Abstract
BACKGROUND
Cholesterol efflux capacity (CEC), a key step in the reverse cholesterol transport pathway, is independently associated with atherosclerotic cardiovascular disease (ASCVD). However, whether it predicts ASCVD beyond validated novel risk markers is unknown.
OBJECTIVE
We assessed whether CEC improved ACSVD risk prediction beyond coronary artery calcium (CAC), family history (FH) and high sensitivity C-reactive protein (hs-CRP).
METHODS
CEC, CAC, self-reported FH, and hs-CRP were assessed among participants without baseline ASCVD enrolled in the Dallas Heart Study (DHS). ASCVD was defined as first nonfatal myocardial infarction (MI) or stroke, coronary revascularization, or cardiovascular death assessed over a median 9.4 years. Risk prediction was assessed using various modeling techniques and improvements in the C-statistic, Integrated Discrimination Index (IDI), and Net Reclassification Index (NRI).
RESULTS
The mean age of the population (N = 1,972) was 45, 52% had CAC (> 0), 31% had FH and 58% had elevated hs-CRP (≥ 2 mg/L). CEC > median was associated with 50% reduced incidence of ASCVD in those with CAC (5.4% vs. 10.5%, p = 0.003), FH (5.8% vs. 10%, p = 0.05) and elevated hs-CRP (3.8% vs. 7.9%, p = 0.004). CEC improved all metrics of discrimination and reclassification when added to CAC (C-statistic p = 0.004; IDI p = 0.02, NRI = 0.38 [95%CI 0.13–0.53]), FH (C-statistic p = 0.006; IDI p = 0.008, NRI = 0.38 [95%CI 0.13- 0.55]), or hs-CRP (C-statistic p = 0.008; IDI p = 0.02, NRI = 0.36 [95%CI 0.12–0.52]).
CONCLUSIONS
CEC improves ASCVD risk prediction beyond CAC, FH, and hs-CRP and warrants consideration as a novel ASCVD risk marker.
Keywords: cholesterol efflux capacity, high density lipoprotein, risk prediction, coronary artery calcium, family history
INTRODUCTION
Low high-density lipoprotein cholesterol (HDL-C) is an important risk marker for atherosclerotic cardiovascular disease (ASCVD). However, recent studies suggest that in the current era of well-treated patients, the association between HDL-C and ASCVD may be attenuated (1–3). In addition, assessment of both low-density and high-density lipoprotein particle composition offsets this association completely (4,5), limiting the role of HDL-C as a biomarker of ASCVD risk.
HDL is a complex lipoprotein with heterogeneous composition and functions (6), and static cholesterol concentration of HDL does not capture the diversity inherent in HDL particles. The classic function attributed to HDL is to promote reverse cholesterol transport from the periphery to the liver for elimination from the body. Cholesterol efflux from the macrophage to HDL is the initial key step of reverse cholesterol transport and is associated with atheroprotection in animal studies. We (and others) have shown that macrophage-specific cholesterol efflux capacity (CEC) measured in large human cohorts is inversely associated with both prevalent coronary disease and incident ASCVD events (5,7,8).
However, it remains unknown whether assessment of the reverse cholesterol transport pathway as reflected by CEC could serve as a clinically relevant biomarker in ASCVD risk prediction. Addressing this knowledge gap would support investigation and development of refined bioassays of HDL function for clinical testing. The presence of coronary artery calcium (CAC), family history (FH) of myocardial infarction (MI) and elevated high sensitivity C-reactive protein (hs-CRP) reflect mediators of ASCVD risk (subclinical atherosclerosis, inherited risk and inflammation, respectively) and are validated biomarkers in clinical use that improve ASCVD risk prediction (9–11). We examined the incremental ability of CEC to improve ASCVD risk prediction beyond CAC, FH and hs-CRP in a low-risk, population-based cohort.
METHODS
The Dallas Heart Study (DHS) is a multiethnic, population-based cohort study of Dallas County residents (12). This random probability sample included intentional oversampling of African Americans to make up 50% of the cohort. Participants 30 to 65 years of age underwent body composition assessment by fasting blood and urine collection and dual-energy x-ray absorptiometry. Detailed cardiovascular phenotyping was accomplished by means of electron-beam computed tomography and magnetic resonance imaging of the heart, and body-fat distribution was evaluated by magnetic resonance imaging of the abdomen. Subjects with a history of cardiovascular disease (self-reported history of MI, stroke, arterial revascularization, heart failure, or arrhythmia) or niacin use were excluded, as were those who died within 1 year after enrollment. Participants (N = 2,971) completed risk factor assessment, laboratory testing, and imaging studies between 2000 and 2002, and 2,744 of them completed CAC scans. Of those, 185 were excluded because of history of CVD, 238 had lack of adequate follow up, 263 lacked valid CEC measurements and 86 had missing covariates, leaving 1972 participants for final analysis of cardiovascular outcomes.
DEFINITIONS
Race/ethnicity, medication usage, FH, and smoking status were self-reported. Detailed definitions of the variables hypertension, metabolic syndrome, and diabetes in the DHS have been previously published (13). FH was defined as any first-degree relative with a history of MI. FH of premature MI was defined as occurring before the age of 50 years in a first-degree male relative or before the age of 55 years in a first-degree female relative (14).
MEASUREMENTS
Analytical methods for the biomarkers reported in this study have been previously described, including lipoprotein assessment and hs-CRP (15,16). Electron-beam CT measurements of CAC were performed in duplicate 1 to 2 minutes apart on an Imatron 150 XP scanner (Imatron Inc., San Bruno, California). CAC scores were determined using the Agatston method and then averaged, with Agatston score > 0 defined as prevalent CAC.
