Abstract
A unique feature of adolescent social re-orientation is heightened sensitivity to peer influence when taking risks. However, positive peer influence effects are not yet well understood. The present fMRI study tested a novel hypothesis, by examining neural correlates of prosocial peer influence on donation decisions in adolescence. Participants (age 12–16 years; N = 61) made decisions in anonymous groups about the allocation of tokens between themselves and the group in a public goods game. Two spectator groups of same-age peers—in fact youth actors—were allegedly online during some of the decisions. The task had a within-subjects design with three conditions: (1) Evaluation: spectators evaluated decisions with likes for large donations to the group, (2) Spectator: spectators were present but no evaluative feedback was displayed and (3) Alone: no spectators nor feedback. Results showed that prosocial behavior increased in the presence of peers, and even more when participants received evaluative feedback from peers. Peer presence resulted in enhanced activity in several social brain regions including medial prefrontal cortex, temporal parietal junction (TPJ), precuneus and superior temporal sulcus. TPJ activity correlated with donations, which suggests similar networks for prosocial behavior and sensitivity to peers. These findings highlight the importance of peers in fostering prosocial development throughout adolescence.
Keywords: peer influence, prosocial behavior, adolescence, social brain network
Introduction
Adolescence is a transition period between childhood and adulthood with major changes in cognitive and social-affective reasoning (Steinberg and Morris, 2001). Social evaluation is highly salient during this time and adolescents become more sensitive to peer influence (Gardner and Steinberg, 2005; Albert et al., 2013; Blakemore and Mills, 2014). Neuroimaging studies have shown that changes in social cognition during adolescence are paralleled by changes in the social brain network (Blakemore, 2008; Van Overwalle, 2009; Crone and Dahl, 2012; Blakemore and Mills, 2014). In this network that supports thinking about self and others, brain regions such as medial prefrontal cortex (mPFC), temporal parietal junction (TPJ) and superior temporal sulcus (STS) show developmental changes in structure and function throughout adolescence (Blakemore, 2008; Mills et al., 2014).
Peer influence has been most extensively studied in the context of adolescent risk-taking and reward-related processing in the brain. Several studies have shown that adolescents engage in more risk-taking behavior when they are being observed or accompanied by peers (Gardner and Steinberg, 2005; Chein et al., 2011; Albert et al., 2013; Smith et al., 2015). Brain regions associated with the affective processing of risks and rewards, such as the ventral striatum, are more activated when peers observe risky choices and this effect is larger in adolescents than in adults (Chein et al., 2011). Very few studies, however, have assessed whether peers can also influence behavior in a prosocial manner. Recent behavioral findings indicate that peers influence donations in a public goods game (PGG) such that adolescents show higher levels of prosocial behavior when peers provide positive feedback to prosocial behavior (Van Hoorn et al., 2014). This study seeks to expand this research and sets out to test how peer influence is associated with the activation of brain regions involved in (pro)social behavior during adolescence.
Previous research in adults has suggested an important role of the mPFC and the ventral striatum in the context of peer evaluation. When adults were asked to report their behavioral tendencies with regards to social norms in the presence of observers, increased activation in mPFC and ventral striatum was found compared with the absence of observers (Izuma et al., 2010a). Moreover, one prior study examining prosocial donation rates in adults has shown that the presence of observers during donations was associated with increased activity in ventral striatum (Izuma et al., 2010b). There is also consistent evidence across imaging studies that mPFC is active when individuals are ‘mentalizing,’ or thinking about thoughts or attributes of the self and others (Frith and Frith, 2006). Finally, mPFC is implicated in social influence and relates to persuasion-induced behavior change after social influence (Falk et al., 2010, 2014; Welborn et al., 2015).
From a developmental perspective, several studies have additionally reported that mPFC is more active in adolescence than in adulthood when performing mentalizing tasks (Blakemore, 2008; Burnett et al., 2008; Gunther Moor et al., 2012). These effects are the largest during early adolescence (Gunther Moor et al., 2012; Van den Bos et al., 2011). Possibly, (early) adolescence is a time window when peers have a heightened influence on mPFC activity (Pfeifer et al., 2009; Somerville et al., 2013; Braams et al., 2014).
Present study
The goal of this study was to investigate the effects of peers on prosocial behavior and neural activity in adolescence. We examined developmental patterns in adolescents of two age groups: 12–13-year-olds and 15–16-year-olds. Peer influence in fMRI studies has previously been examined in a relatively wide age-range of adolescents, ranging from 14 to 19-year-olds (Chein et al., 2011; Smith et al., 2015; Welborn et al., 2015). However, behavioral work shows the strongest peer influence effects in 13–16-year-olds (Gardner and Steinberg, 2005) and increased self-reported peer resistance between ages 14 and 18 years (Steinberg and Monahan, 2007). Moreover, previous fMRI studies that investigated mentalizing and reciprocity in social interactions showed a heightened mPFC response in early adolescents (12–14 years) and a decrease to adult levels in 15–17 year-olds (Van den Bos et al., 2011; Gunter Moor et al., 2012). Therefore, we tested if peer influence effects were different in early (12–13 years) vs mid adolescence (15–16 years).
This study used a novel paradigm to test effects of peer influence on prosocial behavior, in which we aimed to disentangle peer effects on neural activity during the decision-making and feedback phase. For that purpose we adapted the PGG, a well-established method to investigate prosocial behavior, specifically cooperation that benefits one’s group rather than own outcome (Ledyard, 1995; Batson and Powell, 2003; Penner et al., 2005). Behavior in the PGG is not necessarily altruistic, but represents an important dimension of prosocial behavior, which is giving to the group for the benefit of others.
In this social dilemma, participants make decisions in anonymous groups about the allocation of tokens between themselves and the group. Prosocial behavior is measured as the number of tokens donated to the group. Peer influence is examined by asking participants to perform the task with spectators who provided prosocial feedback by giving thumbs up to larger donations (Evaluation condition), with spectators present but no feedback (Spectator condition) and without spectators (Alone condition). The spectator groups were composed of age-matched youth actors who were present at the start of each session. Participants met these peers beforehand, thereby increasing the ecological validity of the design because a ‘real’ social context was created.
We predicted that being observed by spectators would lead to increased activity in mPFC when making PGG donations (Izuma et al., 2010a). In addition, we expected that the impact of peer influence on mPFC activity would be larger for 12–13-year-olds than for 15–16-year-olds, as prior findings have shown that mPFC activity is most malleable in this age range (Van den Bos et al., 2011; Gunther Moor et al., 2012).
