Abstract
Background: Knowledge about influenza transmission in the workplace and whether staying home from work when experiencing influenza-like illness can reduce the spread of influenza is crucial for the design of efficient public health initiatives. Aim: This review synthesizes current literature on sickness presenteeism and influenza transmission in the workplace and provides an overview of sick leave recommendations in Europe for influenza. Methods: A search was performed on Medline, Embase, PsychINFO, Cinahl, Web of Science, Scopus and SweMed to identify studies related to workplace contacts, -transmission, -interventions and compliance with recommendations to take sick leave. A web-based survey on national recommendations and policies for sick leave during influenza was issued to 31 European countries. Results: Twenty-two articles (9 surveys; 13 modelling articles) were eligible for this review. Results from social mixing studies suggest that 20–25% of weekly contacts are made in the workplace, while modelling studies suggest that on average 16% (range 9–33%) of influenza transmission occurs in the workplace. The effectiveness of interventions to reduce workplace presenteeism is largely unknown. Finally, estimates from studies reporting expected compliance with sick leave recommendations ranged from 71 to 95%. Overall, 18 countries participated in the survey of which nine (50%) had issued recommendations encouraging sick employees to stay at home during the 2009 A(H1N1) pandemic, while only one country had official recommendations for seasonal influenza. Conclusions: During the 2009 A(H1N1) pandemic, many European countries recommended ill employees to take sick leave. Further research is warranted to quantify the effect of reduced presenteeism during influenza illness.
Introduction
Each year, seasonal influenza epidemics are widespread and have severe morbidity, mortality and economic consequences. The cost burden of influenza on society are mainly indirect costs resulting from lost productivity.1,2 Influenza is said to account for 10–12%3,4 of total sickness absences from work and yearly productivity losses due to influenza in Europe have been estimated at £1465 million and £131 million in France and Norway, respectively.2
Sick leaves protect employees who need to recover from illness; however, work absences are costly. On the other hand, employees who continue to work while objectively ill (presenteeism) may also impose costs on society. Their productivity at work may be impaired and, if they are infectious, they increase the risk that co-workers and other contacts in the workplace contract influenza. Thus work absence during influenza illness might reduce the influenza attack rate in the workplace and in the population, which would be desirable from both a public health and economic perspective.
Several countries have issued recommendations to reduce presenteeism during influenza illness. For example, during the 2009 H1N1 pandemic the United States Centres for Disease Control and Prevention (CDC) first recommended 7 days of work absence following symptom onset; later, the advice was changed to recommending persons to stay at home for 24 h after fever cessation.5 Similarly, in 2010, the World Health Organization (WHO) advised persons ill with pandemic influenza to remain at home until symptom abatement, despite feeling well enough to work, with the purpose of protecting work colleagues and others in the community.6
However, the extent to which reduced presenteeism can affect attack rates in workplaces and in the general population has not been well documented. The European Centre for Disease Prevention and Control’s public health pandemic influenza guide from 2009 stated that research on influenza transmission in the workplace was absent from the literature.7 The same conclusion has been reached by other researchers.8–10
This article aims to compile current knowledge on workplace transmission, interventions to reduce presenteeism and public compliance (including recent research stimulated by the H1N1 pandemic in 2009) and to survey current European policies on presenteeism during influenza illness. This will provide policy makers and others with an updated overview and may assist in decision making for public health measures and in highlighting research needs.
Methods
The literature review
Studies reporting on (i) proportion and closeness of contacts made in the workplace; (ii) influenza transmission in the workplace; (iii) effectiveness of workplace interventions to reduce presenteeism and (iv) compliance with recommendations to stay at home during influenza illness were included. A search was conducted in Medline, Embase, PsychINFO, Cinahl, Web of Science, Scopus, SweMed and Google Scholar using a wide range of keywords. Secondary sources cited in these articles were also included if relevant. The search was restricted to include articles in English, Norwegian, Swedish, Danish and German.
European survey on sick leave policies for influenza
A survey was conducted among the 31 countries in the European Influenza Surveillance Network, consisting of all European Union nations, Norway, Liechtenstein and Iceland. The national representatives were first contacted by email and informed about the intended purpose of the study. The survey was conducted online via Questback©. The questions addressed national recommendations and policies for work absence during seasonal and pandemic influenza illness, what the recommendation or policy was based upon, the main reasons for having a recommendation or policy, what year they were last published or updated and which bodies issued them. In the final question, respondents were asked to share their thoughts about employers’ opinions or reactions to influenza-associated sick leave; for this particular question, the anonymity of the respondent was guaranteed.
