recent meta-analysis suggested a mortality decrease with heparin treatment during sepsis (OR 0.88)111, although this analysis is derived largely from a single study of low-dose heparin for venous thromboembolism prophylaxis (a dosing regimen of uncertain relevance to the septic microcirculation) in patients receiving activated protein C112. Interestingly, low-dose heparin administration had been previously implicated as detrimental in sepsis studies of antithrombin III14 and tissue factor antagonists13. This complexity may reflect the varied biologic effects of heparin, including the ability of this highly-sulfated GAG to inhibit selectins113, influence growth factor signaling114, and inhibit enzymes implicated in endothelial glycocalyx degradation (i.e. heparanase)28. Indeed, many anticoagulants (including activated protein C and antithrombin) have multiple biologic effects; the failure of these agents to improve sepsis outcomes therefore cannot be viewed as a direct repudiation of the pathophysiological importance of tissue thrombosis to organ injury.
Novel microcirculation-protective therapies
The general failures of microcirculation-targeted therapies to improve patient outcomes highlights a need to identify new therapeutic targets in sepsis. Given the known benefit of early antibiotics in sepsis, studies of the impact of antibiotic administration on microcirculatory function would be instructive as to potential new therapeutic targets115. “Sheddases” implicated in septic glycocalyx degradation may be targeted116, including the use of doxycycline69 or sphingosine-1-phosphate117 to inhibit matrix metalloproteinases responsible for proteoglycan shedding. Alternatively, coagulant or non-anticoagulant variants of heparin can be employed to block heparanase, a heparan sulfate-degrading endoglucuronidase responsible for septic endothelial glycocalyx degradation and lung and kidney injury28,72. Furthermore, interventions aimed at promoting glycocalyx reconstitution may hasten a return of microvascular homeostasis. Rosuvastatin improved glycocalyx reconstitution in patients with familial hyperlipidemia118; however, a randomized trial of statins failed to show benefit as a sepsis therapeutic119
While the general failure of microcirculation-protective interventions to improve clinical outcomes may reflect a lack of novel therapeutic targets, a more compelling explanation might lie in the indiscriminant administration of microcirculation-protective therapies in multicenter trials. Microvascular-protective treatments might only benefit patients who demonstrate baseline abnormalities of microvascular function89. Ideally, future studies will pursue such microvasculature-targeted, “personalized” approaches to sepsis resuscitation. This assessment of baseline microvascular status could be based upon bedside intravital microscopy (with its accompanying technical limitations) or systemic markers of endothelial damage (with their accompanying logistic concerns as point-of-care tests). The promise of such personalized approaches to infection treatment has been demonstrated in recent studies of pneumonia, in which a benefit of adjunctive corticosteroids existed largely in patients with baseline evidence of systemic inflammation120,121.
Summary
The microcirculation is a promising therapeutic target in sepsis. While several techniques allow for the detection of microcirculation dysfunction in humans (including intravital imaging and measures of glycocalyx degradation), these approaches have yet to guide sepsis therapeutics in a manner that demonstrably (in phase III studies) improves patient outcomes. Validating, multicenter patient outcome-focused studies of interventions titrated to improving microcirculation function are needed to create new treatment paradigms in sepsis.
KEY POINTS.
Microcirculatory functions critical for the homeostatic control of infection can become dysregulated and harmful during sepsis.
Microcirculation dysfunction may arise in part from septic degradation of the endothelial glycocalyx, a substantial, glycosaminoglycan-rich layer lining the vascular lumen.
The microcirculation can be measured at the bedside, either directly via intravital microscopy or indirectly via circulating measures of vascular damage. Such evidence of microcirculatory dysfunction is predictive of sepsis outcomes.
Additional human studies are needed to determine if sepsis treatments, when titrated to improvement of microvascular function, improve patient outcomes.
Footnotes
DISCLOSURE STATEMENT
The Authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James F. Colbert, Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine.
Eric P. Schmidt, Division of Pulmonary Sciences and Critical Care Medicine, Department of Medicine, University of Colorado School of Medicine. Department of Medicine, Denver Health Medical Center.
