Abstract
With the use of real-time functional magnetic resonance imaging neurofeedback (NF), amygdala activitiy can be visualized in real time. In this study, continuous amygdala NF was provided to patients with borderline personality disorder (BPD) with the instruction to down-regulate. During four sessions of NF training, patients viewed aversive pictures and received feedback from a thermometer display, which showed the amygdala blood oxygenation level-dependent signal. Conditions of regulation and viewing without regulation were presented. Each session started with a resting-state scan and was followed by a transfer run without NF. Amygdala regulation, task-related and resting-state functional brain connectivity were analyzed. Self-ratings of dissociation and difficulty in emotion regulation were collected. BPD patients down-regulated right amygdala activation but there were no improvements over time. Task-related amygdala-ventromedial prefrontal cortex connectivity was altered across the four sessions, with an increased connectivity when regulating vs viewing pictures. Resting-state amygdala-lateral prefrontal cortex connectivity was altered and dissociation, as well as scores for ‘lack of emotional awareness’, decreased with training. Results demonstrated that amygdala NF may improve healthy brain connectivity, as well as emotion regulation. A randomized-controlled trial is needed to investigate whether amygdala NF is instrumental for improving neural regulation and emotion regulation in BPD patients.
Keywords: borderline personality disorder, psychopathology, prefrontal cortex, dissociation, brain-computer interface, psychotherapy
Introduction
Borderline personality disorder (BPD) is a devastating psychiatric condition with severe deficits in patients’ emotional processing and emotion regulation skills (Sanislow et al., 2002; Schmahl et al., 2014). A key feature of BPD is a hyperactivation of the amygdala in response to emotional stimuli (Schulze et al., 2016). Those with BPD also show reduced lateral prefrontal cortex (PFC) activation in the processing of emotions (Schulze et al., 2011, 2016; Krause-Utz et al., 2012; Lang et al., 2012). This neural pattern likely reflects the emotion regulation deficits observed in BPD patients (Schmahl et al., 2014).
The amygdala is part of the limbic system and located in the medial temporal lobe. Projections from the amygdala to other neural regions play a key role in controlling one’s emotional, motivational and social behavior (Janak and Tye, 2015). Cognitive emotion regulation (i.e. the deliberate control of one’s emotional response) is associated with alterations of amygdala activation (Diekhof et al., 2011; Buhle et al., 2014). According to current models, the lateral and medial PFC is key in effective emotion regulation and the sustainment of one’s mental health (Kalisch, 2009; Ochsner et al., 2012). Neural connectivity between the amygdala and the medial PFC is considered to be a major neural pathway for emotion regulation (Hartley and Phelps, 2010; Diekhof et al., 2011; Viviani, 2014), and aberrant amygdala-prefrontal connectivity patterns have demonstrated to play a role in emotion dysregulation in BPD (New et al., 2007; Kamphausen et al., 2013).
With real-time functional magnetic resonance imaging (rtfMRI), changes in neural activation can be tracked in real time and reported to the patient via a visual feedback display (Weiskopf, 2012). rtfMRI neurofeedback (NF) can enhance the self-control of brain regions showing dysregulated response patterns and also may improve behavioral impairment (Linden et al., 2012; Ruiz et al., 2013; Scheinost et al., 2013; Sitaram et al., 2014; Zilverstand et al., 2015). Recently, research has shown that healthy individuals are able to down-regulate amygdala activity with rtfMRI NF (Brühl et al., 2014; Paret et al., 2014). In our previous study, continuous feedback was given regarding the amygdala response to pictures with affective content. This feedback was provided via a thermometer display with the instruction to down-regulate. Further, brain self-regulation with amygdala feedback was associated with changes in a neural network involving the amygdala, medial and lateral PFC (Paret et al., 2016). Notably, amygdala-ventromedial PFC (vmPFC) connectivity increased with amygdala feedback opposed to sham feedback. Evidence for improved amygdala regulation with rtfMRI NF has also been found in studies investigating amygdala up-regulation (Zotev et al., 2011, 2013; Yuan et al., 2014). While training amygdala up-regulation could promote positive affect in depressed patients (Young et al., 2014; Yuan et al., 2014), training down-regulation may help decrease amygdala hyperactivation and therefore, improve emotion regulation in BPD patients.
Aberrant resting-state brain connectivity between central executive networks, including the lateral PFCs, and networks associated with salience detection, including the amygdala, has been reported in BPD patients (Doll et al., 2013). Initial support has been found for changes in resting-state brain connectivity after rtfMRI NF with depressed patients (Yuan et al., 2014). Comparing resting-state amygdala connectivity before and after treatment is a promising approach to gain insight into changes of the functional architecture of the brain associated with NF training.
Amygdala NF with the instruction to down-regulate has not been assessed in a BPD patient sample and therefore, we investigated this topic with a multi-session rtfMRI NF training. To assess changes in functional network connectivity, a resting-state scan was obtained at the beginning of each of the four NF sessions. We hypothesized that over the four sessions, patients would be able to down-regulate amygdala activation. This was also expected during the transfer runs, during which participants were instructed to apply down-regulation without receiving any feedback. We further hypothesized to see a steady improvement in down-regulation with subsequent training. Based on the previous work (Paret et al., 2016), we hypothesized that BPD patients would increase amygdala-vmPFC functional connectivity in the ‘regulate’ compared to the ‘view’ condition, and we expected this increase to correlate with stimulus arousal. As amygdala-vmPFC connectivity might be dysregulated in patients, we were interested whether connectivity would change with subsequent training.
