Abstract
Background
Frailty is a measure of physiologic reserve that has been used to predict outcomes following surgical procedures in the elderly. We hypothesized that frailty would be associated with outcomes following paraesophageal hernia (PEH) repair.
Methods
The National Surgical Quality Improvement Program (NSQIP) database (2011–2013) was queried for ICD-9 and CPT codes associated with PEH repair in patients ≥ 60 years old. A previously described modified frailty index (mFI), based on 11 clinical variables in NSQIP was used to quantify frailty. Multivariate logistic regression was used to determine the relationship between frailty, complications, and mortality.
Results
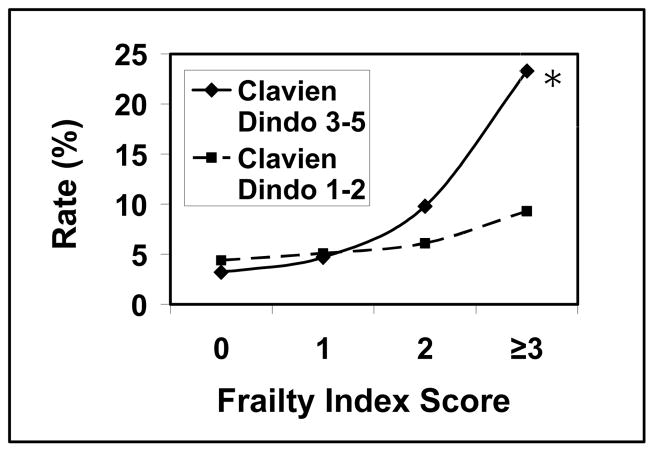
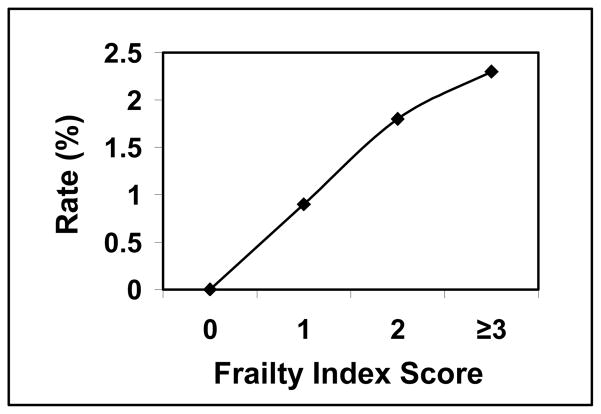
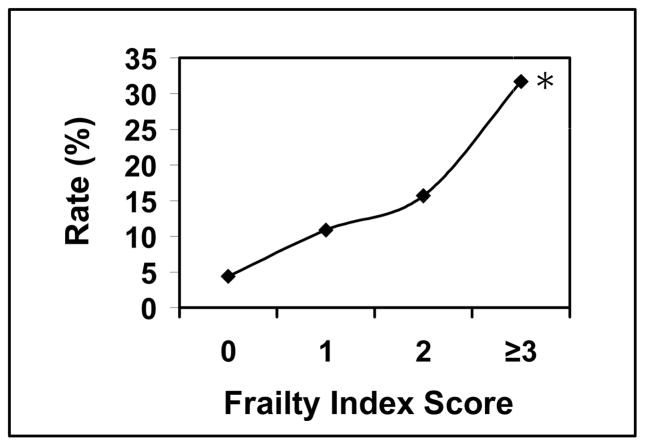
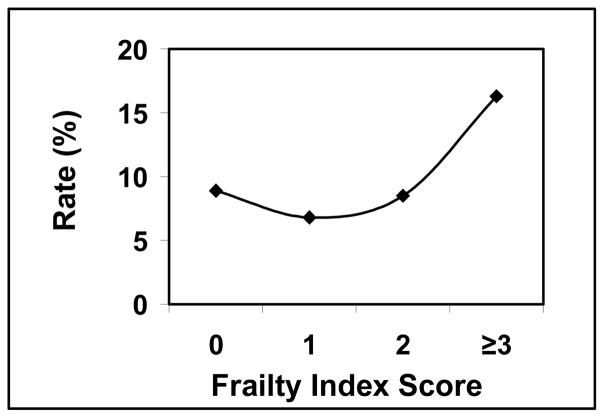
Of the 4434 PEH repairs that met inclusion criteria, 885 records were included in the final analysis (20%). Excluded patients were missing 1 or more variables in the mFI. The rate of complications that were Clavien-Dindo Grade ≥ 3 was 6.1%. Mortality was 0.9%. The readmission rate was 8.2%, and 10.9% of patients were discharged to a facility other than home. Relative to mFI scores of 0, 1, 2, and ≥3, the respective occurrence percentages were as follows; Grade ≥3 complication: 3.2%, 4.7%, 9.8%, and 23.3% (p<0.0001) [OR 3.51; CI 1.46–8.46]; mortality: 0.0%, 0.9%, 1.8%, and 2.3% (p 0.0974); discharge to facility other than home: 4.4%, 10.9%, 15.7%, and 31.7% (p<0.0001) [OR 4.07; CI 1.29–12.82]; and readmission: 8.9%, 6.8%, 8.5%, and 16.3% (p=0.1703) [OR 1.01; CI 0.36–2.84]. Complications and discharge destination were significantly correlated with the mFI.
