Abstract
Acetaminophen has recently been recognized as having impacts that extend into the affective domain. In particular, double blind placebo controlled trials have revealed that acetaminophen reduces the magnitude of reactivity to social rejection, frustration, dissonance and to both negatively and positively valenced attitude objects. Given this diversity of consequences, it has been proposed that the psychological effects of acetaminophen may reflect a widespread blunting of evaluative processing. We tested this hypothesis using event-related potentials (ERPs). Sixty-two participants received acetaminophen or a placebo in a double-blind protocol and completed the Go/NoGo task. Participants’ ERPs were observed following errors on the Go/NoGo task, in particular the error-related negativity (ERN; measured at FCz) and error-related positivity (Pe; measured at Pz and CPz). Results show that acetaminophen inhibits the Pe, but not the ERN, and the magnitude of an individual’s Pe correlates positively with omission errors, partially mediating the effects of acetaminophen on the error rate. These results suggest that recently documented affective blunting caused by acetaminophen may best be described as an inhibition of evaluative processing. They also contribute to the growing work suggesting that the Pe is more strongly associated with conscious awareness of errors relative to the ERN.
Keywords: acetaminophen, ERN, Pe, error
Introduction
Long used as an analgesic and antipyretic, acetaminophen (paracetamol)––or Tylenol, as its brand name is more commonly known––has recently been recognized as having impacts that extend into the affective domain. In particular, double-blind placebo controlled trials have revealed that acetaminophen reduces the magnitude of reactivity to social pain and frustration (Dewall et al., 2010), dissonance and existential anxiety triggered by unsettling experiences (Randles et al., 2013; Dewall et al., 2015), and to both negatively and positively valenced attitude objects (Durso et al., 2015). Given this diversity of consequences, it has recently been proposed that the psychological effects of acetaminophen are not specific to the affective domain, but instead reflect a more widespread blunting of evaluative processing in general (Durso et al., 2015). If this hypothesis is correct, acetaminophen should be able to inhibit or interfere with cognitive signals associated with evaluative processing. We test this by focusing on two event-related potentials (ERPs) that emerge in cortex in quick temporal succession following a choice response error: the error-related negativity (ERN) and positivity (Pe).
Converging evidence suggests that a general conflict detection mechanism in the brain supports cognitive control (Botvinick et al., 2004). Conflict itself is defined as any disagreement or discrepancy between mental representations, response tendencies or actual behavior (Holroyd and Coles, 2002; Botvinick et al., 2004). This system scrutinizes the moment-to-moment representations of action tendencies for potential conflicts, so that inhibitory mechanisms may be engaged to override the unwanted tendency and promote effective goal pursuit. The ERN/Pe ERP complex appears to signal activity of this conflict detection system. The ERN emerges 0–100 ms post-error and is typically maximal at frontal-central midline electrode sites (Falkenstein et al., 1990; Gehring et al., 1993), with the Pe following at 180–350 ms post-response, emerging more clearly along parietal-central midline sites (Hajcak et al., 2003; Santesso et al., 2005; Steinhauser and Young, 2010).
While both ERN and Pe components follow from errors, they are at least partially independent. The ERN is clearly a correlate of evaluative processing (e.g. Hajcak and Foti, 2008; Hobson et al., 2014), but is more strongly associated with an initial, somewhat automatic or implicit monitoring of the error (e.g., Gehring et al., 1993; Falkenstein et al., 2000; Endrass et al., 2005; Overbeek et al., 2005). Some have argued that it is only a precursor to the Pe, which is believed to reflect a more overt awareness and evaluative analysis of the error (e.g., Overbeek et al., 2005; Steinhauser and Yeung, 2010; Boldt and Yeung, 2015). For instance, awareness of an error is often uniquely predicted by the Pe and task salience relates to Pe magnitude, while the ERN is triggered by both aware and unaware errors (Endrass et al., 2005; Overbeek et al. 2005). While both ERP components are associated with post-error slowing, the relationship emerges more consistently for the Pe (e.g. Hajcak et al., 2003; D’Lauro and Curran, 2007; cf. Ullsperger et al., 2010 for a review). Additionally, the Pe appears to be uniquely associated with error-related EMG activity over the corrugator, which has been associated with orienting toward motivationally important events (Elkins-Brown et al., 2015), as well as peripheral sympathetic nervous system activity (Hajcak et al., 2003).