Furthermore, we expected increases in resting-state brain connectivity particularly in amygdala-prefrontal networks. Lastly, we expected patients’ symptomatology, as assessed by their reported dissociation and difficulties in emotion regulation, to improve. Correlation analyses were conducted to explore associations of changes in psychometric measures with changes in neural networks.
Methods
Sample characteristics
Ten female BPD patients were recruited from the BPD inpatient unit at the Department of Psychosomatic Medicine and Psychotherapy at the Central Institute of Mental Health in Mannheim, Germany. Two patients dropped out before the third session and, thus, were excluded from the final analyses, reducing the final sample size to eight. The patients had a mean age of 33.6 ± 9.5 years [±standard deviation (s.d.)]. The patients were diagnosed with BPD according to the International Personality Disorder Examination (Loranger, 1999) and a diagnosis of an Axis I disorder was based on the Structured Clinical Interview (SKID-I) (First et al., 1997). Patients with bipolar disorder, schizophrenia, severe neurological impairment, body weight >120 kg, BMI ≤ 16.5 or who had MR incompatibilities were excluded from participation. A variety of comorbid disorders typical for BPD were found in the sample (Supplementary Table S1). At the time of the first measurement, patients were abstinent from alcohol and drugs for more than 2 months. The patients participated in a 12-week inpatient Dialectical Behavior Therapy program (Bohus and Wolf-Arehult, 2012) throughout study participation but were recruited no earlier than the fourth week of Dialectical Behavior Therapy treatment. All participants were taking stable medication throughout the course of the study (see Supplementary Table S1 for a list of Medication).
This study was conducted in accordance with the declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty Mannheim of the University of Heidelberg. All participants provided written informed consent before participation and received financial compensation for participation.
Procedure
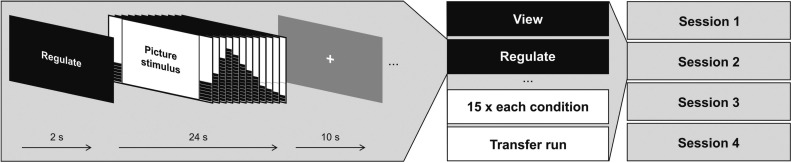
The training paradigm, the rtfMRI analysis steps and feedback preparation have previously been described in detail (see Paret et al., 2014); a graphical overview of the sessions can be obtained from Supplementary Figure S1. BPD patients participated in four rtfMRI NF training sessions with an interval of 2–7 days between subsequent sessions. To start each session, a resting-state scan, with instructions to keep one’s eyes open, was acquired (6 mins), followed by the NF training comprising three runs (9 mins per run). After the training, a run was applied without feedback to assess the transfer of learning (transfer run). Each run comprised three experimental conditions (i.e. ‘regulate’, ‘view’ and ‘neutral’), and each condition was presented five times per run in semi-randomized order (Figure 1). In the ‘regulate’ condition, participants were instructed to down-regulate a thermometer displayed at both sides of an aversive picture presented on a computer monitor. In the ‘view’ condition, an aversive picture was also displayed together with feedback, but participants were instructed to respond naturally to the picture content. In the ‘neutral’ condition, scrambled pictures were presented. Feedback was provided via a thermometer display; an orange line in the lower-half of the display screen indicated patients’ baseline amygdala activation during an 8-s rest period, which preceded the picture presentation.
Fig. 1.
Overview on the experimental procedure.
Feedback was also given to the participants in the control conditions to obtain comparable visual stimulation throughout conditions. The feedback signal was calculated from the temporally smoothed mean BOLD signal activation relative to baseline, obtained from a bilateral anatomical amygdala mask using TurboBrainVoyager 3.0 software (Brain Innovation B.V., Maastricht, the Netherlands). The voxel selection was optimized to 30% showing the best discrimination between the ‘view’ and the ‘neutral’ condition. One hundred pictures were obtained from published sets (Lang et al., 2008; Wessa et al., 2010) and complemented by 40 pictures from the internet, depicting scenes from war and accidents and also scenes of people and animals suffering. Each picture was only presented once to each participant. An eye camera was active during the whole session for visual control, to ensure that patients had their eyes open. Patients were debriefed after each session.
fMRI data
Image acquisition
For brain imaging, a 3 Tesla MRI Scanner (Trio, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil was used. Functional images of the BOLD contrast were acquired with a gradient echo T2* weighted echo-planar-imaging sequence (TE = 30 ms, TR = 2 s, FOV = 192 × 192 mm, flip angle = 80°, inplane resolution = 3 × 3 mm). One volume comprised 36 slices tilted −20° from AC-PC orientation with a thickness of 3 mm and slice gap of 1 mm. Participants’ heads were lightly restrained using soft pads. The experimental runs comprised 284 volumes each, while the resting-state scan comprised 180 volumes. T1-weighted anatomical images were acquired with a Magnetization Prepared Rapid Acquisition Gradient Echo sequence (TE = 3.03 ms, TR = 2.3 s, 192 slices and FOV = 256 × 256 mm).
Preprocessing
The fMRI analyses were conducted with SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). After discarding the 10 initial volumes, the standard preprocessing routine included slice time correction, realignment, unwarping, coregistration of the functional mean image to anatomy and normalization to the Montreal Neurological Institute standard template and smoothing (full width at half maximum = 8 mm).
