Abstract
Kisspeptin (Kiss1) neurons are essential for reproduction, but their role in the control of energy balance and other homeostatic functions remains unclear. Proopiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons, located in the arcuate nucleus (ARC) of the hypothalamus, integrate numerous excitatory and inhibitory inputs to ultimately regulate energy homeostasis. Given that POMC and AgRP neurons are contacted by Kiss1 neurons in the ARC (Kiss1ARC) and they express androgen receptors, Kiss1ARC neurons may mediate the orexigenic action of testosterone via POMC and/or AgRP neurons. Quantitative PCR analysis of pooled Kiss1ARC neurons revealed that mRNA levels for Kiss1 and vesicular glutamate transporter 2 were higher in castrated male mice compared with gonad-intact males. Single-cell RT-PCR analysis of yellow fluorescent protein (YFP) ARC neurons harvested from males injected with AAV1-EF1α-DIO-ChR2:YFP revealed that 100% and 88% expressed mRNAs for Kiss1 and vesicular glutamate transporter 2, respectively. Whole-cell, voltage-clamp recordings from nonfluorescent postsynaptic ARC neurons showed that low frequency photo-stimulation (0.5 Hz) of Kiss1-ChR2:YFP neurons elicited a fast glutamatergic inward current in POMC and AgRP neurons. Paired-pulse, photo-stimulation revealed paired-pulse depression, which is indicative of greater glutamate release, in the castrated male mice compared with gonad-intact male mice. Group I and group II metabotropic glutamate receptor agonists depolarized and hyperpolarized POMC and AgRP neurons, respectively, which was mimicked by high frequency photo-stimulation (20 Hz) of Kiss1ARC neurons. Therefore, POMC and AgRP neurons receive direct steroid- and frequency-dependent glutamatergic synaptic input from Kiss1ARC neurons in male mice, which may be a critical pathway for Kiss1 neurons to help coordinate energy homeostasis and reproduction.
Kisspeptin (Kiss1) neurons play a dominant role in reproduction (1–3), but the degree to which they participate in the control of energy balance and other homeostatic functions remains unclear (4, 5). Kiss1 neurons in both males and females are highly regulated by sex steroids, with the rostral anteroventral periventricular/periventricular preoptic nucleus (AVPV/PeN) being excited, whereas the arcuate nucleus (ARC) Kiss1 (Kiss1ARC) neurons are inhibited by both estrogen and testosterone (6–9). In males, it has been well documented that Kiss1ARC neurons express both estrogen receptor-α and the androgen receptor, and testosterone inhibits the expression of the neuropeptides Kiss1, neurokinin B, and dynorphin via actions on estrogen receptor-α and the androgen receptor (6, 8). Therefore, Kiss1ARC neurons are proposed to be involved primarily in negative feedback regulation of GnRH and LH (8). However, Kiss1ARC neurons in females have been implicated in body weight and temperature regulation. Specifically, ablation of Kiss1ARC neurons prevented 17β-estradiol regulation of body weight and caused cutaneous vasodilation in females (10, 11). In males, food intake and body weight are reduced in gonadectomized as compared with intact or testosterone-replaced male rodents (12, 13), an indication that testosterone-responsive neurons in the hypothalamus such as the Kiss1 neurons may communicate the steroid signal to anorexigenic proopiomelanocortin (POMC) neurons and orexigenic agouti-related peptide (AgRP) neurons, which also coexpress neuropeptide (NPY). It is well known that POMC neurons and AgRP neurons, located within the ARC, are important for metabolic control within the central nervous system (CNS) (14, 15).
In addition to being directly regulated by sex steroids, Kiss1ARC neurons are also direct targets of peripheral metabolic hormones including leptin and insulin. Kiss1ARC neurons express receptors for leptin and insulin (16–19) and are directly depolarized by these hormones, which also links Kiss1 neurons to energy homeostasis (18, 20, 21).
Although Kiss1 neurons are predominantly located in two areas of the ventral forebrain (22), evidence supports the idea that Kiss1ARC neurons directly communicate with neighboring POMC and AgRP neurons (23, 24). Because Kiss1ARC neurons express mRNAs that encode for vesicular glutamate transporter 2 (vGluT2) and glutamic acid decarboxylase 67 (17), they may be able to directly excite or inhibit POMC and AgRP neurons through glutamatergic and/or GABAergic input, respectively. Therefore, we used a combination of optogenetics, molecular biology, and electrophysiology to examine glutamatergic and GABAergic input from Kiss1ARC neurons to POMC and AgRP neurons, as well as the effects of castration on glutamatergic signaling.
Materials and Methods
Mice
Kiss1Cre:GFP (25), Kiss1Cre:GFP::Ai32 (26), PomcEGFP (27), NpyGFP (28), Kiss1Cre:GFP::PomcEGFP, and Kiss1Cre:GFP::NpyGFP male mice were housed under constant temperature (21°C–23°C) and 12-hour light, 12-hour dark cycle schedule (lights on at 6 am and lights off at 6 pm), with free access to food (Lab Diets 5L0D) and water. The Kiss1Cre:GFP strain of mice have a fusion cassette of green fluorescent protein (GFP) and Cre-recombinase driven by the Kiss1 promoter, which allows for specific expression of CreGFP in Kiss1 neurons (25). Kiss1Cre:GFP::Ai32 mice were produced by crossing Kiss1Cre:GFP mice with heterozygous Ai32 mice (C57BL/6 background; The Jackson Laboratory), which carry the ChR2 (H134R)–EYFP gene in their Gt(ROSA)26Sor locus (26). Only Kiss1Cre:GFP::Ai32 male mice that exhibited EYFP distribution similar to that previously described for Kiss1Cre:GFP mice (25) were used in these studies. The PomcEGFP strain of mice express enhanced GFP under the transcriptional control of the Pomc gene (27) and the NpyGFP strain of mice use the Npy promoter to drive the expression of Renilla GFP (28). Kiss1Cre:GFP::PomcEGFP and Kiss1Cre:GFP::NpyGFP mice were produced by crossing Kiss1Cre:GFP mice with PomcEGFP and NpyGFP mice, respectively. Where specified, Kiss1Cre:GFP, Kiss1Cre:GFP::PomcEGFP, and Kiss1Cre:GFP::NpyGFP mice received viral injections to express channelrhodopsin 2 (ChR2) in Kiss1ARC neurons that was driven by the elongation factor 1 (EF1)α promoter. Surgeries were conducted at Oregon Health & Science University according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals with approval for all of the animal use procedures from the Oregon Health & Science University Institutional Animal Care and Use Committee.