References
- 1.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13–19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuebler WM. Inflammatory pathways and microvascular responses in the lung. Pharmacological Reports : PR. 2005;57(Suppl):196–205. [PubMed] [Google Scholar]
- 3.Marki A, Esko JD, Pries AR, Ley K. Role of the endothelial surface layer in neutrophil recruitment. Journal of Leukocyte Biology. 2015 doi: 10.1189/jlb.3MR0115-011R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson IM. Celsus: De medicina, Florence 1478. Part 1. The Journal of the Royal College of Physicians of Edinburgh. 2014;44(3):252–254. doi: 10.4997/JRCPE.2014.314. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt EP, Lee WL, Zemans RL, Yamashita C, Downey GP. On, around, and through: neutrophil-endothelial interactions in innate immunity. Physiology. 2011;26(5):334–347. doi: 10.1152/physiol.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossaint J, Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. Journal of Innate Immunity. 2013;5(4):348–357. doi: 10.1159/000345943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiological Reviews. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 8.Negrini D, Passi A, Moriondo A. The role of proteoglycans in pulmonary edema development. Intensive Care Medicine. 2008;34(4):610–618. doi: 10.1007/s00134-007-0962-y. [DOI] [PubMed] [Google Scholar]
- 9.Levi M, van der Poll T, Schultz M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Seminars in Immunopathology. 2012;34(1):167–179. doi: 10.1007/s00281-011-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon B. The role of microvascular thrombosis in sepsis. Anaesthesia and Intensive Care. 2004;32(5):619–629. doi: 10.1177/0310057X0403200502. [DOI] [PubMed] [Google Scholar]
- 11.Echtenacher B, Weigl K, Lehn N, Mannel DN. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infection and Immunity. 2001;69(6):3550–3555. doi: 10.1128/IAI.69.6.3550-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. The New England Journal of Medicine. 2012;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 13.Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290(2):238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 14.Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 15.Melican K, Boekel J, Mansson LE, et al. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cellular Microbiology. 2008;10(10):1987–1998. doi: 10.1111/j.1462-5822.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 16.Mizgerd JP. Acute lower respiratory tract infection. The New England Journal of Medicine. 2008;358(7):716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nature Reviews. Urology. 2010;7(8):430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 18.Bull TM, Clark B, McFann K, Moss M. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2010;182(9):1123–1128. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostergaard L, Granfeldt A, Secher N, et al. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol Scand. 2015 doi: 10.1111/aas.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. The New England Journal of Medicine. 1994;330(24):1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 21.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. The New England Journal of Medicine. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 22.Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. The New England Journal of Medicine. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 23.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. The New England Journal of Medicine. 2015;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 24.Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. The New England Journal of Medicine. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam C, Tyml K, Martin C, Sibbald W. Microvascular perfusion is impaired in a rat model of normotensive sepsis. The Journal of Clinical Investigation. 1994;94(5):2077–2083. doi: 10.1172/JCI117562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WL, Slutsky AS. Sepsis and endothelial permeability. The New England Journal of Medicine. 2010;363(7):689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 27.Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. Journal of Internal Medicine. 2015;277(3):277–293. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nature Medicine. 2012;18(8):1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Stoppelaar SF, van 't Veer C, van der Poll T. The role of platelets in sepsis. Thrombosis and Haemostasis. 2014;112(4):666–677. doi: 10.1160/TH14-02-0126. [DOI] [PubMed] [Google Scholar]
- 30.Tyml K. Role of connexins in microvascular dysfunction during inflammation. Canadian Journal of Physiology and Pharmacology. 2011;89(1):1–12. doi: 10.1139/y10-099. [DOI] [PubMed] [Google Scholar]
- 31.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of Clinical Investigation. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prowle JR, Bellomo R. Sepsis-associated acute kidney injury: macrohemodynamic and microhemodynamic alterations in the renal circulation. Seminars in Nephrology. 2015;35(1):64–74. doi: 10.1016/j.semnephrol.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Taccone FS, Su F, De Deyne C, et al. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med. 2014;42(2):e114–122. doi: 10.1097/CCM.0b013e3182a641b8. [DOI] [PubMed] [Google Scholar]