Task-related effects
To control for motion-related artifacts, volumes associated with above-threshold movements were ‘censored’ (Siegel et al., 2014). This involved the detection of large movements (>2 mm) and changes in global intensity (z > 9) with the ART software package (www.nitrc.org/projects/artifact_detect). Realignment regressors and nuisance regressors controlling for outlier volumes were included in general linear modeling. Data were high-pass filtered (128 s) and a correction for serial correlations was implemented by autoregressive modeling.
Region-of-interest analysis of amygdala down-regulation
Task-related changes in the BOLD signal were estimated using conventional statistical parametric mapping (SPM) at the subject level with SPM8. Stimulus functions of the three conditions were convolved with the hemodynamic response function to estimate voxel-wise task-related BOLD signal changes. For group-level analysis of variance (ANOVA), beta estimates from the ‘regulate>view’ contrast were extracted from anatomical amygdala masks (Tzourio-Mazoyer et al., 2002) and transferred to SPSS version 23 (IBM Corp. Armonk, NY). Tests of Hemisphere (2) × Session (4) × Run (3) interactions were complemented by analysis of Session × Run interactions for each hemisphere separately. One-sample t-tests of contrast estimates were conducted to assess the main effect of down-regulation. Because this analysis did not differentiate between amygdala subregions, a complementary analysis was conducted to investigate whether there were foci within the amygdalae that were down-regulated by patients. Single-subject beta maps from the ‘view>regulate’ main effect contrast were passed to a voxel-wise one-sample t-test at the group level. Small-volume correction (SVC) using family-wise error (FWE) correction was applied to a bilateral anatomical amygdala mask.
Psychophysiological interaction analysis
Psychophysiological interaction (PPI) analysis estimates the extent to which changes in one brain region predict changes in other regions (i.e. functional connectivity) in a task-related fashion. The deconvolved BOLD signal time course (estimated with the eigenvariate) of the right amygdala, the task-related stimulus functions (convolved with the hemodynamic response function) and the interaction terms were used to estimate task-related functional connectivity of the amygdala with the rest of the brain (McLaren et al., 2012). To assess session-to-session effects, SPM8’s flexible factorial model was used, including a Subject factor and a Session factor (two levels). PPI beta estimates of the ‘regulate>view’ contrast from the last and the first NF session were contrasted [session 4 (‘regulate > view’) > session 1 (‘regulate>view’)], and a SVC analysis with FWE correction was used to identify significant voxels in the vmPFC [Brodmann area (BA) 10, spherical region of interest with radius = 20 mm, center at (0,56,−11)], which we had previously found to be altered by amygdala NF (Paret et al., 2016). An analysis assessing session-to-session effects was done with PPI betas from all four sessions but did not yield any significant results.
Functional resting-state connectivity
Functional connectivity analyses were carried out with the CONN toolbox for SPM (http://www.nitrc.org/projects/conn, Whitfield-Gabrieli and Nieto-Castanon, 2012). For each subject, the CompCor method (Behzadi et al., 2007) was used to identify principal components associated with segmented white matter and cerebrospinal fluid. Global intensity, white matter and cerebrospinal fluid signals, motion parameters and above-threshold movement ‘censor’ regressors were entered as nuisance variables in a first-level analysis. Finally, the data were band-pass filtered to 0.008–0.09 Hz. To determine if resting-state functional connectivity with the amygdala changed over sessions, we conducted a seed-to-voxel analysis with anatomical amygdala masks as seed regions. Temporal associations between the mean time courses of all voxels in the seeds with the rest of the brain were estimated using bivariate correlations. For group statistical analyses, the Fisher z-standardized single-subject correlation coefficients from the four sessions were contrasted in SPM8’s flexible factorial model with inclusion of a Subject random-factor. Linear increases and decreases were assessed with t-contrasts [(−1,−1/3,1/3,1) and vice versa]. A voxel threshold of P < 0.001 was used with a cluster significance threshold of P < 0.05 (k > 44 voxels in cluster), determined by Monte-Carlo simulations (2000 simulations, voxel P < 0.001, uncorrected, indicator of smoothness: residual errors smoothing kernel of group-level analysis) using 3dClustSim implemented in the AFNI software package (Cox, 1996).
Psychometric assessments
Prior to having their MRI scans, patients completed the Difficulties in Emotion Regulation Scale (DERS; Gratz and Roemer, 2004). With the DERS, emotion regulation can be assessed in a comprehensive multi-factorized framework in adult populations (Gratz and Roemer, 2004). To obtain a baseline score, patients were assessed with the DERS three times before the first training session, starting 1 week before the first session and followed by two further assessments with an interval of 2–3 days. These ratings and the rating taken in session 1, where patients were still naïve to NF, were averaged, resulting in one pre-training score. In addition, a follow-up assessment was conducted 3 days after the fourth training session (post-training). As two of the eight patients did not complete the post-training measurement, statistical analyses were done without follow-up. To adapt the DERS to our requirements, the original instruction was changed and patients were asked to rate their emotion regulation over the previous 3 days instead of the previous week. Before conducting the analyses, data were screened visually and one outlier value in the session four measurement of the ‘lack of emotional awareness’ subscale (2 s.d. < group mean) was substituted by the mean value of the previous and subsequent assessments.
At the end of each run, a computerized version of the Dissociation Tension Scale-Short Version (DSS-4, (Stiglmayr et al., 2009), was presented. The DSS-4 comprises four items assessing the degree of dissociative experiences on a 10-level scale (0 = not at all, 9 = very much) and one item on aversive tension. For further analyses, a mean score for dissociation and for aversive tension was obtained from all session ratings. One-way repeated measures ANOVAs were conducted to test for within-subject session-to-session effects. To assess correlations of changes in fMRI results with ratings, regression slopes of the dependent measures were produced with Excel (2010, Microsoft Corporation, Redmond, WA) and transferred to SPSS for correlation analyses.