Adeno-associated viral (AAV) delivery
Fourteen to 21 days before each experiment, Kiss1Cre:GFP, Kiss1Cre:GFP::PomcEGFP or Kiss1Cre:GFP::NpyGFP male mice (>60 d old) received bilateral ARC injections of a Cre-dependent AAV (serotype 1) vector encoding ChR2 fused to yellow fluorescent protein (YFP) (ChR2:YFP) or mCherry (ChR2:mCh) (viral vectors produced by Dr Padilla, University of Washington, Seattle, WA). Using aseptic techniques, anesthetized mice (1.5% isoflurane/O2) were placed in a Kopf stereotaxic apparatus and received a medial skin incision to expose the surface of the skull. Two holes were drilled into the skull at designated coordinates from bregma (x, ± 0.30 mm; y, −1.20 mm) (29). For the first injection, a glass pipette (Drummond Scientific 3-000-203-G/X) with a beveled tip (diameter, 45 μm) was filled with mineral oil, loaded with an aliquot of AAV using a Nanoject II (Drummond Scientific), positioned at −0.30 mm; y, −1.20 mm and lowered to z, −5.80 mm (surface of brain z, 0.0 mm). The AAV (2.0 × 1012 particles/mL) (30) was injected at a rate of 100 nL/min (500 nL total) (31), left in place for 10 minutes after injection, then the pipette was slowly removed from the brain. We found that this injection rate caused minimum damage to ARC neurons as evaluated using electrophysiology. The second injection repeated the first with x = +0.30 mm. The skin incision was closed using skin adhesive, and each mouse received analgesia (Rimadyl; 4 mg/kg, sc) for 2 days after operation.
Gonadectomy
When necessary, 7 days before each experiment, gonadectomies were performed on male mice through bilateral incisions in the scrotum while under isoflurane inhalation anesthesia (Piramal Enterprises Ltd). The vasculature to each testicle was clamped with a small hemostat and sutured using nonabsorbable SOFSILK (MedRep Express). After the removal of each gonad, the skin incision was closed using NYLON nonabsorbable suture (MedRep Express). Each mouse received analgesia (Rimadyl; 4 mg/kg, sc) on the day of operation.
Quantitative Polymerase Chain Reaction (qPCR) and Single Cell Reverse Transcription PCR (scRT-PCR)
qPCR and scRT-PCR were conducted as previously described and validated (32). Briefly, the ARC was microdissected from 240-μm basal hypothalamic coronal slices (3–4 slices per mouse) from gonad-intact and castrated male Kiss1Cre:GFP mice (n = 10 animals/group; qPCR) and AAV1-EF1α-DIO-ChR2:YFP injected, castrated male Kiss1Cre:GFP mice (n = 6 animals, scRT-PCR). Protease treatment and gentle trituration was used to disassociate the ARC neurons as described in details previously (32). The disassociated neurons were dispersed onto a glass bottomed dish and visualized using a Leitz inverted fluorescent microscope. Individual neurons with at least 1 process extending from 1 axis of the cell and having attached to the glass bottom dish were patched, and then harvested with gentle suction to the pipette using a Xenoworks manipulator system (Sutter Instrument) and expelled into a siliconized 0.6-ml microcentrifuge tube containing a solution of 1× Invitrogen Superscript III buffer, 15 U of RNasin (Promega), 10mM dithiothreitol, and diethylpyrocarbonate-treated water in a total of 5 μL for a single cell (1 cell/tube for scRT-PCR) or 8 μL for pooled cells (5 cells/tube for qPCR). cDNA synthesis was performed on single cells and pools of 5 cells as previously described (32) and stored at −20°C. Controls included nonfluorescent cells, artificial cerebrospinal fluid, water blank, single cells without reverse transcriptase, and tissue controls with and without reverse transcriptase. Primers for the genes that encode for Kiss1 (Kiss1), POMC (Pomc), NPY (Npy), AgRP (Agrp), vGluT2 (Slc17a6), vesicular GABA transporter (vGAT, Slc32a1), β-actin (Actb), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Gapdh) were designed using Clone Manager software (Sci Ed Software) to cross at least 1 intron-exon boundary and optimized as previously described (32). See Table 1 for primer sequences and the optimal annealing temperature for each gene. Note that for qPCR, the annealing temperature for all genes was set at 60°C. Primers for qPCR were further tested for efficiency (E = 10(−1/m) − 1; Livak and Schmittgen [33] and Pfaffl [34]), and the results are as follows: Slc17a6, m = −3.29, r2 = 0.92, efficiency = 100%; Slc32a1, m = −3.29, r2 = 0.91, efficiency = 100%; Gapdh, m = −3.35, r2 = 0.99, efficiency = 98.7%. qPCR was performed on a Quantstudio 7 Flex Real-Time PCR System (Life Technologies) using the Power Sybrgreen (Life Technologies) mastermix method according to established protocols (32). The comparative ΔΔCT method (33, 34) was used to determine values from duplicate samples of 4 μL for the target genes Slc17a6 (vGluT2), Slc32a1 (vGAT), and Kiss1 and 2 μl for the reference gene Gapdh (32). The relative linear quantity was determined using the 2−ΔΔCT equation (32). In order to determine the relative expression levels of target genes in Kiss1ARC neurons obtained from intact and castrated animals, the mean ΔCT for the target genes from the castrated male samples were used as the calibrator, and the data are expressed as n-fold change in gene expression normalized to the reference gene Gapdh and relative to the calibrator. scRT-PCR was performed on 3 μl of cDNA in a 30-μl reaction volume and amplified 40–50 cycles using a C1000 Thermal Cycler (Bio-Rad). The PCR product was visualized with ethidium bromide on a 2% agarose gel.
Table 1.
