Abstract
Although behavioral research has shown that positive mood leads to desired outcomes in nearly every major life domain, no studies have directly examined the effects of positive mood on the neural processes underlying reward-related affect and goal-directed behavior. To address this gap, participants in the present fMRI study experienced either a positive (n = 20) or neutral (n = 20) mood induction and subsequently completed a monetary incentive delay task that assessed reward and loss processing. Consistent with prediction, positive mood elevated activity specifically during reward anticipation in corticostriatal neural regions that have been implicated in reward processing and goal-directed behavior, including the nucleus accumbens, caudate, lateral orbitofrontal cortex and putamen, as well as related paralimbic regions, including the anterior insula and ventromedial prefrontal cortex. These effects were not observed during reward outcome, loss anticipation or loss outcome. Critically, this is the first study to report that positive mood enhances reward-related neural activity. Our findings have implications for uncovering the neural mechanisms by which positive mood enhances goal-directed behavior, understanding the malleability of reward-related neural activity, and developing targeted treatments for psychiatric disorders characterized by deficits in reward processing.
Keywords: positive mood, reward, mood induction, nucleus accumbens, corticostriatal circuit
Introduction
A person’s mood can influence a wide variety of phenomena ranging from mental health (World Health Organization, 2004) to cognitive processes, including reward processing and goal-directed behavior (Lyubomirsky et al., 2005). Indeed, positive mood facilitates the attainment of desirable outcomes (Fredrickson, 2001) within all major life domains (Lyubomirsky et al., 2005). Changes in mood can lead to goal reprioritization (Roseman, 2008), and positive mood can motivate and prepare individuals for goal pursuit (Aarts et al., 2008). Although behavioral research demonstrates that positive mood affects goal-directed behavior (Lyubomirsky et al., 2005), no studies have directly examined whether positive mood modulates reward-related neural activity. Examining this question has several important implications. First, it can uncover biological mechanisms by which positive mood enhances reward processing and elevates goal-directed behavior. Second, it informs our understanding of the malleability of reward-related neural activity within a short timeframe. Lastly, it has implications for managing mood disorders characterized by abnormalities in reward processing. Accordingly, this study employed functional magnetic resonance imaging (fMRI) and a positive mood induction paradigm to examine how the neural systems engaged during reward processing are affected by positive mood.
Reward processing refers to the value an individual places on potential rewards, the perceived probability of reward receipt, and how individuals process rewards or goal-relevant cues (Nusslock et al., 2014). Although there is a wealth of research linking positive mood to desired outcomes (Lyubomirsky et al., 2005), the mechanisms by which positive mood modulates reward processing are not well understood. Behaviorally, positive mood is thought to beget successful outcomes by promoting thoughts and actions that facilitate approaching goals and accumulating resources (Lyubomirsky, 2001; Elliot and Thrash, 2002). In these situations, people are able to ‘broaden and build;’ that is, positive mood broadens attention and thought-action repertoires so that individuals can prepare for future challenges and seek new goals (Fredrickson, 2001). Positive mood also cues individuals to environmental rewards through selective attention to positive and rewarding stimuli (Tamir and Robinson, 2007). Indeed, positive mood characterized by high approach motivation has a particularly strong effect on selective attention toward positive stimuli (Gable and Harmon-Jones, 2008).
Positive mood inductions have often been used to examine how mood affects subsequent behavior or functioning. For example, the Velten Mood Induction paradigm (Velten, 1968), in which participants read self-referent statements, is commonly used to examine changes in behavioral interests (Cunningham, 1988). Other methodologies such as musical stimuli, emotional faces, and autobiographical memories have also been used to induce different mood states (Mitterschiffthaler et al., 2007; Kohn et al., 2014). For example, happy, neutral, and sad musical stimuli have been used to identify neural correlates associated with happy and sad affect (Mitterschiffthaler et al., 2007). Thus, positive mood inductions reliably induce positive mood and have been used with fMRI to examine neural correlates of emotion. However, the effect of a positive mood induction on reward-related neural activity has not been directly examined.
The corticostriatal neural circuit is central to reward processing. Reward seeking behavior and proper balance between positive and negative affective states (Coenen et al., 2011) are dependent on connections between striatal regions, including the nucleus accumbens (NAc), caudate, and putamen, with cortical regions, such as the orbitofrontal cortex (OFC) (Ongur and Price, 2000; Knutson et al., 2001; Ongur et al., 2003; Haber and Knutson, 2010). Of the subcortical regions, the putamen facilitates stimulus–action–reward associations (Haruno and Kawato, 2006; Brovelli et al., 2011) through its prominent anatomical connections to sensory-motor related areas (Gerardin et al., 2003). The caudate and NAc, in contrast, have been implicated in reward-prediction error as well as integration of performance and cognitive control demands (Knutson et al., 2001; McClure et al., 2003; O’Doherty et al., 2003, 2004; Haruno and Kawato, 2006; Brovelli et al., 2011). With respect to the OFC, the medial OFC has been implicated in value-based decision-making (Wallis, 2012) and assigning value to external stimuli (Noonan et al., 2010), whereas the lateral OFC is involved in updating stimulus–outcome associations (Gottfried et al., 2003; Valentin et al., 2007) and coordinating behavior changes to achieve one’s goals (Wallis, 2012). Critically, research using a variety of stimuli demonstrates that corticostriatal neural regions are activated during reward processing (Knutson et al., 2001; Mobbs et al., 2003; Menon and Levitin, 2005; Treadway et al., 2012).
The corticostriatal circuit works in concert with paralimbic brain regions, such as the ventromedial prefrontal cortex (VMPFC) and anterior insula, to regulate motivated behavior (Salamone et al., 2007). The role of medial prefrontal cortex projections to the NAc in appetitive conditioning and reward-related behavior has been demonstrated using optogenetics (Deisseroth, 2014). Frontal cortical regions, including the VMPFC, are involved in generating affective meaning, which involves attending to appropriate cues, using past information to guide expectations of future outcomes, evaluating potential outcomes, and triggering or modifying emotional responses (Roy et al., 2012). Additionally, the insula, particularly the anterior insula, is a highly interconnected structure that marks salient events for further processing (Menon and Uddin, 2010), and combines uncertainty with bodily, affective, and sensory information to improve learning and guide decision-making (Singer et al., 2009). In this way, the anterior insula focuses attention onto relevant external stimuli and thereby increases the significance or saliency of that stimulus (Menon and Uddin, 2010). When considered together with behavioral research showing that positive mood redirects attention to elevate reward processing (Tamir and Robinson, 2007; Gable and Harmon-Jones, 2008), the anterior insula likely plays an important role in the effect of positive mood on reward processing.