Using dipole-modeling techniques, the ERN has been most consistently localized in the anterior cingulate cortex (ACC), most likely in the dorsal region (dACC; Herrmann et al., 2004). Less work has focused specifically on the Pe, with the data that exists suggesting a more distributed source. Some research has localized the Pe to the rostral anterior cingulate cortex, (van Veen et al., 2002; Herrmann et al., 2004; Taylor et al., 2007), whereas other work identifies the parietal cortex as responsible for generating the signal (Falkenstein et al., 2000; Davies et al., 2001). In line with the perspective of an integrated conflict-detection system, the ACC shows consistent activation in response to a range of distressing experiences, such as social rejection (Eisenberger, 2015) and pain (cf. Shackman et al., 2011; Lieberman and Eisenberger, 2015). One study has found that acetaminophen inhibits social rejection over a 2-week period, as well as ACC activity following a rejection manipulation in-lab (Dewall et al., 2010). This work, combined with behavioral studies indicating acetaminophen inhibits dissonance reduction (also associated with ACC activity; van Veen et al., 2009; Izuma et al., 2010; DeWall et al., 2015) and broader evaluative processes (Durso et al., 2015), suggests that acetaminophen may inhibit neural processes involved in evaluating any conflict. As the ERN/Pe consistently emerges as a correlate of conflict and error detection, acetaminophen may inhibit this signal following conflict events.
In the following study, we conducted a double-blind placebo controlled acetaminophen trial that had participants perform a two-choice, Go/NoGo target detection task while we measured their brain electrical responses via the electroencephalogram. Given research to date on the ERN/Pe response, we hypothesized that acetaminophen may interfere with early bottom-up processing of error-related cognition, or may only affect downstream processes associated with bringing that information to conscious awareness. As such, we anticipated that acetaminophen may or may not reduce the amplitude of the ERN, but should reduce the amplitude of the Pe elicited by response errors.
Materials and methods
Participants
Sixty-two students (44 women, mean age = 19.42, s.d. = 1.85) were recruited from The University of British Columbia. Participants were mostly East Asian (N = 31) or Western European (N = 17), with the remaining participants from other or mixed ethnic backgrounds. Eight participants’ ERP data were excluded from analysis due to equipment failure, excessive blinking or producing fewer than five no-go errors throughout the task (Olvet and Hajcak, 2009). The mean number of no-go errors was 19.71 (s.d. = 7.47). All 62 participants were used for analyses that only require self-report or behavioral measures. We aimed to collect data for thirty useable participants per condition; data collection was terminated at the end of the academic term. The study finished with n = 33 participants in the acetaminophen condition (n = 28 included in the ERP analysis) and n = 29 in the placebo condition (n = 26 included in the ERP analysis).
Stimuli and task
Participants performed the go/no-go task, which is commonly used to measure response inhibition. Participants were instructed to hit a button as quickly as possible in response to the ‘go’ stimulus, but withhold their response to a similar-looking but different ‘no-go’ stimulus. The ‘go’ stimulus was presented 80% of the time, randomly assigned to each trial with non-replacement, ensuring that all participants completed 240 ‘go’ and 60 ‘no-go’ trials. The 300 trials were broken into six blocks of 50 trials each, with a brief rest period in between each block. The stimuli used were the letters F and E, counter-balanced between participants regarding the stimulus assigned as ‘go’ and ‘no-go’. Each trial began with the presentation of a central fixation dot that randomly varied between 200 and 600 ms, followed by a stimulus presented for 100 ms. Participants had up to 600 ms from the onset of the stimulus to respond, followed by a blank screen used for the inter-trial interval, randomly varied between 0 and 200 ms. Stimulus onset asynchrony therefore varied between 900 and 1500 ms. No feedback was given during the task.
Experimental manipulation
Participants in the experimental condition consumed two capsules containing 500 mg tablets of Kirkland-brand acetaminophen (1000 mg total). The placebo condition consumed two identical-looking capsules that were filled with sugar. Participants were randomly assigned to condition through a double-blind procedure, where each participant’s dose was assigned a unique ID prior to the study that matched it to the correct condition. The researcher running the study did not have direct access to the list that associated IDs with condition, but could request access to identify the condition if it became medically necessary. Participants were sorted into condition beginning at the group averaging stage of analysis.