Primer Sequences and Annealing Temperatures Used for scRT-PCR and qPCR
| Gene Name (Encodes for) | Accession Number | Forward Primer (5′-3′) Reverse Primer (5′-3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Kiss1 (Kiss1)a | NM_178260 | TGCTGCTTCTCCTCTGT | 120 | 57 |
| ACCGCGATTCCTTTTCC | ||||
| Pomc (POMC)a | NM_008895 | GGAAGATGCCGAGATTCTGC | 200 | 60.5 |
| TCCGTTGCCAGGAAACAC | ||||
| Npy (NPY)a | NM_023456 | ACTGACCCTCGCTCTATCTC | 182 | 60 |
| TCTCAGGGCTGGATCTCTTG | ||||
| Agrp (AgRP)a | NM_001271806 | CTCCACTGAAGGGCATCAGAAG | 146 | 59 |
| ACTCAGCACCTCCGCCAAAG | ||||
| Slc17a6 (vGluT2)a | NM_080853 | ATCTGCTAGGTGCAATGG | 136 | 57 |
| TAAGCTGGCTGACTGATG | ||||
| Slc17a6 (vGluT2)b | NM_080853 | TCCCTCGGACAGATCTAC | 113 | 60 |
| CATAGCGGAGCCTTCTTC | ||||
| Slc32a1 (vGAT)ab | NM_009508 | GTCACGACAAACCCAAGATCAC | 137 | 59a, 60b |
| GGCGAAGATGATGAGGAACAAC | ||||
| Actb (β-actin)a | NM_007393 | AAGGCCAACCGTGAAAAGAT | 110 | 60 |
| GTGGTACGACCAGAGGCATAC | ||||
| Gapdh (GAPDH)b | NM_008084 | GCACCACCAACTGCTTAG | 93 | 60 |
| CAGTGATGGCATGGACTG |
Primers used for scRT-PCR.
Primers used for qPCR.
Electron microscopy (EM)
Two Kiss1Cre:GFP castrated male mice (14 d after bilateral ARC injection of AAV1-EF1α-DIO-ChR2:YFP) were overdosed with sodium pentobarbital (150 mg/kg) and perfused through the ascending aorta with the following sequence of solutions: 1) 10 ml of heparinized saline (1000 U/ml); 2) 50 ml of 3.8% acrolein in 2% paraformaldehyde; and 3) 200 ml of 2% paraformaldehyde in 0.1M phosphate buffer (PB) (pH 7.4). Brains were then removed and submersed in 2% paraformaldehyde for 30 minutes, then placed into 0.1M PB. Blocks of tissue were sectioned (40 μm) on a vibrating microtome (Leica) and collected into 0.1M PB. Before immunocytochemical processing, sections were incubated in 1% sodium borohydride solution for 30 minutes and then in 0.5% BSA for 30 minutes in order to increase antigenicity of tissue and to reduce nonspecific binding, respectively. As previously described (35), tissue sections were then processed for dual-labeling immunocytochemistry; immunoperoxidase for vGluT2 and immunogold for YFP. Sections were immersed in cryoprotectant solution (25% sucrose and 3% glycerol in 0.05M PB) then permeabilized by immersion in Freon followed by liquid nitrogen. Tissue sections were rinsed in buffers and then incubated in a cocktail containing both primary antibodies in 0.1% BSA in 0.1M Tris-saline for 40 hours at 4°C. The primary antibodies were a polyclonal rabbit anti-vGluT2 (Synaptic Systems; 1:1000) (36) and a polyclonal chicken anti-GFP (Aves GFP-1020; 1:500, used for YFP detection in mice) (37) The vGluT2 antibody was detected using a biotinylated goat antirabbit IgG (1:400; Vector Laboratories) and visualized using the avidin-biotin detection method (35), whereas the YFP was visualized using a goat antichicken IgG conjugated to colloidal gold (1nm, 1:50; Electron Microscopy Sciences). Sections were then fixed, rinsed in citrate buffer and gold particles were enhanced using a silver enhancement kit (Abcam). All incubations, unless otherwise specified, were carried out at room temperature with continuous agitation and sections were rinsed between incubations with 0.1M Tris-saline (3 × 5 min). After dual-labeling immunocytochemistry, tissue sections were placed in 1.0% osmium tetroxide in 0.1M PB for 15 minutes, washed for 10 minutes in 0.1M PB, dehydrated through a graded series of ethanols, then propylene oxide, and then propylene oxide:EMBed (Electron Microscopy Sciences) (1:1) solution overnight. Tissue sections were then placed in EMBed for 2 hours, embedded between 2 sheets of Aclar plastic, and placed in a 60°C oven for 24–48 hours. Regions of the ARC that contained both labels were glued to plastic blocks formed in Beem capsules, sectioned ultrathin (75 nm), collected onto copper grid, and counterstained with uranyl acetate and Reynolds lead citrate before EM analysis. Images were captured on a Tecnai 12 EM (FEI) interfaced to a CCD camera (XR16; Advanced Microscopy Techniques).
Electrophysiology
Coronal brain slices containing the ARC from AAV1-EF1α-DIO-ChR2:YFP injected Kiss1Cre:GFP male mice (castrated, n = 15; gonad intact, n = 8), Kiss1Cre:GFP::Ai32 male mice (castrated, n = 3; gonad intact, n = 3), PomcEGFP male mice (gonad intact; n = 6), NpyGFP male mice (gonad intact; n = 6), AAV1-EF1α-DIO-ChR2:mCh injected Kiss1Cre:GFP::PomcEGFP male mice (castrated; n = 5), and AAV1-EF1α-DIO-ChR2:mCh injected Kiss1Cre:GFP::NpyGFP male mice (castrated; n = 3) were prepared as previously described (38). Cells were visualized using an Olympus BX51W1 upright microscope equipped with video-enhanced, infrared-differential interference contrast and an Exfo X-Cite 120 Series fluorescence light source. Electrodes were fabricated from borosilicate glass (1.5-mm outer diameter; World Precision Instruments) and filled with a normal internal solution: 128mM potassium gluconate, 10mM NaCl, 1mM MgCl2, 11mM EGTA, 10mM HEPES, 2mM ATP, and 0.25mM GTP (pH was adjusted to 7.3–7.4 with 1N KOH, 290–300 mOsm) or for measurement of GABA postsynaptic currents (PSCs), patch pipettes were filled with a high chloride solution: 140mM KCl, 10mM HEPES, 0.1mM EGTA, 5mM MgCl2, 0.3mM Na-GTP, and 5mM K2-ATP (pH was adjusted to 7.3–7.4 with 1N KOH, 290–300 mOsm). Pipette resistances ranged from 3–5 MΩ. In whole-cell configuration, access resistance was less than 20 MΩ; access resistance was 80% compensated. For optogenetic stimulation, a light-induced response was evoked using a light-emitting diode (LED) 470 blue light source controlled by a variable 2A driver (ThorLabs) at 0.5–20 Hz with the light path directly delivered through an Olympus 40× water-immersion lens. For high-frequency (20 Hz) stimulation the length of stimulation was 10–20 seconds (39). Electrophysiological signals were amplified with an Axopatch 200A amplifier and digitized with Digidata 1322A (Molecular Devices), and the data were analyzed using p-Clamp software (version 9.2; Molecular Devices). The liquid junction potential was corrected for all data analyses. After recording, the cytosol of nonfluorescent recorded cells was harvested and used for post hoc identification by scRT-PCR using the same protocol as for the dispersed single cells (see above). If no product was detected, cDNA was then analyzed for Actb mRNA to assess efficient cytoplasm collection. The lack of Actb expression revealed that the cell cDNA content was insufficient for scRT-PCR identification of the cell.