- 34.Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4(6):507–516. doi: 10.4161/viru.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klijn E, Den Uil CA, Bakker J, Ince C. The heterogeneity of the microcirculation in critical illness. Clinics in Chest Medicine. 2008;29(4):643–654. viii. doi: 10.1016/j.ccm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 36.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11(5):R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold RC, Parrillo JE, Phillip Dellinger R, et al. Point-of-care assessment of microvascular blood flow in critically ill patients. Intensive Care Medicine. 2009;35(10):1761–1766. doi: 10.1007/s00134-009-1517-1. [DOI] [PubMed] [Google Scholar]
- 38.Boerma EC, Mathura KR, van der Voort PH, Spronk PE, Ince C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit Care. 2005;9(6):R601–606. doi: 10.1186/cc3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallisalmi M, Oksala N, Pettila V, Tenhunen J. Evaluation of sublingual microcirculatory blood flow in the critically ill. Acta Anaesthesiol Scand. 2012;56(3):298–306. doi: 10.1111/j.1399-6576.2011.02569.x. [DOI] [PubMed] [Google Scholar]
- 40.Vellinga NA, Boerma EC, Koopmans M, et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med. 2015;43(1):48–56. doi: 10.1097/CCM.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 41.Boerma EC, van der Voort PH, Spronk PE, Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med. 2007;35(4):1055–1060. doi: 10.1097/01.CCM.0000259527.89927.F9. [DOI] [PubMed] [Google Scholar]
- 42.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. American Journal of Respiratory and Critical Care Medicine. 2002;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 43.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 44.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Annals of Emergency Medicine. 2007;49(1):88–98. 98.e81–82. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med. 2012;40(5):1443–1448. doi: 10.1097/CCM.0b013e31823dae59. [DOI] [PubMed] [Google Scholar]
- 46.Paize F, Sarginson R, Makwana N, et al. Changes in the sublingual microcirculation and endothelial adhesion molecules during the course of severe meningococcal disease treated in the paediatric intensive care unit. Intensive Care Medicine. 2012;38(5):863–871. doi: 10.1007/s00134-012-2476-5. [DOI] [PubMed] [Google Scholar]
- 47.Creteur J, De Backer D, Sakr Y, Koch M, Vincent JL. Sublingual capnometry tracks microcirculatory changes in septic patients. Intensive Care Medicine. 2006;32(4):516–523. doi: 10.1007/s00134-006-0070-4. [DOI] [PubMed] [Google Scholar]
- 48.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 49.Natanson C, Esposito CJ, Banks SM. The sirens' songs of confirmatory sepsis trials: selection bias and sampling error. Crit Care Med. 1998;26(12):1927–1931. doi: 10.1097/00003246-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Opal SM, Laterre PF, Francois B, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 51.Rice TW, Wheeler AP, Bernard GR, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38(8):1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 52.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature Reviews. Immunology. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Backer D, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34(2):403–408. doi: 10.1097/01.ccm.0000198107.61493.5a. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt EP, Damarla M, Rentsendorj O, et al. Soluble guanylyl cyclase contributes to ventilator-induced lung injury in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2008;295(6):L1056–1065. doi: 10.1152/ajplung.90329.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biological Chemistry. 2006;387(12):1521–1533. doi: 10.1515/BC.2006.190. [DOI] [PubMed] [Google Scholar]
- 56.Kuebler WM. The Janus-faced regulation of endothelial permeability by cyclic GMP. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2011;301(2):L157–160. doi: 10.1152/ajplung.00192.2011. [DOI] [PubMed] [Google Scholar]
- 57.Boerma EC, Koopmans M, Konijn A, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38(1):93–100. doi: 10.1097/CCM.0b013e3181b02fc1. [DOI] [PubMed] [Google Scholar]
- 58.Trzeciak S, Glaspey LJ, Dellinger RP, et al. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis. Critical Care Medicine. 2014;42(12):2482–2492. doi: 10.1097/CCM.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 59.Vincent JL, Zhang H, Szabo C, Preiser JC. Effects of nitric oxide in septic shock. American Journal of Respiratory and Critical Care Medicine. 2000;161(6):1781–1785. doi: 10.1164/ajrccm.161.6.9812004. [DOI] [PubMed] [Google Scholar]
- 60.Trzeciak S, Cinel I, Phillip Dellinger R, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Academic Emergency Medicine: Official Journal of the Society for Academic Emergency Medicine. 2008;15(5):399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Schmidt EP. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers. 2013;1(1) doi: 10.4161/tisb.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Federation Proceedings. 1966;25(6):1773–1783. [PubMed] [Google Scholar]