Conclusion
Frailty, as assessed by the mFI, is correlated with postoperative complications and discharge to a facility other than home following paraesophageal hernia repair.
Keywords: paraesophageal hernia, NSQIP, frailty, outcomes, geriatric
1. Introduction
Paraesophageal hernias (PEHs) are defined as having both the gastroesophageal junction and gastric fundus displaced above the diaphragm [1]. Over 90% of PEHs are type III hiatal hernias in which the stomach is herniated alongside the esophagus, and the gastroesophageal junction is displaced above the diaphragm [2]. PEHs account for 5–10% of all hiatal hernias, but are increasingly common with advancing age [1,2,3,4]. Generally, PEHs tend to enlarge with time, and the annual incidence of acute symptoms requiring emergency surgery is estimated to be 0.7–7% per year [4,5]. Surgery is indicated for symptomatic PEHs [4,6]. Even though the elderly are more likely to suffer from a symptomatic PEH and experience diminished quality of life, clinicians may be reluctant to seek surgical consultation or offer surgical intervention secondary to fear of increased morbidity and mortality and a perceived lack of symptomatic benefit [7].
Frailty is increasingly recognized as an important predictor of healthcare outcomes, as it is thought to estimate physiologic reserves primarily in older adults [8,9]. With an aging US population, the ability to identify frailty is becoming of paramount importance given the fact that over half of all operations in the US are being performed on patients 60 years and older, and this is the fastest growing segment of the US population [5,8]. Frailty relates to an individual patient’s physiologic reserve and resistance to stressors [8,9,10]. Frailty is defined as a decrease in physiologic reserves giving rise to vulnerability separate from the normal aging process [8,11]. Although there is a universal intuitive recognition of frailty by most physicians caring for older people, there is not a standardized assessment tool to quantify the phenomenon of frailty used frequently in clinical practice [12]. To quantify frailty, two models have been described. The first, a physical phenotype model described by Fried et. al is based on the following characteristics: unintentional weight loss, exhaustion, weakened grip strength, slow walking, and low physical activity [11,13,14]. The second, a multiple domain aggregate model validated by a number of studies including a 70-item scale large population-based study, the Canadian Study of Health and Aging, is based on the concept of cumulative deficits integrating medical, psychological, and functional capabilities [13,15]. A simplified modified frailty index (mFI), based on 11 clinical variables has been derived from the later model and validated in several surgical studies [11,15,16].
To our knowledge, the mFI has not been considered for older adults undergoing paraesophageal hernia repair. We hypothesized that the use of the mFI would be a predictor of adverse occurrences and mortality in patients ≥60 years old undergoing paraesophageal hernia repair.
2. Methods
We utilized the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) participant user file (PUF), years 2011–2013 for this study. Patients were included if they underwent surgery based on CPT and ICD-9 codes (Table 1), and if they were ≥60 years. Exclusion criteria included patients with missing preoperative data in reference to any of the 11 modified frailty variables (Table 2). A mFI based on these 11 variables was calculated for each patient by adding the number of variables present for each patient. Patients with a score of 3 or greater were classified as a high frailty cohort.
Table 1.
CPT Procedure and ICD-9 Diagnosis Codes
| CPT Procedure Codes and Description | |
|---|---|
| 43281 | Laparoscopy, surgical, repair of paraesophageal hernia, without mesh |
| 43282 | Laparoscopy, surgical, repair of paraesophageal hernia, with mesh |
| 43332 | Open, repair of paraesophageal hiatal hernia, via laparotomy, without mesh |
| 43333 | Open, repair of paraesophageal hiatal hernia, via laparotomy, with mesh |
| 43334 | Thoracic repair, repair of paraesophageal hiatal hernia, via thoracotomy, without mesh |
| 43335 | Thoracic repair, repair of paraesophageal hiatal hernia, via thoracotomy, with mesh |
| 43336 | Repair of paraesophageal hiatal hernia, via thoracoabdominal incision, without mesh |
| 43337 | Repair of paraesophageal hiatal hernia, via thoracoabdominal incision, with mesh |
| ICD-9 Diagnosis Codes and Description | |
| 551.3 | Diaphragm hernia with gangrene |
| 552.3 | Diaphragm hernia with obstruction |
| 553.3 | Diaphragm hernia |
Table 2.
The 11 variables of the modified frailty index (mFI)
| COPD, or recent pneumonia | Peripheral vascular disease or ischemic rest pain |
| Congestive heart failure | Functional status (not independent) |
| Myocardial infarction | Hypertension requiring medication |
| PCI, PCS, or angina | TIA or CVA |
| Diabetes mellitus | CVA with neurologic deficit |
| Impaired sensorium |
COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; PCS, prior cardiac surgery; CVA, cerebrovascular accident; TIA, transient ischemic attack; Functional status in the 30 days prior to surgery.