Picture ratings
Subjective ratings of picture valence and arousal were assessed after each training session outside the scanner suite. On a computer laptop, each picture was presented again to participants. After viewing each picture, participants filled out the valence and arousal dimensions on the Self-assessment Manikin assessment (1 = very positive /relaxed, 5 = very negative/highly aroused). The average of all picture ratings provided by the participants in each rating session was used for further analyses.
Results
fMRI data
Task-related effects
Region of interest analysis
Within-subject effects from ANOVAs were not significant (F values < 2.4, P values > 0.1), indicating no improvement in regulation across runs. Taking all runs together, a trend of right amygdala down-regulation was found (t(7) = 2.04, P = 0.08; see Supplementary Table S2 for parameter estimates). Left amygdala activation did not decrease significantly (t(7) = 1.08, P = 0.32). A main effect of right amygdala down-regulation was supported by the voxel-wise analysis [(18,−1,−14), z = 3.25, P < 0.05 SVC] (Figure 2). Statistical tests were not significant in the transfer run (F values/t values < 2.0, P values > 0.1). A visual inspection of the contrast estimates (‘regulate>view’ main effect) showed that on average, patients did not show a down-regulation effect in the transfer run (left amygdala: 0.08 ± 0.75, right amygdala: .27 ± 0.79). Only three patients decreased amygdala activation in the ‘regulate’ vs the ‘view’ condition.
Fig. 2.
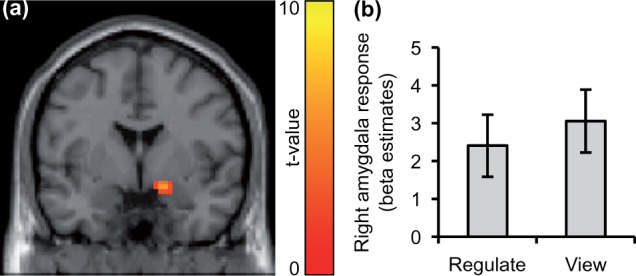
BPD patients decreased right amygdala response with neurofeedback. (A) Significant right amygdala activation was found with the ‘view>regulate’ contrast and SVC. For illustration on an axial slice (y = −1) of the canonical SPM template, a voxel threshold of P < 0.01 (uncorrected) was chosen. (B) Beta estimates from the experimental conditions (‘regulate>neutral’, ‘view>neutral’) extracted from a right amygdala anatomical mask.
PPI analysis
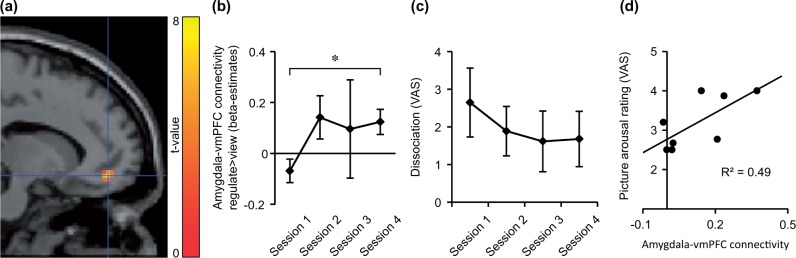
A significant interaction was found in the connectivity between the right amygdala and a vmPFC-cluster, located in the left middle orbital gyrus at the border of BA10 to BA11 [peak at (−12,47,−11), z = 4.21, P < 0.05 field-wise error rate (FWE) corrected, Figure 3a]. A post-hoc analysis of the first-level beta estimates extracted from the peak voxel indicated no significant difference between conditions in session 1 (mean PPI beta estimates ± standard error of mean: ‘regulate>neutral’ = 0.01 ± 0.05, ‘view>neutral’ = 0.08 ± 0.07, t(7) = 1.48, P = 0.18). However, a significant difference between the conditions was found in session 4 (beta estimates: ‘regulate>neutral’ = 0.01 ± 0.22, ‘view>neutral’ = −0.11 ± 0.22, t(7) = 2.51, P < 0.05). The time course parameter estimates of the peak voxel were explored for session-to-session differences. A Session × Condition ANOVA showed a trend for an interaction from the first to the second session (F(1,7) = 5.24, P = 0.06, beta estimates of session 2: ‘regulate>neutral’ = 0.04 ± 0.14, ‘view>neutral’ = −0.11 ± 0.14) and the pattern of larger beta values in the ‘regulate’ compared to the ‘view’ condition remained stable (session 3: ‘regulate>neutral’ = 0.18 ± 0.20, ‘view>neutral = 0.08 ± 0.04, Figure 3b). Taken together, the difference between the condition beta estimates reversed from the first to the other sessions. This trend was mainly driven by a decrease of amygdala-connectivity in the ‘view’ condition.
Fig. 3.
Functional connectivity of right amygdala with vmPFC and dissociation is altered in BPD patients from NF training session 1 to session 4. (A) VmPFC activation detected with the ‘session 4 [regulate>view] > session 1 [regulate>view]’ contrast. Voxels are displayed on a sagittal slice of the canonical SPM template with an uncorrected voxel threshold of P < 0.01 and k > 10. Right is anterior. Crosshairs indicate coordinate of the peak voxel [P < 0.05, FWE-corrected, (−12,47,−11)]. (B) Group mean of PPI beta estimates at peak voxel from the ‘regulate>view’ contrast. Asterisk indicates significant interaction contrast. error bars = standard error of mean (SEM). (C) Group mean of within-session DSS-4 ratings,VAS, visual analogue scale. Error bars = SEM. (D) Picture arousal is correlated with amygdala-vmPFC connectivity in session four. Variance of PPI beta estimates at vmPFC peak voxel (‘regulate>view’ contrast) is predicted by arousal ratings. Line indicates linear association.