Results
Literature review
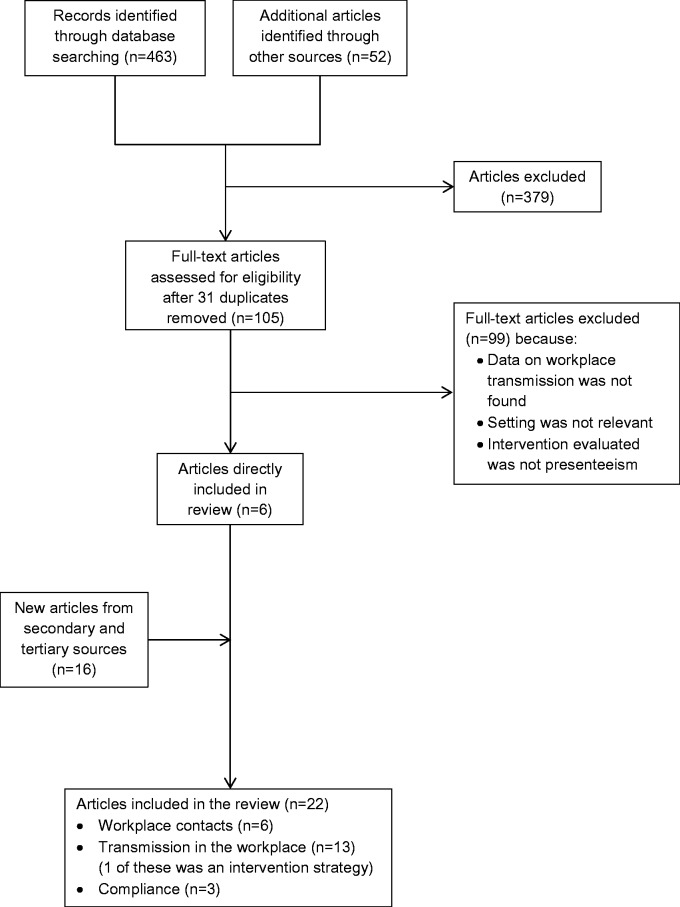
The search identified 514 articles. Many articles had to be excluded due to a lack of quantitative details about workplace interventions or because workplace interventions were combined with other social distancing interventions and no separate effects were reported. Together with other reasons for ineligibility, the majority of papers were excluded and only 22 studies remained for this review (figure 1). Of the included studies, 6 were social mixing studies, 13 were modelling studies and 3 were survey studies on public compliance.
Figure 1.
Outline of the search method used for the review
Workplace contacts
Three population-based studies including data from 10 countries in Europe and Asia were found (table 1). The mean proportion of the total weekly contacts made in the workplace was 20% (range 4–25%). All country-specific estimates in developed settings were relatively similar (19–25%), while the low value of 4% was obtained from a rural setting in Vietnam.12 The average proportion of workplace contacts that were physical was 34% (range 13–48%) and was reported by nine countries. Additionally, we found three studies among university staff and students; in those settings the proportion of workplace contacts ranged from 13% in a Belgian study,14 to 57% and 76%, respectively, in two studies from England covering weekdays only.15,16
Table 1.
Estimated weekly proportion of contacts in the workplace and the proportion of workplace contacts that are close/physical from social mixing studies
| Workplace contacts (%) | Close/physical contacts (%) | Study size (N) | Sample period (year) | Population | Country | Reference |
|---|---|---|---|---|---|---|
| 21 | 45 | 750 | 2006 | Population based | Belgium | Mossong et al.11 |
| 19 | 37 | 1341 | 2006 | Population based | Germany | Mossong et al.11 |
| 22 | 13 | 1006 | 2006 | Population based | Finland | Mossong et al.11 |
| 19 | 27 | 1012 | 2006 | Population based | Great Britain | Mossong et al.11 |
| 22 | 37 | 849 | 2006 | Population based | Italy | Mossong et al.11 |
| 25 | 37 | 1051 | 2005–06 | Population based | Luxembourg | Mossong et al.11 |
| 21 | 48 | 269 | 2006 | Population based | Netherlands | Mossong et al.11 |
| 24 | 43 | 1012 | 2006 | Population based | Poland | Mossong et al.11 |
| 4 | 22 | 865 | 2007 | Rural population | Vietnam | Horby et al. 12 |
| 25 | 1943 | 2010 | Population based | Taiwan | Fu et al.13 | |
| 13 | 26 | 73 | 2003 | Uni. students/staff | Belgium | Beutels et al.14 |
| 57–76 (workdays) | 65 | 1996a | Uni. students/staff | England | Edmunds et al.15 | |
| (7–29) (weekend) | ||||||
| 58 (workdays) | 4 | 49 | 1997–98a | Uni. students/staff | England | Read et al.16 |
aSample period not explicitly provided in text.
Workplace transmission
We identified 13 articles including estimates of the proportion of influenza transmission occurring in the workplace or workplace/schools. All estimates were derived from stochastic spatially structured, individual-based models, set in South East Asia, Australia, the USA and Europe (table 2). With the exception of one article by Kumar et al.,30 all models were for pandemic influenza and commonly fitted to match the age profile of historic pandemic data. The average proportion of workplace transmission was 16.2% (range 8.9–33%). Additionally, four modelling studies reported the combined proportion of transmission in workplaces and schools to be on average 34% (range: 29–37%). Ferguson et al.17,19 were referenced for assumptions on transmission in different settings in five later studies included in this review and were the most commonly cited, while Longini et al. 200518 were referenced in three studies.
Table 2.