Drugs
Stocks of the following drugs at the concentrations indicated were prepared. DL-amino-5-phosphonovaleric acid (AP5) (50mM), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10mM), 4-aminopyridine (4-AP) (100mM), 3,5-dihydroxyphenylglycine (DHPG) (50mM), and (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) (10mM) were purchased from Tocris Bioscience and were dissolved in H2O. Tetrodotoxin (TTX) was purchased from Alomone Labs (1mM) and was dissolved in H2O. Bicuculline methiodide (bicuculline; 20mM) and glutamate (100mM) were purchased from Sigma-Aldrich and were dissolved in dimethyl sulfoxide and H2O, respectively. Picrotoxin (100mM) was purchased from Tocris Bioscience and dissolved in dimethylsulfoxide. Aliquots of the stock solutions were stored at −20°C until needed.
Imaging
Photomicrographs of ARC YFP expression were acquired using and Olympus BX51W1 upright microscope equipped with a Rolera XR Fast 1394 camera. Confocal photomicrographs were acquired using a Zeiss LSM 510 and a Zeiss LSM 780 confocal microscopes, each equipped with a ×20 (numerical aperture 0.8) apochromatic objective with Zen software. EM image was acquired using an FEI Tecnai 12 EM and AMT camera.
Data analysis
For qPCR, 3–9 Kiss1 neuronal pools (5 cells/pool) from each animal were run in duplicate for mRNAs that encode for Kiss1, vGluT2, vGAT, and GAPDH, and the mean value of each gene from each animal was used for statistical analysis. Data are expressed as mean ± SEM and were analyzed using a one-way ANOVA and Newman-Keuls multiple comparison post hoc test. Because mRNA for vGAT was below the level of detectability (CT 36) in ARC Kiss1 pools, we report it as not detectable. For scRT-PCR the number of ARC YFP neurons expressing each transcript was counted for each animal, and the mean number of neurons/animal was determined and used for further analysis of mean, SEM, and percentage expression. Electrophysiology data were expressed as mean ± SEM and were analyzed using either an unpaired Student's t test (paired-pulse experiment) or a one-way ANOVA and Newman-Keuls multiple comparison post hoc test (POMC and AgRP pharmacology experiments).
Results
The mRNA for vGluT2 is up-regulated by castration in Kiss1ARC neurons
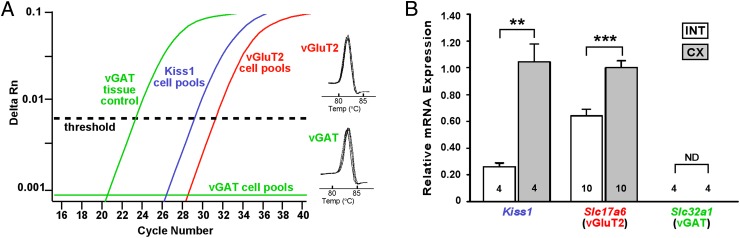
Because Kiss1ARC mRNA levels inversely correlate with circulating concentrations of androgens (6), we performed quantitative analysis of mRNAs that encode for Kiss1, vGluT2 (Slc17a6), and vGAT (Slc32a1) using qPCR on pools of Kiss1Cre:GFP neurons (5 cells/pool; 3–9 pools/animal) from gonad-intact male mice and castrated male mice (Figure 1A). This analysis revealed that Kiss1 mRNA is higher in castrated male mice compared with gonad-intact males (Figure 1B). Furthermore, castrated male Kiss1ARC neurons expressed higher levels of Slc17a6 (vGluT2) mRNA than gonad-intact male mice, whereas levels of Slc32a1 (vGAT) mRNA was essentially undetectable in both groups of male mice (Figure 1B).
Figure 1.
Kiss1, vGluT2, and vGAT expression in Kiss1ARC neurons. A, qPCR assay with amplification curves for Kiss1, vGluT2, and vGAT (all analyzed in 5-cell pools). Cycle number of castrated males was plotted against the normalized fluorescence intensity (ΔRn) to visualize the PCR amplification. The cycle threshold (dashed line) is the point in the amplification at which sample values were calculated. Melting curves depict single-product melting at 82.8°C and 83.7°C for vGluT2 and vGAT, respectively, illustrating that only 1 product was formed from each transcript in the Kiss1ARC neuronal pools. B, Bar graph summarizing mRNA expression of Kiss1, Slc17a6 (vGluT2), and Slc32a1 (vGAT) from pooled Kiss1Cre:GFP neurons relative to the calibrator (see Materials and Methods) of gonad-intact (INT) and castrated (CX) male mice. Animal numbers are indicated. Using Newman-Keuls multiple comparison test; **, P < .01; ***, P < .001. Slc32a1 mRNA in pooled Kiss1ARC neurons was not detectable (ND).
