Synopsis
This review begins with a description of the trends in the incidence of and mortality from sepsis in the United States and globally. Then we discuss the known factors associated with increased risk for the development of sepsis. Finally, we discuss the limitations of the current clinical definition of sepsis and the clinical correlations of the current epidemiology of sepsis.
Keywords: sepsis, severe sepsis, septic shock, epidemiology
Introduction
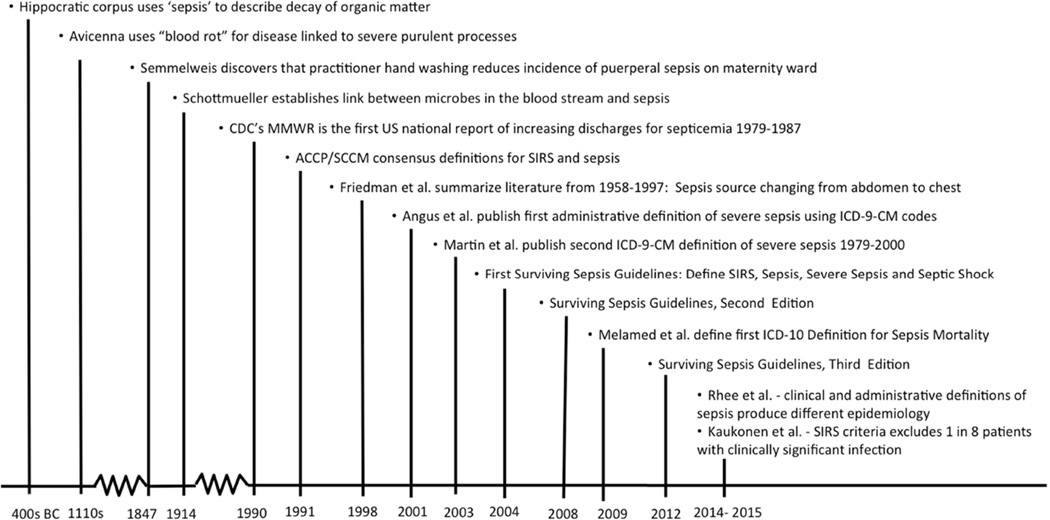
While the first written description of the sepsis syndrome appears in an Egyptian papyrus circa 1600 B.C., the origin of the term sepsis comes from the Ancient Greek word sêpsis that means “putrefaction” or the “decay of organic matter” (1, 2). The Greek word is first encountered in Homer’s Iliad and was also used in the Hippocratic corpus in the 4th century B.C. (2). Since then over two thousand years passed before humankind first approached the etiology and prevention of this syndrome. In the 19th century, Hungarian obstetrician Ignaz Semmelweis recognized that physician handwashing drastically decreased the incidence of puerperal sepsis on the maternity ward (2). While Semmelweiss’s theories were rejected during his lifetime, they were later unknowingly validated by Louis Pasteur and Robert Koch whose works gave birth to the germ theory of infectious diseases (2) (Figure 1). This pivotal achievement paved the way for further developments in defining the spectrum of sepsis syndromes and studying their impact on human life, which will be summarized in this review.
Figure 1. Abbreviated Timeline of the Conceptual Definition of Sepsis.
The following overview of the modern epidemiology of sepsis will begin by discussing the recent epidemiology of sepsis in the United States (US) and globally, followed by a review of the literature on the associated risk factors for sepsis and finishing with a discussion of the clinical utility of current definitions of sepsis and future directions in the field.
United States’ trends in incidence and mortality from sepsis
The epidemiology of sepsis in the US has been primarily based on studies using large, administrative databases. Therefore, some initial discussion of the administrative definitions used is necessary to understand the observed variability in the estimates. Various investigators have defined sepsis cases using different combinations of diagnostic codes listed on hospital discharge records and using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding scheme. Table 1 summarizes details of several ICD-9-CM definitions, demonstrating that there is a wide range of the types of coding schemas used to create a case definition of severe sepsis from various infections and associated organ dysfunctions (3–5). Various attempts have been made to assess the validity of these administrative definitions, which demonstrated the Martin definition to have sensitivity of 17–81% and specificity of 88–100% and the Angus definition to have a sensitivity of 47–50% and specificity of 92%. (5–7). While these data support that the administrative definitions are approximating the clinical definitions of severe sepsis, other studies have revealed problems in the accuracy of these administrative definitions over time. One recent study compared the 2003–2012 trends in severe sepsis between various administrative definitions and cases defined with objective clinical data, demonstrating large increases (54–706%) in rates of severe sepsis based on administrative definitions in the absence of comparable increases in bacteremia and shock based on objective clinical data. This suggests an increase in the use of severe sepsis ICD-9-CM codes on hospital discharge documentation in the absence of objectively proven severe sepsis cases (8). This increasing use of the sepsis codes on hospital discharges was also demonstrated in an analysis that showed an 11% increase in hospital claims with an infection code and a disproportionately higher 49% increase in hospital claims with a sepsis code from 2003–2009 (9). Overall, these data inform us that the epidemiology of sepsis in the US is based on imperfect administrative definitions and these factors must be taken into account as we examine the estimates and trends.
Table 1.
Comparison of Administrative Methodologies for Epidemiology of Sepsis in the United States
| Study Author | Sepsis Spectrum | Patients | ICD | Number of Infection Codes |
Number Organ Dysfunction Codes |
Geography | Years | Incidence1 | Average Annual Trend |
Case-Fatality |
|---|---|---|---|---|---|---|---|---|---|---|
| CDC MMWR | Septicemia | All | 9-CM | 17 | 0 | US | 79–87 | 74–176 | +17% | 25–31% |
| Angus | Severe sepsis | All | 9-CM | 1,286 | 13 | 7 states2 | 95 | 300 | NA | 29% |
| Martin | Severe Sepsis | All | 9-CM | 6 | 32 | US | 79–00 | 83–240 | +9% | 18–28% |
| Dombrovskiy | Severe sepsis | ≥18 | 9-CM | 15–173 | 29–304 | 1 state5 | 95–02 | 135–208 | +7% | 45–51% |
| Melamed | Sepsis mortality | All | 10 | 21 | NA | US | 99–05 | 50–52 | +1% | NA |
{9-CM = ninth revision, clinical modification; CDC = Centers for Disease Control and Prevention; ICD = international classification of disease; MMWR = morbidity and mortality weekly report; NA = not applicable}
Age- and gender adjusted incidence per 100,000 population except for Dombrovskiy and Melamed studies which are crude rate per 100,000 population.