Methods
Participants
This study included participants of two age groups: 12–13 year-old adolescents (Mage = 12.93; N = 31; 15 males) and 15–16-year-old adolescents (Mage = 16.08; N = 30; 14 males). An additional four participants from the original sample (total N = 65) were excluded either due to excessive movement (N = 3; >3 mm in any direction) or technical problems (N = 1). Further background information about the final sample can be found in Supplementary Table 1. Participants were recruited via local secondary schools and through our participant recruitment database. The majority of the participants was born in the Netherlands (93%) and a minority was born elsewhere [England (3%) and USA (1%)], or missing (2%). All participants were fluent in both spoken and written Dutch. We screened participants in a private telephone conversation to ensure that they were free of neurological disorders, psychiatric disorders or any MRI contra indications. When at the lab, all participants and their parents signed an informed consent form prior to the start of the study. The institutional review board of Leiden University Medical Centre approved all procedures.
To obtain an estimate of IQ, we used the subscales Similarities and Block Patterns from the Wechsler Intelligence Scale for Children (WISC-III) for participants under 16 years and the same subscales from the Wechsler Intelligence Scale for Adults (WAIS-III) for participants 16 years and older (Wechsler, 1991, 1997). The estimated IQ scores fell within the normal range for all participants (M = 109.67; SD = 10.72) and did not differ between age groups (t(59) = 1.58, P > 0.05).
Experimental design
Peers Public Goods Game
Participants played an adapted version of the PGG (Ledyard, 1995; Harbaugh and Krause, 2000; Van Hoorn et al., 2014). Participants were explained that they played the PGG online within a group of four anonymous players. These other players allegedly were anonymous same-age peers who were also participating in the study and participants would not meet these players before or after the study. Participants received the instruction that the experiment was about decision-making in groups, and their group would get the opportunity to obtain a monetary bonus. Each round, participants received five tokens with an exchange value of either €1, €1.50 or €2 per token. These different exchange values were included to keep the participants engaged in the task, but were not of main interest in this study.
Participants had to make a decision about the allocation of the tokens between themselves and their fellow group members. If they contributed to the group by giving any portion of the tokens to the public goods pot, then the donated tokens were multiplied by two and divided equally among the group. Therefore, the optimal strategy for the individual group members in this game is to donate nothing to the public goods pot, whereas on the collective level the group would earn most if all members would donate all of their tokens (Harbaugh and Krause, 2000). That is, with individual contributions being multiplied by two and then divided equally over four players, the individual’s net return of contributing one token is negative (one loses 0.50 token). Hedonistic or egoistic motivations thus cannot explain contributions; this is why contributions to the public good are viewed as prosocial behavior (Penner et al., 2005). Participants could not see the decisions of the other players in the group, nor the payoff after each round. This was done to ensure that participants made each choice independently. In addition to the standard endowment, they were informed that one round of the PGG task would be selected by the computer for actual payout.
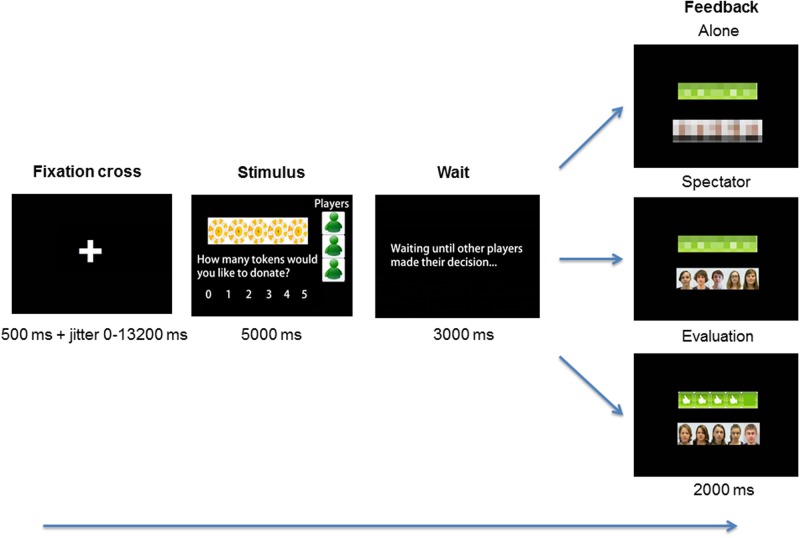
The task consisted of two runs with 36 trials (i.e. 72 trials in total), presented in three feedback conditions. Each run consisted of three blocks with 12 trials in an ‘Alone–Spectator–Evaluation’ fixed order of conditions. We used a fixed order of conditions so that we could avoid social norm induction through feedback before baseline (i.e. playing alone). Subsequent analyses demonstrated that analyses for the two runs separately resulted in very comparable effects to the collapsed analyses presented in the ‘Results’ section. For completeness, these additional analyses are presented in the Supplementary material. At the start of each 12-trial block, a condition start screen was presented for 2 s displaying the text ‘No spectators,’ ‘Spectators’ or ‘Spectators with evaluation.’ Within this set of 12 trials, the sequence of the events was as follows: Each trial started with the stimulus screen which was presented for 5 s, followed by a 3-s waiting screen. Next, the feedback screen was presented for 2 s (see Figure 1 for screens and presentation time).
Figure 1.
Illustration of the peers Public Goods Game. In each round, participants made decisions in a group about the allocation of five tokens between self and the group. The group consisted of three anonymous age-matched peers, displayed in green. These group members were unable to see the decisions of the participant. Participants played three types of rounds: (1) Alone, without spectators or feedback, (2) Spectator, with spectators present who would evaluate their decisions, but feedback was blurred, and (3) Evaluation, with spectators present who would provide prosocial feedback with ‘likes’. In this example, four out of five spectators liked the decision of the participant.
In the Evaluation condition, the feedback screen displayed images of five spectators and feedback through ‘likes’ (thumbs up), reinforcing prosocial behavior. Peer feedback was dependent on the decision that the participant made. When participants donated zero or one token to the group, they received zero thumbs up in the feedback condition. Donating two tokens resulted in two thumbs up, and donating three tokens resulted in four thumbs up. Finally, for a donation of four or five tokens, participants received five thumbs up (cf. Van Hoorn et al., 2014).
In the Spectator condition, the feedback screen displayed pictures of five different peers, and participants were informed that these peers would also evaluate their decisions but these evaluations would not be displayed. Feedback was displayed as a blurred signal and was therefore not informative. In the Alone condition, participants played with their anonymous group members but without spectators, thus both spectators and feedback were blurred. The reason to include the blurred images was to keep the amount of visual stimulation as similar as possible between the conditions. Trials were separated with a 500 ms to 13.2 s jitter during which a fixation cross was presented. If participants did not respond within the time frame of 5 s, the text ‘Too late!’ was displayed, after which a new trial started. These trials on which the participant did not respond in time were modeled separately and were not included in the analyses.