ASSESSMENT OF LIPID VARIABLES AND EFFLUX CAPACITY
Fasting blood samples were collected by venipuncture into EDTA tubes, stored at 4°C for less than 4 hours, and centrifuged. Plasma was removed and stored at −70°C. Plasma lipids, including HDL-C, were measured as described previously (12). CEC was assessed by measuring the efflux of fluorescence-labeled cholesterol from J774 macrophages to apolipoprotein B–depleted plasma in study participants as previously described (5).
CLINICAL END POINTS
The primary end point was a composite of first nonfatal MI, nonfatal stroke, coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), or death from cardiovascular causes. Non-fatal end points were actively ascertained and adjudicated by 2 cardiologists who were unaware of the measurements of CEC, as previously described (17). The National Death Index was used to determine vital status for all the participants through December 31, 2010. Death from cardiovascular causes was defined according to the International Classification of Diseases, 10th Revision, codes I00 to I99.
STATISTICAL ANALYSIS
Baseline categorical variables are reported as percentages and continuous variables as means with standard deviations. Kaplan-Meier curves for CEC below the median (vs. above the median) for the overall cohort were compared by the log-rank test and stratified by presence of prevalent CAC (defined as CAC > 0), FH and elevated hs-CRP (defined as hs-CRP > 2 mg/L). Cox proportional-hazards models were used to assess the association between CEC and the time to first ASCVD event in univariable and multivariable models. The proportional hazards assumption was tested by Schoenfeld residuals. Traditional risk factors included age, sex, race, presence or absence of diabetes, systolic blood pressure, current smoking status, body mass index, total cholesterol level, HDL-C, history of anti-hypertensive medication use and statin use. Model overfitting was tested by calculating the shrinkage coefficient of the full model. The shrinkage estimator of van Houwelingen and le Cessie was 0.92, well above the cutoff of 0.85 as outlined by Harrell and indicating no concern for model overfitting (18). Forward stepwise selection was performed including all traditional risk factors, CAC, FH, hs-CRP, and CEC, with variables with P < 0.05 retained in the model. Finally, the ability of CEC to improve ASCVD risk prediction beyond traditional ASCVD risk factors was assessed