Florida, Maryland, Massachusetts, New Jersey, New York, Virginia, Washington statewide databases used to generate national estimates.
In October 2002, 995.91 (sepsis) and 995.92 (severe sepsis) were added to ICD-9-CM and the study’s definition.
In October 2002, 995.92 (severe sepsis) was added to ICD-9-CM and the study’s definition.
New Jersey
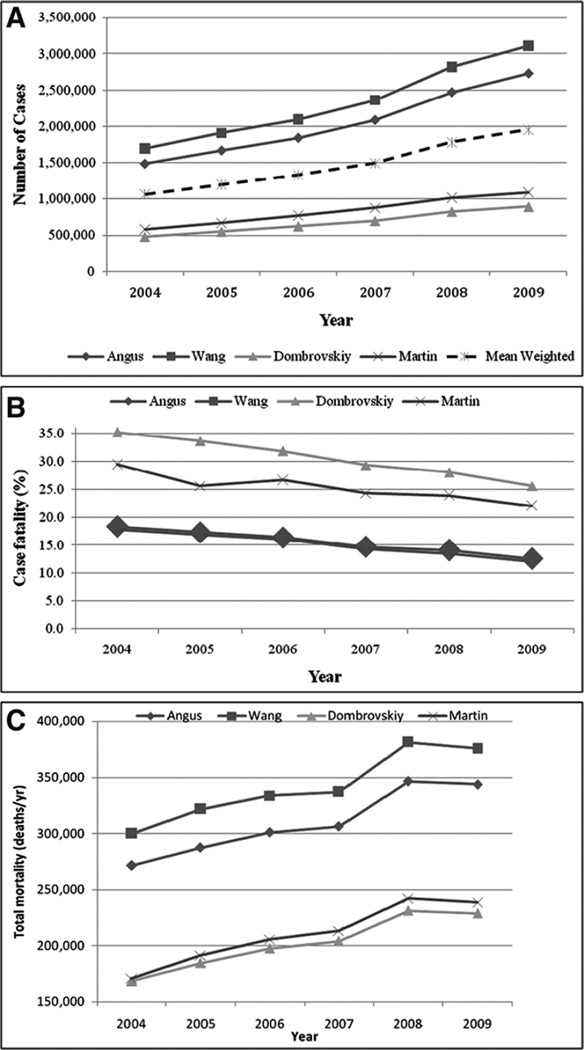
Based on the methodology and years evaluated, studies examining hospital discharges to estimate the incidence and case fatality of sepsis have produced various estimates. The first published report to characterize the national epidemiology of sepsis syndromes was a 1990 Morbidity and Mortality Weekly Report from the CDC that described trends in hospital discharges with ICD-9-CM codes for septicemia (10). From 1979 through 1987, discharges with septicemia codes increased from 74 to 176 per 100,000 persons while case fatality decreased from 31% to 25% (10). Over a decade later, several investigators independently produced varied sepsis incidence estimates, trends and case-fatalities using different datasets and definitions (Table 1). Applying these various definitions to a single national dataset covering 2004–2009, the average annual incidence varies widely by definition, ranging from 300 to 1,031 per 100,000 persons yet all with a similar average annual increase of 13% (Figure 2) (11). Examining sepsis mortality indices, this study demonstrated similar patterns of variation. While case fatality varies up to 2-fold by administrative definition, the case fatality trends over time are similar at a 4–5% decline per year (12). Additionally, the total incidence of mortality from severe sepsis in the US continued to increase nationally even while the case fatality decreased (numbers not reported) (11). Another study specifically looked at sepsis-related mortality with the CDC’s Multiple Causes of Death database from 1999–2005 and using a novel sepsis definition based on ICD-10 coding again demonstrated a small increases in the incidence of sepsis-related mortality over the study period from 50 to 52 per 100,000 (13). One important note on examining sepsis deaths using the National Vital Statistics Reports, is that this report separates out septicemia codes from other infections, notably the combined influenza and pneumonia category which comprise the largest proportion of sepsis sources in the incidence studies (14). Within the National Vital Statistics Reports’ schema, influenza and pneumonia comprised the eighth leading cause of death in the US in 2011 at 17 deaths per 100,000 while septicemia does not make the top ten list for reporting (14).
Figure 2. Comparison of Epidemiological Trends of Severe Sepsis in the United States Between Three Case Definitions.
From Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Critical care medicine 2013;41(5):1167–74; with permission.
From these we can summarize some common trends. While the case fatality of severe sepsis is decreasing, the national incidence of severe sepsis cases is increasing at a larger rate. This larger increase in incident cases seems to be driving the proportionately smaller increase of total sepsis-related deaths despite the trend of improving case fatality from sepsis over the same time period. Potential reasons for the increasing incidence of severe sepsis may be an aging population, a larger number of people with disease comorbidities, greater improvements in disease-specific mortality from other competing causes of death, growing bacterial drug resistance and increasing recognition and more frequent and liberal use of sepsis codes on hospital discharges. Reasons for improving case fatality include scientific advances in care, dissemination of effective protocolized treatment and increasing inclusion of sepsis coding for non-sepsis SIRS patients and less severe sepsis patients.