Adolescent actors as peers
Adolescent actors (N = 44) were recruited through local theater schools and received an endowment of €5 per session. They signed up for at least one or more sessions, so there were different spectator groups for each participant depending on which actors could attend the session. We aimed for each participant to get introduced to six out of 10 spectators (three males and three females) before the start of the experiment. Introduction to six rather than all 10 spectators was for pragmatic reasons, to balance between ecological validity of the design and having enough actors present for each participant. Effort was made to have a sufficient number of actors in each session. In 75% of the sessions there were six actors present, in 20% of the sessions five actors and in 5% of the sessions less than five actors were present.
Pictures of all actors were taken beforehand and we asked the actors to show a neutral facial expression. These pictures were rated by 30 independent adolescent raters (ages 14–16) on neutrality and estimated age. The actors were rated as relatively neutral (M = 4.42, SD = 0.72) on a scale ranging from 1 (not at all neutral) to 7 (very much neutral). The estimated age of the actors fell within the age-range of our participants, (M = 15.17, SD = 0.89), indicating that the pictures of the actors were valid for use within our paradigm. Finally, participants rated the pictures on the dimension of likeability after the scanning session on a scale of 1 (do not like at all) to 10 (like very much). Participants rated the online peers with an overall likeability of M(SD) = 6.37(1.34).
Procedure
Participants arrived with their best friend at the scanning session, as part of a larger study on peer relationships. The best friend participated in a different part of the study, which will be reported elsewhere. They were explained that the experimenters were waiting for more participants, and a couple of minutes later the actors walked into the room accompanied by an experimenter. The participant got introduced to the actors and shook hands (similar to Sanfey et al., 2003). The group was told that the goal of the study was to examine what happens in the brain when you play games with others, and that they would all be playing online games with each other on the Internet, with the participant playing the game in the MRI scanner. It was explained that not all of the peers had arrived yet (i.e. the other four from the spectator group that consisted of 10 peers in total), but that we would already take the participant to the mock-scanner while we waited for the remaining peers to arrive. This procedure was used to balance between feasibility and credibility.
After being introduced, the actors left the facility through a side door and the participant was taken to the mock-scanner to get familiar with the scanning procedure. The participant listened to pre-recorded scanner sounds, received instructions for the task and played five practice trials. It was explicitly addressed that participants would not play with their best friend during the PGG task. In addition, it was explained that the participants would play the game with three other players in another room that they had not met yet and that everyone in the group would be anonymous. The scan session lasted ∼1 h. After the scan session participants filled out questionnaires and the two subtests from the WISC-III/WAIS-III were administered.
Finally, participants received an endowment (€30) for their participation as part of a larger study and €2 additional earnings for the task. They were debriefed about the setup of the larger study and learned that all participants in fact received an amount of €2 for the PGG. None of the participants expressed doubts about the cover story during debriefing.
MRI data acquisition
Data were obtained with a 3-T Philips scanner at the Leiden University Medical Center, using a standard head coil. The subjects saw the visual stimuli projected on a screen through a mirror attached to the head coil. The task consisted of two runs that lasted 7 min each. We collected the functional data using a T2*-weighted echo-planar pulse sequence (38 contiguous 2.75 mm oblique axial slices, using sequential acquisition, FOV = 220 mm, 80 × 80 matrix, TR = 2.2 s, TE = 30 ms, 2.75 ×2.75 mm in-plane resolution). The first two volumes of each run (215 volumes each) were discarded to allow for T1-equilibration effects. To provide anatomical reference, a high-resolution 3D T1-FFE scan was acquired (TR = 9.76 ms; TE = 4.59 ms, flip angle = 8°, 140 slices, 0.875 × 0.875 × 1.2 mm3 voxels, FOV =224 × 168 × 177 mm3). In addition, a high-resolution 3D T1-weighted anatomical image was collected after the functional scans (TR = 9.751 ms, TE = 4.59 ms, flip angle = 8°, 140 slices, 0.875 × 0.875 × 1.2 mm3, and FOV = 224.000 × 168.000 × 177.333 mm3). To prevent head motion, participants were restricted with foam inserts that surrounded the head. The translational movement parameters did not surpass the threshold of one voxel (<3 mm) for all directions, participants and scans. Average head movement was 1.2 mm (SD = 0.92 mm) and did not differ between age groups, t(59) = 1.60, P = 0.114.
fMRI preprocessing and statistical analysis
Data preprocessing and analysis was performed with SPM8 (Wellcome Department of Cognitive Neurology, London). We corrected for rigid body motion and the structural and functional volumes were spatially normalized to T1 templates. The normalization algorithm used a 12-parameter affine transform together with a non-linear transformation involving cosine basis functions and resampled the volumes to 3-mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al., 1997). Functional volumes were spatially smoothed with a 6-mm FWHM isotropic Gaussian kernel.
We conducted statistical analyses on individual subjects’ data using the GLM in SPM8. The fMRI time series were modeled as a series of zero duration events convolved with the hemodynamic response function (HRF). Stimulus onset, i.e. the moment of decision-making, and feedback onset were modeled as separate events of interest. The six start screens showing whether participants were in the alone, spectator or evaluation condition (presented in both runs) were modeled separately. In pair-wise contrasts, the least-squares parameter estimates of height of the best-fitting canonical HRF for each condition were used. We submitted the resulting contrast images, computed on a subject-by-subject basis, to group analyses.
At the group level, we computed two ANOVAs to investigate responses on stimulus onset and feedback onset. We used a 3 (Condition: Alone, Spectator and Evaluation) × 3 (Token value: €1, €1.50 and €2) ANOVA for stimulus onset and feedback onset separately. Task-related responses were considered significant if they exceeded a voxel-wise threshold of P < 0.05 FWE-corrected. This threshold was chosen to minimize Type-I errors. To test for individual differences on a whole brain level, we lowered the threshold to uncorrected P < 0.001 to balance between Type-I and Type-2 errors, because individual differences may not survive stringent corrections across all voxels in the brain (Lieberman and Cunningham, 2009). Using this threshold of uncorrected P < 0.001 with a minimum cluster of contiguous voxels of 10, we examined age group differences in whole brain interaction analyses with the between-subjects factor Age group.
In addition, we performed whole brain regression analyses on stimulus onset with the mean number of tokens donated, to test across the whole brain whether stimulus-related activation correlated with the average number of tokens that were donated per individual. These analyses were also performed at an uncorrected threshold of P < 0.001 with a minimum cluster of 10 contiguous voxels to balance between Type-I and Type-2 errors.