From the NSQIP database, we abstracted details of patient’s age, sex, race, procedure timing [emergent versus non emergent], functional status, ASA classification, wound classification, preoperative serum albumin, and procedure type [laparoscopic versus open]. The occurrence of 30-day postoperative adverse outcomes and mortality were evaluated and analyzed relative to the mFI. The primary outcomes analyzed were 30-day occurrences of superficial surgical site infection, acute renal failure, peripheral nerve injury, deep incisional surgical site infection, organ space surgical site infection, wound disruption, DVT/thrombophlebitis, pulmonary embolism, pneumonia, urinary tract infection, bleeding requiring transfusion, sepsis, reoperation, need for ventilator >48 hours, unplanned intubation, septic shock, myocardial infarction, cardiac arrest requiring CPR, coma, progressive renal insufficiency, and mortality. The secondary outcomes analyzed were discharge destination and readmission.
The severity of the postoperative complications was evaluated using the Clavien-Dindo Classification system. The Clavien-Dindo surgical complication grading system ranks complications based on the magnitude of the interventions required to manage the complication and whether that complication causes permanent disability or death [11,17,18]. The above postoperative complications tracked through NSQIP were grouped based on how they are treated in routine clinical practice under Clavien-Dindo grading criteria (Table 3) [18].
Table 3.
Clavien Classification of Surgical Complications
| Grade | Definition |
|---|---|
| I |
Any postoperative complication that does not require interventions; Superficial SSI, Acute Renal Failure, Renal Insufficiency, Neurologic defecit/peripheral nerve injury |
| II |
Postoperative complications requiring pharmalogic interventions; Deep Incisional SSI, Organ/Space SSI, Wound Disruption/Dehiscence, Deep Venous Thrombosis, Pulmonary Embolism, Pneumonia, Urinary tract infection, Transfusion, Sepsis |
| III |
Postoperative complications requiring surgical, radiologic, or endoscopic interventions; Reoperation |
| IV |
Life-threatening complications requiring intensive care unit management; Ventilator >48 hours, Reintubation/Unplanned intubation, Septic shock, Myocardial infarction, Cardiac Arrest, Coma, Progressive Renal Failure |
| V |
Complications leading to death; Death |
For statistical analysis, univariate analysis of categorical data was performed using Chi-square tests and Fischer exact tests. Multiple logistic regression analysis was presented as Odds Ratio (95% CI) and used to determine each outcome by mFI category after adjusting for the other variables in the model. Logistic regression analysis excluded unknown and expired patients. We chose to omit the records of patients missing one or more of the 11 variables used to calculate the mFI from final analysis. A p value < 0.05 was considered to be statistically significant. Statistical analysis was conducted using SAS 9.2 (SAS Institute, Cary, NC).
3. Results
Of the 4434 PEH repairs performed in patients ≥ 60 years old in the study interval, 885 records were included in the final analysis (20%). The remaining patients were excluded because the NSQIP PUF was missing one or more of the variables used to calculate the 11 item mFI. Of those included in the final analysis, 76.3% were females, 81.9% underwent a laparoscopic procedure, 88% were white, 94.2% were elective procedures, 48.4% had 1/11 mFI variables, 56.9% had an ASA of ≥3, and 56.6% had a clean wound class (Table 4). The primary outcomes were 30-day postoperative complications graded according to the Clavien-Dindo Classification system, and mortality. The secondary outcomes were discharge destination and 30-day readmission rate. There were 6.1% patients with a Clavien-Dindo grade of ≥3, 0.9% mortality, 10.9% were discharged to facility other than home, and 8.2% were readmitted (Table 5). Because only 43 patients recorded to have a mFI of ≥3, these patients were grouped into one cohort for analysis (Table 6).
Table 4.
Patient Characteristics
| Total (N=885) | Total (N=885) | ||
|---|---|---|---|
| Age Category | Procedure Type | ||
| 80+ | 184 (20.8%) | Laparoscopic | 725 (81.9%) |
| 75–79 | 142 (16.0%) | Open | 160 (18.1%) |
| 70–74 | 190 (21.5%) | Surgical Timing | |
| 65–69 | 194 (21.9%) | Emergent | 51 (5.8%) |
| 60–64 | 175 (19.8%) | Non emergent | 834 (94.2%) |
| Gender | ASA Class | ||
| Female | 675 (76.3%) | 4/5, Life threat/Moribund | 28 (3.2%) |
| Male | 210 (23.7%) | 3, Severe Disturb | 475 (53.7%) |
| Race | 1/2, No disturb/Mild Disturb | 382 (43.2%) | |
| White | 779 (88.0%) | Wound Class | |
| Black | 24 (2.7%) | 3/4, Contaminated, Dirty/Infected | 19 (2.1%) |
| Hispanic | 18 (2.0%) | 2, Clean contaminated | 365 (41.2%) |
| Other | 64 (7.2%) | 1, Clean | 501 (56.6%) |
Table 5.