This study examines the effect of a positive mood induction on reward-related neural activation in healthy adults. Participants were first randomly assigned to either a positive or neutral mood induction. Immediately following the mood induction, the monetary incentive delay (MID) task (Knutson et al., 2005) was used to assess neural activation during the anticipation and outcome of uncertain monetary gains and losses. Examining the effect of a positive mood induction on reward-related neural activation can inform our understanding of the biological mechanisms by which positive mood enhances reward processing and goal-directed behavior. Furthermore, it increases our knowledge about the malleability of reward-related brain function in real time, and facilitates the development of targeted interventions for psychiatric disorders characterized by deficits in reward processing. Given the link between positive mood, reward processing, and attention, we predicted that positive mood would increase activity in the corticostriatal circuit and related paralimbic regions during reward processing.
Methods
Participants
Forty-six healthy young adults from the Chicago area were recruited for this study. Six participants were excluded due to poor brain coverage in fMRI data. The remaining 40 participants were randomly assigned to either a positive (10 Female/10 Male; M = 21.19 years, standard deviation (S.D.) = 1.34 years) or neutral mood induction (10 Female/10 Male; M = 20.86 years, S.D. = 1.83 years). The groups did not differ in age or gender, all p > 0.518. At screening, participants had no self-reported history of psychiatric illness or neurological disorders, were not taking psychiatric medication and were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Self-reported depression was assessed using the 9-item Patient Health Questionnaire (PHQ-9) (Kroenke et al., 2001) at the time of the scan. This study was approved by the Institutional Review Board at Northwestern University and participants were treated in accordance with the American Psychological Association Code of Conduct.
Mood induction
All participants completed the mood induction (positive or neutral) in the MRI scanner immediately before the fMRI MID task. The mood inductions combined the use of music and sentences similar to those in the established Velten Mood Induction paradigm (Velten, 1968). The positive mood induction consisted of reading 30 positive sentences (e.g., ‘I feel amazing today!’), each presented individually for 8 s, while upbeat music (i.e., Strauß’ Radetzky march) was simultaneously presented through earphones. Participants in the neutral mood induction read 30 neutral sentences (e.g., ‘The doorkeeper was dressed in red.’), also presented individually for 8 s, while listening to neutral music (i.e., Schumann’s L’oiseau prophete). To maximize comparisons between induction groups, all participants were instructed to try and remember a time during which they felt similar to what the sentence describes. The music pieces (i.e., Strauß’ Radetzky march for the positive mood induction and Schumann’s L’oiseau prophete for the neutral mood induction) were selected according to music ratings obtained in a previous study that examined neural, activity of positive, neutral, and sad mood states induced by various classical music pieces (Mitterschiffthaler et al., 2007). All participants used a visual analog scale ranging from ‘Not at all’ (0) to ‘Very’ (100) to provide happy, sad, excited, and tense ratings immediately before and after the mood induction.
fMRI MID
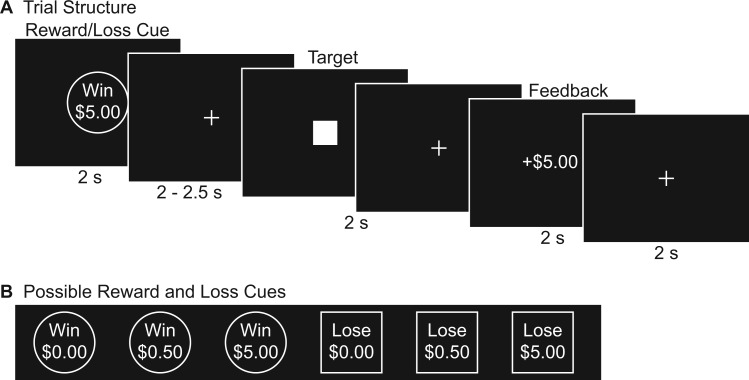
Immediately following the mood induction, participants completed the MID task (Figure 1) (Samanez-Larkin et al., 2007a). A circle cue signifying the opportunity to win money (Win $0.00, Win $0.50, Win $5.00) or a square cue indicating the possibility of losing money (Lose $0.00, Lose $0.50, Lose $5.00) was first presented for 2 s. Then, a jittered fixation followed by a solid white square was presented. Participants were required to make a button response when the solid white square appeared. For reward trials, participants won money if they hit the solid white target and did not earn money if the target was missed; for loss trials, participants avoided losing money if they hit the solid white target and lost money if the target was missed. Feedback depicting the amount of money won or lost on each trial was then displayed for 2 s. Finally, a fixation cross was presented for 2 s as an intertrial interval. The initial duration of the target square was based on each participant’s mean hit reaction time (RT) during a MID practice phase performed before entering the scanner and undergoing the mood induction. The target duration then dynamically updated throughout the fMRI MID task to maintain task difficulty such that participants successfully hit the target on approximately 66% of the trials, calculated separately for each trial type (i.e., Win $0.00, Win $0.50, Win $5.00, Lose $0.00, Lose $0.50, Lose $5.00). The six trial types were each presented 15 times in random order, totaling to 90 trials lasting 10 s each.
Fig. 1.
The (A) trial structure and (B) possible reward and loss cues of the monetary incentive delay (MID) task used to examine reward and loss anticipation and outcome.
After every 30 trials of the MID, a mood induction booster session was given. The booster session lasted 40 s and consisted of the presentation of 5 positive or neutral sentences along with positive or neutral music, depending on the assigned group. These mood booster sessions were included to help participants sustain a positive or neutral mood throughout the MID task.
fMRI data acquisition and analysis
Acquisition and preprocessing
Neuroimaging data were collected using a Siemens TRIO 3T scanner with a standard 32-channel head coil. The following parameters were used to collect whole-brain gradient-recalled echo-planar images: 32 axial 3 mm slices, 0 mm gap, repetition time (TR) = 2000 ms, echo time (TE) = 20 ms; flip angle = 80°; field of view (FOV) = 220 × 220 mm. The resulting voxel size was 3.44 × 3.44 × 3 mm.