No significant interaction effects were detected with the left amygdala seed in an exploratory analysis, and no main effects were found in connectivity with both seeds.
Functional resting-state connectivity
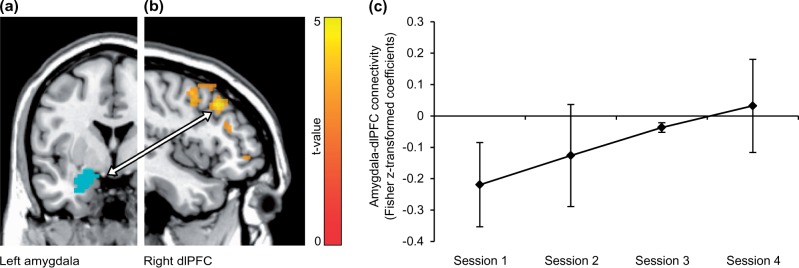
A significant linear increase of left amygdala connectivity was observed in the right middle frontal gyrus in the dorsolateral PFC (dlPFC) (Figure 4). An increase in connectivity was found between the right amygdala and pre- and paracentral gyrus. Linear decreasing connectivity was found between the left amygdala, a hippocampal-parahippocampal-thalamic cluster and the cerebellum (Table 1).
Fig. 4.
Resting-state functional connectivity from left amygdala to right dlPFC was altered in BPD patients over sessions. (A) For illustration, the amygdala mask (blue) is displayed on an anatomical template in axial view (left is left). (B) DlPFC cluster showing a linear functional connectivity increase with left amygdala. Voxels are displayed on a saggital slice (x = 45) of an anatomical template with an uncorrected voxel threshold of P < 0.05 and k > 10 for visualization purposes. Right is anterior. (C) Beta estimates from amygdala-dlPFC connectivity, extracted from the peak voxel (45,23,40). Error bars = SEM.
Table 1.
Results from the resting-state functional connectivity analysis
| Region | BA | Peak voxel |
z | k* | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left amygdala seed: linear increase | ||||||
| Right dlPFC | 9 | 45 | 23 | 40 | 4.13 | 47 |
| Left amygdala seed: linear decrease | ||||||
| Left parahippocampal gyrus, extending to thalamus and hippocampus | −12 | −31 | −11 | 5.02 | 131 | |
| Right cerebellum | 33 | −52 | −26 | 4.62 | 64 | |
| Right hippocampus, extending to thalamus and parahippocampal gyrus | 24 | −31 | −5 | 3.71 | 50 | |
| Right amygdala seed: linear increase | ||||||
| Precentral gyrus/WM | 30 | −22 | 49 | 4.65 | 48 | |
| Right paracentral lobe | 4 | 6 | −34 | 76 | 3.91 | 47 |
| Right amygdala seed: linear decrease | ||||||
| No significant clusters | ||||||
Note. BA, Brodmann area; dlPFC, dorsolateral prefrontal cortex; WM = white matter. Montreal Neurological Institute (MNI) coordinates.
*A cluster size of k > 44 adjacent voxels was expected for significance (P < 0.05).
Psychometric assessments
For the DERS subscale ‘lack of emotional awareness’, a large session effect size (η2 = 0.54) was observed, though this effect was not significant after correction for multiple comparisons (Table 2). The effect resulted from a linear decrease of scores from pre-training to session four (linear contrast: (F(1,7) = 8.26, P < 0.05, uncorrected, pre-training: 25.36 ± 2.59, session 2: 24.88 ± 3.52, session 3: 22.88 ± 3.27, session 4: 23.00 ± 3.42). A visual inspection of the follow-up completers indicated stability of the effect (23.00 ± 3.52, N = 6). Additionally, in the subscale ‘lack of emotional clarity’, a trend effect was found (P = 0.09, uncorrected). Though group mean scores decreased during training, the statistical test of the linear trend was not significant [pre-training: 17.77 ± 2.00, session 2: 17.75 ± 2.25, session 3: 16.38 ± 2.83, session 4: 16.00 ± 4.75; follow-up (N = 6): 16.17 ± 6.31]. Results from the DERS analyses can be obtained from Table 2.
Table 2.
Results from the DERS analysis
| DERS subscale | ANOVA within-subject effects (4 levels; pre-training, session 2, 3, 4) |
||
|---|---|---|---|
| F(3,21) | Effect size (η2) | P | |
| Nonacceptance of emotional responses | 0.46 | 0.06 | 0.71 |
| Difficulties engaging in goal-directed behavior | 0.38 | 0.05 | 0.77 |
| Impulse control difficulties | 0.40 | 0.05 | 0.76 |
| Lack of emotional awareness | 4.27 | 0.38 | 0.02* |
| Limited access to emotion regulation strategies | 0.24 | 0.03 | 0.87 |
| Lack of emotional clarity | 2.46 | 0.26 | 0.09 |
| DERS total score | 0.96 | 0.12 | 0.43 |
Note. DERS, Difficulties in Emotion Regulation Scale.
*Significant without correction for multiple comparisons.