Region of interest analysis
The whole brain results across all participants were further examined in region of interest (ROI) analyses to test for age group differences. The reason for using ROI analyses is because the age group differences are typically subtle and may not survive correction across all voxels of the brain. The MarsBar toolbox for SPM8 (Brett et al., 2002; http://marsbar.sourceforge.net/) was used to conduct ROI analyses for examination of activation patterns found in the clusters from the whole-brain analyses. Activation patterns that spanned across several regions of the brain were masked with the anatomical regions of the Marsbar Anatomical Toolbox. ROIs were averaged across the activated voxels in these regions and the coordinates of the center of mass are reported in the text.
Results
Behavioral results
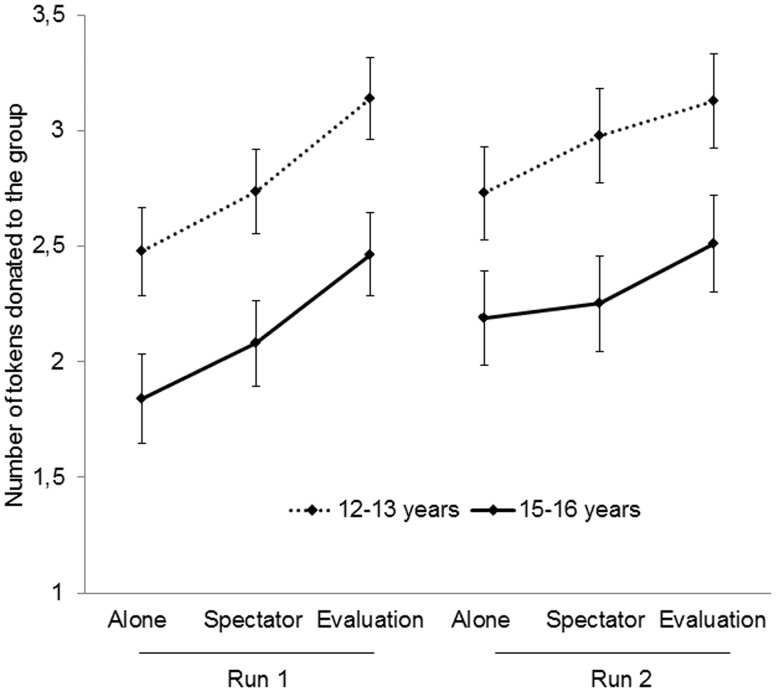
To test peer effects on prosocial behavior, we examined the number of tokens donated to the group in the three conditions. The mean number of tokens donated per condition was submitted to a repeated measures ANOVA with Condition (3), Run (2) and Token value (3) as within-subject factors and Age group (2) as between-subject factor. This analysis yielded significant main effects for all three within-subject factors, Condition (F(2, 118) = 50.08, P < 0.001, partial η2 = 0.459), Run (F(1, 59) = 7.03, P = 0.01, partial η2 = 0.107) and Token value F(2, 118) = 28.73, P < 0.001, partial η2 = 0.327).
The main effect of Condition showed that participants donated more tokens to the group when there were spectators present and even more when spectators were present who provided feedback (P’s < 0.001). This main effect was qualified by a Run × Condition interaction (F(2, 118) = 7.45, P = 0.001, partial η2 = 0.112). Post hoc comparisons showed that the same condition pattern was present in run 1 (F(2, 118) = 54.37, P < 0.001, partial η2 = 0.48) and run 2 (F(2, 118) = 16.29, P < 0.001, partial η2 = 0.216), but this pattern was more differentiated in run 1 than in run 2. Based on the observation that the pattern was similar, in the fMRI analyses we collapsed across runs to increase power. Separate fMRI analyses per run are presented in the Supplementary material.
The main effect of Token value showed the highest donations for the €1 tokens, lower donations for the €1.50 tokens (P = 0.001) and least for the €2 tokens (P < 0.001). The between-subject comparisons resulted in a main effect of Age group showing that the younger age group donated more tokens in all conditions, F(1, 59) = 6.21, P = 0.016, partial η2 = 0.095. There were no Age group × Condition interactions, no Token value × Age group or Token value × Condition interactions, nor a three-way interaction between Token value × Age group × Condition (see Figure 2).
Figure 2.
Mean (SE) number of tokens donated to the group in the PGG displayed for the two age groups separately. Error bars represent standard error of the mean. Dotted line indicates 12–13 year-olds and full line indicates 15–16 year-olds.
fMRI analyses at stimulus onset
Stimulus onset: whole brain analysis
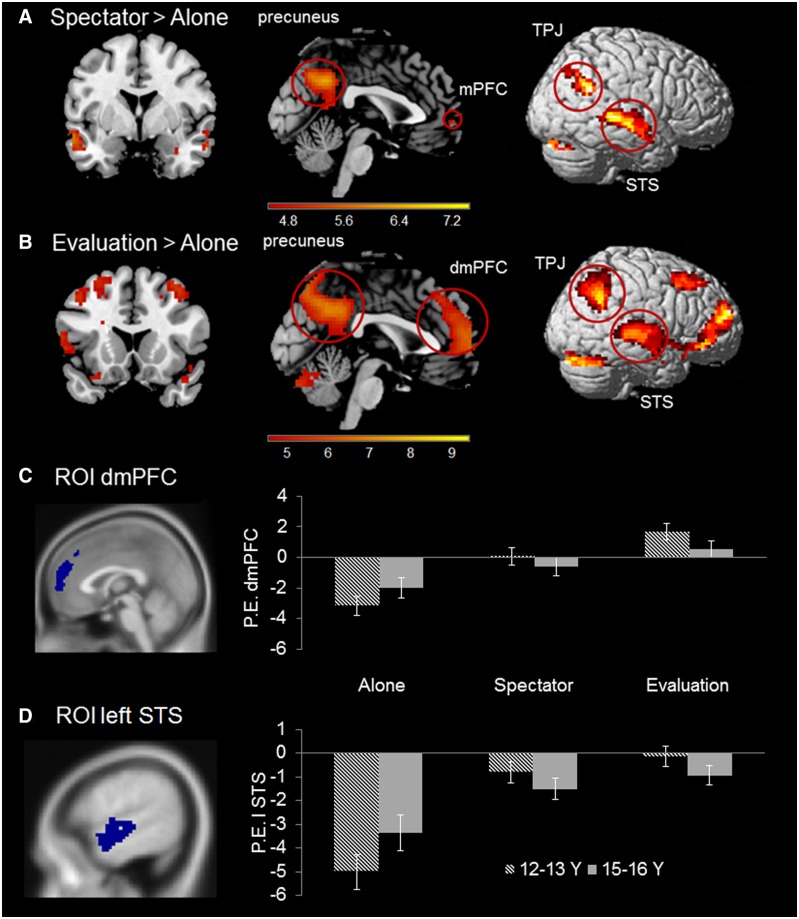
The first set of fMRI analyses examined neural responses at stimulus onset, when the participants made their decision about the allocation of tokens between themselves and the group. A repeated measures ANOVA with factors Condition (three levels: Alone, Spectator and Evaluation) and Token value (three levels: €1, €1.50 and €2) yielded a significant main effect of Condition in a widespread network of brain areas, including dorsomedial PFC, precuneus, superior temporal sulcus (STS) and temporoparietal junction (see Figure 3). There was no effect for Token value, nor a Condition × Token value interaction suggesting that the activation patterns were similar for all the three types of tokens.