Data were analyzed using a general linear model implemented in the Standard Parametric Program (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK). Functional images were realigned, corrected for errors in slice-timing, spatially normalized to MNI space and smoothed with a 6 mm full width at half maximum (FWHM) Gaussian kernel. Translational movement in millimeters (x, y, z) and rotational motion in degrees (pitch, roll, yaw) were calculated based on SPM8 parameters for motion correction of the functional images in each subject. The final sample of 40 subjects had less than 3 mm of movement.
Statistical analysis
A general linear model identifying the six trial types (i.e., Win $0.00, Win $0.50, Win $5.00, Lose $0.00, Lose $0.50, Lose $5.00) during the anticipation and outcome phases was used to deconvolve the hemodynamic signal. The anticipation phase of the MID task was defined as the period after participants saw the cue signifying the possibility to win or lose money but had not yet responded to the target square (2–2.5 s). The outcome phase was defined as the period after participants received feedback indicating whether they won or lost money for that trial (2 s). Six variables of no interest for motion were included. First-level voxel-wise t-statistics were generated for each participant in contrasting reward (i.e., Win $0.50, Win $5.00) vs non-reward (i.e., Win $0.00) trials to assess reward anticipation and outcome, and loss (i.e., Lose $0.50, Lose $5.00) vs non-loss (i.e., Lose $0.00) trials to assess loss anticipation and outcome (Samanez-Larkin et al., 2007b).
The main effects of reward vs non-reward and loss vs non-loss during the anticipation and outcome phases were first examined to ensure that the MID task activated expected regions. Next, two separate full factorial 2 × 2 analyses of variance (ANOVAs) for the anticipation and outcome phases were conducted on whole-brain activation. In both these models, Group (positive vs neutral mood induction) was a between subjects factor, Value (reward vs loss) was a within subjects factor and pre-induction sadness ratings and depression scores were included as covariates of no interest. Although previous literature has often used the term ‘Valence’ to refer to the reward vs loss factor (Knutson et al., 2008), we used the term ‘Value’ for this factor to avoid confusion with the valenced nature of the mood induction. Significant clusters of activation at the whole-brain level were determined at a voxel-wise height threshold of p < 0.01, with family-wise error (FWE) correction for multiple spatial comparisons (p < 0.01, k = 140 voxels). The whole-brain FWE correction was determined using 3dClustSim in AFNI (Cox, 1996).
A series of t-tests were performed to follow-up significant Group and Value main effects and Group × Value interactions during the anticipation and outcome phases. Within regions that showed a significant Group main effect, a follow-up t-test comparing positive and neutral mood inductions was conducted to determine the direction of effects. Similarly, reward and loss were compared in a t-test within regions that showed a significant Value main effect. Finally, the following t-tests were conducted within regions that showed a significant Group × Value interaction: (i) reward vs loss in the positive mood induction group, (ii) reward vs loss in the neutral mood induction group, (iii) positive vs neutral mood induction groups during reward processing, and (iv) positive vs neutral mood induction groups during loss processing. Results from the t-tests were thresholded at a height of p < 0.01.
Results
Mood induction ratings
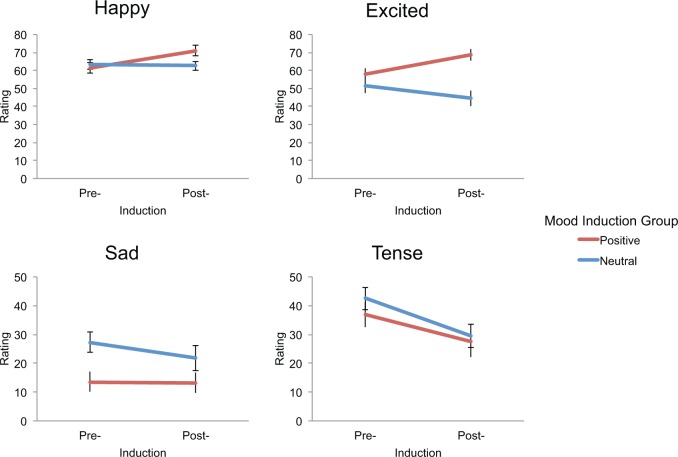
To assess the effectiveness of the mood induction paradigm, four mixed-effects ANOVAs were first conducted on happy, excited, sad, and tense self-report ratings with Group (positive vs neutral mood induction) as a between subjects factor and Time (pre- vs post-induction) as a within subjects factor (Figure 2). There was a main effect of Group for excited, F(1,38) = 8.90, p = 0.005, and sad ratings, F(1,38) = 5.46, p = 0.025, such that the positive mood induction group was more excited and less sad than the neutral mood induction group. There was also a main effect of Time for happy, F(1,38) = 6.44, p = 0.015, and tense ratings, F(1,38) = 34.17, p < 0.001, such that all participants were more happy and less tense after the induction. No other significant main effects were found. The main effects for excited and happy ratings were qualified by a Group × Time interaction [excited, F(1,38) = 29.68, p < 0.001; happy, F(1,38) = 9.20, p = 0.004], in which the groups were equivalent in these ratings before the induction, all p > 0.23, but the positive mood induction group was significantly more excited and happier after the induction, [excited, t(38) = 4.406, p < 0.001; happy, t(38) = 2.152, p = 0.038]. Furthermore, within the positive mood induction group alone, there was a significant increase in excited, F(1,19) = 20.739, p < 0.001, and happy ratings, F(1,19) = 12.338, p = 0.002, from pre- to post-mood induction. Thus, the positive mood induction was successful in increasing excitement and happiness. Notably, because the positive mood induction group was significantly less sad than the neutral mood induction group prior to the mood induction, t(38) = −2.81, p = 0.008, pre-induction sadness ratings were included as a covariate of no interest in all fMRI analyses. Pre-induction sadness was not related to activity in neural regions central to this study during the anticipation phase and related only to activity in the caudate during outcome phase (Supplementary Figure S1 and Table S1).
Fig. 2.
Happy, excited, sad, and tense ratings pre- and post- mood induction for positive and neutral mood induction groups. The efficacy of the positive mood induction is demonstrated by increased happy and excited ratings after induction in the positive mood induction group, and unchanged ratings in the neutral mood induction group. Note: error bars represent standard error of the mean (SEM).
Depression scores
Because self-reported depression scores were highly skewed, a Mann–Whitney U-test was conducted to determine group differences in self-reported depression. The neutral mood induction group had higher depression scores at baseline (Median = 2.5) than the positive mood induction group (Median = 1), U = 124.5, p = 0.04. Importantly, all participants except one were in the minimal or mild range for depression. Nevertheless, given that the two groups differed in self-reported depression, PHQ-9 scores were used as a covariate of no interest for all analyses. Self-reported depression was related only to activity in the right superior frontal gyrus during the anticipation phase (Supplementary Figure S1 and Table S1).