using indices of discrimination as measured by the Harrell’s c statistic and Integrated Discrimination Improvement (IDI), and reclassification as measured by the category-less net reclassification index (NRI) (19–21). The Gronnesby and Borgan test was used for assessing model calibration (22). Several pre-specified sensitivity analyses were performed, including removing PCI/CABG from the primary end point, restricting CV death to fatal MI or fatal stroke, restricting the cohort to age >45, using risk thresholds for calculation of categorical NRI, continuous CEC, using categorical definitions of CAC at varying thresholds, and using premature FH instead of FH. Two-sided P values of 0.05 or less were considered to indicate statistical significance. All statistical analyses were performed with the use of SAS software, version 9.3 (SAS Institute. Raleigh, North Carolina).
RESULTS
The baseline demographic and clinical characteristics of study participants are displayed in Table 1. The mean age of the study population was 44 ± 9.2 years, with 44% men and 47% African Americans. The percentage of participants with prevalent CAC (CAC >0) was 52%. FH and premature FH were reported in 31% and 10% of the participants, respectively. Hs-CRP >2 mg/L was noted in 58% of the participants.
Table 1.
Baseline Demographic and Clinical Characteristics*
| Characteristic* | Frequency (n = 1972) |
|---|---|
| Age, years | 44.9 (9.2) |
| Men | 44% |
| African American | 47% |
| White | 35% |
| Hispanic | 18% |
| Prevalent diabetes mellitus | 9% |
| Systolic blood pressure, mm of Hg | 125 (16) |
| Anti-hypertensive medication | 19% |
| Smoking | 26% |
| Body Mass Index, kg/m2 | 28.9 (6.0) |
| Total cholesterol, mg/dl | 183 (40) |
| HDL-C, mg/dl | 50 (15) |
| Statin use | 6% |
| CAC (>0) | 52% |
| Family history of myocardial infarction | 31% |
| Premature family history of myocardial infarction | 10% |
| Elevated hs-CRP (>2 mg/L) | 58% |
All continuous measures are reported as means with standard deviation and categorical measures as percentages
HDL-C = High density Lipoprotein cholesterol; CAC = Coronary artery calcium; Hs-CRP = High sensitivity C-reactive protein
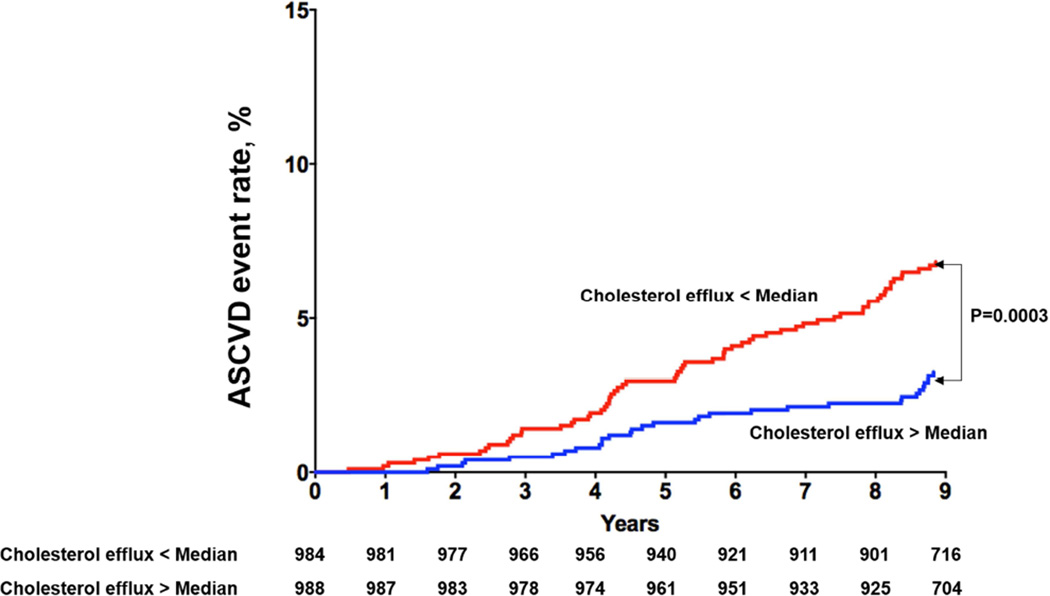
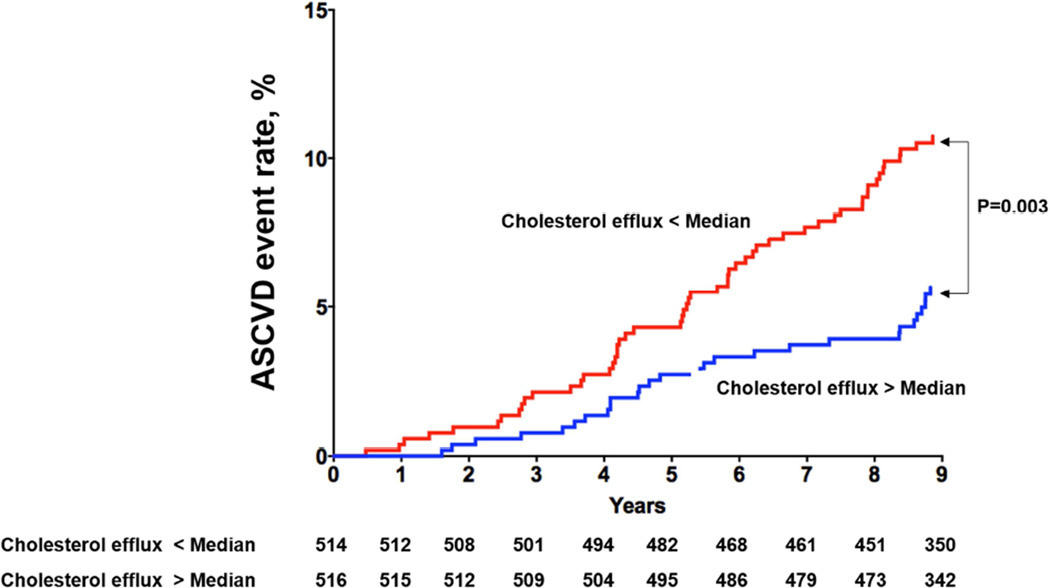
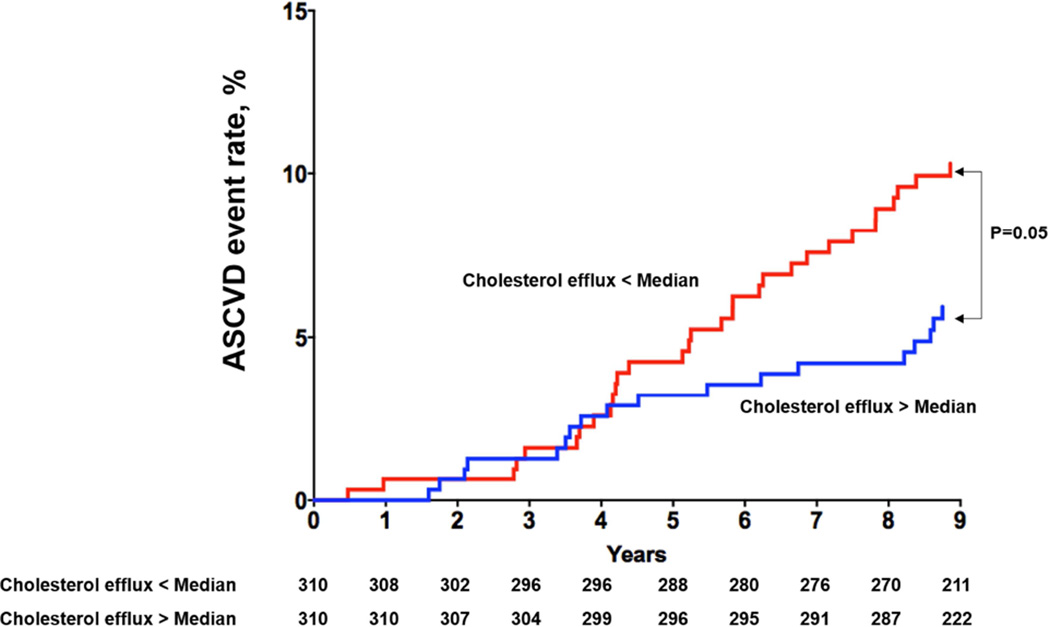
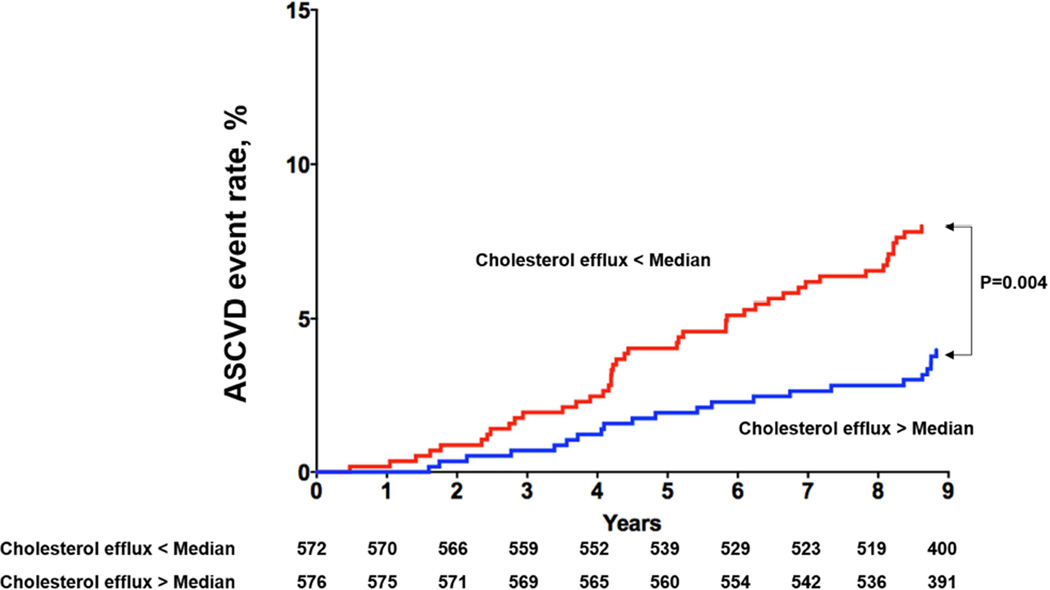