Model-based estimates of the proportion of influenza transmission occurring in the workplace
| Workplace transmission (%) | Scenario | Setting | Reference | Secondary reference(s) |
|---|---|---|---|---|
| 33.3a | Pandemic, avian | SE-Asia | Ferguson et al.17 | |
| 18 | Pandemic, avian | SE-Asia | Longini et al.18 | |
| 37a | Pandemic | USA/UK | Ferguson et al.19 | Ferguson et al.17 |
| 12.6 | Pandemic, avian H5N1 | USA | Germann et al.20 | |
| 29–33 | Pandemic | USA | Lewis et al.21 | |
| 13 | Pandemic | USA | Stroud et al.22 | |
| 21.8 | Pandemic | USA | Halloran et al.(2008)-I23 | Ferguson et al.19 |
| 14.5 | Pandemic | USA | Halloran et al.(2008)-II23 | German et al.20 |
| 26.6 | Pandemic | USA | Halloran et al. (2008)-III23 | Lewis et al.21 |
| 29a | Pandemic | Australia | Milne et al.24 | Ferguson et al.19 |
| 11.2 | Pandemic, H1N1 2009 | USA | Yang et al.25 | German et al.,20 Longini et al.18 |
| 37a | Pandemic, H1N1 2009 | Europe | Merler et al.26 | Ferguson et al.19 |
| 8.9 | Pandemic | USA | Cooley et al.27 | Ferguson et al.,19 Halloran et al.,23 Longini et al.18 |
| 9 | Pandemic, H1N1 | Europe | Merler et al.28 | Ferguson et al.,17,19 Cauchemez et al. 200929 |
| 11.5 | Seasonal | USA | Kumar et al.30 | Longini et al.18 |
aWorkplaces and schools together.
A total of nine studies addressed workplace interventions to reduce presenteeism. In eight of these studies, other types of interventions, such as reactive workplace closure or school closure, were considered in combination with other measures, but no quantitative estimates of the isolated effects of presenteeism reduction were reported. Only one study from the USA by Kumar et al.31 exclusively explored the effect of sickness absence on transmission. In this study it was found that 11.5% of transmission occurs in the workplace and that liberal sick leave or 1–2 additional days of paid sickness absence during influenza can reduce workplace transmission by ∼6%, 25% and 39%, respectively.
Compliance with recommendations to take sick leave
We found three articles addressing compliance with public health recommendations to stay at home when experiencing symptoms of influenza. All the studies were population based and measured self-reported anticipated compliance with recommendations to stay at home for at least seven days during pandemic influenza. A US study by Blendon et al.32 found that 94% would comply with these recommendations. Among full-time employees, 35% said they would continue to work if their employer requested it, despite public recommendations to remain at home. In a study from Australia, Brown et al.33 show that compliance with recommendations during seasonal influenza illness (71.3%) was lower compared with the expected compliance in the case of H1N1/Avian influenza illness (95%). Another study from Australia by Eastwood et al.34 found that 94.1% would willingly comply with the recommendation to stay at home had they been exposed to pandemic influenza.
Questionnaire on European sick leave policies for influenza
Among the 31 European Union/European Economic Area countries invited, 18 countries (58%) participated in the study, namely Austria, Belgium, Bulgaria, Czech Republic, Denmark, France, Greece, Hungary, Iceland, Ireland, Italy, Malta, the Netherlands, Portugal, Spain, Sweden, Norway and the UK.
National recommendations or policies for sick leave during the 2009 H1N1 influenza pandemic
In total, nine countries (50%) reported having official recommendations or policies for sick leave during the 2009 A(H1N1) pandemic (table 3). Among these, six countries had introduced recommendations for employees to take sick leave, and three countries had introduced more flexible sick leave policies.
Table 3.
A country-specific overview of the recommendations and policies on sickness absence issued during the H1N1 2009 pandemic and during seasonal influenza, the year the recommendation or policy was introduced, the main reasons for having introduced the recommendation or policy, the grounds on which the recommendations were based and the issuing body
| Country | National recommendation or policy for sick leave during the H1N1 2009 pandemic | Year issued | Reasons for introducing recommendations or policies for sickness absence during influenza | Grounds for the recommendation or policy | Issuing body |
|---|---|---|---|---|---|
| BEL | Containment phase: symptomatic persons and close contacts advised to stay home until disease confirmation. After containment phase: symptomatic persons advised to stay at home. | 2009/2010 |
|
National expertsWHO | MOH |
| NIPH | |||||
| RHAs | |||||
| CZE | GP certified sick-leave could be delayed for up to 3 days and could be obtained via telephone consultation. | 2009/2010 |
|
No response | MOH |
| MOLSA | |||||
| DNK | Stay at home from school work (etc.) if experiencing influenza-like illness. | No response |
|
Other countries and national experts | MOH |
| NIPH | |||||
| ESP | Legal consideration of ‘short-term disability' for cases in preventive isolation due to pandemic influenza A(H1N1) | 2009/2010 |
|
WHO | MOEI |
| GRC | Symptomatic persons should stay at home until defervescence | 2009/2010 |
|
No response | No response |
| IRL | Days of uncertified sick leave permitted was increased from 2 to 10 days during the period of the pandemic. | 2009/2010 |
|
Not known | HSE |
| MLT | Stay home until symptoms subside before returning to work or school. | 2009/2010 |
|
WHO, National and international experts | MOH |
| NIPH | |||||
| NLD | Stay home in case of acute fever >38 °C in combination with other influenza-like symptoms. | 2009/2010 |
|
WHO | NIPH |
| NOR | Before November: 7 days of sick leave was recommended. After November: stay at home for at least 24 h after defervescence (except health care personnel who were advised to stay at home for 7 days). | 2009/2010 |
|
National experts | NIPH |
| Country | National recommendation or policy for sick leave during seasonal influenza | Year issued | Reasons for introducing recommendations or policies for sickness absence during influenza | Grounds for the recommendation or policy | Issuing body |
| BEL | Symptomatic persons should stay at home and seek health advice | 2013 |
|
National Experts | MOH |
| RHAs |
MOH: Ministry of Health, NIPH: National Public Health Institute, MOEI: Ministry of Employment and Immigration, RHA: Regional Health Authority, MOLSA: Ministry of Labour and Social Affairs, HSE: Health Services Executive.