MID behavioral results
There were no significant differences between the positive and neutral mood induction groups in either accuracy or RT on the MID task when examining (i) all reward and loss trials, all p > 0.16, (ii) incentive trials only (i.e., $0.50 and $5.00 trials), all p > 0.23 and (iii) comparisons between incentive and non-incentive trials (i.e., $0.50 and $5.00 vs $0.00), all p > 0.15. These lack of behavioral differences are consistent with previous studies using the MID to examine current depression (Knutson et al., 2008), cocaine dependence (Jia et al., 2011) and schizophrenia (Simon et al., 2010).
fMRI results
MID task engaged expected regions
In line with previous research (Knutson et al., 2000, 2001), there were main effects of reward vs non-reward (i.e., Win $5.00, Win $0.50 > Win $0.00), and loss vs non-loss (i.e., Lose $5.00, Lose $0.50 > Lose $0.00) during the anticipation phase in canonical regions including the caudate, NAc, and anterior insula (Supplementary Figure S2 and Table S2). No regions showed significant activation during reward or loss outcome. Therefore, the MID task activated expected regions during the anticipation phase in our sample.
Anticipation phase: Group main effect
There was no significant main effect of Group.
Anticipation phase: Value main effect
There was a main effect of Value in corticostriatal regions, including the right NAc, right caudate, and right OFC, as well as paralimbic regions, including the right subcallosal cortex (Supplementary Figure S3, Table 1). Follow-up t-tests indicated that the value main effect was driven by greater activity during reward anticipation in comparison to loss anticipation.
Table 1.
Neural regions that showed significant Group and Value main effects during the anticipation phase
| Size of cluster (voxels) | Peak F-score | Peak MNI coordinates (mm) |
|||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Group main effect | |||||
| n/a | |||||
| Value main effect | |||||
| L paracingulate gyrus | 267 | 16.74 | −10 | 40 | −2 |
| R inferior parietal lobule | 1283 | 19.67 | 50 | −38 | 54 |
| R lateral occipital cortex | 248 | 19.09 | 30 | −78 | 34 |
| R subcallosal cortex | 471 | 18.03 | 4 | 20 | −6 |
| R nucleus accumbens | 10.47 | 14 | 16 | −6 | |
| R caudate | 10.32 | 10 | 22 | 0 | |
| R orbitofrontal cortex | 9.44 | 16 | 18 | −18 | |
| L lateral occipital cortex | 298 | 16.02 | −30 | −86 | 36 |
| L frontal pole | 178 | 12.52 | −28 | 56 | 6 |
Results are for whole-brain analyses, and controlled for pre-mood induction sadness ratings and depression scores. Follow-up t-tests demonstrated that Value main effects were driven by greater activity during reward than loss.
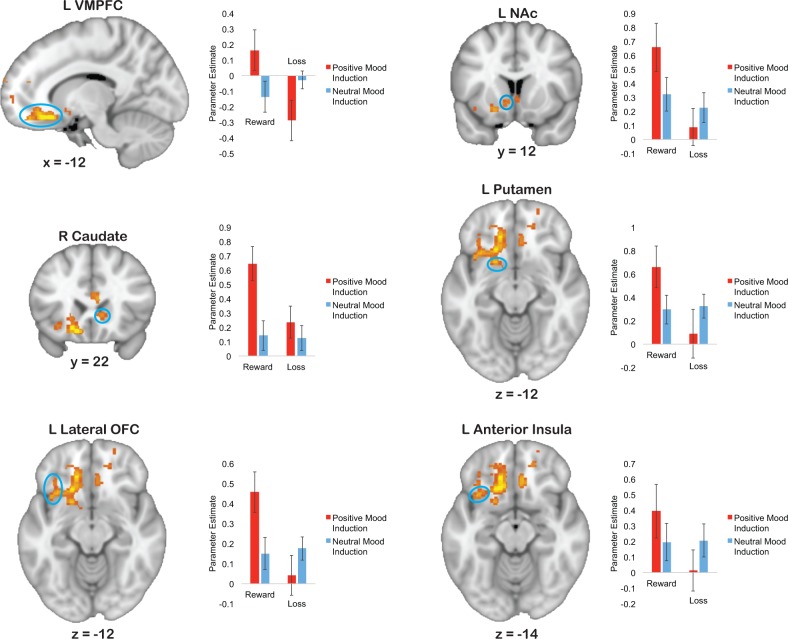
Anticipation phase: Group × Value interaction
The main effect of Value was qualified by a Group × Value interaction in key regions of the corticostriatal circuit including the left NAc, right caudate, left putamen, left lateral OFC, and left anterior insula, as well as related paralimbic regions such as the left VMPFC (Figure 3, Table 2). Follow-up t-tests (Table 2) examining positive and neutral mood induction groups separately demonstrated that this interaction was driven by greater activity during reward anticipation than loss anticipation in the positive mood induction group, but equivalent activity in the neutral mood induction group. Furthermore when examining reward and loss anticipation separately, the positive mood induction group showed greater activity than the neutral mood induction group during reward anticipation, but equivalent activity to the neutral mood induction group during loss anticipation.
Fig. 3.
Brain regions that showed a significant Group (positive mood induction vs neutral mood induction) × Value (reward vs loss) interaction during the anticipation period. The positive mood induction group showed greater activity than the neutral mood induction group during reward anticipation in the left ventromedial prefrontal cortex (VMPFC), left nucleus accumbens (NAc), right caudate, left putamen, left lateral orbitofrontal cortex (OFC), and left anterior insula. The positive mood induction group, in contrast, showed reduced or equivalent activity than the neutral mood induction group during loss anticipation. Note: error bars represent standard error of the mean (SEM).
Table 2.