Procedure
Participants provided written consent and then consumed their assigned capsules. After a brief waiting period (30 min) the researcher placed the electroencephalogram electrodes over the participants’ scalp, and sat them in front of a computer monitor to begin the go/no-go task. This task always began 60 min after consuming the pills, ensuring that most participants experienced peak pharmacological activation; typically requiring 45–60 min for adults consuming acetaminophen orally (Bertolini et al., 2006). When this task was completed, participants completed the positive and negative affect schedule (PANAS) asking how they felt at that moment (∝ = 0.87 for positive and ∝ = 0.77 for negative items), exploratory measures, suspicion-check items, and finally a second administration of the PANAS (∝ = .88 for positive and ∝ = 0.87 for negative items) that asked how they were feeling specifically while completing the go/no-go. We additionally included three extra items in both versions of the PANAS: Frustrated, anxious, and unpleasant (along with ‘attentive’ which is already in the PANAS). The second PANAS administration and added words were used to address concerns raised by Spunt et al. (2012) that people are better able to articulate their feelings relative to an actual event and that these particular words were more informative than a broad survey of negative affect.
EEG recording and analysis
Continuous EEG was recorded during the task using 64 Ag/AgCl active electrodes (BioSemi Active-Two amplifier system) in accordance with the international 10–20 system. Two additional electrodes located over the medial-parietal cortex (Common Mode Sense and Driven Right Leg) were used as ground electrodes. Recordings were digitized at 256 Hz, digitally filtered offline between 0.1 and 30 Hz (zero phase-shift Butterworth filter) and then referenced offline to the average of two mastoid electrodes. EEG data processing was performed using ERPLAB, a toolbox within MATLAB used in conjunction with EEGLAB. To ensure proper eye fixation and allow for the removal of events associated with eye movement artifacts, vertical and horizontal electrooculograms (EOGs) were also recorded—the vertical EOGs from an electrode inferior to the right eye, and the horizontal EOGs from two electrodes on the right and left outer canthus. Offline, computerized artifact rejection was used to eliminate trials during which detectable eye movements and blinks occurred. These eye artifacts were detected by identifying the minimum and maximum voltage values on all recorded EOG channels from − 200 to 800 ms post-stimulus for each event epoch, and then removing the trial from subsequent signal averaging if that value exceeded 200 μV, a value calibrated to capture all blinks and saccades. This was followed by visual inspection of the data. If additional artifacts (e.g. muscle movements and loose connections) were observed, the threshold was reduced by 25 μV until artifacts were not present or a minimum of 100 μV was reached. An average of 13.34% (s.d. = 12.94%) of the total number of correct trials across participants and 21.62% (s.d. = 19.7%) of the incorrect trials were rejected due to these signal artifacts. The percentage of trials rejected did not significantly differ between the acetaminophen and placebo conditions (both ts < 1, Ps > 0.25). Continuous data were segmented into 800 ms epochs, time-locked to response and were baseline-corrected by subtracting the average voltage during the time period −200 to −50 ms prior to response. This baseline window was used to avoid subtracting out the ERN component, which often is visible up to 50 ms prior to recording of the response (e.g. Inzlicht and Al-Khindi, 2012). These epochs were averaged within participants independently for correct button-presses (i.e. correct ‘go’s) and incorrect button presses (i.e. responding to ‘no-go’s; commission errors) and then grand-averaged across participants within the respective conditions.
Results
Behavior
Data and analysis scripts are available for download from the Open Science Framework at https://osf.io/d5zc4/. Reaction times (RTs) and error rates are reported in Table 1 by group. To assess effects on the number of errors made, we used negative binomial regression with a log link function for all analyses. This analysis employs a distribution that fits count data well (such as number of errors made), anticipating a distribution that is extremely right-skewed and over-dispersed (i.e. the variance of errors is greater than the mean; as was the case in our study). Regarding errors of commission (a go response on no-go trials), acetaminophen did not predict more erroneous button presses, χ2(1,60) = 1.56, P = 0.21, ln(b) = −0.13 CI0.975[−0.33 to 0.07]. However, the acetaminophen group made significantly more errors of omission, allowing a larger number of ‘go’ trials to pass by without entering a response, χ2(1,60) = 11.56, P < 0.001, ln(b) = 0.76 CI0.975[0.32 to 1.19]. Finally, to examine post-error slowing (a common response in cognitive RT tasks that reflects behavioral adjustment in response to the error), we calculated the average RT for go trials following a no-go error, minus the average RT for a go trial following a no-go success. Although a clear post-error slowing effect emerged for the entire sample, (tested against 0) t(61) = 9.39, P < 0.001, d = 2.4 CI0.975[1.89 to 2.91], there was no significant difference between groups, t(59.7) = 1.28, P = 0.20 d = 0.33 CI0.975[−0.19 to 0.85].
Table 1.