The country respondents were asked to state one or more reasons for putting forward the recommendations or policies. The reasons stated were (i) reducing transmission in the population (9/9); (ii) protecting high-risk persons from contracting influenza (4/9); (iii) protecting sick persons (3/9); (iv) raising awareness among schools and employers (2/9); (v) providing physicians with recommendations (1/9); (vi) spreading awareness in the population (1/9) and (vii) reducing the number of persons on sick leave (11%). The recommendations and policies were based upon advice from one or more of the following: national experts (4/6), international experts (2/6), expert advice or the WHO (4/6).
Of the nine countries that had implemented strategies to encourage sickness absence among employees with influenza, six had issued recommendations and three had made alterations to current sick leave policies. The countries that had issued recommendations disseminated these via the National Public Health Institute (5/5), the Ministry of Health (3/5) and Regional Health Authorities (2/5). One country did not provide a response to this question. The three countries that made changes to sick leave policies communicated these changes via the Ministry of Health and the Ministry of Labour and Social Affairs (1/3), Health Services Executive (1/3) or the Ministry of Employment and Immigration (1/3).
Recommendations or policies for sick leave during seasonal influenza
Only one country (Belgium) reported having national recommendations for sick leave during seasonal influenza. Symptomatic persons were advised to stay at home and seek health advice. This was based on national expert advice and advice from the WHO. The recommendation was issued via the Belgian Ministry of Health and the Regional Health Authorities.
Respondent perceptions of employer attitudes and opinions
Respondents from 11 countries replied to the question concerning perceived employer attitudes and opinions regarding sickness absence. Respondents from two countries believed that influenza was considered a valid reason to take sickness absence from work. The remaining impressions of employer attitudes were varied, the perceptions were that (i) attitudes and opinions among employers varied depending on the specific workplace/employer and symptom severity; (ii) public employers were perceived to be more accepting than private employers; (iii) acceptance of health-care workers and educators taking sickness absence was perceived to be lower than for other occupational groups; (iv) employers were perceived to be more accepting during pandemic influenza than during seasonal influenza unless symptoms were severe, and (v) some employers were perceived to consider absence during influenza to be misuse of social benefits. Two respondents reported that they believed perceptions among employers vary significantly, and three respondents stated that their information on this topic was lacking.
Discussion
Social mixing studies eligible for this review indicate that one in four or one in five of all weekly contacts in developed countries are made in the workplace. Results from individual-based simulation models suggest that almost one in six influenza transmission events occur in the workplace. To the best of our knowledge, only a single modelling study from the USA has explicitly explored the effectiveness of interventions to reduce presenteeism during influenza illness, suggesting that workplace transmission may be reduced by 6–40%, depending on the particular policy implemented. We found the population-level anticipated compliance with public health recommendations to stay at home during pandemic influenza to be high, roughly 95%. Half of the European countries that participated in our survey had issued recommendations or policies to reduce presenteeism during the 2009 H1N1 pandemic. Only Belgium had issued such guidelines for seasonal influenza. One of the primary reasons stated for advising work absence during influenza illness was to reduce transmission in the population.
In this review we included social mixing studies reporting the proportion of weekly contacts in the workplace. Alternatively, the importance of the workplace may be studied by comparing the daily contacts during weekdays to those in weekends14 or in holiday periods.35 Overall, the findings from this review are in line with such estimates. In the pan-European POLYMOD study, the number of contacts increased by a factor of 1.3–1.4 during weekdays compared to sundays,11,35 and the reproductive number on weekdays was higher compared to weekends and holidays by factors of 1.21 and 1.17, respectively.35 Recently, also synthetic social mixing matrices have been estimated from detailed census data; however, in this approach, the proportions of contacts in various settings, including the workplace, are assumed.36,37 Although it should be noted that proportions of contacts do not automatically translate into proportions of transmission. Estimating the proportion of transmission occurring in the workplace is difficult. The spread of influenza in the population is a complex outcome of population mixing, population demography and population behavioural changes during illness. Furthermore, from the point of view of the individual, transmission is the result of competing risks of infection in various settings, wherefore the reduction or elimination of risk in one setting may have a complex effect on the total risk of infection. Hence, work absence of an infectious individual may increase the risk of that person infecting members in the household or elsewhere in the community. On the positive side, however, influenza is commonly assumed to have a low reproductive number of ∼1.2–1.8, and therefore any reduction in the force of infection can have quite substantial effects on the attack rate. Although there are case studies confirming influenza transmission in the workplace, in particular in health care settings38 it is difficult to generalize those findings. Time-use surveys is yet another method that has been used to assess social contact patterns39 and could also be used to quantify workplace transmission.