In addition to incidence and mortality trends, the infection sources and causative pathogens for sepsis in the US have changed over time. From 1979 through 2000 in the US, while the proportions of hospital discharges for severe sepsis with concomitant recording pathogens increased for all classes of organisms, gram-positive bacterial infections increased the greatest with an average 26% per year, surpassing gram-negative organisms in 1987 as the predominant class of organism associated with severe sepsis (5). Furthermore, the proportion of severe sepsis cases with concomitant fungal infections increased 207% over this 22-year time period (5). In the year 2000, gram-positive organisms accounted for 52% of severe sepsis cases while gram-negative and fungal organisms comprised 38% and 5% of cases respectively (5). Several reports have demonstrated the respiratory tract to be the most commonly identified (29–42%) source of infection in US sepsis, followed by either primary bacteremia or genitourinary sources depending on the study (3, 15–17).
Global epidemiology of sepsis
The studies describing the epidemiology of sepsis outside of the US are summarized in Table 2 and in general utilize intensive care unit-based observational cohort study designs and clinical definitions rather than administrative databases and definitions. In those studies that estimate a population incidence of severe sepsis we observe a range from 38 to 110 per 100,000 persons; on the lower range of the estimates produced in the US (3–5, 18–27). The case fatality rates of reported severe sepsis range from 22–55% and are comparable to those observed in US studies of similar years (3–5, 18–21, 23–33). With a few exceptions, the respiratory tract is the most common source of infection while the pathogen distribution appears variable observed in US studies (20, 26, 28–30, 34–37).
Table 2.
Summary of Studies of the Epidemiology of Sepsis Outside of the United States
| Country | Study Design | Definition | Time period | Number of Patients (ICUs) |
Attack Rate per 100 ICU Admissions (per 100,000 population/year) |
Sources | Organisms | Mortality |
|---|---|---|---|---|---|---|---|---|
| Europe | ||||||||
| France(1) | Cohort | Consensus(2) | 1 - 2/93 | 11,828 (170) |
Severe Sepsis 6% | Pulmonary 40% Abdomen 32% |
Gram-positive 51% Gram-negative 59% |
28-day Severe sepsis 56% |
| Italy(3) | Cohort | Consensus | 4/93 - 3/94 | 1,101 (99) |
Severe Sepsis 2% | NA | NA | Hospital Severe Sepsis 52% |
| England, Wales and Northern Ireland(4) | Cohort | PROWESS(5) | 95 - 00 | 56,673 (91) |
Severe Sepsis 27% (51) |
NA | NA | Hospital Severe Sepsis 47% |
| England, Wales and North Ireland(6) | Cohort | PROWESS | 12/95 – 1/05 | 343,860 (172) |
Severe sepsis (46–66) |
NA | NA | Hospital Severe Sepsis 48–45% |
| 8 countries(7)6 | Cohort | Consensus | 5/97 – 5/98 | 3,9467 (28) |
Severe Sepsis 7% | Pulmonary 62% Abdominal 14% |
Gram-positive 39% Gram-negative 49% |
NA |
| Croatia(8) | Cohort | Consensus | 1/00 – 12/05 | 5,022 (1) |
Sepsis 6% | Urinary 54% Pulmonary 14% |
Gram-positive 32% Gram-negative 64% |
Hospital Sepsis 17% |
| Norway(9) | Cohort | ICD-10 septicemia codes | 1 – 12/99 | (NA)8 700,107 |
Severe sepsis (50) |
NA | NA | Hospital Severe sepsis 27% |
| Netherlands(10) | One day Point Prevalence | PROWESS | 12/01 | 455 (47) |
Severe sepsis 30% (54) |
Pulmonary 47% Abdomen 34% |
NA | NA |
| France(11) | 2-week point prevalence | Consensus | 11/01 | 3,738 (206) |
Severe sepsis9 15% (95) |
NA | NA | 30-day Severe sepsis 35% |
| Slovakia(12) | Cohort | PROWESS | 7/02 – 12/02 | 1,533 (12) |
Severe sepsis 8% (80–90) |
Pulmonary 55% Abdomen 39% |
NA | Hospital Severe Sepsis 51% |
| 24 European Countries(13) | Two-week prevalence | Consensus | 5/02 | 3,147 (198) |
Sepsis 37% | Pulmonary 68% Abdomen 22% |
Gram-positive 40% Gram-negative 38% |
Hospital Sepsis 30% |
| Germany(14) | 1-day point prevalences | Consensus | 03 | 3,877 (454) |
Severe sepsis10 11% (76–110) |
Pulmonary 63% Abdominal 25% |
NA | Hospital Severe sepsis9 55% |
| Finland(15) | Cohort | Consensus | 11/04 – 2/05 | 4,500 (24) |
Severe sepsis NA (38) |
Pulmonary 43% Abdominal 32% |
Gram-positive 59% Gram-negative 33% |
Hospital Severe sepsis 28% |
| Republic of Macedonia(16) | Cohort | Consensus | 1/08 – 12/10 | 875 (1) |
Severe sepsis 21% | Pulmonary 66% Meningitis 9% |
Gram-positive 78% Gram-negative 22% |
Hospital Severe sepsis 52% |
| South America | ||||||||
| Brazil(17) | Cohort | PROWESS | 5/01 – 1/02 | 1,383 (5) |
Severe sepsis 17% | Pulmonary 66% Urinary 6% |
NA | 28-day Severe sepsis 47% |
| Colombia(18) | Cohort | Modified IHI definition | 9/07 – 2/08 | 49,739 (10)11 |
Sepsis 4%10 | NA | NA | 28-day Severe sepsis 22% |
| Pacific and Asia | ||||||||
| Australia and New Zealand(19) | Cohort | Consensus | 5/99 – 7/99 | 5,878 (23) |
Severe Sepsis 12% (77) |
Pulmonary 50% Abdominal 19% |
Gram-positive 48% Gram-negative 39% |
Hospital Severe Sepsis 38% |
| Australia and New Zealand(20) | Cohort | Consensus | 1/00 –12/12 | 1,037,11 5(171) |
10% | NA | NA | Hospital Severe Sepsis 24% |
| South Korea(21) | Cohort | Consensus | 4/05 – 2/09 | NA (22) |
NA | Pulmonary 30% Urinary 26% |
Gram-positive 20% Gram-negative 43% |
28-day Severe sepsis 23% |
| South Vietnam(22) | Cohort | Positive blood culture | 6/93 – 5/94 | 437 (1)13 |
Bacteremia 2%10 | NA | Gram-positive NA Gram-negative 90% |
Hospital Bacteremia 6% |
| Middle East | ||||||||
| Kuwait(23) | Cohort | Positive blood culture | 1/82 – 6/83 | 3,845 (1)13 |
Bacteremia 11%10 | NA | Gram-positive 20% Gram-negative 80% |
NA |
This study was included in the European subsection as it contained mostly European countries: Italy, France, Spain, Germany, Portugal, United Kingdom, Canada and Israel.