Figure 3.
(A) Areas identified in the contrast Spectator > Alone at stimulus onset. (B) Areas identified in the contrast Evaluation > Alone at stimulus onset. Evaluation > Spectator yielded no significant findings at stimulus onset. All contrasts are FWE-corrected, P < 0.05. (C and D) Graphs illustrate two follow-up ROI analyses performed on dmPFC (MNI: 0 52 19) and left STS (MNI: −60 −12 3) extracted from the main effect of Condition. Both regions show a Condition × Age group interaction, see text for explanation. Striped bars indicate means for 12–13 year olds and grey bars indicate means for 15–16 year olds. Error bars represent standard error of the mean.
Next, we tested each of the contrasts separately using repeated measures ANOVAs. Spectator > Alone resulted in activation in precuneus, bilateral TPJ and bilateral STS; these regions were more active when participants made a decision with spectators present than when playing alone (Figure 3A). Evaluation > Alone resulted in overlapping patterns of activation and additional activation in the dmPFC (Figure 3B; Supplementary Table S2 for the complete list of activated regions). The contrasts Evaluation > Spectator and Spectator >Evaluation yielded no significant results.
We performed separate whole brain interaction analyses including the between-subjects factor Age group, to test for differences in neural activity between the two adolescent groups. None of these analyses resulted in significant interactions with Age group (also not when the threshold was lowered to P < 0.001 uncorrected for multiple comparisons, at least 10 contiguous voxels). However, the differences between groups may not survive corrections across all the voxels in the brain. Given that we had a priori hypotheses regarding age group differences in social brain regions, we followed up the analyses reported above with ROI analyses, in which we tested for each region specifically for interaction between Condition and Age group.
Stimulus onset: post hoc ROI analyses for age group differences
To investigate interactions between Age group and Condition at stimulus onset, we extracted ROIs based on the F-test showing a main effect of Condition, collapsed across all participants. This analysis was not biased for age differences or direction of contrast differences. The selected ROIs were dorsomedial PFC (masked with the anatomical superior medial frontal cortex, collapsed across left and right), left and right TPJ and left and right STS. For each ROI, an Age group (2) × Condition (3) ANOVA was conducted. We collapsed across Token value because there were no effects of Token value in the whole brain analyses.
First, in the dmPFC the Alone, Spectator and Evaluation conditions all differed significantly from each other (Alone >Spectator, P < 0.001; Spectator > Evaluation, P = 0.02). Moreover, the ANOVA yielded an Age group × Condition interaction, F(2,118) = 3.31, P = 0.04. Post hoc comparisons revealed that the difference between the Alone and Evaluation condition was larger for early adolescents (age 12–13) than for late adolescents (age 15–16) (Figure 3C). Second, in left STS, the Alone condition was significantly different from the Spectator condition (P < 0.001), but Spectator was not significantly different from the Evaluation condition (P = 0.121). There was also a significant Age group × Condition interaction, F(2, 118) = 5.69, P = 0.004. The difference between the Alone and Evaluation condition was again larger for the younger than the older age group, but this difference was only significant in the interaction term and not within conditions (Figure 3D).
For right STS and right TPJ, the Alone condition was significantly different from the Spectator condition (P’s < 0.001), but Spectator was not significantly different from the Evaluation condition (PrSTS = 0.124 and PrTPJ = 0.273). Finally, for left TPJ the Alone, Spectator and Evaluation conditions were all significantly different from each other (Alone > Spectator, P < 0.001; Spectator > Evaluation, P = 0.031). The right STS, left TPJ and right TPJ ROIs did not result in Age group × Condition interactions.
Stimulus onset: relations with task behavior
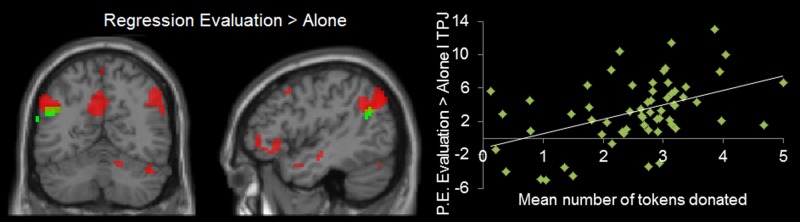
For each contrast, we then tested the relation between brain activation and average task donations per individual with whole-brain regressions analyses. In the Evaluation > Alone contrast, a positive relation with donating behavior on the task was found in left TPJ and left STS. Interestingly, there was an overlap between the brain regions activated in the regression contrast Evaluation > Alone (displayed in green) and the main contrast Evaluation > Alone (displayed in red) (see Figure 4). This might indicate that adolescents who act more prosocial are also more sensitive to being evaluated.
Figure 4.
An overlap between the brain regions (including left TPJ: −48 −60 24) that became active in the regression with mean tokens donated for Evaluation > Alone displayed in green (uncorrected,>10 voxels, P < 0.001) and contrast Evaluation > Alone displayed in red from the main ANOVA (FWE-corrected, P < 0.05).
For Evaluation > Spectator, we also found a positive relation between mean number of tokens donated and right TPJ and right STS (see Supplementary Table S3 for a full list of activated regions). The network of brain activation from this regression did not show an overlap with the main contrast Evaluation >Spectator. Spectator > Alone regression analyses yielded no results in relevant brain areas.