We only identified quantitative estimates of influenza transmission in the workplace derived from modelling studies. In these models, influenza transmission was partitioned into households, schools, workplaces and the general community, following Ferguson et al.19 In some of the models, a hospital setting was also added, as introduced by Longini et al.18 Ferguson et al.17,19 used household studies to gauge the proportion of household transmission to be ∼30%. They noted that no information was available to distinguish between transmission in the workplace/school and in the general community, and arbitrarily attributed 33–37% and 30–33% of the transmission to these settings, respectively. Despite the lack of empirical evidence, the aforementioned articles are commonly referenced in other modelling studies for assumptions on transmission outside households. This practice neglects the hypothetical nature pertaining to these parameters. However, some of the later papers discuss the uncertainty associated with the transmission in various settings. Merler et al.26 address the uncertainty of transmission in workplaces/schools versus community and show that increasing the proportion of transmission in the general community (while keeping the proportion of household transmission fixed) results in a model similar to homogenous mixing models with a faster epidemic and an increased attack rate. Cooley et al.40 assume in their baseline scenario that within-school transmission is twice that of within-workplace transmission but also investigate consequences of assuming equal transmission rates in schools and workplaces. They find that the latter assumption generates flatter infection curves with lower peak attack rates but with comparable cumulative incidence and find no clear evidence for favouring one alternative over the other when fitting the model to 1957–58 pandemic data.
There was variability across the modelling studies in their estimate of the proportion of influenza transmission in the workplace ranging from 9% and up to 33%. School-age children have been identified as drivers of local spread.41,42,43 Several models assumed that the susceptibility of children was 2–3 times larger than that of adults22,24,28 or that transmission in schools was 2–3 times higher than in workplaces.19,26,27 The model by Lewis et al.21 provided the highest estimate of workplace transmission used. Here an age-uniform susceptibility was assumed, which may have led to an overestimation of transmission among adults and consequently workers.
The potential effect of reduced presenteeism on population transmission will be limited if a substantial part of influenza transmission stems from asymptomatic individuals or from the presymptomatic phase of infection. All models in this review that distinguished between symptomatic and asymptomatic infection assumed that the proportion developing asymptomatic infection was either 33% or 50%. A cohort study from the UK conducted during the H1N1 pandemic suggests that asymptomatic infections may be even more common as only 23% (95% confidence interval 13–34%) of infections were symptomatic.44 The models consistently assumed that asymptomatic infection was half as infectious as symptomatic infection; however, references for this assumption were not provided. In fact, the evidence for asymptomatic or pre-symptomatic transmission is scarce and more definitive studies are needed.45 The models also varied in their assumptions concerning the infectiousness of symptomatically infected: Ferguson et al.17,19 assumed a mean latency/incubation period of 1.48 days, re-used by later models,26,28 while the infectivity profile was assumed to be lognormal, resulting in a generation time of 2.6 days based on analyses of data from household and exposure studies. Longini et al.18 assumed a mean latency, incubation and infectious period of 1.2, 1.9 and 4.1 days, respectively, resulting in a longer generation time of ∼4 days. These assumptions were adopted in several other models.20–22,25,27
The majority of the included models had implemented ad hoc assumptions on baseline withdrawal rates for people with symptomatic infection, most often with higher withdrawal rates in children compared to working adults. Two modelling studies reference a study by Elveback et al. from 1976, although the choice of withdrawal rates in that paper are guessed due to lack of information.46
Only a single article by Kumar et al.30 specifically modelled the effect of changed presenteeism. Unfortunately, this article does not provide adequate information about important modelling assumptions, such as the proportion of asymptomatic infections, the natural history of influenza and population-wide effects, making the study difficult to interpret. Several models consider workplace-based interventions, such as reactive workplace closure17,18,20 or liberal sick leave23; however, these interventions were combined with other social distancing measures, and no quantitative results related to presenteeism were provided. It is noteworthy that modelling studies have not focused on presenteeism reduction, despite this being a commonly implemented workplace intervention during the 2009 H1N1 pandemic. Other social distancing measures, such as school closures,24,35,47–50 restrictions on public gatherings,51 quarantine and isolation,52,53 have been studied somewhat more extensively and have been recommended in several European countries.8,9,49,53–56
The effectiveness of behaviour-based interventions for influenza is affected by adherence, which varies widely depending on the setting and type of intervention.47,52,56–58 The number of employees that take sick leave will depend on whether a recommendation is in place to encourage work absence. We considered studies evaluating compliance with recommendations to stay at home when sick with influenza. All studies were prospective and most were population-based and not focused on the working population, using these estimates as predictors of worker compliance may overestimate the true adherence level. A literature review from 2008 found that the mean number of workdays lost in Europe during seasonal influenza ranged from <1 to 5.9 days per influenza case.59 These disparities could be due to country-specific sick leave policies, nonetheless the variation indicates that some individuals continue to work while ill. Another study based on the 2007/2008 influenza season found that 87% of respondents worked for 3.1 days (SD = 2.9; median = 2), on average, while experiencing influenza-like symptoms) and 72% of respondents worked for 1.3 days (SD = 1.5; median = 1), on average, while experiencing severe influenza.60
Usefulness of increasing knowledge on influenza transmission
This study shows that there is a widespread belief among European public health policy makers that reduced presenteeism may limit the circulation of influenza in the population. Studies are needed to evaluate the effectiveness of policies and recommendations targeting presenteeism. These studies must also be coupled with cost-effectiveness analyses to inform policy makers on different strategies for sickness absence during influenza. Most previous studies have focused on the costs of influenza-associated absences without assessing the possible benefits.1–4,10,59
As this review demonstrates, mathematical modelling is commonly used to evaluate intervention effects, also in the workplace. However, many modelling studies use parameters and assumptions from previously published articles without differentiating between the assumptions that are based on best-guess estimates and the ones based on data. It would be of great scientific value if the degree of evidence supporting each parameter was clearly described in modelling papers.