This the total number of patients that stayed in ICU > 24 hours which was just one strata of the study but the one with data on sepsis density
The cohort includes all Norwegian hospitals but an exact number is not supplied in the publication.
This definition also included severe sepsis and septic shock.
This definition also included severe sepsis and septic shock.
This refers to total number of hospitals in the study rather than intensive care units.
{ICU = intensive care unit; PROWESS = Prospective Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis trial; NA = not available
. Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. Jama. 1995;274(12):968-74.
. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644-55.
. Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, et al. The Italian SEPSIS study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive care medicine. 1995;21 Suppl 2:S244-9.
. Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Critical care medicine. 2003;31(9):2332-8.
. Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. The New England journal of medicine. 2001;344(10):699–709.
. Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Critical care. 2006;10(2):R42.
. Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive care medicine. 2002;28(2):108-21.
. Degoricija V, Sharma M, Legac A, Gradiser M, Sefer S, Vucicevic Z. Survival analysis of 314 episodes of sepsis in medical intensive care unit in university hospital: impact of intensive care unit performance and antimicrobial therapy. Croatian medical journal. 2006;47(3):385-97.
. Flaatten H. Epidemiology of sepsis in Norway in 1999. Critical care. 2004;8(4):R180-4.
. van Gestel A, Bakker J, Veraart CP, van Hout BA. Prevalence and incidence of severe sepsis in Dutch intensive care units. Critical care. 2004;8(4):R153-62.
. Brun-Buisson C, Meshaka P, Pinton P, Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive care medicine. 2004;30(4):580-8.
. Zahorec R, Firment J, Strakova J, Mikula J, Malik P, Novak I, et al. Epidemiology of severe sepsis in intensive care units in the Slovak Republic. Infection. 2005;33(3):122-8.
. Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study*. Critical care medicine. 2006;34(2):344-53.
. Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive care medicine. 2007;33(4):606-18.
. Karlsson S, Varpula M, Ruokonen E, Pettila V, Parviainen I, Ala-Kokko TI, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive care medicine. 2007;33(3):435-43.
. Grozdanovski K, Milenkovic Z, Demiri I, Spasovska K. Prediction of outcome from community-acquired severe sepsis and septic shock in tertiary-care university hospital in a developing country. Critical care research and practice. 2012;2012:182324.
. Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian Sepsis Epidemiological Study (BASES study). Critical care. 2004;8(4):R251-60.
. Rodriguez F, Barrera L, De La Rosa G, Dennis R, Duenas C, Granados M, et al. The epidemiology of sepsis in Colombia: a prospective multicenter cohort study in ten university hospitals. Critical care medicine. 2011;39(7):1675-82.
. Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive care medicine. 2004;30(4):589-96.
. Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. Jama. 2014;311(13):1308-16.
. Park DW, Chun BC, Kim JM, Sohn JW, Peck KR, Kim YS, et al. Epidemiological and clinical characteristics of community-acquired severe sepsis and septic shock: a prospective observational study in 12 university hospitals in Korea. Journal of Korean medical science. 2012;27(11):1308-14.
. Hoa NT, Diep TS, Wain J, Parry CM, Hien TT, Smith MD, et al. Community-acquired septicaemia in southern Viet Nam: the importance of multidrug-resistant Salmonella typhi. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92(5):503-8.
. Elhag KM, Mustafa AK, Sethi SK. Septicaemia in a teaching hospital in Kuwait--I: Incidence and aetiology. The Journal of infection. 1985;10(1):17–24.
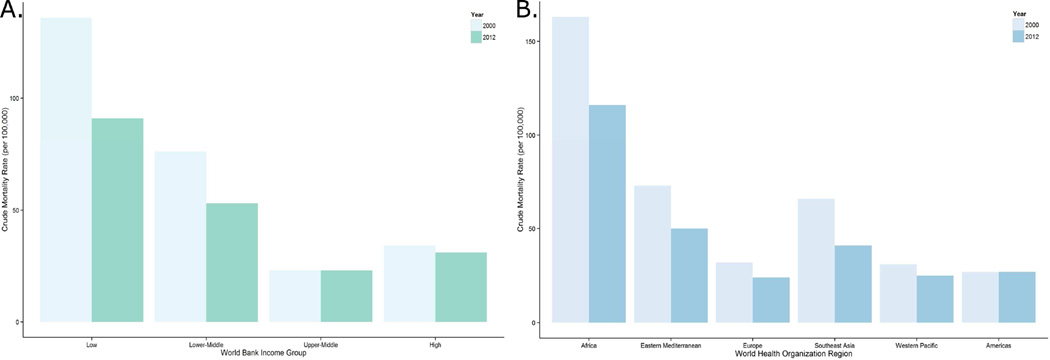
Data regarding the epidemiology of sepsis incidence from developing countries is exceedingly scarce; therefore to make estimations, this review will extrapolate from the World Health Organization (WHO) reports on causes of global mortality. While the WHO does not individually record sepsis or septicemia as a cause of death, it does report deaths due to lower respiratory tract infections, a leading cause of sepsis. From this data both the WHO Africa Region and countries designated as low-income by the World Bank disproportionately suffer higher mortality rates from lower respiratory. More specifically, in 2012 lower respiratory tract infections were the leading cause of death among low-income countries at 91 deaths per 100,000 persons, three-fold higher than the rate among high-income countries (38, 39). Additionally of the WHO geographic regions in 2012, the Africa region suffered the highest mortality rate from lower respiratory tract infections at 116 per 100,000 persons, over four-fold higher than the European Region at 24 deaths per 100,000 persons (Figure 3) (38, 39). While using deaths due to lower respiratory infection as a surrogate for sepsis-related deaths is imperfect and likely underestimates measures of sepsis epidemiology, it is clear that this is large global problem with a disproportionately heavy burden on developing nations.