Among the 1,972 participants included in analysis, 97 had a first ASCVD event (28 MIs, 32 strokes, 5 coronary artery bypass graft surgeries, 11 percutaneous coronary interventions and 21 cardiovascular deaths) over a median follow up of 9.4 years (95% CI 9.0, 9.8). Those with CEC > median versus < median had decreased risk of ASCVD (3.1% vs. 6.7%, p = 0.0003; Figure 1A). Among those with prevalent CAC (n = 1030), those with CEC > median versus < median had decreased risk of ASCVD (5.4% vs. 10.5%, p = 0.003) (Figure 1B). Similar findings were seen among those with FH (n = 621; CEC > median vs. < median: 5.8% vs. 10%, p = 0.05) (Figure 1C), and elevated hs-CRP (n = 1148; CEC > median vs. < median: 3.8% vs. 7.9%, p = 0.004) (Figure 1D).
Figure 1. Kaplan-Meier Curves for ASCVD events in Participants with Cholesterol Efflux.
Figure 1A. Kaplan-Meier Curves for ASCVD events in Participants with Cholesterol Efflux below the Median vs. above the Median Among the Overall Study Population.
Figure 1B. Kaplan-Meier Curves for ASCVD events in Participants with Cholesterol Efflux below the Median vs. above the Median Among those with Prevalent CAC (> 0).
Figure 1C. Kaplan-Meier Curves for ASCVD events in Participants with Cholesterol Efflux below the Median vs. above the Median Among those with Prevalent Family History of Myocardial Infarction.
Figure 1D. Kaplan-Meier Curves for ASCVD events in Participants with Cholesterol Efflux below the Median vs. above the Median Among those with Elevated Hs-CRP (> 2).
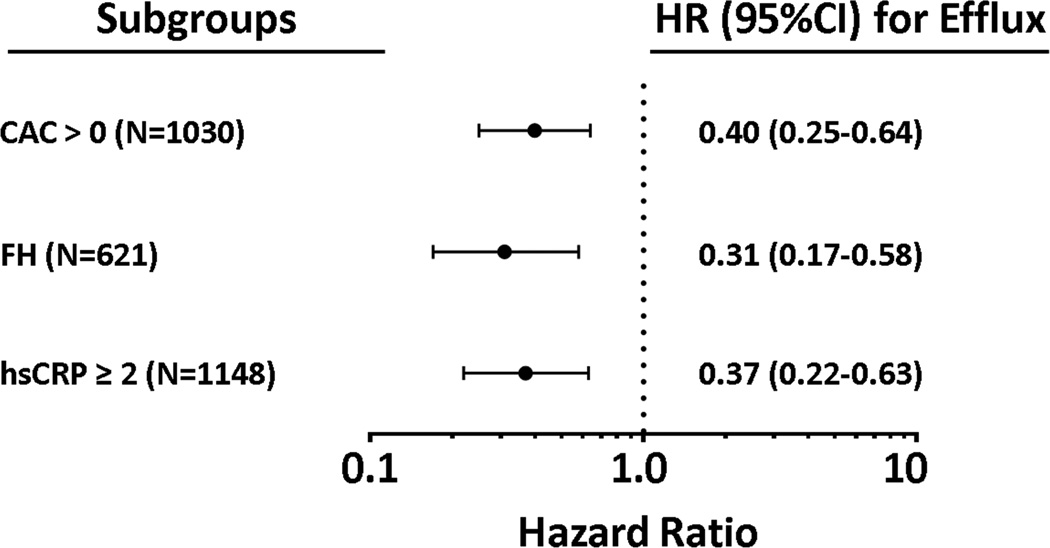
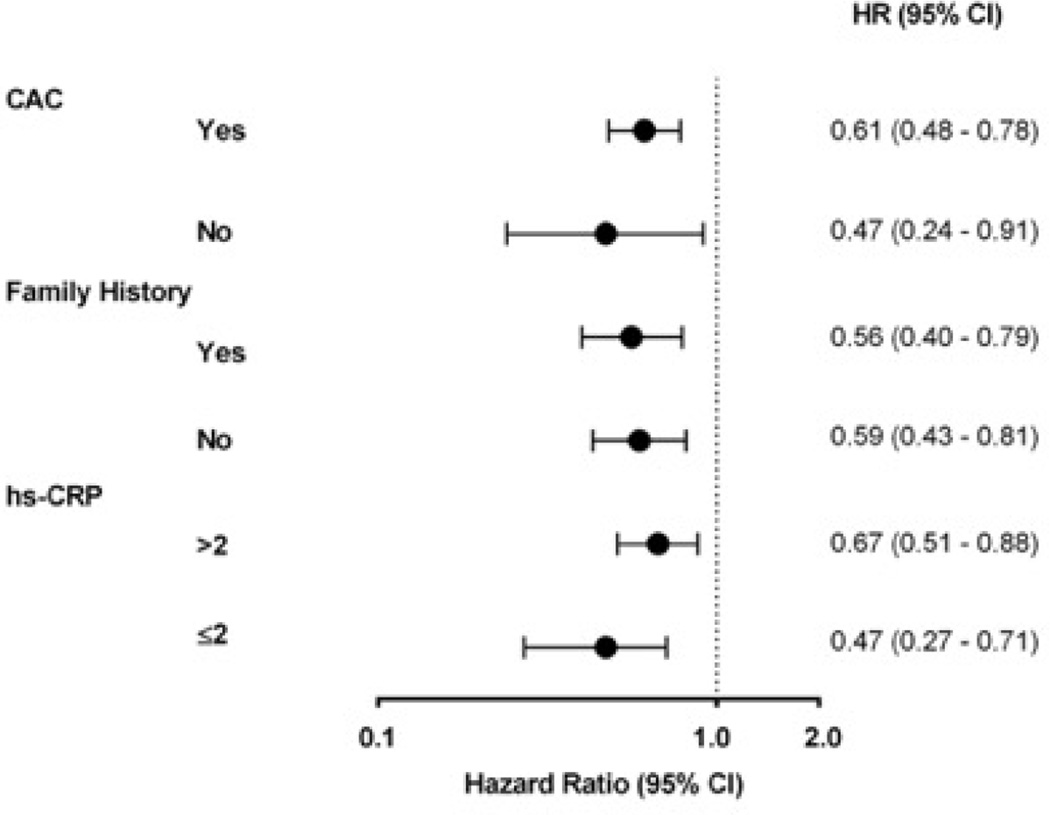
In a fully adjusted model including all traditional risk factors, prevalent CAC, FH, and elevated hs-CRP, CEC remained inversely associated with incident ASCVD without attenuation (adjusted HR: 0.35, 95% CI 0.23 – 0.55). Forward stepwise selection retained CEC along with prevalent CAC and FH (Table 2). Subgroup analyses revealed that CEC was inversely associated with incident ASCVD among those with prevalent CAC (adjusted HR 0.40, 95% CI 0.25 – 0.64), among those with FH (adjusted HR 0.31, 95% CI 0.17 – 0.58), and among those with elevated hs-CRP (adjusted HR 0.37, 95% CI 0.22–0.63) (Figure 2). Statistical interaction tests between CEC and CAC, FH and hs-CRP were performed and were non-significant.
Table 2.
Forward Stepwise Selection Model for Incident ASCVD
| Variable | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Age (per 1 SD) | 1.70 (1.32–2.19) | < 0.0001 |
| Diabetes | 2.62 (1.67–4.13) | < 0.0001 |
| Male sex | 2.41 (1.56–3.72) | < 0.0001 |
| Smoking | 2.38 (1.58–3.59) | < 0.0001 |