To develop a dependable model, more precise information about the natural history of influenza is required. The parameters that, presumably, will have the largest effect on the efficacy of sickness absence recommendations are the fraction of infectivity occurring before symptom onset, the fraction of asymptomatic infections, individual variation in the interpretation of symptom onset and compliance with recommendations. Studies addressing pre-symptomatic and asymptomatic infectivity are challenging to conduct, and viral shedding studies are often used as a surrogate. More detailed studies, especially on naturally infected individuals would be an important contribution to the literature on presymptomatic and asymptomatic infections. These studies would need to test for influenza using, for instance, a nasopharyngeal real-time polymerase chain reaction test from the point of exposure and subsequently go on to monitor the presence of symptoms. The latter can be done using either a diary approach or post-infection interviews or questionnaires. Behavioural variables would be best studied through interviews or questionnaires both before and after disease. As far as we have been able to ascertain in this study, very few empirical studies exist on behaviour during sickness absence, and such studies would be of high value for future research on the present subject. Furthermore, the foundation of almost all modelling work concerning influenza transmission and mitigation strategies is an age/sex/category matrix, of the type currently known as a POLYMOD-matrix.11 Attempts have been made to reconstruct such matrices using demographical or time-use data due to a lack of observational data. This is an important area for future research and should be coupled with analyses of the number of contacts made in various settings and the frequency, duration and closeness of contact.
There are some limitations associated with this study. First, although we believe that the search procedure has been thorough, workplace transmission is one of many assumptions used in model-based studies and may not have been uncovered in a database search based on keywords. Second, the questionnaire on European public health recommendations and workplace policies for influenza was addressed to a subset (31 countries) of the ∼50 European countries, of which 12 countries did not respond. The results may therefore not be representative of the whole region and it is possible that countries without recommendations or policies were less likely to participate. In addition, some respondents pointed out that the information they provided was quite general due to the complexity of the systems. Third, the formulation of the questions on recommendations could have led to incomplete responses. For example, Norway reported having issued ‘advice’ rather than specific recommendations for work absence during seasonal influenza illness and the same might be the case for other countries. The results should be interpreted with these limitations in mind.
Conclusions
Several European countries introduced guidelines to reduce presenteeism as a way of mitigating transmission during the 2009 A(H1N1) pandemic; however, evidence that sick leaves effectively limit the spread of influenza is lacking in the literature.
Supplementary data
Supplementary data are available at EURPUB online.
Acknowledgements
We thank Siri Helene Hauge, Norwegian Institute of Public Health, for help with the survey questionnaire, contact information and useful discussions.
Conflicts of interest: None declared.
Key points
Evidence that work absence can mitigate the spread of influenza in the population is lacking in the literature.
Available studies are based on mathematical models; the complex nature of influenza transmission pathways make RCTs and other real life studies are unsuitable.
Improved knowledge about social mixing patterns during illness, timing of sick leave and adherence with sick leave recommendations is needed to develop dependable models.
Of the 18 European countries in our study, sickness absence during influenza was recommended by 9 (50%) countries during the 2009 A(H1N1) pandemic and by one country (6%) during seasonal epidemics.
References
- 1.Szucs T. The socio-economic burden of influenza. J Antimicrob Chemother 1999;44:11–5. [DOI] [PubMed] [Google Scholar]
- 2.Peasah SK, Azziz-Baumgartner E, Breese J, et al. Influenza cost and cost-effectiveness studies globally—a review. Vaccine 2013;31:5339–48. [DOI] [PubMed] [Google Scholar]
- 3.Szucs T, Nichol K. Economic and social impact of epidemic and pandemic influenza. Vaccine 2006;24:6776–8. [DOI] [PubMed] [Google Scholar]
- 4.Keech M, Scott A, Ryan P. The impact of influenza and influenza-like illness on productivity and healthcare resource utilization in a working population. Occup Med 1998;48:85–90. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). CDC Recommendations for the Amount of Time Persons with Influenza-Like Illness Should be Away from Others. 2009. http://www.cdc.gov/h1n1flu/guidance/exclusion.htm. [accessed: 06.01.2016].