Figure 3. Crude Mortality from Lower Respiratory Tract Infections by World Health Organization Region and World Bank Income Group.
Data from World Health Organization. Global Health Data Repository: Death Rates [August 12, 2015]. Available from: http://apps.who.int/gho/data/node.main.CODRATE?lang=en.
Factors associated with an increased incidence of sepsis
There are no longitudinal cohort studies examining pre-hospital risk factors for the subsequent development of sepsis and the below factors associated with sepsis are based on hospital cohorts and cross-sectional administrative data.
Age
Several studies have demonstrated that older age is a risk factor for sepsis (3, 10, 18, 21, 27, 28, 40–42).
The risk for sepsis has a bimodal age distribution, with increased age-adjusted incidences in infants that decreases through childhood to increase again in adulthood with a steep inflection upwards around 50–60 years of age (3, 27, 41).
The average annual increases in sepsis incidence interact with age, with greatest increases in older age groups. From 1979 to 2002 in the US, the average annual increase of sepsis incidence was 20% faster in adults > 65 years old when compared to the younger group (10, 41).
Sex
While there is some variation in the distribution of sexes in the prevalence of sepsis, male sex is consistently associated with higher incidence of sepsis (3, 5, 15, 18, 22–27, 31, 33, 37, 40, 43–45).
There appears to be interaction between sex and age in the incidence of severe sepsis, such that men have a similar age-adjusted incidence of women 5 years older (3, 5).
Race
In the US, Black race has been associated with an approximately two-fold increase in the incidence of severe sepsis when compared to Whites (5, 15, 43, 46, 47).
The racial disparity in severe sepsis in the US is dependent on age and greatest among the 35–44 year-old age groups (5, 13, 16, 47).
The distribution of comorbid illnesses may be different among races with some evidence that Non-whites have a higher proportion of diabetes mellitus, HIV, chronic renal failure and alcohol abuse while Whites more often have pulmonary disease and cancer (15, 16).
The distribution of pathogens may be different by race with some evidence that Blacks had higher rates of gram positive infections and higher rates of invasive pneumococcal disease (15, 16).
Comorbid Conditions
There is some evidence that individuals with a higher number of comorbid illnesses are at higher risks of sepsis (42).
Comorbid illnesses that have been associated with sepsis include: diabetes mellitus, congestive heart failure, chronic pulmonary disease, immunosuppression, liver disease, cancer, chronic renal failure (15, 28, 33, 41, 48)
One study demonstrated that the number of high-risk organ transplantations that the institution performed was associated with a higher sepsis attack rate (40).
Geography and season
There is evidence for increased incidences of respiratory infections, streptococcal and pneumococcal sepsis diagnoses in the winter but not other causes of septicemia (46, 49).
In the US from 1979–1987, septicemia increased most greatly in the US West (10). In a study of the US from 1979–2003, the West had the lowest average annual sepsis rate when compared to the South and the Northeast but was not significantly different from the Midwest (49).
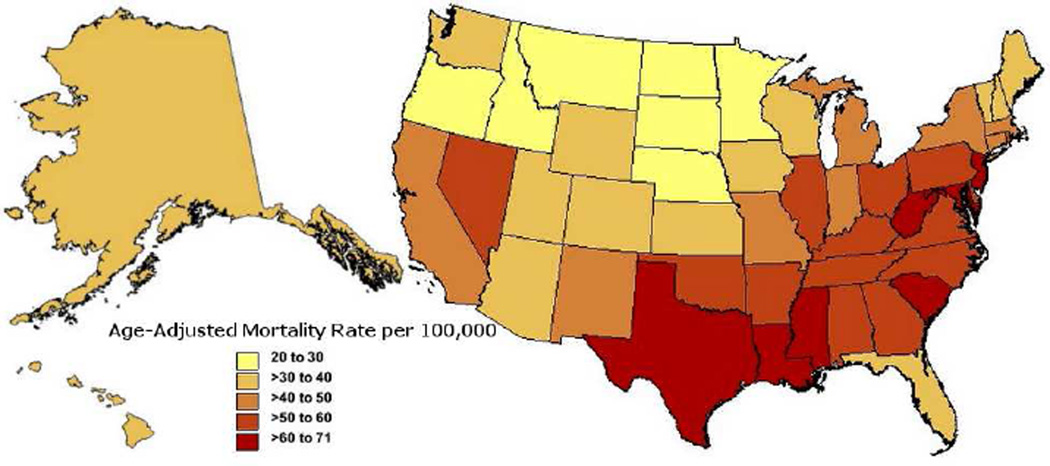
A data extraction performed for this publication shows the average annual incidence of sepsis-related mortality from 1999 to 2013 by US state, revealing higher incidence of mortality among southeastern states (Figure 4).
Figure 4. Average Annual Age-Adjusted Incidence of Sepsis-Related Mortality per 100,000 over 1999–2013 by State in the United States.