| African American race/ethnicity | 1.82 (1.17–2.83) | 0.008 |
| Systolic blood pressure (per 1 SD) | 1.25 (1.05–1.49) | 0.01 |
| Statin medication | 2.32 (1.30–4.14) | 0.004 |
| CAC (> 0) | 2.19 (1.22–3.93) | 0.008 |
| Family history | 1.92 (1.27–2.90) | 0.002 |
| Cholesterol efflux (> median) | 0.35 (0.23–0.54) | < 0.0001 |
Variables retained in Cox proportional hazards model for incident ASCVD using forward stepwise selection with a p-value < 0 .05 are shown. Variables not retained include total cholesterol, HDL-C, history of blood pressure medication use, body mass index, and hs-CRP ≥ 2 mg/L.
SD = standard deviation; CAC = coronary artery calcium.
Figure 2. Association Between Cholesterol Efflux and Incident ASCVD Among Subgroups with Abnormal Novel Risk Factors.
Hazard ratios and 95% CI derived from Cox proportional hazards models for cholesterol efflux > vs. < median among the listed subgroups. CAC = coronary artery calcium; FH = family history; hsCRP = high sensitivity C-reactive protein.
The ability of CEC to improve ASCVD risk prediction beyond CAC, FH, and hs-CRP was assessed using metrics of calibration, discrimination, and reclassification. All models including CEC were well-calibrated. CEC > median versus < median improved discrimination indices as determined by the C-statistic and IDI when added to risk factor-adjusted models including either prevalent CAC, FH, or elevated hs-CRP (Table 3). With respect to reclassification as determined by the NRI, the addition of CEC led to significant reclassification for all models including prevalent CAC, FH, and elevated hs-CRP (Table 4). The improvement in reclassification with the addition of CEC was driven by upward reclassification in those with events with minimal change in those without events.
Table 3.
Change in Discrimination with Adding Cholesterol Efflux Capacity to Risk Factors for Incident ASCVD
| Risk Prediction Models | Harrell’s C-statistic |
IDI |
|---|---|---|
| TRF + CAC > 0* | 0.825 | |
| TRF + CAC > 0* + efflux | 0.848 (p = 0.004) |
0.02 p = 0.02 |
| TRF + FH | 0.824 | |
| TRF + FH + efflux | 0.846 (p = 0.006) |
0.02 p = 0.008 |
| TRF + hs-CRP ≥ 2 mg/L* | 0.817 | |
| TRF + hs-CRP ≥ 2 mg/L* + efflux | 0.839 (p = 0.008) |
0.02 p = 0.02 |
Harrell’s C-statistic and Integrated Discrimination Index calculated for the addition of cholesterol efflux > vs. < median to base models: 1) TRF + CAC > 0; 2) TRF + FH; 3) TRF + hs-CRP ≥ 2 mg/L.