- 6.World Health Organization (WHO). What can I do? 2010. http://www.who.int/csr/disease/swineflu/frequently_asked_questions/what/en/index.html#v (6 January 2016, date last accessed).
- 7.European Centre for Disease Prevention and Control (ECDC). Guide to public health measures to reduce the impact of influenza pandemics in Europe: ‘the ECDC Menu'. P. 24–25. Stockholm: 2009.
- 8.Aledort JE, Lurie N, Wasserman J, et al. Non-pharmaceutical public health interventions for pandemic influenza: an evaluation of the evidence base. BMC Public Health 2007;7:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabtree A, Henry B. Non-pharmaceutical Measures to Prevent Influenza Transmission: The Evidence for Individuals Protective Measures National Collaborating Center for Infectious Diseases, Winnipeg 2011.
- 10.O'Reilly F, Stevens A. Sickness absence due to influenza. Occup Med 2002;52:265–9. [DOI] [PubMed] [Google Scholar]
- 11.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008;5:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horby P, Thai PQ, Hens N, et al. Social contact patterns in Vietnam and implications for the control of infectious diseases. PLoS One 2011;6:e16965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y-c, Wang D-W, Chuang J-H. Representative contact diaries for modeling the spread of infectious diseases in Taiwan. PLoS One 2012;7:e45113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beutels P, Shkedy Z, Aerts M, et al. Social mixing patterns for transmission models of close contact infections: exploring self-evaluation and diary-based data collection through a web-based interface. Epidemiol Infect 2006;134:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edmunds WJ, O'callaghan C, Nokes D. Who mixes with whom? A method to determine the contact patterns of adults that may lead to the spread of airborne infections. Proc R Soc Lond Ser B: Biol Sci 1997;264:949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read JM, Eames KT, Edmunds WJ. Dynamic social networks and the implications for the spread of infectious disease. J R Soc Interface 2008;5:1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005;437:209–14. [DOI] [PubMed] [Google Scholar]
- 18.Longini IM, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science 2005;309:1083–7. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson NM, Cummings DA, Fraser C, et al. Strategies for mitigating an influenza pandemic. Nature 2006;442:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA 2006;103:5935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis B, Beckman R, Kumar VS, et al. Simulated pandemic influenza outbreaks in Chicago. Virginia Bioinformatics Institute at Virginia Tech 2007; Contract No.: NDSSL-TR-07-004. [Google Scholar]
- 22.Stroud P, Del Valle S, Sydoriak S, et al. Spatial dynamics of pandemic influenza in a massive artificial society. J Artificial Soc Soc Simul 2007;10:9. [Google Scholar]
- 23.Halloran ME, Ferguson NM, Eubank S, Longini IM, Cummings DA, Lewis B, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci USA 2008;105:4639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milne GJ, Kelso JK, Kelly HA, et al. A small community model for the transmission of infectious diseases: comparison of school closure as an intervention in individual-based models of an influenza pandemic. PLoS One 2008;3:e4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, Matrajt L, et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science 2009;326:729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merler S, Ajelli M. The role of population heterogeneity and human mobility in the spread of pandemic influenza. Proc R Soc B: Biol Sci 2010;277:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooley P, Brown S, Cajka J, et al. The role of subway travel in an influenza epidemic: a New York City simulation. J Urban Health 2011;88:982–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merler S, Ajelli M, Pugliese A, et al. Determinants of the spatiotemporal dynamics of the 2009 H1N1 pandemic in Europe: implications for real-time modelling. PLoS Comput Biol 2011;7:e1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauchemez S, et al. Household Transmission of 2009 Pandemic Influenza A (H1N1) Virus in the United States. New England Journal of Medicine 2009;361(27):2619–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Grefenstette JJ, Galloway D, et al. Policies to reduce influenza in the workplace: impact assessments using an agent-based model. Am J Public Health 2013;103:1406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Quinn SC, Kim KH, et al. The impact of workplace policies and other social factors on self-reported influenza-like illness incidence during the 2009 H1N1 pandemic. American Journal of Public Health, 102 (2012), pp. 