Descriptive statistics for errors and RTs
| Behavioral measures | Placebo | Acetaminophen |
|---|---|---|
| Full Sample | ||
| Commission error rate (%) | 33.79 (10.56) | 29.70 (14.96) |
| Omission error rate (%) | 2.62 (2.27) | 5.58 (5.06)*** |
| RT excluding errors (ms) | 198.17 (31.86) | 202.72 (36.19) |
| Post-error slowing (ms) | 91.35 (86.26) | 120.33 (91.54) |
| Sample with unusable ERP participants removed | ||
| Commission error rate (%) | 33.63 (10.51) | 32.28 (14.01) |
| Omission error rate (%) | 2.63 (2.36) | 6.21 (5.07)** |
| RT excluding errors (ms) | 198.56 (32.73) | 198.93 (32.05) |
| Post-error slowing (ms) | 86.6 (85.51) | 122.31 (80.84) |
Note: Mean values are presented, standard deviations are in brackets. RT only includes Go trials. Post-error slowing is the difference between the RT on a Go trial following a NoGo error, vs RT on a Go trial following a NoGo success.
**P < 0.01, ***P < 0.001.
Electrophysiology
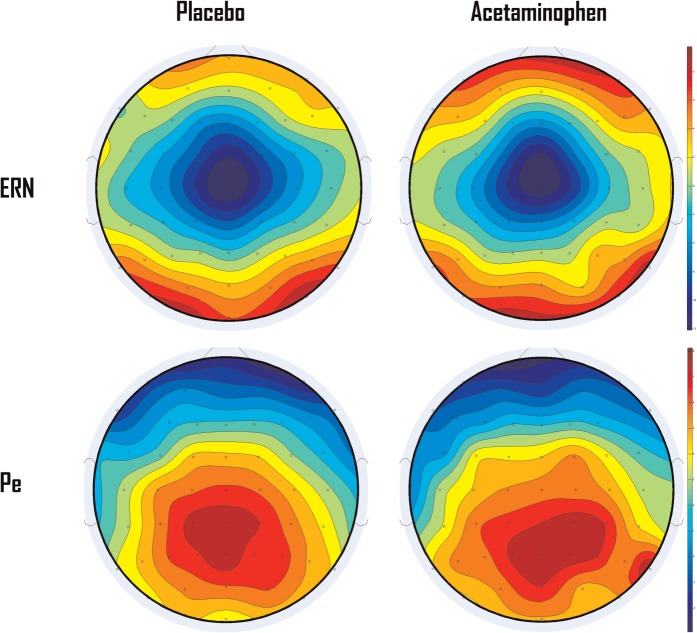
The ERN was defined as the mean amplitude of the negative deflection occurring 0–100 ms post-response, at FCz (e.g., Hajcak et al., 2003; Santesso et al., 2005; Steinhauser and Yeung, 2010; Inzlicht and Al-Khindi, 2012). The Pe was measured as the mean amplitude of the positive deflection from 180 to 350 ms post-response, using the average of CPz and Pz (Hajcak et al., 2003; Santesso et al., 2005). In order to isolate the neural response specifically elicited by an error, we computed difference waveforms to control for activity elicited by a motor response. Difference waveforms were obtained by subtracting the waveforms for correct presses from the waveforms for incorrect presses (Luck, 2005). Unprocessed ERP waveforms are available in the supplementary online material (Supplementary Figures S1 and S2). Topographical maps of the difference waves confirm the choice of electrodes used in the analyses (see Figure 1) As per standard for the ERN waveform, all measures were made relative to a −200 to −50 pre-stimulus baseline.
Fig. 1.
Topographical maps of the difference waves. Three participants from the acetaminophen group and one from the placebo were removed before constructing topographical maps. They had at least one electrode failure not critical to the primary hypothesis, with average magnitudes over 4 s.d. from the group mean (Failed electrodes ranged between 172 and 7746 absolute µV).