Data pulled from Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple Cause of Death 1999–2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999–2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/mcd-icd10.html on Aug 27, 2015 7:00:28 PM
Clinical correlations and the future of sepsis epidemiology
The current clinical definitions of sepsis syndromes have a nonlinear history and some current opinion suggests that they may be in need of revision to improve clinical utility and accuracy (Figure 1) (50). By the time the American College of Chest Physicians and the Society of Critical Care Medicine (ACCP/SCCM) published the first consensus definition of sepsis syndromes in 1992, they recognized that there had been a century’s worth of proliferation of various terms describing these syndromes including: “infection, bacteremia, sepsis, septicemia, septic syndrome, and septic shock” (51). In an effort to “improve [the] ability to make early bedside detection of the disease possible … [and allow] the standardization of research protocols…” the ACCP/SCCM consensus committee: 1) specified the clinical criteria for the systemic inflammatory response syndrome (SIRS); 2) defined sepsis as SIRS in the presence of a known or suspected infection; and 3) identified severe sepsis and septic shock as the potential progression of sepsis to multiple organ dysfunction and death (51).
The basic structure of the 1992 ACCP/SCCM consensus definitions of SIRS, sepsis, severe sepsis and septic shock has gone largely unchanged in the three editions of the Surviving Sepsis Campaign Guidelines published in 2003, 2008 and 2012 (52–54). Now in their third decade, these definitions have demonstrated clinical endurance and been critical to research developments in the field nevertheless, there is a shifting perception that the SIRS definition may be overly sensitive and nonspecific (50). In 2006, a survey of European intensive care units demonstrated the SIRS criteria to be 100% sensitive but only 18% specific for severe infections (37). Furthermore, in 2012 a prospective observational study in the Netherlands demonstrated that minor variations in the timing and method of capture of SIRS criteria such as manual versus automated data collection significantly changed the measured incidences of sepsis syndromes (55). Furthermore, this study demonstrated that 6–17% (depending on the method of SIRS capture) of infected intensive care unit (ICU) patients did not meet SIRS criteria (55). More recently, a study of a 14-year survey of all ICUs in New Zealand highlighted the significant potential problems with the clinical definition of sepsis. This study found that the SIRS criteria missed 1 in 8 patients with severe infections and that these missed cases were associated with substantial hospital morbidity and mortality (56). More specifically, the SIRS-negative sepsis patients still had high rates of organ failure with 42% having septic shock, 55% requiring mechanical ventilation and 12% experiencing acute renal failure and (56). Furthermore, when compared to SIRS-positive sepsis patients the SIRS-negative sepsis patients had a lower but still substantial hospital mortality (16% vs. 23%) (56). These data call into question the utility of the SIRS component of the sepsis diagnostic criteria in aiding in timely recognition and treatment of severe infections. Furthermore, this complicates our current understanding of the epidemiology of sepsis, as definitions may both overestimate the incidence of sepsis by mistakenly including non-infectious SIRS and underestimate the incidence, morbidity and mortality of severe infections by excluding these SIRS-negative infections. Such nuances serve as a cautionary reminder that the reliance on a clinical case definition that is syndromic rather than pathologically defined will likely introduce some misclassification error in epidemiological surveillance.
Despite these issues, overall the epidemiological data is encouragingly consistent in demonstrating that despite the increasing incidence of sepsis cases, there is a decreasing case fatality. While case fatality was improving before the development and dissemination of the 1992 ACCP/SCCM Consensus definitions and long before the landmark early goal directed therapy trial by Rivers et al. in 2001, it is reasonable to consider that the monumental efforts of the Surviving Sepsis Campaign to disseminate evidence-based improvements in sepsis and critical illness care have contributed to the continued improvements in case fatality (57).
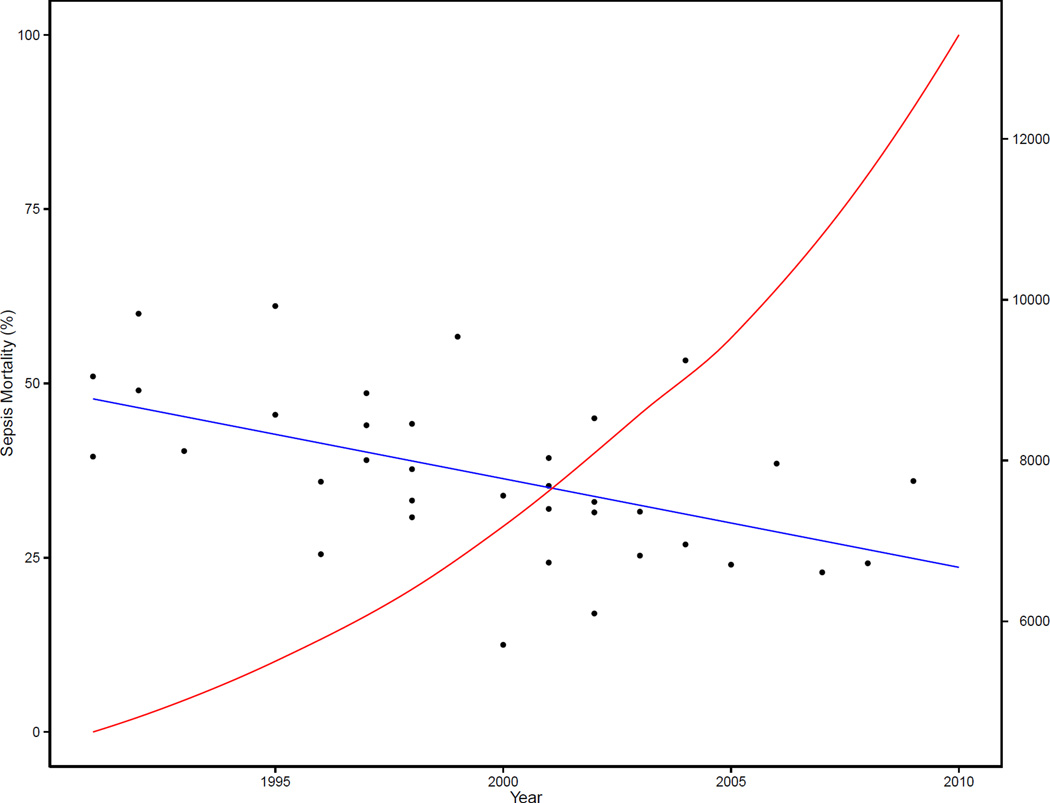
The improving case fatality of sepsis also has implications for the future of research into effective sepsis therapies. As the baseline mortality of the standard care of sepsis improves, it becomes increasingly difficult to statistically demonstrate the benefit of newer therapies. As the baseline mortality approaches 25%, sample sizes greater than 10,000 participants are required to show relative risk differences of 10%, thus making future research much more time, cost and labor intensive (Figure 5). This statistical effect of decreasing case fatality from sepsis helps to contextualize the null findings of the recent trials re-examining the efficacy of early goal-directed therapy when compared to standard care a decade after the original trial (58–60).