Select vector expression in Kiss1ARC neurons
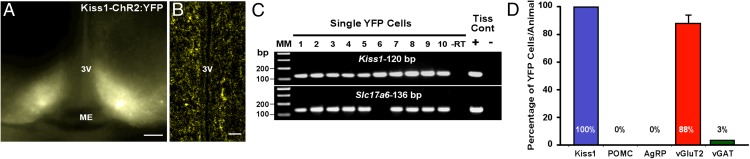
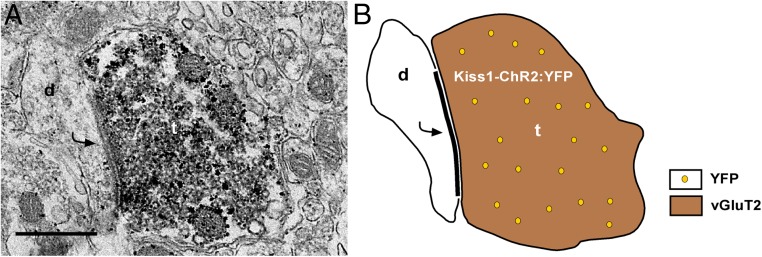
Before assessing functional release of glutamate or GABA from Kiss1ARC neurons, we first characterized select expression of our viral vector. After bilateral injection of AAV1-EF1α-DIO-ChR2:YFP into the ARC of Kiss1Cre:GFP male mice (n = 6), we observed a robust fluorescent signal of neuronal cell bodies and fibers in the ARC (Figure 2A), with only fluorescent fibers in the AVPV/PeN (Figure 2B). To verify that fluorescent cells were Kiss1 neurons, we harvested individual YFP-expressing cells and performed scRT-PCR for Kiss1 mRNA. Indeed, 100% of YFP harvested neurons (30–35 cells/animal) (Figure 2, C and D) expressed Kiss1 mRNA, whereas nonfluorescent harvested cells (n = 8), although Actb positive, did not express Kiss1 mRNA. To exclude the possibility that these Kiss1-YFP expressing cells were POMC or AgRP neurons, we examined Pomc and Agrp mRNA expression in a subset of YFP harvested cells (n = 5 cells/animal) and found that neither transcript was expressed (Figure 2D). We also examined the percentage of Kiss1-YFP cells (17 cells/animal) that expressed mRNA that encodes for vGluT2 and found that 88 ± 6% of Kiss1-YFP neurons contained Slc17a6 mRNA (Figure 2, C and D). However, only 1 out of 30 Kiss1-YFP neurons expressed mRNA that encodes for vGAT. To verify that Kiss1-YFP neurons form glutamatergic synapses, we conducted dual-labeling immunoelectron microscopy studies. The EM analysis revealed that Kiss1ARC-labeled axon terminals contain immunoreactivity for vGluT2 and also form asymmetric synapses with dendrites of other ARC neurons (Figure 3).
Figure 2.
ARC YFP cells contain mRNA for Kiss1 and vGluT2. A, Photomicrograph of the ARC 14 days after bilateral ARC injection of AAV1-EF1α-DIO-ChR2:YFP in a Kiss1Cre:GFP castrated male mouse at a low-power magnification (scale bar, 200 μm). 3V, third ventricle; ME, median eminence. B, Photomicrograph of the periventricular nucleus illustrating YFP fibers (scale bar, 20 μm). 3V, third ventricle. C, Representative gels illustrating mRNA expression of Kiss1 and Slc17a6 (vGluT2) in single YFP cells. The expected base pair (bp) sizes for Kiss1 and Slc17a6 are 120 and 136 bp, respectively. Exclusion of reverse transcriptase (−RT) in a reacted cell was used as a negative control. RNA extracted from the medial basal hypothalamic tissue was also included as positive (+, with RT) and negative (−, without RT) tissue controls. MM, molecular marker. D, Bar graph summarizing the percentage (mean ± SEM) of YFP cells that expressed mRNAs that encode for Kiss1, POMC, AgRP, vGluT2, and vGAT. Note: only 1 out of 30 cells expressed mRNA for vGAT.
Figure 3.
EM revealed vGluT2-immunoreactivity in Kiss1ARC axon terminals. A, A dual-labeled ARC Kiss1-ChR2:YFP axon terminal (t) containing both vGluT2 (immunoperoxidase) and YFP (immunogold particles) and forms an asymmetrical synapse with a postsynaptic density (curved arrow) onto an unlabeled ARC dendrite (d). Scale bar, 500 nm. B, Schematic representation of A with a Kiss1ARC terminal (containing vGluT2 and YFP) forming an asymmetrical (glutamatergic) synapse with an unlabeled ARC dendrite.
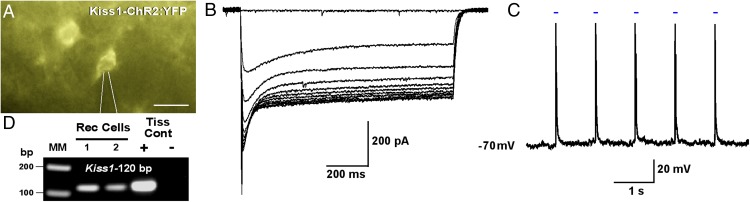
ARC Kiss1ChR2:YFP neurons follow photo-stimulation with high fidelity
We did whole-cell recordings (n = 18 cells) in hypothalamic slices using an LED blue light (470 nm) stimulus to excite ARC YFP-positive neurons from AAV1-EF1α-DIO-ChR2:YFP injected male Kiss1Cre:GFP mice (Figure 4A). In whole-cell, voltage clamp (Vh = −60 mV), we measured light-induced graded inward currents (Figure 4B) with increasing intensities from 33 to 330 μW. The mean induced peak current was 478 ± 83 pA with a mean steady-state current of 308 ± 54 pA. Using whole-cell, current-clamp recordings (current clamped at −70 mV), 1-Hz photo-stimulation (intensity, 660 μW) resulted in the generation of action potentials in Kiss1-ChR2:YFP cells (Figure 4C). Post hoc analysis of a subset of recorded YFP-positive neurons (n = 5) verified that 100% expressed Kiss1 mRNA (Figure 4D).
Figure 4.