Neural regions that showed a significant Group (positive vs neutral mood induction) × Value (reward vs loss) interaction during the anticipation phase, and follow-up t-tests to determine the direction of the interaction
| Size of cluster (voxels) | Peak F-score | Peak MNI coordinates (mm) |
|||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Group × Value interaction | |||||
| R ventromedial prefrontal cortex | 247 | 11.03 | 2 | 38 | −8 |
| L ventromedial prefrontal cortex | 784 | 22.91 | −12 | 24 | −16 |
| L putamen | 15.57 | −20 | 10 | −12 | |
| L lateral orbitofrontal cortex | 15.30 | −32 | 20 | −12 | |
| L anterior insula | 14.49 | −36 | 16 | −14 | |
| L nucleus accumbens | 11.60 | −8 | 12 | −6 | |
| R caudate | 11.34 | 10 | 22 | 0 | |
| L nucleus accumbens | 8.53 | −6 | 6 | −8 | |
| R frontal pole | 337 | 19.44 | 12 | 62 | 36 |
| R frontal pole | 579 | 18.51 | 36 | 56 | −2 |
| R frontal pole | 185 | 17.43 | 38 | 54 | 28 |
| R inferior parietal lobule | 154 | 15.09 | 56 | −40 | 52 |
| L frontal pole | 406 | 14.02 | −8 | 60 | 2 |
| R anterior cingulate cortex | 143 | 12.80 | 4 | 38 | 10 |
| L frontal pole | 158 | 13.02 | −44 | 52 | −6 |
| Peak T-score | |||||
| Within-group post hoc effects | |||||
| Positive mood induction: reward anticipation > loss anticipation | |||||
| R paracingulate gyrus | 215 | 3.77 | 12 | 42 | −6 |
| L orbitofrontal cortex | 728 | 4.76 | −22 | 28 | −16 |
| R caudate | 4.37 | 10 | 22 | 0 | |
| L putamen | 4.25 | −18 | 10 | −12 | |
| L nucleus accumbens | 4.10 | −6 | 12 | −4 | |
| R frontal pole | 569 | 4.86 | 26 | 66 | 6 |
| R frontal pole | 182 | 4.59 | 48 | 48 | 18 |
| R frontal pole | 55 | 4.46 | 46 | 38 | 38 |
| R supramarginal gyrus | 146 | 4.18 | 58 | −38 | 50 |
| L frontal pole | 324 | 4.03 | −8 | 68 | 20 |
| R anterior cingulate cortex | 93 | 3.66 | 4 | 38 | 10 |
| R frontal pole | 113 | 3.65 | 10 | 54 | 44 |
| L frontal pole | 145 | 3.27 | −38 | 54 | −8 |
| R frontal pole | 41 | 3.42 | 10 | 66 | 28 |
| L frontal pole | 12 | 2.90 | −30 | 46 | 18 |
| R frontal pole | 1 | 2.42 | 16 | 48 | −18 |
| Positive mood induction: loss anticipation > reward anticipation | |||||
| n/a | |||||
| Neutral mood induction: reward anticipation > loss anticipation | |||||
| n/a | |||||
| Neutral mood induction: loss anticipation > reward anticipation | |||||
| R frontal pole | 52 | 3.39 | 10 | 62 | 36 |
| R frontal pole | 10 | 2.86 | 22 | 50 | 46 |
| L frontal pole | 2 | 2.47 | −44 | 52 | −6 |
| Between-group post hoc effects | |||||
| Reward anticipation: positive mood induction > neutral mood induction | |||||
| R frontal pole | 71 | 4.24 | 24 | 60 | 32 |
| R frontal pole | 48 | 3.40 | 42 | 58 | −4 |
| R frontal pole | 61 | 3.37 | 38 | 56 | 26 |
| R frontal pole | 56 | 3.35 | 32 | 66 | 10 |
| R frontal pole | 45 | 3.30 | 2 | 64 | 4 |
| R paracingulate gyrus | 3.02 | 2 | 56 | 6 | |
| R lateral occipital cortex | 15 | 3.25 | 44 | −58 | 54 |
| R caudate | 41 | 3.03 | 12 | 20 | 8 |
| R supramarginal gyrus | 47 | 3.12 | 56 | −44 | 50 |
| R anterior cingulate cortex | 11 | 2.95 | 2 | 40 | 12 |
| R frontal pole | 11 | 2.85 | 42 | 44 | 36 |
| R frontal pole | 2 | 2.78 | 4 | 66 | 26 |
| R paracingulate gyrus | 9 | 2.75 | 10 | 42 | −4 |
| R frontal pole | 1 | 2.72 | 36 | 48 | 38 |
| L orbitofrontal cortex | 3 | 2.70 | −24 | 36 | −18 |
| R frontal pole | 2 | 2.65 | 8 | 56 | 46 |
| L orbitofrontal cortex | 2 | 2.63 | −22 | 44 | −12 |
| L frontal pole | 10 | 2.63 | −14 | 22 | −16 |
| L insula | 5 | 2.58 | −38 | 16 | −6 |
| R frontal pole | 1 | 2.54 | 6 | 58 | 44 |
| R frontal pole | 2 | 2.47 | 22 | 56 | −12 |
| R frontal pole | 1 | 2.42 | 32 | 50 | 38 |
| L insula | 1 | 2.42 | −32 | 20 | −8 |
| Reward anticipation: neutral mood induction > positive mood induction | |||||
| n/a | |||||
| Loss anticipation: positive mood induction > neutral mood induction | |||||
| n/a | |||||
| Loss anticipation: neutral mood induction > positive mood induction | |||||
| L orbitofrontal cortex | 32 | 3.23 | −22 | 22 | −16 |
| R frontal pole | 41 | 3.20 | 34 | 54 | 0 |
| L orbitofrontal cortex | 25 | 3.04 | −36 | 16 | −14 |
| L nucleus accumbens | 6 | 2.77 | −8 | 6 | −10 |
| R paracingulate gyrus | 7 | 2.71 | 4 | 52 | 20 |
| L putamen | 8 | 2.67 | −20 | 10 | −12 |
| R frontal pole | 5 | 2.57 | 54 | 38 | 16 |
| R superior frontal gyrus | 2 | 2.56 | 4 | 54 | 34 |
| L frontal pole | 4 | 2.54 | −44 | 52 | −6 |
| R superior frontal gyrus | 6 | 2.50 | 4 | 50 | 42 |
| R frontal pole | 1 | 2.46 | 28 | 46 | 46 |
| L nucleus accumbens | 1 | 2.44 | −8 | 12 | −8 |
| L orbitofrontal cortex | 1 | 2.41 | −34 | 28 | −16 |
Results are for whole-brain analyses and controlled for pre-mood induction sadness ratings and depression scores. Follow-up t-tests show that the Group × Value interaction was driven primarily by greater activity during reward than loss anticipation in the positive mood induction group, but equivalent activity across reward and loss anticipation in the neutral mood induction group.