IDI = Integrated Discrimination Index; TRF = Traditional Risk Factors; CAC = Coronary Artery Calcium; FH = Family History; hs-CRP = high-sensitivity C-reactive protein
Table 4.
Net Reclassification of Events and Non-Events with Adding Cholesterol Efflux Capacity to Risk Factors for Incident ASCVD
| Model | Reclassified Up, % | Reclassified Down, % | NRI | |
|---|---|---|---|---|
| Event | 67.6 | 32.3 | 0.35 | |
| TRF+CAC+Efflux | Nonevent | 48.8 | 51.2 | 0.02 |
| Total | 0.38 (0.13 – 0.53) | |||
| Event | 67.6 | 32.3 | 0.35 | |
| TRF+FH+Efflux | Nonevent | 48.5 | 51.5 | 0.03 |
| Total | 0.38 (0.13 – 0.55) | |||
| Event | 66.5 | 33.4 | 0.33 | |
|
TRF+Hs- CRP+Efflux |
Nonevent | 48.6 | 51.4 | 0.03 |
| Total | 0.36 (0.12 – 0.52) |
Category-less NRI (95%CI) calculated for the addition of efflux > vs. < median to base models: 1) TRF + CAC>0; 2) TRF + FH; 3) TRF + hs-CRP≥2 mg/L. Reclassification separated by those with incident ASCVD and those without incident ASCVD during follow up.
NRI = Net Reclassification Index; TRF = Traditional Risk Factors; CAC = Coronary Artery Calcium; FH = Family History; hs-CRP = high-sensitivity C-reactive protein
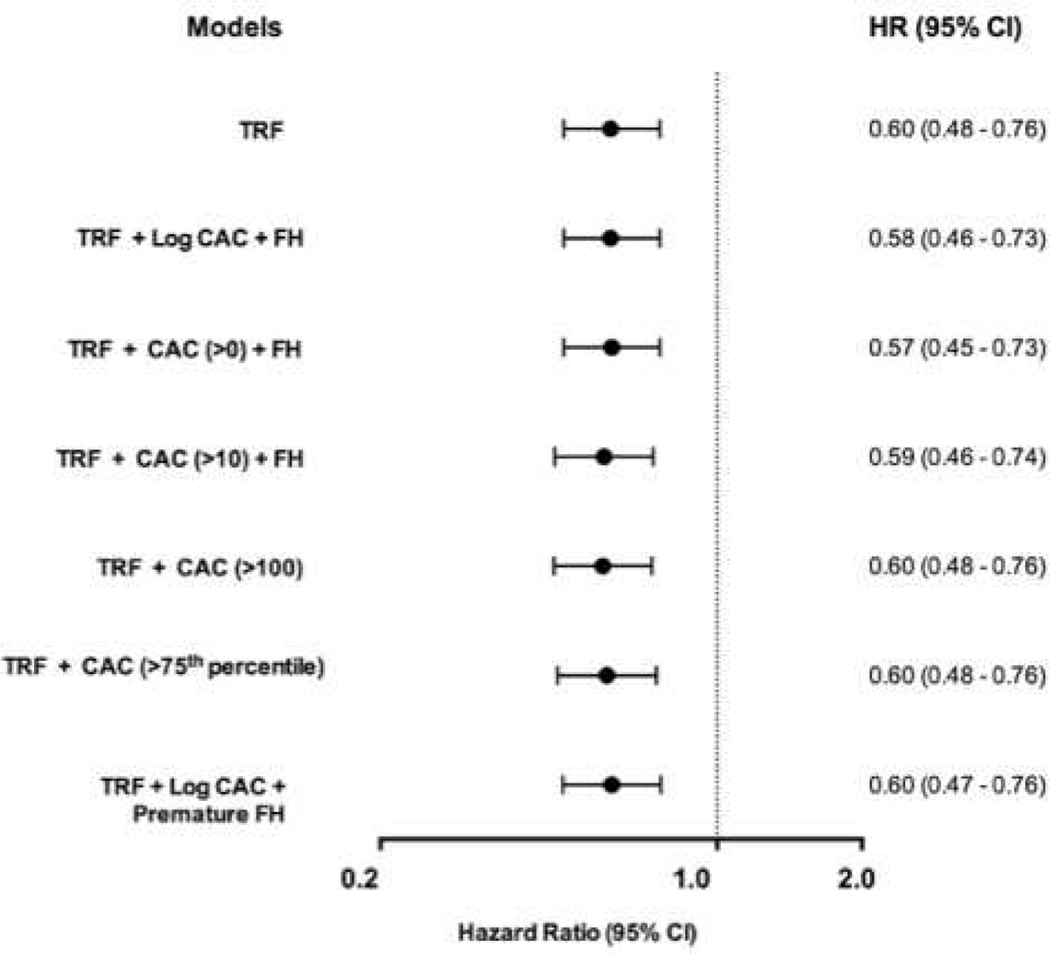
Several sensitivity analyses were conducted with no overall effect on the above findings. Excluding coronary revascularization (PCI and CABG: 16 out of 97 events) and restricting death to fatal MI or stroke did not alter the association of CEC with ASCVD when added to prevalent CAC, FH, or elevated hs-CRP. Similarly, findings were unchanged using varying categorical and continuous definitions of CAC, replacing FH with premature FH, or serial adjustment for self-reported exercise activity. Restricting the cohort to age > 45 did not alter the association between CEC and ASCVD when adjusted for CAC (fully adjusted HR for cholesterol efflux: 0.38, 95% CI 0.23 – 0.64). Analysis of CAC and hs-CRP as log-transformed continuous variables did not alter the findings of any of the risk prediction indices. Using continuous CEC did not alter any findings (Online Figures 1 and 2) and improved both upward and downward reclassification (Online Table 1). NRI calculated using risk thresholds did not alter the findings (Online Tables 2–4).