Direct ChR2 activation of Kiss1ARC neurons. A, Photomicrograph showing a recording pipette (outlined by white lines) patched onto an ARC Kiss1-ChR2:YFP cell. Scale bar, 20 μm. B, Whole-cell voltage clamp recording (Vh = −60 mV) of light-evoked inward currents with LED intensities increasing from 33 to 330 μW. C, Whole-cell current clamp recording of depolarizations/action potentials induced by 1-Hz photo-stimulation (intensity, 660 μW). Blue bar above recording indicates LED stimulus. D, Representative gel illustrating Kiss1 mRNA expression in ChR2:YFP recorded cells (Rec Cells). RNA extracted from the medial basal hypothalamic tissue was included as positive (+, with RT) and negative (−, without RT) controls. MM, molecular marker.
Glutamate, not GABA, is released locally from Kiss1ARC neurons
Using whole-cell, voltage-clamp recording (Vh = −60 mV), we examined PSCs in nonfluorescent ARC neurons adjacent to Kiss1-ChR2:YFP neurons/fibers (Figure 5, A and C) in gonad-intact and castrated male mice. At the light-microscopic level, we could not predetermine whether the cells selected for recording had synaptic contact with KissARC neurons. However, after light stimulation, 51% (total n = 53) of the targeted neurons exhibited an inward current (Figure 5D) with a mean amplitude of 37.4 ± 7.5 pA (gonad intact, 31.1 ± 12.9 pA; castrated, 40.5 ± 10 pA), a mean delay to peak of 4.04 ± 0.3 milliseconds (gonad intact, 5.14 ± 1.0 ms; castrated, 3.59 ± 0.3 ms), and an average decay time constant of 6.4 ± 1.4 milliseconds (gonad intact, 8.08 ± 3.5 ms; castrated, 5.61 ± 1.5 ms). The light-induced inward current was abrogated with the application of AMPA and NMDA glutamate receptor antagonists (49.6 ± 29.4 pA control, 2.0 ± 0.3 pA CNQX plus AP5; n = 5) (Figure 5E). We did not detect an outward GABA-mediated current in any neurons after light stimulation.
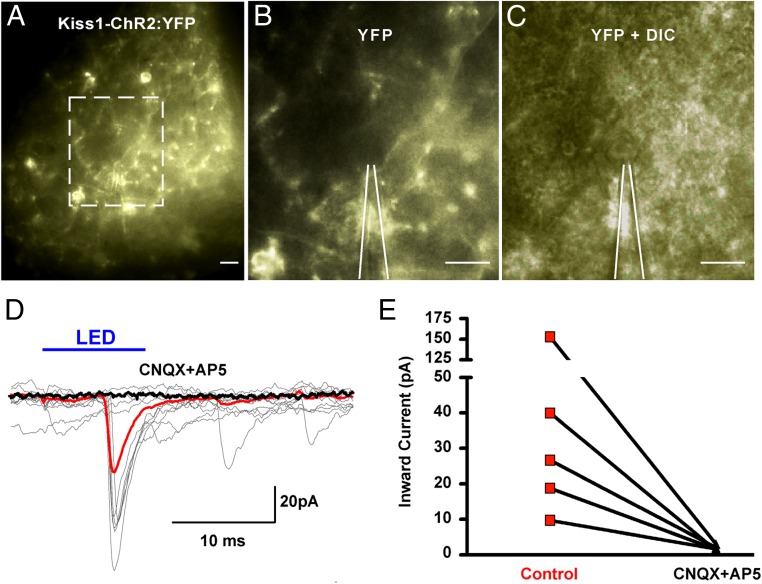
Figure 5.
Photo-stimulation of ARC Kiss1-ChR2:YFP neurons evoked excitatory glutamatergic PSCs. A, Low-power magnification photomicrograph of Kiss1-ChR2:YFP neurons in the ARC. Outlined area in A (scale bar, 20 μm) enlarged to show Kiss-ChR2:YFP projections (B) (YFP; scale bar, 20 μm) near a nonfluorescent postsynaptic cell (C) (YFP + DIC; scale bar, 20 μm). White lines outline recording pipette (B and C). D, Overlay of individual (gray) whole-cell recordings where photo-stimulation (intensity, 660 μW; duration, 10 ms) of ARC Kiss1-ChR2:YFP neurons induced PSCs in a nonfluorescent, ARC neuron. The averaged responses before (red trace) and after (black trace) the application of CNQX (10μM) and AP5 (50μM). Solid blue line above recordings indicates light stimulus. E, Line graph illustrates light-induced inward currents before (control) and after the application of CNQX and AP5 in 5 nonfluorescent ARC cells.
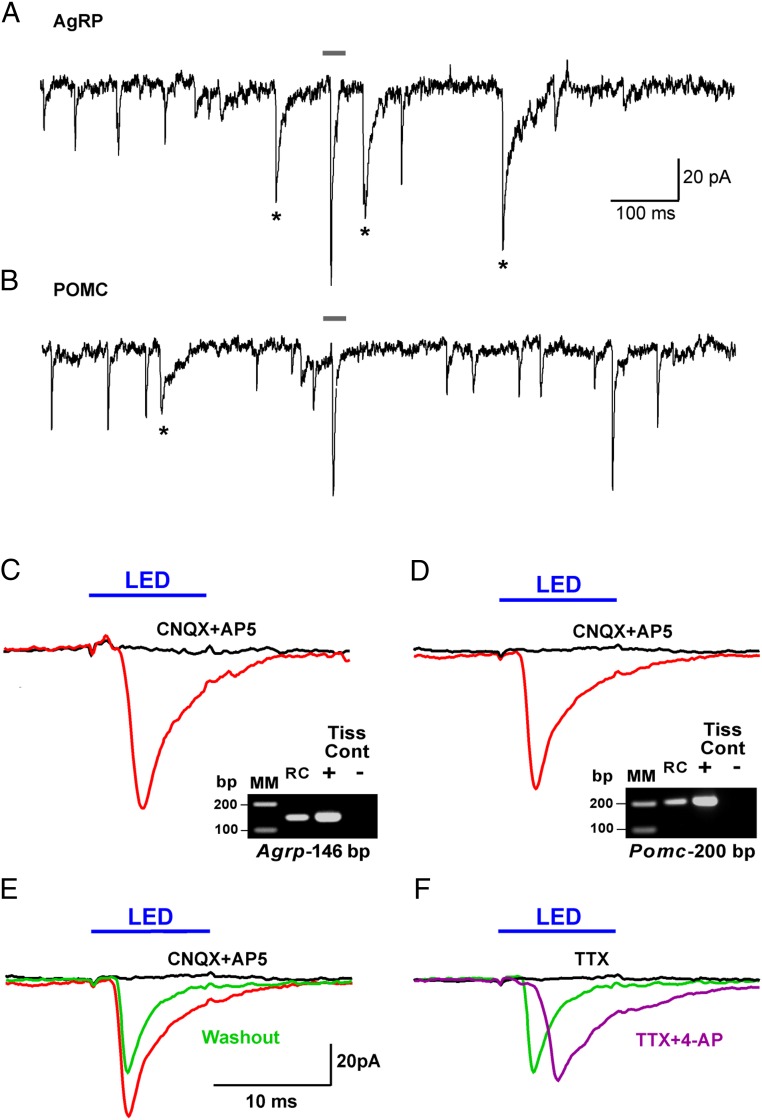
In an attempt to augment any potential GABA-mediated release from Kiss1ARC neurons, 10 additional neurons were recorded using a high chloride internal solution (Figure 6). Both glutamate and GABA spontaneous PSCs were evident in AgRP and POMC neurons (post hoc identified) (Figure 6, A and B), and GABA PSCs (mean decay time constant of 13.1 ± 1.8 ms) could be blocked with the addition of bicuculline (20μM; data not shown). Seven of 10 cells showed an inward current after photo-stimulation with a mean amplitude of 45.3 ± 12.2 pA, a mean delay to peak of 2.9 ± 0.3 milliseconds, and an average decay time constant of 7.9 ± 5.0 milliseconds, whereas the other 3 cells did not respond. This light-induced response was blocked with CNQX and AP5 in 2 cells (83.1 ± 17.8 pA control, 0.5 ± 0.7 pA CNQX plus AP5) (Figure 6, C and D). After washout of CNQX and AP5 (Figure 6E), the light-induced inward current partially recovered. Moreover, although TTX blocked the