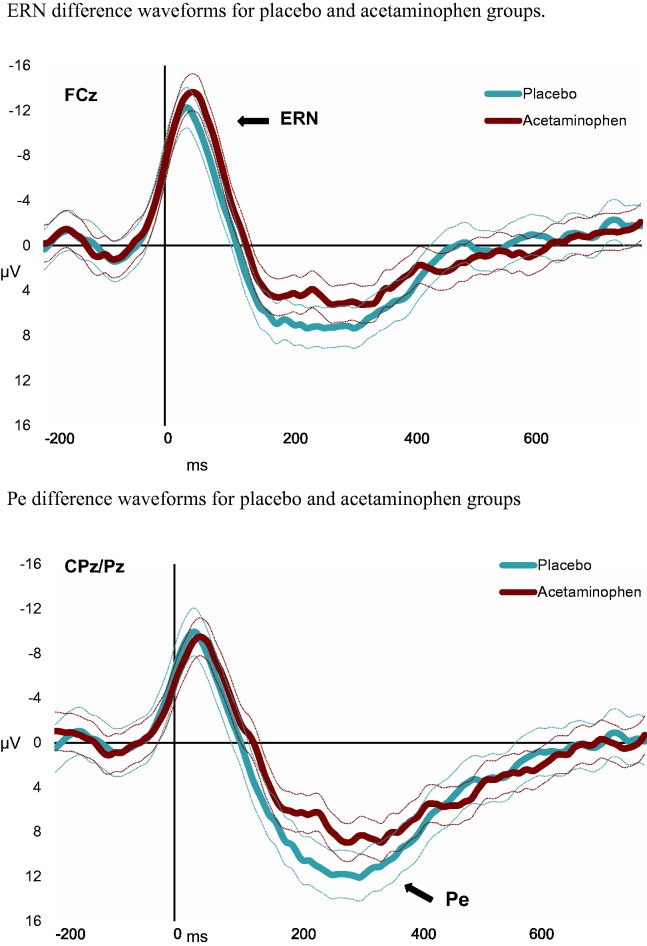
The ERN and Pe difference waveforms are shown in Figure 2 by group, and mean amplitude measures are reported in Table 2. For all tests, a correction for non-equal variance was applied to the degrees of freedom, although in most cases the assumption of equal variance was met. Comparing between the acetaminophen and placebo groups, there was no significant difference in the ERN measured at FCz, t(48.3) = 1.56, P = 0.13, d = 0.45 CI0.975[−0.13 to 1.03], but there was a significant reduction in the magnitude of the Pe measured at CPz/Pz in the acetaminophen condition, t(51.8) = 2.32, P = 0.02, d = 0.65 CI0.975[0.09 to 1.2].
Fig. 2.
Difference waveforms for placebo and acetaminophen groups.
Note for both figures: Difference waveforms were constructed by subtracting the ERP for a correct button press from the ERP for an incorrect button press. Dashed lines represent 95% CI. Mean amplitude for the ERN was measured at FCz across 0–100 ms. Mean amplitude of Pe (180–350 ms) measured at CPz and Pz.
Table 2.
Descriptive statistics for ERPs
| ERP measures | Placebo | Acetaminophen |
|---|---|---|
| ERN at FCz (μV) | −9.05 (3.34) | −10.80 (4.82) |
| Pe at Cpz/Pz (μV) | 10.94(5.05) | 7.5(5.81)* |
Note: Mean values are presented, standard deviations are in brackets. ERN and Pe mean amplitudes are constructed by first subtracting the average wave for a correct Go response from the wave for an incorrect NoGo response.
*P < 0.05
Toward understanding the relationship between observed behavioral and ERP effects, we conducted several additional analyses. First, errors of commission were not predicted by the amplitude of an individual’s ERN (χ2(1,52) = 0.22, P > 0.25, ln(b) = 0.01 CI0.975[−0.02 to 0.03]) or Pe (χ2(1,52) = 0.07, P > 0.25, ln(b) = 0.00 CI0.975[−0.02 to 0.02]). However, individual differences in Pe amplitude predicted fewer errors of omission as amplitude increased (χ2(1,52) = 11.02, P < 0.001, ln(b) = −0.06 CI0.975[−0.10 to −0.03]. While this effect remained marginally significant when controlling for condition χ2(1,52) = 3.31, P = 0.07, ln(b) = −0.03 CI0.975[−0.07 to 0.003], a bootstrap analysis of the indirect effect of acetaminophen on errors through inhibited Pe activity was significant (10 000 resamples, bias corrected and accelerated CI0.975[0.012 to 1.37]; Preacher and Hayes, 2008), indicating partial mediation of acetaminophen’s effect on omission error rates via reduced Pe activity. In contrast, individual differences in ERN magnitude failed to predict these errors, χ2(1,51) = 1.43, P > 0.25, ln(b) = 0.03 CI0.975[−0.05 to 0.07]. Further, there was no correlation between post-error slowing and either magnitude of the ERN, P > 0.25, r = −0.01 CI0.975[−0.29 to 0.27]), or Pe, P > 0.25, r = −0.08 CI0.975[−0.36 to 0.20].