30 Day Outcomes
| Total (N=885) | Total (N=885) | ||
|---|---|---|---|
| Clavien Dindo Grade | Bleeding Requiring Transfusion | ||
| 0 | 784 (88.6%) | Yes | 19 (2.1%) |
| 1 | 6 (0.7%) | No | 866 (97.9%) |
| 2 | 41 (4.6%) | Sepsis | |
| 3 | 19 (2.1%) | Yes | 8 (0.9%) |
| 4 | 27 (3.1%) | No | 877 (99.1%) |
| 5 | 8 (0.9%) | Return to Operating Room | |
| Superficial Surgical Site Infection | Yes | 29 (3.3%) | |
| Yes | 7 (0.8%) | No | 856 (96.7%) |
| No | 878 (99.2%) | Ventilator > 48 Hours | |
| Progressive Renal Insufficiency | Yes | 13 (1.5%) | |
| Yes | 1 (0.1%) | No | 872 (98.5%) |
| No | 884 (99.9%) | Unplanned Intubation | |
| Peripheral Nerve Injury | Yes | 13 (1.5%) | |
| No | 885 (100%) | No | 872 (98.5%) |
| Deep Incisional Surgical Site | Septic Shock | ||
| Infection | |||
| Yes | 3 (0.3%) | Yes | 9 (1.0%) |
| No | 882 (99.7%) | No | 876 (99.0%) |
| Organ Space Surgical Site Infection | Myocardial Infarction | ||
| Yes | 8 (0.9%) | Yes | 12 (1.4%) |
| No | 877 (99.1%) | No | 873 (98.6%) |
| Wound Disruption | Cardiac Arrest | ||
| Yes | 1 (0.1%) | Yes | 3 (0.3%) |
| No | 884 (99.9%) | No | 882 (99.7%) |
| DVT/Thrombophlebitis | Coma > 24 Hours | ||
| Yes | 9 (1.0%) | No | 885 (100%) |
| No | 876 (99.0%) | Acute Renal Failure | |
| Pulmonary Embolism | Yes | 1 (0.1%) | |
| Yes | 5 (0.6%) | No | 884 (99.9%) |
| No | 880 (99.4%) | Mortality | |
| Pneumonia | Alive | 877 (99.1%) | |
| Yes | 27 (2.6%) | Dead | 8 (0.9%) |
| No | 858 (96.9%) | Discharge Disposition | |
| Urinary Tract Infection | Home | 776 (89.1%) | |
| Yes | 23 (2.6%) | Not Home | 95 (10.9) |
| No | 862 (97.4%) | Readmission | |
| Yes | 68 (8.2%) | ||
| No | 765 (91.8%) |
Table 6.
Frailty Index Score Distribution
| Frailty Index Score | Total (n=885) |
|---|---|
| 3+ | 43 (4.9%) |
| 2 | 163 (18.4%) |
| 1 | 428 (48.4%) |
| 0 | 251 (28.4%) |
3.1. Clavien-Dindo Outcomes
Univariate analysis with Chi-square testing revealed a higher frailty score was predictive of a higher Clavien-Dindo complication grade. The percentage of patients with a Clavien-Dindo grade ≥3 complication relative to a mFI score of 0, 1, 2, and ≥3 were 3.2%, 4.7%, 9.8%, and 23.3% respectively (p <0.0001) (Graph 1). Ordinal logistic regression revealed a mFI score of ≥3 was the strongest predictor for Clavien-Dindo complications grade ≥3 (OR 3.51; CI 1.46–8.46) when compared to age ≥80 years (OR 3.31; CI 1.41–7.78), gender (OR 1.03; CI 0.63–1.69), Hispanic or other race (OR 1.55; CI 0.70–3.42), surgical timing [emergent versus non emergent] (OR 1.42; CI 0.67–3.01), ASA class ≥3 (OR 1.71; CI 0.65–4.48), wound class ≥3 (OR 1.99; CI 0.56–7.10), prealbumin less ≤3 (OR 2.39; CI 1.06–5.38), and procedure type [laparoscopic versus open] (OR 0.42; CI 0.25–0.69) (Table 7).
Graph 1.
Clavien-Dindo Complication rate relative to frailty index score*. * indicates p-value <0.05
Table 7.
Predictors of outcomes based on logistic regression. Presented as Odds Ratio (95% CI). Logistic regression was not performed on mortality.
| Complications Grade ≥3 |
Discharge Destination | Readmission | |
|---|---|---|---|
| Frailty Index score ≥3 | 3.51 (1.46–8.46)* | 4.07 (1.29–12.82)* | 1.01 (0.36–2.84) |
| Age ≥80 years | 3.31 (1.41–7.78)* | 16.28 (4.68–56.72)* | 2.32 (0.98–5.47) |
| Gender (female) | 1.03 (0.63–1.69) | 1.60 (0.86–2.97) | 0.66 (0.38–1.16) |
| Race (Hispanic) | 1.55 (0.70–3.42) | 1.43 (0.32–6.37) | -- |
| Race (Black) | -- | -- | 2.81 (0.96–8.24) |
| Surgical timing (emergent) | 1.42 (0.67–3.01) | 2.58 (1.13–5.89)* | 1.89 (0.81–4.43) |
| ASA class ≥3 | 1.71 (0.65–4.48) | 3.14 (1.05–9.39)* | 2.21 (0.66–7.38) |
| Wound class ≥3 | 1.99 (0.56–7.10) | 5.41 (1.54–19.02)* | 1.25 (0.26–5.92) |
| Prealbumin ≤3 | 2.39 (1.06–5.38)* | 4.64 (1.93–11.14)* | 0.62 (0.17–2.31) |
| Procedure type (open) | 0.42 (0.25–0.69) | 0.51 (0.28–0.95) | 1.06 (0.56–2.03) |
indicates p-value <0.05
3.2 Mortality
Univariate analysis with Fischer exact testing revealed a higher frailty score correlated with a higher mortality rate, but was not statistically significant. The percentage of patients who experienced 30-day mortality relative to a mFI score of 0, 1, 2, and ≥3 were 0.0%, 0.9%, 1.8%, and 2.3% respectively (p 0.0974) (Graph 2). Logistic regression could not be performed due to a low event rate in the study (N=8).