Figure 5. Total Sample Size Required to Show a Relative Risk Difference of 10% Based on Mortality From Control Arms of Randomized Controlled Trials of Severe Sepsis and Septic Shock.
The y-axis corresponds to the years of publication of respective randomized control trials. The left-side y-axis corresponds to the reported case-fatality of the control arms of trials enrolling patients with severe sepsis or septic shock; with dots representing individual trials and the blue line representing a simple unweighted linear regression of case-fatality by year. The right-side y-axis represents the total sample size (both experimental and control arms) that would be required to show a relative mortality risk difference of 10% with a power of 90% at an alpha of 0.05 for a hypothetical experimental trial with the control arm’s mortality rate estimated from the aforementioned regression of that given year. The red line is a simple plot of this total sample size calculated from the control arm’s mortality estimated from the blue regression line for each year.
Data from supplement of Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Critical care medicine. 2014;42(3):625–31.
Another inference from the epidemiology of sepsis is that while the case fatality of sepsis is decreasing, the incidence of sepsis cases continues to increase and drive a smaller, but still significant increase in national rates of sepsis-related mortality (13). Within this context, in addition to improving the treatment of sepsis cases, the next paradigms in curbing the growing problem of sepsis may focus on identifying and treating pre-hospital risk factors for sepsis. More longitudinal studies are needed to identify high impact, corrigible pre-hospital risk factors for sepsis and the highest incidence subpopulations in which to study ambulatory interventions. In addition to exploring these general risk factors, examination of the specific risk factors mediating the racial and gender disparities in the incidence of sepsis is needed in order to take the next steps towards eliminating these inequalities.
Key Points.
In the epidemiology of sepsis in the United States, different case definitions have produced varied results, with recent estimates of an average annual age-adjusted incidence between approximately 300 to 1,000 sepsis cases per 100,000 persons. Estimates consistently show trends towards an increasing incidence in sepsis with a decreasing case fatality.
In the United States and globally, respiratory tract infections are consistently the most common source of sepsis while there is more variability in the microbiological distribution of common pathogens.
While evidence regarding the epidemiology of sepsis in developing countries is scarce, they appear to have 3- to 4-fold increased incidence of mortality from sepsis-related infections.
While data for longitudinal risk factors for sepsis is lacking, in the United States this syndrome disproportionately affects the very young and old, males, Blacks and the southeastern states.
Outline.
United States’ trends in incidence and mortality from sepsis
Global epidemiology of sepsis
Factors associated with an increased incidence of sepsis
Clinical Correlations and the future of sepsis epidemiology
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have nothing to disclose
References
- 1.Moss M. Epidemiology of sepsis: race sex, chronic alcohol abuse. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(Suppl 7):S490–S497. doi: 10.1086/432003. [DOI] [PubMed] [Google Scholar]
- 2.Botero JSH, Pérez MCF. The History of Sepsis from Ancient Egypt to the XIX Century 2012. Available from: http://www.intechopen.com/books/export/citation/EndNote/sepsis-an-ongoing-and-significant-challenge/the-history-of-sepsis-from-ancient-egypt-to-the-xix-century. [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Critical care medicine. 2005;33(11):2555–2562. doi: 10.1097/01.ccm.0000186748.64438.7b. [DOI] [PubMed] [Google Scholar]
- 5.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The New England journal of medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker SA, Mikkelsen ME, Gaieski DF, Koshy S, Kean C, Fuchs BD. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Critical care medicine. 2013;41(4):945–953. doi: 10.1097/CCM.0b013e31827466f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Odden A, Rohde J, Bonham C, Kuhn L, Malani P, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Medical care. 2014;52(6):e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee C, Murphy MV, Li L, Platt R, Klompas M, et al. for the Centers for Disease C. Comparison of Trends in Sepsis Incidence and Coding Using Administrative Claims Versus Objective Clinical Data. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 doi: 10.1093/cid/ciu750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walkey AJ, Wiener RS. Trends in infection source and mortality among patients with septic shock. American journal of respiratory and critical care medicine. 2014;190(6):709–710. doi: 10.1164/rccm.201406-1043LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.From the Centers for Disease Control. Increase in National Hospital Discharge Survey rates for septicemia--United States, 1979–1987. Jama. 1990;263(7):937–938. [PubMed] [Google Scholar]
- 11.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Critical care medicine. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Critical care medicine. 2014;42(3):625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Critical care. 2009;13(1):R28. doi: 10.1186/cc7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heron M. Deaths: Leading Causes for 2011. In: Services HaH, editor. 2015. [Google Scholar]
- 15.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Critical care medicine. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. Jama. 2010;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Critical care medicine. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 18.Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Critical care medicine. 2003;31(9):2332–2338. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 19.Brun-Buisson C, Meshaka P, Pinton P, Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive care medicine. 2004;30(4):580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 20.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive care medicine. 2004;30(4):589–596. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 21.Flaatten H. Epidemiology of sepsis in Norway in 1999. Critical care. 2004;8(4):R180–R184. doi: 10.1186/cc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Gestel A, Bakker J, Veraart CP, van Hout BA. Prevalence and incidence of severe sepsis in Dutch intensive care units. Critical care. 2004;8(4):R153–R162. doi: 10.1186/cc2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahorec R, Firment J, Strakova J, Mikula J, Malik P, Novak I, et al. Epidemiology of severe sepsis in intensive care units in the Slovak Republic. Infection. 2005;33(3):122–128. doi: 10.1007/s15010-005-4019-2. [DOI] [PubMed] [Google Scholar]