Control analyses
Given our study design and question, we conducted two additional control analyses. First, as a check of the double-blind protocol, we asked participants to report what type of pill they believed they had received, and whether they were confident in their guess. Only 6.7% claimed they were confident (three in the acetaminophen group, and one in the placebo group), and guesses were correct 56.45% of the time (not different from chance, P > 0.25). Although neither group was more accurate at guessing their condition (both Ps > 0.25), all participants who were confident guessed correctly, suggesting that for a small minority (possibly those experiencing mild pain before the study) it was at least somewhat possible to detect whether they had been given a placebo or the drug. Using participants’ perceived group, we found no significant differences on any of our key dependent variables (i.e. no placebo effect; see Supplementary Table S1 in the supplementary online materials).
Second, to account for possible impacts of acetaminophen on affect, we had participants complete the PANAS. Regarding conscious change in affect, there were no differences between conditions regarding positive or negative affect, regardless of whether the PANAS was presented immediately after the go/no-go task and referred to their mood in general, or whether it was presented later and had the participant report their mood in reference to when they were completing the go/-no-go task, all ts < 0.5, Ps > 0.25. There was also no difference for either measure when focusing on just the new items recommended by Spunt et al. (2012), both ts < 1.0, Ps > 0.25. While participants may have been experiencing brief immediate affective reactions to errors, acetaminophen did not impact their affective state either immediately after the entire task, or after an additional delay.
Discussion
Our study assessed the hypothesis that acetaminophen, previously linked to a range of affect-related impacts (e.g. Dewall et al., 2010, 2015; Randles et al., 2013), inhibits evaluative processing in a more domain-general manner (Durso et al., 2015). In this regard, we found that not only was acetaminophen associated with reduced Pe activity in a visual go/no-go task, it also led to a systematic reduction in the propensity to detect and respond to targets relative to a placebo control group, possibly indicating inhibited evaluative analysis of errors. This suggests that the psychological effects of acetaminophen are not limited to the social-affective domain, but consistent with the predictions of Durso et al. (2015), they extend to more basic evaluative processes in cortex, and in particular, those associated with performance monitoring. Beyond this immediate conclusion, several additional points follow.
First, our ERP data confirm an important theoretical prediction regarding basic cognitive functioning that, paradoxically, has arisen from the social psychology literature. Specifically, our results build on the growing evidence pointing to the role of aversive arousal motivating cognitive control (Elkins-Brown et al., 2015; Inzlicht et al., 2015; Saunders et al., 2015). Given that acetaminophen has previously been shown to attenuate social strife and dissonance associated with situational uncertainty (DeWall et al., 2010, 2015; Randles et al., 2013) the demonstration here is that error monitoring is similarly impacted. This indicates that, while this trio of domains can involve very different content and modalities, they share a common underlying challenge of updating information and altering behavior, and thus may be processed in a similar neural manner as a result.
Second, our findings provide novel insight into the basic cortical mechanisms associated with error and performance monitoring. In particular, there has been intense on-going debate regarding what specifically the ERN and Pe respectively capture about these vital cortical processes (e.g. Falkenstein et al., 2000; Holroyd and Coles, 2002; Endrass et al., 2005; Overbeek et al., 2005; O'Connell et al., 2007; Vocat et al., 2008; Shalgi et al., 2009). Toward resolving this debate, recent evidence using signal-detection modeling has shown that whereas the Pe is sensitive to one’s subjective criteria for a perceptual decision and confidence in the decision made, the ERN is not (Steinhauser and Yeung, 2010; Boldt and Yeung, 2015). As such, our results expand on this behavioral dissociation in two critical ways. We show that the Pe and ERN can be dissociated psychopharmacologically in a manner consistent with this general functional interpretation. Additionally, the sensitivity of the Pe to acetaminophen we report here suggests these processes may have at least some functional overlap with the broader array of affective-evaluative processes previously linked to attenuation via acetaminophen (Dewall et al., 2010; Randles et al., 2013; Durso et al., 2015; Inzlicht et al., 2015). With regards to this distinction however, additional follow-up work should be completed. As this was the first ERP study to use acetaminophen as a manipulation, and given our null ERN finding, we may have failed to detect an effect for the ERN that may be revealed with larger samples.
Third, the effect of acetaminophen on errors of omission, while robust, was unanticipated. Given that RTs between groups were not different for Go trials (once omissions were excluded), it suggests that acetaminophen may be affecting the depth of our attentional engagement with events in the external environment, in a manner akin to what is observed during episodes of mind wandering, when our cognitive and affective sensitivity to task-relevant events decreases (e.g. Kam and Handy, 2013; Handy and Kam, 2015). This possibility is consistent with evidence from functional neuroimaging indicating that brief attentional lapses in simple choice RT tasks are associated with transient decreases in pre-stimulus activity in the ACC (Weissman et al., 2006), the key brain region presumably affected by acetaminophen. However, without further evidence, this remains a speculative interpretation.