Graph 2.
Mortality
3.3 Discharge Destination
Univariate analysis with Chi-square testing revealed a higher frailty score was predictive of a discharge destination to a facility other than home. The percentage of patients discharged to a facility other than home relative to a mFI score of 0, 1, 2, and ≥3 was 4.4%, 10.9%, 15.7%, and 31.7% respectively (p <0.0001) (Graph 3). Logistic regression revealed a mFI score of ≥3 was the strongest predictor for discharge to a facility other than home (OR 4.07; CI 1.29–12.82) when compared to gender (OR 1.60; CI 0.86–2.97), Hispanic race (OR 1.43; CI 0.32–6.37), surgical timing [emergent versus non emergent] (OR 2.58; CI 1.13–5.89), ASA class ≥3 (OR 3.14; CI 1.05–9.39), and procedure type [laparoscopic versus open] (OR 0.51; CI 0.28–0.95) (Table 7). Three other variables were stronger predictors for discharge to a facility other than home when compared to the mFI score and are as follows; Age of ≥80 years (OR 16.28; CI 4.68–56.72), wound class ≥3 (OR 5.41; CI 1.54–19.02), and prealbumin less ≤3 (OR 4.64; CI 1.93–11.14) (Table 7).
Graph 3.
Discharge Destination rate relative to frailty index score*. * indicates p-value <0.05
3.4 Readmission
Univariate analysis with Chi-square testing revealed a higher frailty score correlated to a higher likelihood for readmission, but was not statistically significant. The percentage of the 30-day readmission rate relative to a mFI score of 0, 1, 2, and ≥3 was 8.9%, 6.8%, 8.5%, and 16.3%% respectively (p 0.1703) (Graph 4). Using logistic regression a mFI score of ≥3 had a non significant higher odds ratio for readmission (OR 1.01; CI 0.36–2.84) when compared to gender (OR 0.66; CI 0.38–1.16) and prealbumin ≤3 (OR 0.62; CI 0.17–2.31) (Table 7). Other variables retained non significant higher odds ratios for readmission when compared to the mFI score of ≥3 and are as follows; age ≥80 years (OR 2.32; CI 0.98–5.47), Black race (OR 2.81; CI .96–8.24), surgical timing [emergent versus non emergent] (OR 1.89; CI 0.81–4.43), ASA class ≥3 (OR 2.21; CI 0.66–7.38), wound class ≥3 (OR 1.25; CI 0.26–5.92), and procedure type [laparoscopic versus open] (OR 1.06; CI 0.56–2.03) (Table 7).
Graph 4.
Readmission rate relative to frailty index score
4. Discussion
Generally speaking, older adult patients carry an increasing burden of chronic illnesses affecting overall health and wellbeing [16]. Geriatric surgery patients have unique physiologic vulnerability requiring assessment beyond traditional preoperative evaluation of older adults [10]. Frailty is a unique domain of health status that can be a marker of decreased reserves and resultant vulnerability in older patients [9]. Most surgeons recognize that an 80 year old may have a lifestyle and physical appearance that makes him/her seem more like a 60 year old, and another patient may appear and act greater than his/her actual age. Frailty might help explain why some older patients recover better than expected and others fare worse [9]. In addition, frailty may improve our understanding of the heterogeneity of vulnerability in the elderly. As the population ages and the incidence of symptomatic paraesophageal hernias increases with age, identifying frail patients at high risk for postoperative morbidity and mortality is becoming increasingly important.
Paraesophageal hernias account for about 5–10% of all hiatal hernias but are an important clinical entity because of their presentation and greater prevalence in older individuals [1,2,3]. Herniation of the stomach into the chest in elderly patients is a dilemma to many physicians, often compromising surgical consultation secondary to fear of increased morbidity and mortality, a perceived lack of symptomatic benefit from intervention, and poor understanding of the natural history of untreated paraesophageal hernias [7,22]. Symptomatic paraesophageal hernias represent a disease process for which surgery remains the best treatment [6,19]. But how to manage non-symptomatic or minimally symptomatic PEHs has been a topic of controversy.