fMRI analyses at feedback onset
Feedback onset: whole brain analyses
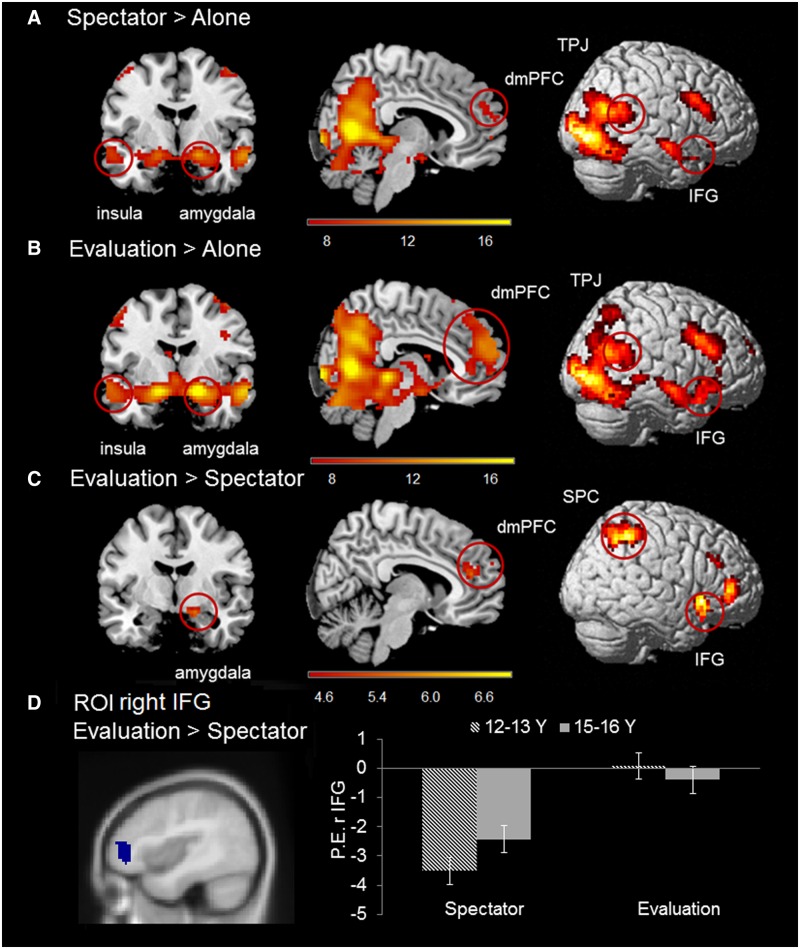
With the second set of fMRI analyses we examined neural responses at feedback onset, i.e. when the feedback screen was presented. Whereas participants received feedback from the spectator group about their decision in the Evaluation condition, they received blurred feedback in the Spectator condition and no feedback nor spectators (blurred images) in the Alone condition. A repeated measures ANOVA with factors Condition (three levels: Alone, Spectator and Evaluation) and Token Value (three levels: €1, €1.50 and €2) yielded a significant main effect of Condition in a widespread network of brain areas, including bilateral insula, right amygdala, right IFG, right TPJ and dmPFC. There was no effect for Token Value, nor a Condition × Token value interaction suggesting that the activation patterns were similar for all the three types of tokens.
The contrasts Evaluation > Alone and Spectator > Alone showed, as expected, activation in a wider brain network involving fusiform face area, amygdala and insula, which are core brain regions for face processing (Figure 5A and B). The contrast Evaluation > Spectator revealed increased activity in dmPFC, right amygdala, and bilateral insula, as well as right IFG and right superior parietal cortex (SPC) (Figure 5C; see Supplementary Table S4 for the complete list of activated regions).
Figure 5.
(A) Areas identified in contrast Spectator > Alone at feedback onset. (B) Areas identified in contrast Evaluation > Alone at feedback onset. (C) Areas identified in contrast Evaluation > Spectator at feedback onset. All contrasts are FWE-corrected, P < 0.05. (D) Graph illustrates follow-up ROI analysis performed on right IFG (MNI: 42 45 3) extracted from contrast Evaluation > Spectator. The STS region showed a Condition × Age Group interaction, see text for explanation. Striped bars indicate means for 12–13 year olds and grey bars indicate means for 15–16-year olds. Error bars represent standard error of the mean.
To investigate the age-effects in the Evaluation > Spectator condition, we performed a 2-sample t-test across the whole brain, but this contrast did not result in Age group × Condition interactions (also not when the threshold was lowered to P < 0.001 uncorrected for multiple comparisons, at least 10 contiguous voxels).
Feedback onset: post hoc ROI analyses for age group differences
Given that we were specifically interested in the effects of peer feedback (thumbs) vs peer presence (scrambled thumbs), we extracted ROIs from the Evaluation > Spectator contrast across all participants (Figure 5C). We explored whether there were age differences in activity patterns of each of these ROIs. For each ROI, an Age group (2) × Condition (2) ANOVA was performed. Only the right IFG showed an interaction effect between Age group and Condition (F(1, 59) = 4.16, P = 0.046), revealing that the younger age group showed a larger differentiation between the Spectator and Evaluation conditions than the older age group (Figure 5D).
Discussion
This study examined the neural correlates of peer influence on prosocial behavior with an adapted PGG in 12–16-year-old adolescents. Neural correlates were investigated during donation choices and peer feedback. During donation choices, peer presence resulted in higher donations and enhanced activity in several social brain regions including the dmPFC, TPJ, precuneus and STS. This social brain network is involved in mentalizing and social cognitive processes (Blakemore, 2008; Blakemore and Mills, 2014). Interestingly, individual differences analyses resulted in two important findings. First, TPJ activity correlated positively with the donation amounts, suggesting similar networks for prosocial behavior and sensitivity to peers. Second, during donation decisions peer presence effects were larger in dmPFC and STS for the younger adolescents (12–13 year-olds).
Adolescence is a time of major social re-orientation, with changes in social behavior paralleled by changes in the social brain network (Nelson et al., 2005; Blakemore, 2008; Blakemore and Mills, 2014). A unique feature of adolescent social behavior is heightened sensitivity to peer evaluation, as peer feedback becomes an increasingly important tool to navigate the complex social world (Albert et al., 2013; Somerville, 2013). The current findings revealed that peer presence increases prosocial behavior, even more when peers provide prosocial feedback (see also Van Hoorn et al., 2014). These results are consistent with previous work in adults, in which the mere presence of observers increased donations to charity (Izuma et al., 2010b).
In terms of neural activity, the social brain network (dmPFC, TPJ, STS) becomes active when making donating choices in the presence of peers. These findings resonate with past work on prosocial behavior in a family context and public goods contributions in adults (Telzer et al., 2011; Bault et al., 2015). Within the social brain network, the mPFC is thought to incorporate salient contextual cues such as social evaluation with emotional valuation processes (Blakemore, 2008; Frith and Frith, 2012; Somerville et al., 2013). It was previously found that even the most basic form of social evaluation—being looked at without performing any task—elicited increased mPFC activation in adolescents (Somerville et al., 2013). In addition, mPFC is implicated in social influence (Falk et al., 2010, 2014; Welborn et al., 2015). The current findings concur with these prior studies by showing that dmPFC is more active when making donating choices when peers are observing these choices (Izuma et al., 2010a; Somerville, 2013; Somerville et al., 2013). Interestingly, there were no neural differences between the Evaluation and Spectator conditions, which may imply that anticipation of active and passive peer influence rely on the same neural mechanisms of social cognition. Participants in both conditions were instructed that the spectators would judge their decisions, but the evaluation was only visible in the Evaluation condition. Speculatively, this may suggest that neural activity during stimulus onset represents the feeling of being judged, independent of receiving feedback.