Finally, our findings also raise a pair of more practical implications regarding the neurocognitive consequences of acetaminophen. For one, as our study and others have now demonstrated, it represents a potentially novel and previously unrecognized approach for psychopharmacologically manipulating cortical evaluative processes in the laboratory, relevant to reinforcement schedules (Pfabigan et al., 2010), directed attention (Hauswald et al., 2011) and other tasks known to be associated with Pe activity. For another, our results suggest a need for greater awareness regarding the potential neurocognitive side-effects of acetaminophen. Participants made more errors under the influence of acetaminophen, an effect that was partially mediated by reduced Pe magnitude. Cognitive control is constantly required throughout an individual’s day. Whether operating complex machinery, coordinating teams of individuals, or driving a car, cognitive control is important for quickly and accurately responding to everyday challenges. Additional studies monitoring different types of tasks, and under more ecologically valid conditions, will help to verify the magnitude of the real-world effect of this medication.
Funding
This work was supported by a Social Sciences and Humanities Research Council of Canada grant, administered to the Third author [435-2014-0025]; an NSERC discovery grant administered to the fifth author; and a Social Sciences and Humanities Research Council Banting Postdoctoral Fellowship administered to the first author [BPF-SSHRC-01045].
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Bertolini A., Ferrari A., Ottani A., Guerzoni S., Tacchi R., Leone S. (2006). Paracetamol: new vistas of an old drug. CNS Drug Reviews, 12(3-4), 250–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt A., Yeung N. (2015). Shared neural markers of decision confidence and error detection. The Journal of Neuroscience, 35(8), 3478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Science , 8(12), 539–46. [DOI] [PubMed] [Google Scholar]
- Davies P.L., Seaglowitz S.J., Dywan J., Pailing P.E. (2001). Error-negativity and positivity as they relate to other ERP indices of attentional control and stimululs processing. Biological Psychology , 56(3), 191–206. [DOI] [PubMed] [Google Scholar]
- D’Lauro C., Curran T. (2007). Cross-task individual differences in error processing: neural, electrophysiological, and genetic components. Cognitive, Affective, and Behavioral Neuroscience, 7(4), 297–308. [DOI] [PubMed] [Google Scholar]
- DeWall N.C., Chester D.S., White D.S. (2015). Can acetaminophen reduce the pain of decision-making? Journal of Experimental Social Psychology, 56, 117–20. [Google Scholar]
- DeWall N.C., MacDonald G., Webster G.D., et al. (2010). Acetaminophen reduces social pain: behavioral and neural evidence. Psychological Science, 21(7), 931–7. [DOI] [PubMed] [Google Scholar]
- Durso G.R.O., Luttrell A., Way B.M. (2015). Over-the-counter relief from pains and pleasures alike: acetaminophen blunts evaluation sensitivity to both negative and positive stimuli. Psychological Science, 26(6), 750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N. (2015). Social pain and the brain: controversies, questions, and where to go from here. Annual Review of Psychology , 66(1), 601–29. [DOI] [PubMed] [Google Scholar]
- Elkins-Brown N., Saunders B., Inzlicht M. (2015). Error-related electromyographic activity over the corrugator supercilii is associated with neural performance monitoring. Psychophysiology, 53(2), 159–70. [DOI] [PubMed] [Google Scholar]
- Endrass T., Franke C., Kathmann N. (2005). Error awareness in a saccade countermanding task. Journal of Psychophysiology, 19(4), 275–80. [Google Scholar]
- Falkenstein M., Hoormann J., Christ S., Hohnsbein J. (2000). ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology, 51(2), 87–107. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoorman J., Blnake L. (1990). Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia C.H.M., Gaillard A.W.K., Kok A., editors. Psychophysiological Brain Research, Vol. 1 Tilburg, The Netherlands: Tilburg University Press, 192–5. [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. (1993). A neural system for error detection and compension. Psychological Science, 4(6), 385–90. [Google Scholar]
- Hajcak G., Foti D. (2008). Errors are aversive: defensive motivation and the error-related negativity. Psychological Science, 19(2), 103–8. [DOI] [PubMed] [Google Scholar]
- Hajcak G., McDonald N., Simons R.F. (2003). To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40(6), 895–903. [DOI] [PubMed] [Google Scholar]
- Handy T.C., Kam J.W.Y. (2015). Mind wandering and selective attention to the external world. Canadian Journal of Experimental Psychology , 69, 183–9. [DOI] [PubMed] [Google Scholar]
- Hauswald A., Schulz H., Iordanov T., Kissler J. (2011). ERP dynamics underlying successful directed forgetting of neutral but not negative pictures. Social Cognitive and Affective Neuroscience, 6(4), 450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M.J., Römmler J., Ehlis A., Heidrich A., Fallgatter A.J. (2004). Source localization (LORETA) of the error-related-negativity (ERN/NE) and positivity (Pe). Cognitive Brain Research , 20, 294–9. [DOI] [PubMed] [Google Scholar]