- 63.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102(7):770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 64.Chappell D, Jacob M, Paul O, et al. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009;104(11):1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 65.Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(8):1908–1915. doi: 10.1161/ATVBAHA.111.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiesinger A, Peters W, Chappell D, et al. Nanomechanics of the Endothelial Glycocalyx in Experimental Sepsis. PLoS One. 2013;8(11):e80905. doi: 10.1371/journal.pone.0080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Annals of Biomedical Engineering. 2012;40(4):828–839. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan Sulfate Proteoglycan Is a Mechanosensor on Endothelial Cells. Circulation Research. 2003;93(10):e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 69.Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation. 2009;16(8):657–666. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 70.Cabrales P, Vazquez BY, Tsai AG, Intaglietta M. Microvascular and capillary perfusion following glycocalyx degradation. Journal of Applied Physiology (Bethesda, Md: 1985) 2007;102(6):2251–2259. doi: 10.1152/japplphysiol.01155.2006. [DOI] [PubMed] [Google Scholar]
- 71.Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. Journal of Applied Physiology (Bethesda, Md: 1985) 2005;99(4):1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]
- 72.Lygizos MI, Yang Y, Altmann CJ, et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep. 2013;1(6):e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marechal X, Favory R, Joulin O, et al. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock. 2008;29(5):572–576. doi: 10.1097/SHK.0b013e318157e926. [DOI] [PubMed] [Google Scholar]
- 74.Nieuwdorp M, Meuwese MC, Mooij HL, et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202(1):296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30(6):623–627. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 76.Sallisalmi M, Tenhunen J, Yang R, Oksala N, Pettila V. Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesthesiol Scand. 2012;56(3):316–322. doi: 10.1111/j.1399-6576.2011.02578.x. [DOI] [PubMed] [Google Scholar]
- 77.Ostrowski SR, Berg RMG, Windeløv NA, et al. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: A prospective study. Journal of Critical Care. 2013;28(5):586–596. doi: 10.1016/j.jcrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt EP, Li G, Li L, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289(12):8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelson A, Berkestedt I, Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol Scand. 2014;58(1):36–43. doi: 10.1111/aas.12223. [DOI] [PubMed] [Google Scholar]
- 80.Berg S, Brodin B, Hesselvik F, Laurent TC, Maller R. Elevated levels of plasma hyaluronan in septicaemia. Scandinavian Journal of Clinical and Laboratory Investigation. 1988;48(8):727–732. doi: 10.3109/00365518809088752. [DOI] [PubMed] [Google Scholar]
- 81.Steppan J, Hofer S, Funke B, et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. The Journal of Surgical Research. 2011;165(1):136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 82.Yagmur E, Koch A, Haumann M, Kramann R, Trautwein C, Tacke F. Hyaluronan serum concentrations are elevated in critically ill patients and associated with disease severity. Clinical Biochemistry. 2012;45(1–2):82–87. doi: 10.1016/j.clinbiochem.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 83.Oragui EE, Nadel S, Kyd P, Levin M. Increased excretion of urinary glycosaminoglycans in meningococcal septicemia and their relationship to proteinuria. Crit Care Med. 2000;28(8):3002–3008. doi: 10.1097/00003246-200008000-00054. [DOI] [PubMed] [Google Scholar]
- 84.Sun X, Li L, Overdier KH, et al. Analysis of Total Human Urinary Glycosaminoglycan Disaccharides by Liquid Chromatography–Tandem Mass Spectrometry. Analytical Chemistry. 2015;87(12):6220–6227. doi: 10.1021/acs.analchem.5b00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. The Journal of Trauma and Acute Care Surgery. 2012;73(1):60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 86.Nieuwdorp M, Meuwese MC, Mooij HL, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. Journal of Applied Physiology (Bethesda, Md: 1985) 2008;104(3):845–852. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- 87.Michel CC, Curry FR. Glycocalyx volume: a critical review of tracer dilution methods for its measurement. Microcirculation. 2009;16(3):213–219. doi: 10.1080/10739680802527404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donati A, Damiani E, Domizi R, et al. Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvascular research. 2013;90:86–89. doi: 10.1016/j.mvr.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 89.Shapiro NI, Angus DC. A review of therapeutic attempts to recruit the microcirculation in patients with sepsis. Minerva Anestesiologica. 2014;80(2):225–235. [PubMed] [Google Scholar]
- 90.De Backer D, Verdant C, Chierego M, Koch M, Gullo A, Vincent JL. Effects of drotrecogin alfa activated on microcirculatory alterations in patients with severe sepsis. Crit Care Med. 2006;34(7):1918–1924. doi: 10.1097/01.CCM.0000220498.48773.3C. [DOI] [PubMed] [Google Scholar]