DISCUSSION
In a large, multi-ethnic population-based cohort, we evaluated the clinical relevance of a measure of reverse cholesterol transport, CEC, on ASCVD in the context of coronary artery calcium (CAC), FH of MI, and hs-CRP. The inverse association of CEC with incident ASCVD was not attenuated when accounting for all 3 risk markers combined. Furthermore, among patients with prevalent CAC, FH or hs-CRP, CEC was able to meaningfully stratify ASCVD risk.
CAC is a non-invasive measure of calcified coronary atherosclerosis, and increasing CAC score is directly proportional to coronary plaque burden and short-term ASCVD risk. Multiple prospective studies have demonstrated its ability to improve risk prediction for incident coronary heart disease beyond traditional risk factors, including the DHS (23–26). Based on the consistency of these data, CAC is often used clinically in those where statin eligibility is uncertain, a practice given a class IIb recommendation in the recent 2013 ACC/AHA guidelines on risk assessment (9).
Similarly, multiple large population-based studies have demonstrated that FH also improves ASCVD risk prediction after accounting for common cardiovascular risk factors (27,28). FH represents a heritable risk for ASCVD and is easily obtainable by self-report. Along with CAC, FH was given a class IIb recommendation in those whose statin eligibility remains unclear (9,29). In the DHS, FH was found to be additive to CAC in identifying individuals at significantly increased risk, demonstrating that FH and CAC represent distinct pathways leading to ASCVD events (26).
Hs-CRP is a circulating biomarker that represents inflammatory pathways leading to and part of ASCVD. Multiple studies have demonstrated an association of elevated hs-CRP levels with cardiovascular events and its ability to appropriately reclassify intermediate risk individuals (30). Given these data, it has been incorporated in the Reynolds Risk Score in addition to FH (29,31) and the current ACC/AHA guidelines give a IIB recommendation for incorporating hs-CRP levels in those with unclear cardiovascular risk (9).
Reverse cholesterol transport is the key anti-atherosclerotic function of HDL, and cholesterol efflux from the macrophage to the circulation is the key first step of this pathway. Unfortunately, circulating HDL-C levels and HDL particle concentration are poor surrogates for CEC (5). Bioassays measuring CEC have been applied to a few large cohorts, demonstrating inverse associations with prevalent coronary disease and incident CV death among high risk individuals (7,32). Within the DHS and EPIC-Norfolk (European Prospective Investigation of Cancer--Norfolk) study, 2 large population-based cohorts at low baseline risk, CEC was shown to be inversely associated with incident ASCVD, independent of HDL-C and HDL particle composition (5,8).
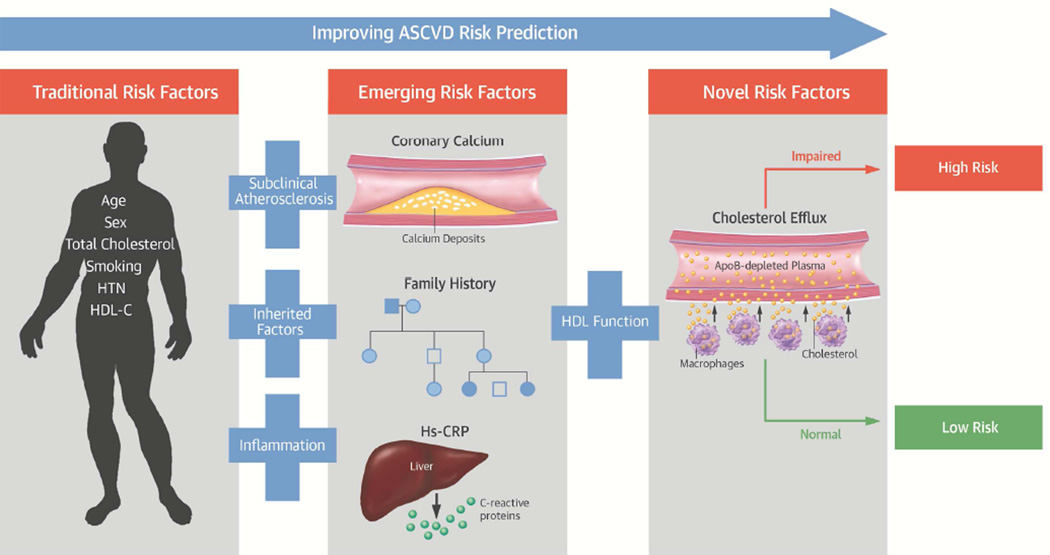
The unexpected magnitude of the association with ASCVD in these studies and the lack of attenuation with traditional risk factors prompted us to investigate further the impact of CAC, FH and hs-CRP on CEC and ASCVD. When CEC, CAC, FH and hs-CRP were added to traditional risk factors, efflux remained associated with ASCVD regardless of how predictors were analyzed. Analysis of the survival curves demonstrated that CEC improved risk stratification among those with either prevalent CAC, FH or elevated hs-CRP. Those with CEC above the median had a halving or more of their ASCVD risk over almost 10 years. Risk prediction performance measures confirmed that adding CEC to CAC, FH and hs-CRP improved the ability to predict incident events. Taken together, these findings demonstrate that the pathways that mediate the association between efflux and ASCVD in this study population are not reflected by atherosclerotic pathways reflected by CAC, inherited risk reflected by FH, or inflammatory pathways reflected by CRP (Central Illustration).