Thirty-four percent of within-subject variance was explained by the DSS-4 ANOVA model (one factor, four levels; F(3,21) = 3.57, P < 0.05, η2 = 0.34; Figure 3c). The effect was driven by a decrease in dissociation from early to late training sessions (session 1: 2.65 ± 0.91, session 2: 1.89 ± 0.66, session 3: 1.61 ± 0.80, session 4: 1.68 ± 0.73; linear contrast: F(1,7) = 10.22, P < 0.05, η2 = 0.59). Though ‘aversive tension’ scores decreased over sessions at the group level (session 1: 3.75 ± 2.53, session 2: 3.50 ± 2.51, session 3: 2.54 ± 2.55, session 4: 2.63 ± 2.14), the within-subject effect was not significant (F(3,21) = 1.02, P = 0.41, η2 = 0.13).
Correlations of session-to-session changes in psychometrics with parameter estimates of amygdala down-regulation and connectivity were not significant (Supplementary Table S5).
Picture ratings
In line with the results from Paret et al. (2016), a correlation of arousal ratings with amygdala-vmPFC connectivity was found in session four (Spearman’s ρ = 0.62, P < 0.05 one-tailed, Figure 3d).
To explore changes in ratings over sessions, Session (4) × Condition (2, ‘regulate’, ‘view’) ANOVAs were conducted. Arousal ratings of training and transfer stimuli decreased significantly over training sessions and condition main effects and interactions were not significant (see Supplementary Table S3 for group statistics and Supplementary Table S4 for ANOVA results).
Discussion
Amygdala NF via rtfMRI NF during the presentation of aversive pictures was associated with successful down-regulation of right dorsal amygdala activation in BPD patients. Contrary to our hypotheses, successful down-regulation did not persist into the transfer run, which tested for regulation of neural responding without feedback. Task-dependent right amygdala-vmPFC connectivity was altered over the course of training, resulting in a pattern comparable to that observed in healthy participants (Paret et al., 2016). Furthermore, an analysis of functional connectivity at rest revealed an increase in amygdala connectivity with the dlPFC and a decrease of connectivity with other limbic regions. Complementing the fMRI results, self-reports showed a decline in patients’ dissociative experiences, and modest evidence was also found for improvements in emotion regulation after training.
Limbic hyperreactivity and lateral PFC hypoactivity are considered to be neural correlates of emotion dysregulation in BPD patients (Niedtfeld et al., 2010; Schulze et al., 2016). Therefore, not surprisingly, psychotherapy aiding in the reduction of amygdala hyperreactivity to emotional cues is associated with an improvement in emotion regulation in BPD patients (Goodman et al., 2014). This is consistent with the view that effective amygdala top-down control is a neural mechanism associated with the recovery from emotion dysregulation symptoms. In this pilot study, this mechanism was targeted using rtfMRI with the aim to establish appropriate NF training for BPD patients and to elucidate neural and psychometric changes associated with training. We could show that BPD patients generally achieved down-regulation; however, they did not improve their ability to down-regulate with repeated training sessions. Although earlier findings pointed to healthy individuals being able to down-regulate amygdala activation during the transfer run (Paret et al., 2014), BPD patients were not successful in reducing their amygdala response when feedback was no longer provided. Successful down-regulation with feedback in training, but not without in the transfer run, points to the importance of providing NF to improve amygdala regulation in patients. However, the supporting evidence is rather weak because comparisons of training with transfer runs are prone to time effects due to the fixed run order. Based on the experimental design and results, no conclusions are possible on the necessity of providing feedback to achieve regulation. Therefore, further research is needed. Negative findings in the transfer run may be interpreted as a lack of transferability, particularly in patients with severe emotion regulation difficulties. It may also reflect that patients were not able to identify a successful strategy for amygdala regulation during training. This conclusion, however, has to be considered with some caution, due to the small sample size and because we did not systematically assess control strategies. Group-level analyses need to be interpreted with caution as well, as some patients may have benefitted from training, while others did not. Indeed, we found that three patients down-regulated amygdala activation in the transfer run. The small sample size, however, does not allow informative statistical comparisons of learners to non-learners.
In accordance with previous studies (Zotev et al., 2011, 2013; Paret et al., 2016), amygdala-vmPFC functional connectivity was altered with NF. In line with previous work (Scheinost et al., 2013), connectivity patterns changed already during the second session, suggesting a relatively early effect. The extent to which the patients altered amygdala-vmPFC connectivity during the task was associated with subjective arousal ratings of the stimulus material, supporting the involvement of emotion processing. As discussed by Paret et al. (2016), connectivity in this network may represent neural information flow of affective value and may inform NF control and learning. Amygdala-vmPFC connectivity is associated with the cognitive regulation of emotions (Diekhof et al., 2011) and differences in limbic-prefrontal connectivity between BPD patients and controls have been reported (New et al., 2007; Kamphausen et al., 2013). This provides initial evidence that rtfMRI NF may tackle impaired brain connectivity patterns associated with emotion regulation deficits in BPD patients.