- Hobson N.M., Saunders B., Al-Khindi T., Inzlicht M. (2014). Emotion down-regulation diminishes cognitive control: a neurophysiological investigation. Emotion, 14(6), 1014–26. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. [DOI] [PubMed] [Google Scholar]
- Kam J.W.Y., Handy T.C. (2013). The neurocognitive consequences of the wandering mind: a mechanistic account of sensory-motor decoupling. Frontiers in Perception Science , 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M., Al-Khindi T. (2012). ERN and the placebo: a misattribution approach to studying the arousal properties of the error-related negativity. Journal of Experimental Psychology: General, 141(4), 799–808. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Bartholow B.D., Hirsh J.B. (2015). Emotional foundations of cognitive control. Trends in Cognitive Science, 19(3), 126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Matsumoto M., Murayama K., et al. (2010). Neural correlated of cognitive dissonance and choice-induced preference change. Proceedings of the National Academy of Science of the United States of America , 107(51), 22014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I. (2015). The dorsal anterior cingulate cortex is selective for pain: results from large-scale reverse inference. Proceedings of the National Academy of Science of the United States of America , 112(49), 15250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J. (2005). An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press. [Google Scholar]
- O'Connell R.G., Dockree P.M., Bellgrove M.A., et al. (2007). The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. European Journal of Neuroscience, 25(8), 2571–9. [DOI] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. (2009). The stability of error-related brain activity with increasing trials. Psychophysiology , 46, 957–61. [DOI] [PubMed] [Google Scholar]
- Overbeek T.J.M., Nieuwenhuis S., Ridderinkhof K.R. (2005). Dissociable components of error processing. Journal of Psychophysiology, 19(4), 319–29. [Google Scholar]
- Pfabigan D.M., Alexopoulos J., Bauer H., Sailer U. (2010). Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event-related brain potentials. Psychophysiology, 48(5), 656–64. [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Hayes A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods , 40(3), 879–91. [DOI] [PubMed] [Google Scholar]
- Randles D., Heine S.J., Santos N. (2013). The common pain of surrealism and death: acetaminophen reduces compensatory affirmation following meaning threats. Psychological Science, 24(6), 966–73. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. (2005). ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology, 70(2), 79–87. [DOI] [PubMed] [Google Scholar]
- Saunders B., Milyavskaya M., Inzlicht M. (2015). What does cognitive control feel like? Effective and ineffective cognitive control is associated with divergent phenomenology. Psychophysiology , 52, 1205–17. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., et al. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience , 12(3), 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalgi S., Barkan I., Deouell L.Y. (2009). On the positive side of error processing: error-awareness positivity revisited. European Journal of Neuroscience, 29(7), 1522–32. [DOI] [PubMed] [Google Scholar]
- Spunt R.P., Lieberman M.D., Cohen J.R., Eisenberger N.I. (2012). The phenomenology of error processing: the dorsal ACC response to stop-signal errors tracks reports of negative affect. Journal of Cognitive Neuroscience, 24(8), 1753–65. [DOI] [PubMed] [Google Scholar]
- Steinhauser M., Yeung N. (2010). Decision processes in human performance monitoring. Journal of Neuroscience, 30(46), 15643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Stern E.R., Gehring W.J. (2007). Neural systems for error monitoring: recent findings and theoretical perspectives. The Neuroscientist, 13(2), 160–72. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Harsay H.A., Wessel J.R., Ridderinkhof K.R. (2010). Conscious perception of errors and its relation to the anterior insula. Brain Structure and Function, 214(5-6), 629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. (2002). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience , 14(4), 593–602. [DOI] [PubMed] [Google Scholar]
- van Veen V., Krug M.K., Schooler J.W., Carter C.S. (2009). Neural activity predicts attitude change in cognitive dissonance. Nature Neuroscience , 12(11), 1469–74. [DOI] [PubMed] [Google Scholar]
- Vocat R., Pourtois G., Vuilleumier P. (2008). Unavoidable errors: a spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia, 46(10), 2545–55. [DOI] [PubMed] [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. (2006). The neural bases of momentary lapses in attention. Nature Neuroscience , 9, 971–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.