Earlier work in the domain of peer influence also implicates the involvement of the ventral striatum, such that ventral striatum activity is enhanced during the peer presence condition (Izuma et al., 2010b; Chein et al., 2011; Smith et al., 2015). One commonly used interpretation is that peer presence makes behavior (i.e. risk-taking or donating) more rewarding. Contrary to this literature, this study did not reveal enhanced striatum activity. Our findings suggest that the presence of peers may not be specifically related to activation of the ventral striatum, but instead heightens activity in brain areas that are already involved in that particular behavior. In our task, this implies more activity in mentalizing areas such as dmPFC, TPJ and STS.
An important additional goal of this study was to examine how neural activity to peer presence overlaps with individual differences in donation choices. Here we found that TPJ activity in the Evaluation > Alone contrast was positively correlated with donation amounts. These findings indicate that adolescents who act more prosocial show higher activation in this brain region when being evaluated by others. These results fit with prior findings from Van den Bos et al. (2011), who showed that increased involvement of the TPJ was associated with more advanced forms of social perspective-taking behavior. Moreover, TPJ activity has been related to self-reported altruism and charitable giving (Tankersly et al., 2007; Hare et al., 2010). A tentative hypothesis is that heightened perspective-taking when peers are present during decision-making may result in more prosocial behavior. This question should be addressed in more detail in future studies.
Second, we addressed the question if younger adolescents are more susceptible to peer influence in terms of behavior and neural activity. Behaviorally, younger adolescents (age 12–13 years) gave larger donations to the group in all task conditions—alone, with spectators present and evaluation. These findings fit with recent studies showing that younger adolescents are more prosocial towards unknown others (Güroğlu et al., 2014; Burnett Heyes et al., 2015; Meuwese et al., 2015). Nevertheless, other studies have suggested that in general prosocial behavior does not necessarily increase across adolescence, but that there is an increase in sensitivity to the perspective of others in prosocial decision-making (Van den Bos et al., 2011). The exact developmental trajectory of prosocial behavior is possibly sensitive to task demands and social context.
The social influence effects are consistent with results from prior research. In an earlier study, participants were asked to rate the riskiness of scenarios, subsequently shown either peers’ or adult’ opinions about these scenarios, and then asked to rate the scenarios again (Knoll et al., 2015). The 12–14-year-olds showed more sensitivity to social influence from peers than adults, whereas older adolescents (age 15–18 years) showed similar sensitivity for peers and adults. Another behavioral study illustrated that younger adolescents (12–13-year-olds) were more sensitive to social exclusion during the experimental paradigm Cyberball than older adolescents (14–16-years) (Sebastian et al., 2010). Thus, younger adolescents may be more susceptible to contextual cues.
Consistent with this hypothesis, on a neural level we found larger effects in younger adolescents in dmPFC and left STS when being evaluated by peers relative to being alone. Even though there were Age group × Condition interactions in the core brain regions of interest, there were only main effects of age with respect to donating behavior. Future studies should examine the brain behavior relations in more detail across adolescent development. Heightened dmPFC activity in the current task shows an interesting overlap with past work in young adolescents during social emotions and prosocial decision-making tasks (Burnett et al., 2008; Van den Bos et al., 2011; Gunther Moor et al., 2012). Specifically, one prior study focused on prosocial changes by examining the neural correlates of reciprocity in 12–14-year-olds, 15–17-year-olds and young adults. In the 12–14-year-olds, mPFC activity was elevated when participants showed reciprocal behavior, whereas the other age groups showed similar levels of mPFC activity also when defecting others (Van den Bos et al., 2011). Thus, together with the prior behavioral studies these results indicate that younger adolescents may be more sensitive to social context (Wolf et al., 2015).
Finally, we explored neural responses to peer evaluation at feedback onset. This analysis resulted in increased activity in lateral PFC and SPC, a network typically related to learning and cognitive control, as well as dorsal mPFC, IFG, insula and amygdala, related to mentalizing and emotional processing (Blakemore, 2008; Nelson and Guyer, 2011; Nelson et al., 2014; Peters et al., 2014). Within the process of peer feedback, learning and cognitive control areas may be involved to regulate own actions and adapt to the opinions or behaviors of others, which is typically associated with increased activity in lateral PFC and SPC (Peters et al., 2014). Although past work has related the IFG to a wide range of functions, one interpretation of IFG activity may be that it plays a role in (re)appraisal of social stimuli and updating expectancies that result from social feedback (Nelson and Guyer, 2011; Guyer et al., 2012). Similarly, insula activity has been related to prediction error fluctuations in the social learning context, specifically in adolescents (Jones et al., 2014). These exploratory analyses need to be replicated but provide interesting starting points for future research.
It should be noted that in the current paradigm it is difficult to entirely disentangle the neural correlates of feedback from donation choice, because there was no jitter between donation choice and feedback. We addressed this issue by having a relatively long stimulus display (5 s) and waiting period (3 s) for each trial. Nonetheless, this affects the interpretation of a direct comparison between these moments in the task.
In conclusion, we show that peer presence when making donations consistently evoked activity in the social brain network, and that monitoring of peer feedback is associated with activity in a network of regions associated with learning and control, mentalizing and emotional processing. Developmental differences during decision-making suggest that 12–13-year-olds are more sensitive to peer influence on prosocial behavior than 15–16-year-olds, and that dmPFC may play an important role in the social evaluation process. These findings shed light on how peers may positively impact outcomes in adolescence. Stimulating prosocial development in adolescents might set the stage for adaptive development extending into adulthood (Jones et al., 2014). These findings have implications for society, as they may indicate that introducing community services to early adolescents—when prosocial behavior is especially malleable—can possibly foster prosocial development over time.
Supplementary Material
Acknowledgements
The authors would like to thank all participants and their parents for participation in this study. We want to thank Jeugd Theaterhuis Leiden and Jeugd Theaterhuis Leiderdorp for the great collaboration and the youth actors who were confederates in this study. In addition, the authors would like to thank the entire Peers Project team for help with data collection, especially Aafke Snelting.