Resting-state connectivity of the dlPFC and the pre- and paracentral gyrus with the amygdala was altered with repeated training, following a linear increase of connectivity estimates over sessions. The lateral prefrontal cortices and the dlPFC, in particular, are implicated in cognitive emotion regulation (Kalisch, 2009; Buhle et al., 2014). Lower connectivity between the left amygdala and dlPFC at rest is associated with negative affect and decreased executive control (Rohr et al., 2015). In light of this, this finding may reflect an improved involvement of a neurocircuitry supporting affective control. Increases in resting-state connectivity of the dlPFC and decreases within limbic networks were recently reported in fMRI NF associated with a change in contamination anxiety (Scheinost et al., 2013). These findings are nicely complemented by the present study’s results. The observed decrease of connectivity within the limbic system may reflect changes in emotion information processing associated with NF training. Left compared to right amygdala resting-state connectivity effects were more pronounced and congruent with the expected topography based on the literature (Rohr et al., 2015). Apparently, task-dependent and resting-state connectivity effects differed between left and right amygdala. Furthermore, both connectivity analyses showed changes over time and down-regulation did not improve over time. Amygdala down-regulation was previously also observed with sham-feedback, although task-dependent connectivity was only altered with amygdala and not sham-feedback (Paret et al., 2016). Descriptively, the present results appear to complement these findings, as patients increased connectivity in session four in absence of a significant down-regulation effect. This is worth mentioning, as it may suggest that the observed neural effects relate to different processes associated with emotion regulation and brain-computer interface control.
Dissociative experiences are often reported by BPD patients (Zanarini et al., 2000; Stiglmayr et al., 2001) and have been discussed as a psychopathological response to (traumatic) stress (Schmahl et al., 2014). In this study, DSS-4 scores dropped significantly. The decrease in dissociation may be explained by a reduced need to recruit pathological emotion regulation mechanisms with repeated exposure to the task. This was accompanied by a reduction of scores in the ‘lack of emotional awareness’ subscale in the DERS. However, the DERS has six subscales and this finding was not significant after correction. Exploring correlations of changes in psychometric scores with amygdala down-regulation and brain connectivity did not yield significant results. A possible explanation is that our psychometric instruments lack sensibility for the processes involved in the training itself. Furthermore, the changes in the DERS and DSS-4 could rather reflect secondary processes arising from improved affective processing and control. Last but not least, it cannot be ruled out that the changes did not relate to NF training and further studies with larger sample sizes are needed.
This study has important implications for potential improvements of NF training. In general, the lack of a transfer effect suggests poor salience of NF, calling for improvements of the signal-to-noise ratio. During debriefing, subjects stated that they were sometimes confused by the feedback during the control conditions. Therefore, we suggest excluding feedback during control conditions in future studies. Descriptively, neural effects of training were observed already in the first two sessions, emphasizing quick training effects. Dose–response effects of NF treatment need to be addressed in future. In our investigation, we did not provide strategies for amygdala down-regulation, but future studies could address the question of the potential benefits of providing participants with specific instructions.
Limitations stem from the study design and the small sample size. It cannot be concluded that the results are specific to NF training, because no control group was assessed. It is possible that the observed changes relate to the repeated exposure to any element of the protocol (e.g. aversive picture viewing, attention from staff or the attractiveness of testing a new treatment). In addition, patients were undergoing psychotherapeutic treatment; therefore changes may also represent a response to psychotherapy. However, the relatively fast response speaks against this assumption. The sample size is large enough to assess the expected neural and self-rating effects at the pilot stage, especially considering the longitudinal design. However, without replication from another study, caution is recommended in the generalization of the results. Further, all patients were female. Thus, the results might not generalize to male BPD patients. Finally, the discussion on cognitive functions associated with the observed neural effects remains speculative because no cognitive tasks were administered to patients.
In summary, BPD patients decreased their level of amygdala activation with the instruction to regulate the feedback signal, though no transfer of learning was found. Task-related and resting-state brain connectivity in limbic-prefrontal networks was altered in BPD patients over the course of the four sessions. This was accompanied by improvements in dissociation and emotion regulation. Taken together, the results support the hypothesis that amygdala NF training might be beneficial in ameliorating emotion regulation deficits in BPD patients. A replication of findings in a randomized-controlled trial design is needed and currently under preparation.
Funding
The work was part of the Clinical Research Unit 256, funded by the German Research Foundation (DFG, SCHM 1526/14-1, EN 361/13-1).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Behzadi Y., Restom K., Liau J., Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohus M., Wolf-Arehult M. (2012). Interaktives Skillstraining für Borderline-Patienten: Das Therapeutenmanual - Inklusive Keycard zur Programmfreischaltung - Akkreditiert vom Deutschen Dachverband DBT. 2. korrigierter Nachdruck 2016 der 2., überarb. Aufl. Schattauer. [Google Scholar]
- Brühl A.B., Scherpiet S., Sulzer J., Stämpfli P., Seifritz E., Herwig U. (2014). Real-time neurofeedback using functional MRI could improve down-regulation of amygdala activity during emotional stimulation: a proof-of-concept study. Brain Topography, 27, 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–73. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage, 58, 275–85. [DOI] [PubMed] [Google Scholar]
- Doll A., Sorg C., Manoliu A., et al. (2013). Shifted intrinsic connectivity of central executive and salience network in borderline personality disorder. Frontiers in Human Neuroscience, 7, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. (1997). User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders: Scid-1 Clinician Version. 1st edn Arlington: American Psychiatric Publishing. [Google Scholar]