Outcome phase: Group main effect
During the outcome phase, there was a main effect of Group in the left insula, anterior cingulate cortex, and other regions unrelated to the corticostriatal circuit (Supplementary Figure S4A and Table S3). Follow-up t-tests revealed that the group main effects were driven by greater deactivation in the positive compared with the neutral mood induction group.
Outcome phase: Value main effect
There was a main effect of Value in the right amygdala, left insula, and other regions unrelated to corticostriatal circuits, such as the superior parietal lobule and posterior cingulate gyrus (Supplementary Figure S4B and Table S3). Follow-up t-tests indicated that these main effects were driven by greater activation during loss in comparison to reward outcome.
Outcome phase: Group × Value interaction
No regions showed a significant Group × Value interaction during the outcome period.
Discussion
This study examined the effect of positive mood on the neural mechanisms subserving reward and loss processing. In line with prediction, positive mood modulated corticostriatal regions central to reward processing, and paralimbic regions that help regulate motivated behavior. Specifically, positive mood increased activity during reward anticipation in comparison to loss anticipation in the NAc, caudate, putamen, lateral OFC and anterior insula. In contrast, those in the neutral mood condition showed largely equivalent activity in these regions during reward vs loss anticipation. Positive mood also increased activity in the VMPFC during reward anticipation but deactivated this region during loss anticipation. These interactions were specific to the anticipation period, as there was no unique effect of positive mood on neural activity during the outcome period. Furthermore, neural effects were not driven by behavioral differences in the MID task given there were no significant differences between the positive and neutral mood induction groups in either accuracy or RT in the MID task. Critically, our study suggests an underlying neural mechanism for how positive mood affects reward processing and is an important first step towards understanding the biological pathways by which positive mood leads to desired outcomes.
Positive mood increases activity in neural regions that assign meaning and value
Previous research demonstrates that value judgments about expected outcomes are represented in the VMPFC during reward anticipation (Kim et al., 2011). More broadly, the VMPFC is critical for determining the affective meaning of a stimulus. That is, the VMPFC represents conceptual information and is essential for extracting meaning that is relevant for one’s physical and social well-being as well as future prospects (Roy et al., 2012). Under this affective meaning framework, our results suggest that enhanced VMPFC activity during reward anticipation by individuals in a positive mood may reflect greater affective meaning generated for rewarding stimuli. In contrast, deactivation of the VMPFC during loss anticipation by individuals in a positive mood may reflect reduced affective meaning to loss stimuli, although future research is needed to test this hypothesis. These findings are consistent with research showing that positive mood increases the value of already positive stimuli. For example, individuals in a positive mood were willing to forego a greater monetary payout in order to retrieve a positive memory (Speer et al., 2014). Positive mood also increased activity in the NAc and caudate, which are important for processing the subjective value of a stimulus (O’Doherty, 2004; Delgado, 2007; Salimpoor et al., 2011). Taken together, positive mood increases activity in regions that determine the subjective value of already rewarding stimuli.
Positive mood increases activity in neural regions important for motivation
Positive mood also increased activity in corticostriatal and paralimbic regions that subserve motivation to obtain potential rewards. Previous research indicates that engagement of the NAc, caudate and putamen energizes behavior, even without conscious awareness. For example, in a study of unconscious motivation, individuals engaged striatal regions including the NAc, caudate, and putamen, and exerted more effort during trials that included an unconscious reward (Pessiglione et al., 2007). Similarly, a positron emission tomography (PET) study demonstrated that increased dopamine in the caudate, VMPFC, and insula, all regions that were elevated in the positive mood induction group during reward anticipation in this study, was associated with a greater willingness to expend energy to obtain the reward (Treadway et al., 2012). Collectively, this suggests that positive mood elevates corticostriatal and paralimbic neural activity, which may enhance motivation to obtain rewards.
Positive mood increases activity in neural regions important for orienting attention to salient stimuli
Another proposed mechanism by which positive mood enhances reward anticipation is through attention selection (Tamir and Robinson, 2007). Our finding that positive mood elevates activity in the anterior insula during reward anticipation provides support for this hypothesis. The anterior insula is particularly sensitive to salient environmental stimuli and is responsible for detecting behaviorally relevant stimuli, marking salient events for additional processing, and coordinating neural resources (Menon and Uddin, 2010; Uddin, 2015). Our finding that the positive mood induction group, in comparison to the neutral mood induction group, showed greater activity in the anterior insula during reward anticipation suggests that positive mood enhances the salient properties of reward. This is consistent with behavioral literature indicating that individuals focus on rewarding stimuli during a positive mood (Tamir and Robinson, 2007; Gable and Harmon-Jones, 2008). Collectively, our findings suggest that positive mood enhances activity in salience regions, which may increase attention allocated to these stimuli. Thus, increased anterior insula activity may be a neural mechanism subserving the behavioral finding that positive mood elevates selective attention for rewarding stimuli.
Positive mood increases activity in neural regions that facilitate behavior in order to attain reward
Finally, we demonstrated that positive mood elevated activity during reward anticipation in two neural regions implicated in decision-making and action-based strategies that maximize reward and/or minimize loss. The first region is the lateral OFC, which is involved in updating stimulus–outcome learning (Valentin et al., 2007) and coordinating behavior to achieve goals (Wallis, 2007). Because the success rate was fixed in the MID task, the target duration changed throughout the task. Accordingly, participants needed to continuously learn and adapt their responses in order to obtain the reward. Thus, increased lateral OFC activity in the positive mood induction group may reflect additional effort expended on planning future behavior, particularly if positive mood increased the subjective value of the stimulus and motivation to obtain the reward.
Second, the reward-related functions of the putamen are thought to be more action-based given its unique anatomical connections with the corticostriatal reward system and sensorimotor, pre-motor, and motor structures (Wise et al., 1996; Middleton and Strick, 2000; Nachev et al., 2008; Ashby et al., 2010). For example, the putamen has been proposed to be the striatal hub underlying habit learning, a process that begins with goal-directed action (Yin and Knowlton, 2006; Graybiel, 2008; Ashby et al., 2010; Balleine and O’Doherty, 2010; Brovelli et al., 2011). The putamen is also involved in making associations between stimuli, actions, and rewards (Haruno and Kawato, 2006). The MID task used in this study has clear stimulus–action–reward associations, in which participants see a stimulus, make a motor response, and are either rewarded or punished (Figure 1). As a result, the involvement of the putamen is expected and consistent with previous reward studies that have used the MID task (Knutson et al., 2000, 2001). We extend these findings by demonstrating that positive mood enhances putamen activity during reward anticipation. Taken together, positive mood may enhance reward processing by strengthening activity in regions responsible for stimulus–action–reward associations, thereby facilitating action-based strategies that can help individuals attain rewards.