photo-stimulated postsynaptic inward current (Figure 6F), we could rescue the light-induced response with the addition of 4-AP (40, 41), which is electrophysiological evidence for direct synaptic contact between Kiss1ARC neurons and the postsynaptic responsive neuron. We could segregate the recordings between the higher input resistance (∼1.5 GΩ), lower capacitance (∼12 pF) AgRP neurons (42) vs the relatively lower input resistance (∼1 GΩ), higher capacitance (∼20 pF) POMC neurons (43). Moreover, after recording (average recording time, ∼20 min), the cytoplasm of the responsive cells was (routinely) collected and analyzed for mRNA transcripts (Pomc, Npy, Agrp, Kiss1, and Actb) using scRT-PCR to confirm our segregation, and 34% of the neurons (total n = 29) expressed only Pomc mRNA, 28% of the neurons expressed only Npy and Agrp mRNA, and none of the cells expressed Kiss1 mRNA. In 34% of neurons that did not express Pomc or Agrp mRNA, we also did not detect Actb mRNA, an indication that there was insufficient cDNA content to identify these neurons. There was no difference in the amplitude of the photo-stimulated fast PSCs between POMC and AgRP neurons (post hoc identified) using normal internal solution (27.4 ± 5.5 pA in POMC neurons vs 37.7 ± 10.7 pA in AgRP neurons). Therefore, it appears that Kiss1ARC neurons in the male firing at low frequencies provide equivalent fast (ionotropic) glutamatergic input to both POMC and AgRP neurons in the male. Whether this is from the same Kiss1ARC or from 2 different Kiss1ARC populations is unknown at the present time.
Figure 6.
Photo-stimulation of glutamate, not GABA, release from Kiss1ARC neurons onto AgRP and POMC neurons. Whole-cell, voltage-clamp recording (Vh = −60 mV) using high chloride internal solution from an AgRP neuron (A) and a POMC neuron (B) near Kiss-ChR2:YFP fibers. Based on the decay time constant (13.1 ± 1.8 ms), GABA PSCs are noted with an asterisk. Averaged traces for photo-stimulation (intensity, 660 μW; duration, 10 ms) of ARC Kiss1-ChR2:YFP neurons while recording (high chloride internal solution) from an AgRP neuron (C) and a POMC neuron (D) before (red trace) and after (black trace) CNQX (10μM) and AP5 (50μM) application. E, Photo-stimulation after the washout (green trace) of CNQX and AP5 from (D). F, Photo-stimulation in the presence of TTX (black trace; 1μM) followed by photo-stimulation with addition of 4-AP (purple trace; 100μM). The average of recordings for each experiment is displayed as a trace line with color (red, black, green, or purple). Cells were identified post hoc using scRT-PCR. RC, recorded cell. Gray bar above recordings in A and B correspond to recordings in C and D. Blue bar above recordings indicate LED stimulus (C–F). Scale in E also applies to C–F.
Steroid-dependent glutamate release from Kiss1ARC neurons
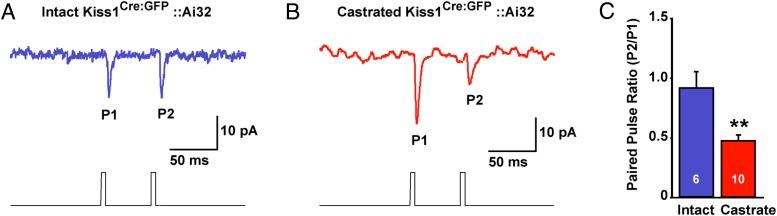
Because castration dramatically increased the expression of vGluT2 in Kiss1ARC neurons and we know that synaptic glutamate release is tightly coupled to vGluT2 expression (44), we explored the differences in evoked glutamate release from Kiss1ARC neurons using paired-pulse ratio (PPR) (ratio of the amplitude of the second pulse over the amplitude of the first pulse) (45) in gonad-intact and castrated Kiss1Cre:GFP::Ai32 and AAV ARC injected Kiss1Cre:GFP male mice. Using a photo-stimulation PPR protocol of 2 5-ms LED stimulations separated by 50 milliseconds, we found that AgRP neurons (post hoc identified) in castrated male mice had a 2-fold lower PPR compared with gonad-intact males (Figure 7), which indicates that there was a greater glutamate release with the first stimulus (ie, a higher probability of glutamate release) in castrated males and is congruent with higher mRNA expression of vGluT2 in Kiss1ARC neurons after castration.