- 91.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. The New England Journal of Medicine. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 92.Abraham E, Laterre PF, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. The New England Journal of Medicine. 2005;353(13):1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- 93.Thooft A, Favory R, Salgado DR, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15(5):R222. doi: 10.1186/cc10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13(3):R92. doi: 10.1186/cc7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. The New England Journal of Medicine. 2014;370(17):1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 96.Russell JA. Adding fuel to the fire--the supranormal oxygen delivery trials controversy. Crit Care Med. 1998;26(6):981–983. doi: 10.1097/00003246-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 97.Buchele GL, Silva E, Ospina-Tascon GA, Vincent JL, De Backer D. Effects of hydrocortisone on microcirculatory alterations in patients with septic shock. Crit Care Med. 2009;37(4):1341–1347. doi: 10.1097/ccm.0b013e3181986647. [DOI] [PubMed] [Google Scholar]
- 98.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. The New England Journal of Medicine. 1984;311(18):1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 99.Group VASSCS. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. The New England Journal of Medicine. 1987;317(11):659–665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 100.Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23(8):1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 101.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. The New England Journal of Medicine. 2008;358(2):111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 102.Berlot G, Bianco N, Tomasini A, Vassallo MC, Bianco F. Changes in microvascular blood flow during coupled plasma filtration and adsorption. Anaesthesia and Intensive Care. 2011;39(4):687–689. doi: 10.1177/0310057X1103900426. [DOI] [PubMed] [Google Scholar]
- 103.Ruiz C, Hernandez G, Godoy C, Downey P, Andresen M, Bruhn A. Sublingual microcirculatory changes during high-volume hemofiltration in hyperdynamic septic shock patients. Crit Care. 2010;14(5):R170. doi: 10.1186/cc9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301(23):2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 105.Ospina-Tascon G, Neves AP, Occhipinti G, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Medicine. 2010;36(6):949–955. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 106.Dubin A, Pozo MO, Casabella CA, et al. Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care. 2010;25(4):659 e651–658. doi: 10.1016/j.jcrc.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 107.Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, Norton R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Medicine. 2011;37(1):86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 108.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. The New England Journal of Medicine. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 109.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. The New England Journal of Medicine. 2012;367(20):1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 110.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. The New England Journal of Medicine. 2012;367(2):124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 111.Zarychanski R, Abou-Setta AM, Kanji S, et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43(3):511–518. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 112.Levi M, Levy M, Williams MD, et al. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated) American Journal of Respiratory and Critical Care Medicine. 2007;176(5):483–490. doi: 10.1164/rccm.200612-1803OC. [DOI] [PubMed] [Google Scholar]
- 113.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nature Immunology. 2005;6(9):902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 114.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nature Reviews. Molecular Cell Biology. 2013;14(3):166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Al-Banna NA, Pavlovic D, Bac VH, et al. Acute administration of antibiotics modulates intestinal capillary perfusion and leukocyte adherence during experimental sepsis. International Journal of Antimicrobial Agents. 2013;41(6):536–543. doi: 10.1016/j.ijantimicag.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 116.Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. British Journal of Clinical Pharmacology. 2015 doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. American Journal of Physiology. Heart and Circulatory Physiology. 2014;306(3):H363–372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meuwese MC, Mooij HL, Nieuwdorp M, et al. Partial recovery of the endothelial glycocalyx upon rosuvastatin therapy in patients with heterozygous familial hypercholesterolemia. Journal of Lipid Research. 2009;50(1):148–153. doi: 10.1194/jlr.P800025-JLR200. [DOI] [PubMed] [Google Scholar]
- 119.Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. American Journal of Respiratory and Critical Care Medicine. 2013;187(7):743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 120.Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet (London, England) 2015;385(9977):1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 121.Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313(7):677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]