Reward-related increases in neural activity due to positive mood is specific to the anticipation phase
Although positive mood increased activity in corticostriatal and paralimbic regions during reward anticipation, no significant interactions were found during the outcome phase. Thus, while positive mood enhances numerous processes that occur before reward attainment, it does not appear to modulate neural activity during reward consumption. Future research is needed, however, to assess whether positive mood has a different effect on reward outcome in mood disorder patients, as two studies showed that depressed patients display blunted reward-related brain activity to reward outcomes but not to reward anticipation (Knutson et al., 2008; Pizzagalli et al., 2009).
Implications
In addition to informing our understanding of the biological mechanisms by which positive mood elevates reward processing and goal-directed behavior, this study also demonstrates the malleability of reward-related brain function. Although treatment research demonstrates that interventions can generate trait-like changes in reward-related brain function over long periods of time (Lam et al., 2003; Dichter et al., 2009), notable changes in mood and reward processing occur on a much shorter time scale over the course of the day. Results from this study suggest that these moment-to-moment changes in mood are likely characterized by measurable changes in reward-related brain function. Our results may also help inform the development of targeted interventions for psychiatric disorders characterized by abnormal reward processing. For example, whereas unipolar depression involves abnormally reduced reward-related brain function, reflecting anhedonia and decreased approach motivation, bipolar disorder involves abnormally elevated reward-related brain function, reflecting increased approach motivation and risk for mania (Forbes et al., 2009; Nusslock et al., 2012, 2014). Interventions that help patients manage positive affect on a moment-to-moment basis may regulate reward-related brain function and attenuate, or ideally prevent, symptom onset (Nusslock et al., 2009).
Limitations
There are a few limitations to note in this study. First, the mood induction groups differed in depression scores and pre-induction sadness ratings. This limitation is mitigated, however, by the inclusion of these variables as covariates of no interest in all analyses, and by the fact that sadness and depression scores did not relate to neural activity in a meaningful way. Second, our study did not include a negative mood induction. Assessing negative mood inductions will provide a more comprehensive understanding of how emotions differentially affect reward-related brain function. Third, participants were not explicitly asked to guess the purpose of the mood manipulation. Thus, we are unable to determine if knowledge about the mood manipulation affected the results.
Conclusions
This study examined the biological mechanisms by which positive mood enhances reward processing and elevates goal-directed behavior. We suggest that elevated corticostriatal and paralimbic activity during reward anticipation amongst individuals in a positive mood reflects enhanced subjective value and meaning of reward stimuli, motivation to obtain the reward, attention orientation, and stimulus–action–reward associations. We further propose that these are the neural mechanisms by which positive mood facilitates goal pursuit and the attainment of positive outcomes, although further research is needed to directly assess this. This study is an important step in bridging neuroimaging methods with research on positive mood and reward processing. Findings have important implications for understanding how positive mood facilitates positive life outcomes and can aid the development of interventions for managing abnormalities in positive affect.
Funding
This work was supported by the National Science Foundation Graduate Research Fellowship Program [DGE-0824162 to C.B.Y.]; the National Institute of Mental Health [R01 MH100117-01, R01 MH077908-01A1 to R.N.]; the Ryan Licht Sang Bipolar Foundation [Young Investigator Grant to R.N.] and the Chauncey and Marion D. McCormick Family Foundation [Young Investigator Grant to R.N.].
Supplementary Material
Acknowledgements
The authors thank Todd Parrish and Sandra Shi for their assistance with MRI data acquisition. They also thank Mutahir Rauf for his assistance with data collection and dedicate this manuscript in his memory.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Aarts H., Custers R., Veltkamp M. (2008). Goal priming and the affective-motivational route to nonconscious goal pursuit. Social Cognition, 26(5), 555–77. [Google Scholar]
- Ashby F.G., Turner B.O., Horvitz J.C. (2010). Cortical and basal ganglia contributions to habit learning and automaticity. Trends in Cognitive Sciences, 14(5), 208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., O’Doherty J.P. (2010). Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology, 35(1), 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A., Nazarian B., Meunier M., Boussaoud D. (2011). Differential roles of caudate nucleus and putamen during instrumental learning. Neuroimage, 57(4), 1580–90. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Schlaepfer T.E., Maedler B., Panksepp J. (2011). Cross-species affective functions of the medial forebrain bundle-implications for the treatment of affective pain and depression in humans. Neuroscience and Biobehavioral Reviews, 35(9), 1971–81. [DOI] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Cunningham M. (1988). What do you do when you’re happy or blue? Mood, expectancies, and behavioral interest. Motivation and Emotion, 12(4), 309–31. [Google Scholar]
- Deisseroth K. (2014). Circuit dynamics of adaptive and maladaptive behaviour. Nature, 505(7483), 309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Felder J.N., Petty C., Bizzell J., Ernst M., Smoski M.J. (2009). The effects of psychotherapy on neural responses to rewards in major depression. Biological Psychiatry, 66(9), 886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot A.J., Thrash T.M. (2002). Approach-avoidance motivation in personality: approach and avoidance temperaments and goals. Journal of Personality and Social Psychology, 82(5), 804–18. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Hariri A.R., Martin S.L., et al. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166(1), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B.L. (2001). The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. American Psychologist, 56(3), 218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable P.A., Harmon-Jones E. (2008). Approach-motivated positive affect reduces breadth of attention. Psychological Science, 19(5), 476–82. [DOI] [PubMed] [Google Scholar]
- Gerardin E., Lehericy S., Pochon J.B., et al. (2003). Foot, hand, face and eye representation in the human striatum. Cerebral Cortex, 13(2), 162–9. [DOI] [PubMed] [Google Scholar]
- Gottfried J.A., O’Doherty J., Dolan R.J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301(5636), 1104–7. [DOI] [PubMed] [Google Scholar]
- Graybiel A.M. (2008). Habits, rituals, and the evaluative brain. Annual Review of Neuroscience, 31, 359–87. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M., Kawato M. (2006). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology, 95(2), 948–59. [DOI] [PubMed] [Google Scholar]
- Jia Z., Worhunsky P.D., Carroll K.M., et al. (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry, 70(6), 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Shimojo S., O’Doherty J.P. (2011). Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex, 21(4), 769–76. [DOI] [PubMed] [Google Scholar]