- 24.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Critical care. 2006;10(2):R42. doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive care medicine. 2007;33(4):606–618. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson S, Varpula M, Ruokonen E, Pettila V, Parviainen I, Ala-Kokko TI, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive care medicine. 2007;33(3):435–443. doi: 10.1007/s00134-006-0504-z. [DOI] [PubMed] [Google Scholar]
- 27.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Critical care medicine. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 28.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. Jama. 1995;274(12):968–974. [PubMed] [Google Scholar]
- 29.Grozdanovski K, Milenkovic Z, Demiri I, Spasovska K. Prediction of outcome from community-acquired severe sepsis and septic shock in tertiary-care university hospital in a developing country. Critical care research and practice. 2012;2012:182324. doi: 10.1155/2012/182324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park DW, Chun BC, Kim JM, Sohn JW, Peck KR, Kim YS, et al. Epidemiological and clinical characteristics of community-acquired severe sepsis and septic shock: a prospective observational study in 12 university hospitals in Korea. Journal of Korean medical science. 2012;27(11):1308–1314. doi: 10.3346/jkms.2012.27.11.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez F, Barrera L, De La Rosa G, Dennis R, Duenas C, Granados M, et al. The epidemiology of sepsis in Colombia: a prospective multicenter cohort study in ten university hospitals. Critical care medicine. 2011;39(7):1675–1682. doi: 10.1097/CCM.0b013e318218a35e. [DOI] [PubMed] [Google Scholar]
- 32.Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, et al. The Italian SEPSIS study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive care medicine. 1995;21(Suppl 2):S244–S249. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- 33.Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian Sepsis Epidemiological Study (BASES study) Critical care. 2004;8(4):R251–R260. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degoricija V, Sharma M, Legac A, Gradiser M, Sefer S, Vucicevic Z. Survival analysis of 314 episodes of sepsis in medical intensive care unit in university hospital: impact of intensive care unit performance and antimicrobial therapy. Croatian medical journal. 2006;47(3):385–397. [PMC free article] [PubMed] [Google Scholar]
- 35.Elhag KM, Mustafa AK, Sethi SK. Septicaemia in a teaching hospital in Kuwait--I: Incidence and aetiology. The Journal of infection. 1985;10(1):17–24. doi: 10.1016/s0163-4453(85)80004-9. [DOI] [PubMed] [Google Scholar]
- 36.Hoa NT, Diep TS, Wain J, Parry CM, Hien TT, Smith MD, et al. Community-acquired septicaemia in southern Viet Nam: the importance of multidrug-resistant Salmonella typhi. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92(5):503–508. doi: 10.1016/s0035-9203(98)90891-4. [DOI] [PubMed] [Google Scholar]
- 37.Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study*. Critical care medicine. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. The Top 10 Causes of Death. [august 12, 2015]; Available from: http://www.who.int/mediacentre/factsheets/fs310/en/index1.html.
- 39.World Health Organization. Global Health Data Repository: Death Rates. [August 12, 2015]; Available from: http://apps.who.int/gho/data/node.main.CODRATE?lang=en.
- 40.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. Jama. 1997;278(3):234–240. [PubMed] [Google Scholar]
- 41.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis*. Critical care medicine. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 42.Banta JE, Joshi KP, Beeson L, Nguyen HB. Patient and hospital characteristics associated with inpatient severe sepsis mortality in California, 2005–2010. Critical care medicine. 2012;40(11):2960–2966. doi: 10.1097/CCM.0b013e31825bc92f. [DOI] [PubMed] [Google Scholar]
- 43.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. American journal of respiratory and critical care medicine. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. Jama. 1995;273(2):117–123. [PubMed] [Google Scholar]
- 45.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive care medicine. 2002;28(2):108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 46.Baine WB, Yu W, Summe JP. The epidemiology of hospitalization of elderly Americans for septicemia or bacteremia in 1991–1998. Application of Medicare claims data. Annals of epidemiology. 2001;11(2):118–126. doi: 10.1016/s1047-2797(00)00184-8. [DOI] [PubMed] [Google Scholar]
- 47.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Critical care medicine. 2007;35(3):763–768. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 48.McBean M, Rajamani S. Increasing rates of hospitalization due to septicemia in the US elderly population, 1986–1997. The Journal of infectious diseases. 2001;183(4):596–603. doi: 10.1086/318526. [DOI] [PubMed] [Google Scholar]
- 49.Danai PA, Sinha S, Moss M, Haber MJ, Martin GS. Seasonal variation in the epidemiology of sepsis. Critical care medicine. 2007;35(2):410–415. doi: 10.1097/01.CCM.0000253405.17038.43. [DOI] [PubMed] [Google Scholar]
- 50.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381(9868):774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 52.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive care medicine. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical care medicine. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 54.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Critical care medicine. 2004;32(3):858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 55.Klein Klouwenberg PM, Ong DS, Bonten MJ, Cremer OL. Classification of sepsis, severe sepsis and septic shock: the impact of minor variations in data capture and definition of SIRS criteria. Intensive care medicine. 2012;38(5):811–819. doi: 10.1007/s00134-012-2549-5. [DOI] [PubMed] [Google Scholar]
- 56.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. The New England journal of medicine. 2015;372(17):1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 57.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 58.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, et al. Goal-directed resuscitation for patients with early septic shock. The New England journal of medicine. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 59.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. The New England journal of medicine. 2015;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]