- Goodman M., Carpenter D., Tang C.Y., et al. (2014). Dialectical behavior therapy alters emotion regulation and amygdala activity in patients with borderline personality disorder. Journal of Psychiatric Research, 57, 108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz K.L., Roemer L. (2004). Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26, 41–54. [Google Scholar]
- Hartley C.A., Phelps E.A. (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology, 35, 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. (2009). The functional neuroanatomy of reappraisal: time matters. Neuroscience & Biobehavioral Reviews, 33, 1215–26. [DOI] [PubMed] [Google Scholar]
- Kamphausen S., Schröder P., Maier S., et al. (2013). Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. World Journal of Biological Psychiatry, 14, 307–318, S1–4. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A., Oei N.Y.L., Niedtfeld I., et al. (2012). Influence of emotional distraction on working memory performance in borderline personality disorder. Psychological Medicine, 42, 2181–92. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2008). International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical report A-8. Gainsville, FL: University of Florida. [Google Scholar]
- Lang S., Kotchoubey B., Frick C., Spitzer C., Grabe H.J., Barnow S. (2012). Cognitive reappraisal in trauma-exposed women with borderline personality disorder. Neuroimage, 59, 1727–34. [DOI] [PubMed] [Google Scholar]
- Linden D.E.J., Habes I., Johnston S.J., et al. (2012). Real-time self-regulation of emotion networks in patients with depression. PLoS One, 7, e38115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger A. (1999). International Personality Disorder Examination (IPDE): DSM-IV and ICD-10 Modules. Odessa: Psychological Assessment Resources. [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61, 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New A.S., Hazlett E.A., Buchsbaum M.S., et al. (2007). Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology, 32, 1629–40. [DOI] [PubMed] [Google Scholar]
- Niedtfeld I., Schulze L., Kirsch P., Herpertz S.C., Bohus M., Schmahl C. (2010). Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biological Psychiatry, 68, 383–91. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C., Kluetsch R., Ruf M., et al. (2014). Down-regulation of amygdala activation with real-time fMRI neurofeedback in a healthy female sample. Frontiers in Behavioral Neuroscience, 8, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C., Ruf M., Gerchen M.F., et al. (2016). fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal–limbic brain connectivity. NeuroImage, 125, 182–8. [DOI] [PubMed] [Google Scholar]
- Rohr C.S., Dreyer F.R., Aderka I.M., et al. (2015). Individual differences in common factors of emotional traits and executive functions predict functional connectivity of the amygdala. Neuroimage, 120, 154–63. [DOI] [PubMed] [Google Scholar]
- Ruiz S., Lee S., Soekadar S.R., et al. (2013). Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Human Brain Mapping, 34, 200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow C.A., Grilo C.M., Morey L.C., et al. (2002). Confirmatory factor analysis of DSM-IV criteria for borderline personality disorder: findings from the collaborative longitudinal personality disorders study. American Journal of Psychiatry, 159, 284–90. [DOI] [PubMed] [Google Scholar]
- Scheinost D., Stoica T., Saksa J., et al. (2013). Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Translational Psychiatry, 3, e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl C., Herpertz S.C., Bertsch K., et al. (2014). Mechanisms of disturbed emotion processing and social interaction in borderline personality disorder: state of knowledge and research agenda of the German Clinical Research Unit. Borderline Personality Disorder and Emotion Dysregulation, 1, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L., Domes G., Krüger A., et al. (2011). Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biological Psychiatry, 69, 564–73. [DOI] [PubMed] [Google Scholar]
- Schulze L., Schmahl C., Niedtfeld I. (2016). Neural correlates of disturbed emotion processing in borderline personality disorder: a multimodal meta-analysis. Biological Psychiatry, 79, 97–106. [DOI] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., et al. (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping, 35, 1981–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R., Caria A., Veit R., Gaber T., Ruiz S., Birbaumer N. (2014). Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: a pilot study. Frontiers in Behavioral Neuroscience, 8, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiglmayr C., Schmahl C., Bremner J.D., Bohus M., Ebner-Priemer U. (2009). Development and psychometric characteristics of the DSS-4 as a short instrument to assess dissociative experience during neuropsychological experiments. Psychopathology, 42, 370–4. [DOI] [PubMed] [Google Scholar]
- Stiglmayr C., Shapiro D.A., Stieglitz R.D., Limberger M.F., Bohus M. (2001). Experience of aversive tension and dissociation in female patients with borderline personality disorder—a controlled study. Journal of Psychiatric Research, 35, 111–8. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Viviani R. (2014). Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Frontiers in Psychiatry, 5, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N. (2012). Real-time fMRI and its application to neurofeedback. Neuroimage, 62, 682–92. [DOI] [PubMed] [Google Scholar]
- Wessa M., Kanske P., Neumeister P., Bode K., Heissler J., Schönfelder S. (2010). EmoPics: subjektive und psychophysiologische evaluation neuen bildmaterials für die klinisch-bio-psychologische Forschung. Zeitschrift für Klinische Psychologie und Psychotherapie, Supplementum, 1/11, 77. [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2, 125–41. [DOI] [PubMed] [Google Scholar]
- Young K.D., Zotev V., Phillips R., et al. (2014). Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One, 9, e88785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Young K.D., Phillips R., Zotev V., Misaki M., Bodurka J. (2014). Resting state functional connectivity modulation and sustained changes after real-time fMRI neurofeedback training in depression. Brain Connectivity, 4, 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini M.C., Ruser T., Frankenburg F.R., Hennen J. (2000). The dissociative experiences of borderline patients. Comprehensive Psychiatry, 41, 223–7. [DOI] [PubMed] [Google Scholar]
- Zilverstand A., Sorger B., Sarkheil P., Goebel R. (2015). fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Frontiers in Behavioral Neuroscience, 9, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Krueger F., Phillips R., et al. (2011). Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One, 6, e24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Young K.D., Drevets W.C., Bodurka J. (2013). Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One, 8, e79184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.