- Knutson B., Bhanji J.P., Cooney R.E., Atlas L.Y., Gotlib I.H. (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport, 12(17), 3683–7. [DOI] [PubMed] [Google Scholar]
- Knutson B., Taylor J., Kaufman M., Peterson R., Glover G. (2005). Distributed neural representation of expected value. Journal of Neuroscience, 25(19), 4806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage, 12(1), 20–7. [DOI] [PubMed] [Google Scholar]
- Kohn N., Falkenberg I., Kellermann T., Eickhoff S.B., Gur R.C., Habel U. (2014). Neural correlates of effective and ineffective mood induction. Social Cognitive and Affective Neuroscience, 9(6), 864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D.H., Watkins E.R., Hayward P., et al. (2003). A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome of the first year. Archives of General Psychiatry, 60(2), 145–52. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S. (2001). Why are some people happier than others? The role of cognitive and motivational processes in well-being. American Psychologist, 56(3), 239–49. [PubMed] [Google Scholar]
- Lyubomirsky S., King L., Diener E. (2005). The benefits of frequent positive affect: does happiness lead to success? Psychological Bulletin, 131(6), 803–55. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Berns G.S., Montague P.R. (2003). Temporal prediction errors in a passive learning task activate human striatum. Neuron, 38(2), 339–46. [DOI] [PubMed] [Google Scholar]
- Menon V., Levitin D.J. (2005). The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage, 28(1), 175–84. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5-6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. (2000). Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain and Cognition, 42(2), 183–200. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler M.T., Fu C.H., Dalton J.A., Andrew C.M., Williams S.C. (2007). A functional MRI study of happy and sad affective states induced by classical music. Human Brain Mapping, 28(11), 1150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Greicius M.D., Abdel-Azim E., Menon V., Reiss A.L. (2003). Humor modulates the mesolimbic reward centers. Neuron, 40(5), 1041–8. [DOI] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience, 9(11), 856–69. [DOI] [PubMed] [Google Scholar]
- Noonan M.P., Walton M.E., Behrens T.E., Sallet J., Buckley M.J., Rushworth M.F. (2010). Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proceedings of the National Academy of Sciences, 107(47), 20547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Abramson L.Y., Harmon-Jones E., Alloy L.B., Coan J.A. (2009). Psychosocial interventions for bipolar disorder: perspective from the behavioral approach system (BAS) dysregulation theory. Clinical Psychology, 16(4), 449–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Almeida J.R., Forbes E.E., et al. (2012). Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders, 14(3), 249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Young C.B., Damme K.S.F. (2014). Elevated reward-related neural activation as a unique biological marker of bipolar disorder: assessment and treatment implications. Behaviour Research and Therapy, 62, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J., Dayan P., Schultz J., Deichmann R., Friston K., Dolan R.J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304(5669), 452–4. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P. (2004). Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology, 14(6), 769–76. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P., Dayan P., Friston K., Critchley H., Dolan R.J. (2003). Temporal difference models and reward-related learning in the human brain. Neuron, 38(2), 329–37. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Ongur D., Ferry A.T., Price J.L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology, 460(3), 425–49. [DOI] [PubMed] [Google Scholar]
- Ongur D., Price J.L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10(3), 206–19. [DOI] [PubMed] [Google Scholar]
- Pessiglione M., Schmidt L., Draganski B., et al. (2007). How the brain translates money into force: a neuroimaging study of subliminal motivation. Science, 316(5826), 904–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., et al. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry, 166(6), 702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman I.J. (2008). Motivations and emotivations: approach, avoidance, and other tendencies in motivated and emotional behavior. In: Elliot A.J., editor. Handbook of Approach and Avoidance Motivation, New York: Psychology Press. [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J.D., Correa M., Farrar A., Mingote S.M. (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology, 191(3), 461–82. [DOI] [PubMed] [Google Scholar]
- Salimpoor V.N., Benovoy M., Larcher K., Dagher A., Zatorre R.J. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nature Neuroscience, 14(2), 257–62. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Gibbs S.E., Khanna K., Nielsen L., Carstensen L.L., Knutson B. (2007a). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience, 10(6), 787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Gibbs S.E.B., Khanna K., Nielsen L., Carstensen L.L., Knutson B. (2007b). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience, 10(6), 787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J.J., Biller A., Walther S., et al. (2010). Neural correlates of reward processing in schizophrenia — relationship to apathy and depression. Schizophrenia Research, 118(1–3), 154–61. [DOI] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13(8), 334–40. [DOI] [PubMed] [Google Scholar]
- Speer M.E., Bhanji J.P., Delgado M.R. (2014). Savoring the past: positive memories evoke value representations in the striatum. Neuron, 84(4), 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir M., Robinson M.D. (2007). The happy spotlight: positive mood and selective attention to rewarding information. Personality and Social Psychology Bulletin, 33(8), 1124–36. [DOI] [PubMed] [Google Scholar]
- Treadway M.T., Buckholtz J.W., Cowan R.L., et al. (2012). Dopaminergic mechanisms of individual differences in human effort-based decision-making. Journal of Neuroscience, 32(18), 6170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Valentin V.V., Dickinson A., O’Doherty J.P. (2007). Determining the neural substrates of goal-directed learning in the human brain. Journal of Neuroscience, 27(15), 4019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten E., Jr. (1968). A laboratory task for induction of mood states. Behaviour Research and Therapy, 6(4), 473–82. [DOI] [PubMed] [Google Scholar]
- Wallis J.D. (2007). Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience, 30, 31–56. [DOI] [PubMed] [Google Scholar]
- Wallis J.D. (2012). Cross-species studies of orbitofrontal cortex and value-based decision-making. Nature Neuroscience, 15(1), 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise S.P., di Pellegrino G., Boussaoud D. (1996). The premotor cortex and nonstandard sensorimotor mapping. Canadian Journal of Physiology and Pharmacology, 74(4), 469–82. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2004). The global burden of disease: 2004 update, World Health Organization, Geneva. http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/. [Google Scholar]
- Yin H.H., Knowlton B.J. (2006). The role of the basal ganglia in habit formation. Nature Reviews Neuroscience, 7(6), 464–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.