Central Illustration. Cholesterol Efflux Improves ASCVD Risk Prediction Beyond Traditional and Emerging Risk Factors.
In low risk populations, validated emerging risk factors reflecting subclinical coronary atherosclerosis, inherited factors, and inflammation improve risk prediction and are used clinically. Our study demonstrates that cholesterol efflux, a measure of HDL function, further improves prediction of incident ASCVD events beyond both traditional and clinically relevant emerging risk factors.
Several limitations to our study deserve comment. The relatively young age of the DHS study population limits generalizability to older age groups. Given the overall low cardiovascular risk of our study population, there were a small number of ASCVD events. The race/ethnicity distribution of our study sample, with oversampling of African-Americans, does not represent the general population. FH in our study population was self-reported and hence making it liable to recall bias and misclassification. However, this is the same information available to practicing clinicians and literature has demonstrated that self-reported parental history has a sensitivity > 80% and specificity > 90%, and any misclassification would bias towards the null (33). Ankle-brachial index, the fourth nontraditional risk factor given a Class IIb recommendation for risk factor assessment in addition to CAC, FH, and hs-CRP, was not available in this cohort. Interpretation of the NRI is associated with several documented limitations (34).
CONCLUSIONS
Cholesterol efflux represents the first critical step of reverse cholesterol transport, the key anti-atherosclerotic action of HDL. Among low risk individuals, efflux adds to ASCVD risk prediction beyond CAC, FH and hs-CRP, 3 well-validated and clinically used markers (Central Illustration). Our findings support efforts to standardize CEC methods and develop assays amenable for clinical use.
Supplementary Material
CLINICAL PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Cholesterol efflux capacity (CEC), a measure of high density lipoprotein function, improves risk prediction beyond FH of MI infarction, hs-CRP levels and detection of coronary calcification.
TRANSLATIONAL OUTLOOK
The development of efficient, standardized assays of CEC could provide a practical method for assessment of cardiovascular risk and serve as a surrogate target for the evaluation of novel therapeutic strategies.
Acknowledgments
Disclosures: The Dallas Heart Study is supported by grants from the Donald W. Reynolds Foundation and the National Center for Advancing Translational Sciences of the NIH (UL1TR001105). Anand Rohatgi is supported by the National Heart, Lung, and Blood Institute of the NIH under Award Number K08HL118131 and by the American Heart Association under Award Number 15CVGPSD27030013. Research grant, Merck, Significant. Advisory board, Cleveland HeartLab, modest. Consultant, Vascular Strategies, modest. Consultant, CSL Limited, modest.
Abbreviation List
- ASCVD
Atherosclerotic cardiovascular disease
- CAC
Coronary artery calcium
- CEC
Cholesterol efflux
- DHS
Dallas Heart Study
- FH
Family history of myocardial infarction
- HDL-C
High density lipoprotein cholesterol
- Hs-CRP
High-sensitivity C-reactive protein
- IDI
Integrated discrimination index
- MI
myocardial infarction
- NRI
Net reclassification index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 3.Guyton JR, Slee AE, Anderson T, et al. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1580–1584. doi: 10.1016/j.jacc.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallejo-Vaz AJ, Ray KK. Cholesterol efflux capacity as a novel biomarker for incident cardiovascular events: has high-density lipoprotein been resuscitated? Circ Res. 2015;116:1646–1648. doi: 10.1161/CIRCRESAHA.115.305938. [DOI] [PubMed] [Google Scholar]
- 7.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 11.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 13.Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Amer Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 14.Philips B, de Lemos JA, Patel MJ, McGuire DK, Khera A. Relation of family history of myocardial infarction and the presence of coronary arterial calcium in various age and risk factor groups. Am J Cardiol. 2007;99:825–829. doi: 10.1016/j.amjcard.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Khera A, Vega GL, Das SR, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maroules CD, Rosero E, Ayers C, Peshock RM, Khera A. Abdominal aortic atherosclerosis at MR imaging is associated with cardiovascular events: the Dallas heart study. Radiology. 2013;269:84–91. doi: 10.1148/radiol.13122707. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gronnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 23.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. New Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 24.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erbel R, Mohlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Paixao AR, Berry JD, Neeland IJ, et al. Coronary artery calcification and family history of myocardial infarction in the Dallas heart study. JACC Cardiovasc Imaging. 2014;7:679–686. doi: 10.1016/j.jcmg.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen M, Andersson C, Gerds TA, et al. Familial clustering of myocardial infarction in first-degree relatives: a nationwide study. Eur Heart J. 2013;34:1198–1203. doi: 10.1093/eurheartj/ehs475. [DOI] [PubMed] [Google Scholar]
- 28.Ranthe MF, Petersen JA, Bundgaard H, Wohlfahrt J, Melbye M, Boyd HA. A detailed family history of myocardial infarction and risk of myocardial infarction--a nationwide cohort study. PloS one. 2015;10:e0125896. doi: 10.1371/journal.pone.0125896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. 4p following 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 32.Ritsch A, Scharnagl H, Marz W. HDL cholesterol efflux capacity and cardiovascular events. N Engl J Med. 2015;372:1870–1871. doi: 10.1056/NEJMc1503139. [DOI] [PubMed] [Google Scholar]
- 33.Murabito JM, Nam BH, D'Agostino RB, Sr, Lloyd-Jones DM, O'Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 34.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160:122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.