Funding
This research was funded by a NWO ResearchTalent Grant (406-11-019) awarded to E.A.C. and J.v.H. and a NWO Veni Grant (451-10-021) awarded to B.G.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Albert D., Chein J., Steinberg L. (2013). The teenage brain: peer influences on adolescent decision making. Current Directions in Psychological Science , 22(2), 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson C.D., Powell A.A. (2003). Altruism and prosocial behavior. In Millon T., Lerner M.J., editors. Handbook of Psychology: Personality and Social Psychology (pp. 463–84). New York: Wiley. [Google Scholar]
- Bault N., Pelloux B., Fahrenfort J.J., Ridderinkhof K.R., Van Winden F. (2015). Neural dynamics of social tie formation in economic decision-making. Social Cognitive and Affective Neuroscience , 10, 877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J. (2008). The social brain in adolescence. Nature Reviews Neuroscience , 9(4), 267–77. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. (2014). Is adolescence a sensitive period for sociocultural processing?. Annual Review of Psychology , 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Braams B.R., Peters S., Peper J.S., Güroğlu B., Crone E.A. (2014). Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. NeuroImage , 100, 281–9. [DOI] [PubMed] [Google Scholar]
- Brett M., Johnsrude I.S., Owen A.M. (2002). The problem of functional localization in the human brain. Nature Reviews Neuroscience , 3, 243–9. [DOI] [PubMed] [Google Scholar]
- Burnett S., Bird G., Moll J., Frith C., Blakemore S.-J. (2008). Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience , 21(9), 1736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett Heyes S., Jih Y-R., Block P., Hiu C-F., Holmes E.A., Lau J.Y.F. (2015). Relationship reciprocation modulates resource allocation in adolescent social networks: developmental effects. Child Development , 86(5), 1489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science , 14(2), F1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco R.A., Kollokian V., Kwan R.K.S., Evans A.C. (1997). Brain web: online interface to a 3D MRI simulated brain database. NeuroImage , 5, S452. [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience , 13, 636–50. [DOI] [PubMed] [Google Scholar]
- Falk E.B., Berkman E.T., Mann T., Harrison B., Lieberman M.D. (2010). Predicting persuasion-induced behavior change from the brain. Journal of Neuroscience , 30(25), 8421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Cascio C.N., O’Donnell M.B., et al. (2014). Neural responses to exclusion predict susceptibility to social influence. Journal of Adolescent Health , 54, S22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron , 50(4), 531–4. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2012). Mechanisms of social cognition. Annual Review of Psychology, 63, 287–313. [DOI] [PubMed] [Google Scholar]
- Gardner M., Steinberg L. (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology , 41(4), 625–35. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B., Op de Macks Z.A., Güroğlu B., Rombouts S.A.R.B., Van der Molen M.W., Crone E.A. (2012). Neurodevelopmental changes of reading the mind in the eyes. Social Cognitive and Affective Neuroscience , 7, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güroğlu B., van den Bos W., Crone E.A. (2014). Sharing and giving across adolescence: an experimental study examining the development of prosocial behavior. Frontiers in Psychology , 291(5), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Pine D.S., Nelson E.E. (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience , 7(1), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh W. T., Krause K. (2000). Children’s altruism in public good and dictator experiments. Economic Inquiry , 38(1), 95–109. [Google Scholar]
- Hare T.A., Camerer C.F., Koepfle D.T., O’Doherty J.P., Rangel A. (2010). Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. Journal of Neuroscience , 30(2), 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2010a). The roles of the medial prefrontal cortex and striatum in reputation processing. Social Neuroscience, 5(2), 133–47. [DOI] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2010b). Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience , 22(4), 621–31. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Somerville L.H., Li J., et al. (2014). Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive Affective and Behavioral Neuroscience, 14(2), 683–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L.J., Magis-Weinberg L., Speekenbrink M., Blakemore S.-J. (2015). Social influence on risk perception during adolescence. Psychological Science , 26, 583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledyard J.O. (1995). Public goods: a survey of experimental research. In: Roth A.E., Kagel J., editors. Handbook of Experimental Economics (pp. 111–94). Princeton: Princeton University Press. [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience , 4, 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwese R., Crone E.A., de Rooij M., Güroğlu B. (2015). Development of equity preferences in boys and girls across adolescence. Child Development , 86, 145–58. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.-J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Guyer A.E. (2011). The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience , 1, 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Lau Y.F., Jarcho J.M. (2014). Growing pains and pleasures: how emotional learning guides development. Trends in Cognitive Sciences , 18(2), 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine , 35, 163–74. [DOI] [PubMed] [Google Scholar]
- Penner L.A., Dovidio J.E., Piliavin J.A., Schroeder D.A. (2005). Prosocial behavior: multilevel perspectives. Annual Review of Psychology , 56, 356–92. [DOI] [PubMed] [Google Scholar]
- Peters S., Braams B.R., Raijmakers M.E., Koolschijn P.C.M.P., Crone E.A. (2014). The neural coding of feedback learning across child and adolescent development. Journal of Cognitive Neuroscience, 26(8), 1705–20. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. (2009). The neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development , 80(4), 1016–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey A.G., Rilling J.K., Aronson J.A., Nystrom L.E., Cohen J.D. (2003). The neural basis of economic decision-making in the ultimatum game. Science , 300(5626), 1755–8. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., Viding E., Williams K.D., Blakemore S.-J. (2010). Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition , 72(1), 134–45. [DOI] [PubMed] [Google Scholar]
- Smith A.R., Steinberg L., Strang N., Chein J. (2015). Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Developmental Cognitive Neuroscience, 11, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H. (2013). The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science , 22(2), 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science , 24(8), 1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Monahan K.C. (2007). Age differences in resistance to peer influence. Developmental Psychology , 43(6), 1531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Morris A.S. (2001). Adolescent development. Annual Review of Psychology , 52, 83–110. [DOI] [PubMed] [Google Scholar]
- Tankersley D., Stowe C.J., Huettel S.A. (2007). Altruism is associated with an increased neural response to agency. Nature Neuroscience , 10, 150–1. [DOI] [PubMed] [Google Scholar]
- Telzer E., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. (2011). Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage , 58(1), 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos W., Van Dijk E., Westenberg P.M., Rombouts S.A.R.B., Crone E.A. (2011). Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychological Science , 22(1), 60–70. [DOI] [PubMed] [Google Scholar]
- Van Hoorn J., Van Dijk E., Meuwese R., Rieffe C., Crone E.A. (2014). Peer influence on prosocial behavior in adolescence. Journal of Research on Adolescence, 26(1), 90–100. [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1991). The Wechsler Intelligence Scale for Children—third edition. Administration and Scoring Manual. San Antonio: The Psychological Corporation. [Google Scholar]
- Wechsler D. (1997). Wechsler Adult Intelligence Scale—third edition. Administration and Scoring Manual. San Antonio: The Psychological Corporation. [Google Scholar]
- Welborn B.L., Lieberman M.D., Goldenberg D., Fuligni A.J., Galvan A., Telzer E.H. (2015). Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience , 11, 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf L.K., Bazargani N., Kilford E.J., Dumontheil I., Blakemore S.-J. (2015). The audience effect in adolescence depends on who’s looking over your shoulder. Journal of Adolescence , 43, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.