The mortality from emergent surgical intervention for PEHs had traditionally been overestimated, with studies suggesting a mortality ranging from 17–40% [23,24,25]. This had led to a trend towards prophylactic repairing of non-symptomatic and minimally symptomatic PEHs to prevent emergent scenarios. However, more recent data has challenged this approach of management, favoring watchful waiting for non-symptomatic and minimally symptomatic PEHs [22]. Contemporary mortality rates following emergent PEH repair are estimated at 5.4–8%, and 0.8–1.4% following elective PEH repair [22,26,27]. The decrease in mortality incidence has largely been attributed to the widespread adoption of laparoscopic interventions and advances in perioperative care. Our data showed 0.0% of the 51 patients who underwent emergent intervention experienced 30-day mortality. Of those experiencing mortality, 1.1% were ≥ 80 years old. Mortality occurred in 0.7% of laparoscopic cases and in 1.9% of open surgical repairs. Higher frailty had a tendency to predict higher mortality, though the data failed to reach statistical significance. In the dataset we chose, mortality was an infrequent event, making definitive conclusions with regards to mortality not possible.
In previous work using the Nationwide Inpatient Sample (NIS) we demonstrated that for PEH repair, younger age, elective case status, and a laparoscopic approach were all associated with a lower probability of complications and morbidity [28]. We also determined that patients undergoing emergent repair were more likely to be older than those having an elective repair. What the current and cited studies are not able to discern is what happened to those patients deemed too old or frail to have surgery that were managed expectantly at first. Perhaps some of these patients with a good functional status would have been offered surgery at an earlier stage of disease avoiding a more urgent or emergent operation. Conversely, a patient demonstrating a high degree of frailty and poor functional status may have been counseled to avoid surgery despite symptoms related to the paraesophageal hernia.
The lack of clinically practical tools of assessing frailty and robust objective data on predicting PEH repair outcomes based on frailty in the above scenarios has likely contributed to the lack of informed consensus in management patterns. Widespread adoption of objective frailty assessment in preoperative patients has been slow due to various limitations including the lack of time to complete frailty assessments [20]. The mFI appears to be a promising tool of assessing frailty. The use of the mFI is practical because the variables are easily available in the clinical setting; it is easily applied to national databases, and requires less time in assessing frailty [16,21]. The mFI uses patient factors found by simple history alone, and disposes the need for additional trained personnel to evaluate and calculate a patient’s frailty index [21]. The mFI is also easily adaptable to an acute setting where variables like gait speed or grip strength would be difficult to ascertain [21].
Our data verifies the work of others showing that the mFI is useful in predicting postoperative complications [11,16]. A higher frailty score was statistically significant in predicting severe complications. In relation to Clavien Dindo complications, a higher frailty score (mFI ≥3) was the strongest predictor of outcome relative to age, gender, race, surgical timing [emergent versus non emergent], ASA class ≥3, wound class ≥3, prealbumin ≤3, and procedure type [laparoscopic versus open].
We defined the frail cohort as mFI ≥ 3, even though it resulted in a relatively small portion of frail patients. We could have lowered the frailty threshold to mFI ≥ 2 resulting in a larger cohort of frail patients, but had several concerns of creating a frailty threshold that was too liberal. Since the 11-point mFI is based on the model of accumulating deficits, setting the number of variables used to define the most frail cohort at a low number runs the risk that patients who are not actually frail are assessed as such. We would have liked to set the bar even higher, but the number of patients with 4 and 5 items in the mFI was extremely low. The reason we ended up with a relatively small frail population is largely secondary to the exclusion of a relatively high number of patients who otherwise met the study inclusion criteria but were missing 1 or more variables used to calculate the mFI. This is a limitation of the study, and we are working on a study where we examine which of the 11 items used to create the frailty index may be unnecessary in an attempt to create a frailty index that is valid with even fewer items to assess. A larger percentage of patients could then be included in the analysis, limiting these concerns.
Some data following surgical intervention has suggested that old age, emergency admission, raised Charlson co-mobidity index, polypharmacy, poor admission mobility, ASA class of ≥4, obesity, large surgical procedure, and length of stay ≥9 are associated with discharge to a facility other than home [29,30]. Frailty has not been robustly studied in this regard. Our data showed the mFI is useful in predicting discharge destination to home versus facility other than home. A higher frailty score was statistically significant in predicting discharge to a destination other than home. In relation to discharge destination other than home, a higher frailty score (mFI ≥3) was the strongest predictor of outcome relative to gender, Hispanic or Black race, surgical timing [emergent versus non emergent], ASA class ≥3, and procedure type [laparoscopic versus open]. Nonetheless, it is noteworthy to recognize that age > 80 years was a strong predictor of outcomes, especially discharge destination, in patients undergoing PEH repair. Although this was not the primary objective of our study, the data is congruent with above literature. For that reason age should be factored in assessing perioperative outcomes. However, this should be done with caution as we think that age is more of a surrogate marker for frailty in our study. The incidence of frailty increases with age, and our data corroborates this notion as 9.8% of patients > 80 years had a mFI ≥ 3 compared to 3.6% for patients < 80 years in this study (p < 0.01). Therefore, an alternate way of considering this data would be such that frailty helps explain age related outcomes; it is perhaps why one patient > 80 years old fares better than another of similar age following PEH repair.