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blendon RJ, Koonin LM, Benson JM, et al. Public response to community mitigation measures for pandemic influenza. Emerg Infect Dis 2008;14:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown L, Aitken P, Leggat P, et al. Self-reported anticipated compliance with physician advice to stay home during pandemic (H1N1) 2009: results from the 2009 Queensland Social Survey. BMC Public Health 2010;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eastwood K, Durrheim D, Francis JL, et al. Knowledge about pandemic influenza and compliance with containment measures among Australians. Bull World Health Organ 2009;87:588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hens N, Ayele G, Goeyvaerts N, et al. Estimating the impact of school closure on social mixing behaviour and the transmission of close contact infections in eight European countries. BMC Infect Dis 2009;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fumanelli L, Ajelli M, Manfredi P, et al. Inferring the structure of social contacts from demographic data in the analysis of infectious diseases spread. PLoS Comput Biol 2012;8:e1002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iozzi F, Trusiano F, Chinazzi M, et al. Little Italy: an agent-based approach to the estimation of contact patterns-fitting predicted matrices to serological data. PLoS Comput Biol 2010;6:e1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eibach D, Casalegno JS, Bouscambert M, et al. Routes of transmission during a nosocomial influenza A(H3N2) outbreak among geriatric patients and healthcare workers. J Hosp Infect 2014;86:188–93. [DOI] [PubMed] [Google Scholar]
- 39.Zagheni E, Billari FC, Manfredi P, et al. Using time-use data to parameterize models for the spread of close-contact infectious diseases. Am J Epidemiol 2008;168:1082–90. [DOI] [PubMed] [Google Scholar]
- 40.Cooley P, Lee BY, Brown S, et al. Protecting health care workers: a pandemic simulation based on Allegheny County. Influenza Other Respir Viruses 2010;4:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viboud C, Boëlle P-Y, Cauchemez S, et al. Risk factors of Influenza Transmission in Households. The British Journal of General Practice. 2004;54(506):684–689. [PMC free article] [PubMed] [Google Scholar]
- 42.Glass LM, Glass RJ. Social contact networks for the spread of pandemic influenza in children and teenagers. BMC Public Health 2008;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol 2006;164:936–44. [DOI] [PubMed] [Google Scholar]
- 44.Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patrozou E, Mermel LA. Does influenza transmission occur from asymptomatic infection or prior to symptom onset? Public health reports. 2009;124(2):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elveback LR, Fox JP, Ackerman E, Langworthy A, Boyd M, Gatewood L. An Influenza Simulation Model for Immunization Studies. American Journal of Epidemiology. 1976;103(2):152–65. [DOI] [PubMed] [Google Scholar]
- 47.Borse RH, Behravesh CB, Dumanovsky T, Zucker JR, Swerdlow D, Edelson P, et al. Closing Schools in Response to the 2009 Pandemic Influenza A H1N1 Virus in New York City: Economic Impact on Households. Clinical Infectious Diseases. 2011;52(suppl 1):S168–S72. doi: 10.1093/cid/ciq033. [DOI] [PubMed] [Google Scholar]
- 48.Cauchemez S, Valleron A-J, Boelle P-Y, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452(7188):750–4. doi: http://www.nature.com/nature/journal/v452/n7188/suppinfo/nature06732_S1.html. [DOI] [PubMed] [Google Scholar]
- 49.Xue Y, Kristiansen IS, de Blasio BF. Dynamic modelling of costs and health consequences of school closure during an influenza pandemic. BMC public health. 2012;12(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizzo C, Lunelli A, Pugliese A, Bella A, Manfredi P, Tomba GS, et al. Scenarios of diffusion and control of an influenza pandemic in Italy. Epidemiology and infection. 2008;136(12):1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.PH-HPER-ID&BP 10200. Impact of Mass Gatherings on an Influenza Pandemic. Scientific Evidence Base Review. In: Health Do, editor. 2014.
- 52.Kavanagh A, Bentley R, Mason K, McVernon J, Petrony S, Fielding J, et al. Sources, perceived usefulness and understanding of information disseminated to families who entered home quarantine during the H1N1 pandemic in Victoria, Australia: a cross-sectional study. BMC infectious diseases. 2011;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee VJ, Lye DC, Wilder-Smith A. Combination strategies for pandemic influenza response-a systematic review of mathematical modeling studies. BMC medicine. 2009;7(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mounier-Jack S, Coker RJ. How prepared is Europe for pandemic influenza? Analysis of national plans. The Lancet. 367(9520):1405–11. doi: http://dx.doi.org/10.1016/S0140-6736(06)68511-5. [DOI] [PubMed] [Google Scholar]
- 55.Bell D, Nicoll A, Fukuda K, Horby P, Monto A, Hayden F, et al. Non-pharmaceutical interventions for pandemic influenza, international measures. Emerging infectious diseases. 2006;12(1):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haber MJ, Shay DK, Davis XM, Patel R, Jin X, Weintraub E, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerging infectious diseases. 2007;13(4):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blake KD, Blendon RJ, Viswanath K. Employment and compliance with pandemic influenza mitigation recommendations. Emerging infectious diseases. 2010;16(2):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simonsen KA, Hunskaar S, Wensaas K-A, Rørtveit S, Cox R, Njølstad G, et al. Influenza-like illness in Norway: clinical course, attitudes towards vaccination and preventive measures during the 2009 pandemic. Family practice. 2012;29(2):139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keech M, Beardsworth P. The impact of influenza on working days lost. Pharmacoeconomics. 2008;26(11):911–24. [DOI] [PubMed] [Google Scholar]
- 60.Rousculp MD, Johnston SS, Palmer LA, Chu B-C, Mahadevia PJ, Nichol KL. Attending Work While Sick: Implication of Flexible Sick Leave Policies. Journal of Occupational and Environmental Medicine. 2010;52(10):1009–13. [DOI] [PubMed] [Google Scholar]