The NSQIP data is prospectively collected hence, biases associated with retrospective data collection was minimized [16]. Weaknesses of this study include its retrospective nature, as demonstrated by missing variables needed to calculate the mFI. NSQIP hospitals self-select to participate in this quality improvement program. Outcomes at NSQIP hospitals may therefore not be representative of care throughout the United States, especially when it comes to a complex operation like paraesophageal hernia repair. This phenomenon has been demonstrated in literature, for example noting significant differences when comparing outcomes of PEH repair and esophageal cancer resection between the NIS data (an administrative database) and the NSQIP data (a clinical database) [26,31]. Additional limitations of this study include the fact that a large numbers of otherwise eligible patients were excluded from the current analysis, as the more recent participant user files of the NSQIP database did not capture all the 11 preoperative variables used to construct the mFI. Because so many patients were excluded, a relatively small number of patients had a mFI ≥ 3, limiting our ability to evaluate outcomes in patients at the extremes of frailty based on the accumulating deficits model. It is possible that relatively few of these patients undergo PEH repair, but further study will be necessary to determine if this is the case. Further, the NSQIP PUF does not contain information regarding the symptoms related to the hernia, the size of the hernia, the contents of the hernia, nor the exact type of hernia, preventing the complexity of the case from being adequately captured. Future investigation is needed to better quantify frailty based on the clinical variables contained in the NSQIP dataset. A more concise version of the frailty index with fewer variables more consistently collected in NSQIP may allow a larger cohort of patients to be studied, and may simplify the application of a clinical frailty index.
5. Conclusions
In conclusion, the mFI is predictive of 30-day postoperative complications and discharge to a facility other than home after surgery for paraesophageal hernia. The mFI is suggestive of a trend of increased mortality and readmission with increasing frailty scores. Identifying the frail elderly patient continues to be of paramount importance, as it identifies the patient at higher risk for poor outcomes, and thus allows for specialized preoperative and postoperative planning in care and disposition of such a patient to improve outcomes. However, the 11-point mFI may not be the ideal measure to assess frailty using the NSQIP dataset due to significant portions of otherwise eligible patients being excluded from this analysis.
Acknowledgments
Matthew Frelich, Matthew Bosler, Lisa Rein, Aniko Szabo and Jon Gould for contributions towards project. The American College of Surgeons National Surgical Quality Improvement Program for the data. Authors supported, IN PART, by the National Center for Advancing Translational, Sciences, National Institutes of Health, through Grant Number 8UL1TR000055.
Footnotes
Author Contributions
Munyaradzi Chimukangara, Matthew Frelich, and Matthew Bosler facilitated all project related tasks. Lisa Rein and Aniko Szabo conducted statistical analysis. Jon Gould conceived of the study, provided oversight, and conducted critical review and revision of the final manuscript.
Disclosures
Munyaradzi Chimukangara, Matthew Frelich, Matthew Bosler, Lisa Rein, and Aniko Szabo declare no conflicts of interests. Jon Gould is a consultant for Torax Medical.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhayani N, Kurian A, Sharata A, Reavis K, Dunst C, Swanstrom L. Wait only to resuscitate: early surgery for acutely presenting paraesophageal hernias yields better outcomes. Surg Endosc. 2013;27:267–71. doi: 10.1007/s00464-012-2436-8. [DOI] [PubMed] [Google Scholar]
- 2.Gangopadhyay N, Perrone J, Soper N, Matthews B, Eagon J, Klingensmith M, et al. Outcomes of laparoscopic paraesophageal hernia repair in elderly and high-risk patients. Surgery. 2006;140(4):491–9. doi: 10.1016/j.surg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Polomsky M, Jones C, Sepesi B, O’Connor M, Matousek A, Hu R, et al. Should Elective Repair of Intrathoracic Stomach be Encouraged? J Gastrointest Surg. 2010;14(2):203–10. doi: 10.1007/s11605-009-1106-1. [DOI] [PubMed] [Google Scholar]
- 4.Sihvo E, Salo J, Räsänen J, Rantanen T. Fatal complications of adult paraesophageal hernia: A population-based study. J Thorac Cardiovasc Surg. 2009;137(2):419–24. doi: 10.1016/j.jtcvs.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Census Bureau. Projections of the population by sex and selected age groups for the United States: 2015 to 2060. Retrieved March 25, 2015 from http://www.census.gov/population/projections/data/national/2014/summarytables.html.
- 6.Kubasiak J, Hood K, Daly S, Deziel D, Myers J, Millikan K, et al. Improved Patient Outcomes in Paraesophageal Hernia Repair Using a Laparoscopic Approach: A Study of the National Surgical Quality Improvement Program Data. Am Surg. 2014;80(9):884–9. [PubMed] [Google Scholar]
- 7.Louie B, Blitz M, Farivar A, Orlina J, Aye R. Repair of Symptomatic Giant Paraesophageal Hernias in Elderly (>70 Years) Patients Results in Improved Quality of Life. J Gastrointest Surg. 2011;15(3):389–96. doi: 10.1007/s11605-010-1324-6. [DOI] [PubMed] [Google Scholar]
- 8.Karam J, Tsiouris A, Shepard A, Velanovich V, Rubinfeld I. Simplified Frailty Index to Predict Adverse Outcomes and Mortality in Vascular Surgery Patients. Ann Vasc Surg. 2013;27(7):904–8. doi: 10.1016/j.avsg.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Makary M, Segev D, Pronovost P, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a Predictor of Surgical Outcomes in Older Patients. J Am Coll Surg. 2010;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Robinson T, Eiseman B, Wallace J, Church S, McFann K, Pfister S, et al. Redefining geriatric preoperative assessment using frailty, disability, and co-morbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 11.Uppal S, Igwe E, Rice L, Spencer R, Rose S. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101. doi: 10.1016/j.ygyno.2015.01.532. [DOI] [PubMed] [Google Scholar]
- 12.Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The Assessment of Frailty in Older Adults. Clin Geriatr Med. 2010;26(2):275–86. doi: 10.1016/j.cger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Pilotto A, Rengo F, Marchionni N, Sancarlo D, Fontana A, Panza F, et al. Comparing the Prognostic Accuracy for All-Cause Mortality of Frailty Instruments: A Multicentre 1-Year Follow-Up in Hospitalized Older Patients. In: Vina J, editor. PLoS One. 1. Vol. 7. 2012. p. e29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulminski A, Ukraintseva S, Kulminskaya I, Arbeev K, Land K, Yashin A. Cumulative Deficits Better Characterize Susceptibility to Death in the Elderly than Phenotypic Frailty: Lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodari A, Hammoud Z, Borgi J, Tsiouris A, Rubinfeld I. Assessment of Morbidity and Mortality After Esophagectomy Using a Modified Frailty Index. Ann Thorac Surg. 2013;96(4):1240–45. doi: 10.1016/j.athoracsur.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104–110. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma V, Aggarwal A, McGuire B, Rambachan A, Matulewicz R, Kim J, et al. Open vs Minimally Invasive Partial Nephrectomy: Assessing the Impact of BMI on Postoperative Outcomes in 3685 Cases from National Data. J Endourol. 2015;29(5):561–7. doi: 10.1089/end.2014.0608. [DOI] [PubMed] [Google Scholar]
- 19.Poulose B, Gosen C, Marks J, Khaitan L, Rosen M, Onders R, et al. Inpatient Mortality Analysis of Paraesophageal Hernia Repair in Octogenarians. J Gastrointest Surg. 2008;12:1888–92. doi: 10.1007/s11605-008-0625-5. [DOI] [PubMed] [Google Scholar]
- 20.Revenig L, Canter D, Kim S, Liu Y, Sweeney J, Sarmiento J, et al. Report of a Simplified Frailty Score Predictive of Short-Term Postoperative Morbidity and Mortality. J Am Coll Surg. 2015;220(5):904–11. doi: 10.1016/j.jamcollsurg.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 21.Farhat J, Velanovich V, Falvo A, Horst H, Swartz A, Patton J, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526–31. doi: 10.1097/TA.0b013e3182542fab. [DOI] [PubMed] [Google Scholar]
- 22.Stylopoulos N, Gazelle G, Rattner D. Paraesophageal Hernias: Operation or Observation? Ann Surg. 2002;236(4):492–501. doi: 10.1097/00000658-200210000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner D, Belsey R. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg. 1967;53:33–54. [PubMed] [Google Scholar]
- 24.Hill L. Incarcerated paraesophageal hernia. A surgical emergency. Am J Surg. 1973;126(2):286–91. doi: 10.1016/s0002-9610(73)80165-5. [DOI] [PubMed] [Google Scholar]
- 25.Beardsley J, Thompson W. Acutely Obstructed Hiatal Hernia. Ann Surg. 1964;159:49–62. doi: 10.1097/00000658-196401000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augustin T, Schneider E, Alaedeen D, Kroh M, Aminian A, Reznick D, et al. Emergent Surgery Does Not Independently Predict 30-Day Mortality After Paraesophageal Hernia Repair: Results from the ACS NSQIP Database. J Gastrointest Surg. 2015;19:2097–2104. doi: 10.1007/s11605-015-2968-z. [DOI] [PubMed] [Google Scholar]
- 27.Luketich J, Nason K, Christie N, Pennathur A, Jobe B, Landreneau R, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg. 2010;139(2):395–404. doi: 10.1016/j.jtcvs.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jassim H, Seligman J, Felich M, Goldblatt M, Kastenmeier A, Wallace J, et al. A population-based analysis of emergent versus elective paraesophageal hernia repair using the Nationwide Inpatient Sample. Surg Endosc. 2014;28(12):3473–78. doi: 10.1007/s00464-014-3626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark D, Ostrander K, Cushing B. A Multistate Model Predicting Mortality, Length of Stay, and Readmission for Surgical Patients. BMC Health Serv Res. 2015 doi: 10.1111/1475-6773.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambler G, Brooks D, Al Zuhir N, Ali A, Gohel M, Hayes P, et al. Effect of frailty on short- and mid-term outcomes in vascular surgical patients. Br J Surg. 2015;102:638–45. doi: 10.1002/bjs.9785. [DOI] [PubMed] [Google Scholar]
- 31.Lapar D, Stukenborg G, Lau C, Jones D, Kozower B. Differences in reported esophageal cancer resection outcomes between national clinical and administrative databases. J Thorac Cardiovasc Surg. 2012;144(5):1152–57. doi: 10.1016/j.jtcvs.2012.08.010. [DOI] [PubMed] [Google Scholar]