Figure 7.
Steroid-dependent change in glutamate release from Kiss1ARC neurons. Whole-cell, voltage-clamp recording (Vh = −60 mV) of nonfluorescent AgRP neurons (post hoc identified) using a photo-stimulation paired-pulse paradigm (pulse duration, 5 ms; interpulse interval, 50 ms) in male Kiss1Cre:GFP::Ai32 mice and AAV-EF1α-DIO-ChR2:YFP injected Kiss1Cre:GFP mice. A representative trace that averages 10 sequential paired-pulse recordings is shown for a gonad-intact (A) and castrated (B) male Kiss1Cre:GFP::Ai32 mouse. C, Bar graph summarizes the averaged (±SEM) PPR (pulse 2 amplitude to pulse 1 amplitude) of the induced EPSC from gonad-intact and castrated male Kiss1Cre:GFP::Ai32 and AAV injected Kiss1Cre:GFP mice. Cell numbers are indicated. Using an unpaired Student's t test; **, P < .01.
Group I metabotropic glutamate receptor (mGluR) agonist excites POMC neurons and group II mGluR agonist inhibits AgRP neurons
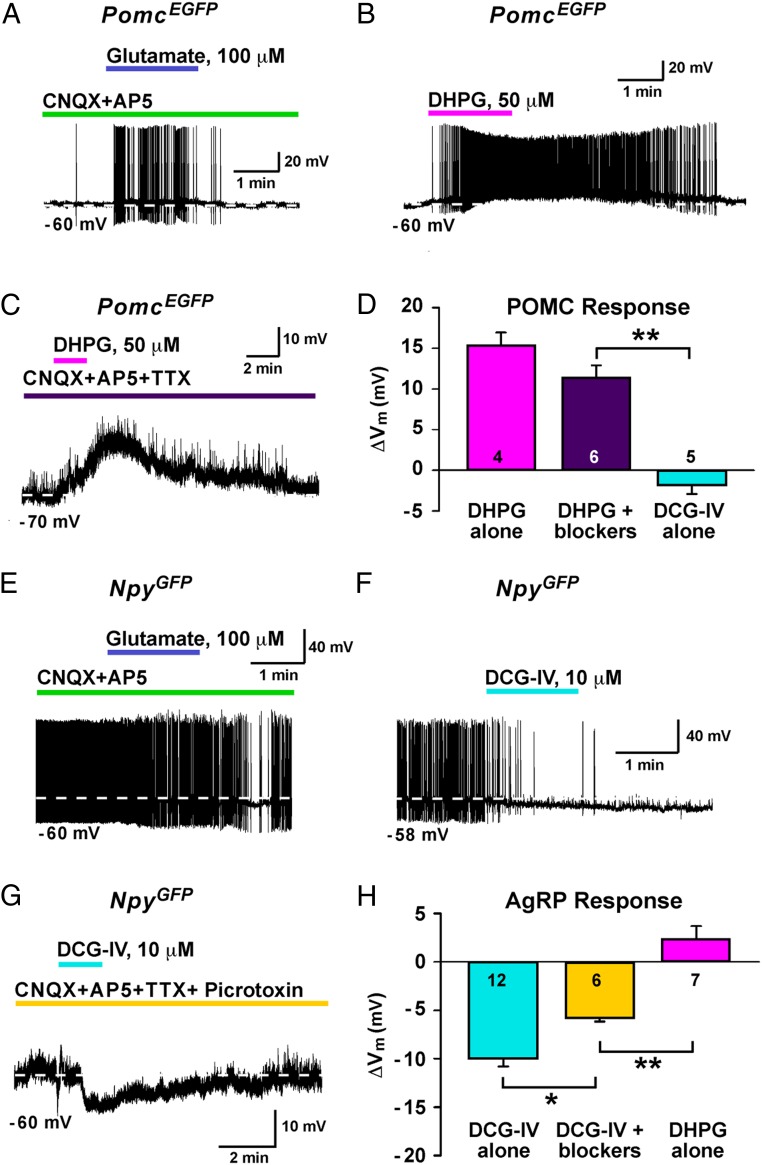
Given that both POMC and AgRP neurons receive glutamatergic input from Kiss1ARC neurons, we were left with the question of how Kiss1 neurons could use glutamate to differentially regulate POMC and AgRP neurons. Because glutamate signaling via group I (Gq/G11 coupled) and group II (Gi/Go coupled) mGluRs results in cellular activation and inhibition, respectively (46), we decided to address our question pharmacologically. Using whole-cell, current-clamp recordings of PomcEGFP neurons, we fast applied glutamate (100μM) in the presence of CNQX and AP5, and glutamate initiated a slow depolarization and stimulated action potential firing in PomcEGFP neurons (Figure 8A). Next, we again targeted PomcEGFP neurons and applied DHPG, a group I mGluR agonist. In all PomcEGFP neurons (n = 4), addition of DHPG (50μM) depolarized and stimulated action potential firing (Figure 8B). Furthermore, DHPG depolarized PomcEGFP neurons (n = 6) in the presence of CNQX, AP5, and TTX (Figure 8C). However, PomcEGFP neurons (n = 5) perfused with DCG-IV, a group II mGluR agonist, showed little to no response (Figure 8D). Then using whole-cell, current-clamp recordings of ARC NpyGFP neurons, which are AgRP neurons (28), we fast applied glutamate (100μM) in the presence of CNQX and AP5. In contrast to PomcEGFP neurons, glutamate in the presence of CNQX and AP5 hyperpolarized ARC NpyGFP neurons (Figure 8E). Next, we again targeted ARC NpyGFP neurons and applied DCG-IV. In all ARC NpyGFP neurons (n = 12), addition of DCG-IV (10μM) hyperpolarized and reduced action potential firing (Figure 8F). Furthermore, DCG-IV hyperpolarized ARC NpyGFP neurons (n = 6) in the presence of CNQX, AP5, TTX, and picrotoxin (Figure 8G). However, ARC NpyGFP neurons (n = 7), showed little to no response to DHPG (Figure 8H).
Figure 8.
Glutamate differentially regulates POMC and AgRP neurons via mGluR signaling. A, Whole-cell current clamp recording of a PomcEGFP neuron in the presence of CNQX (10μM) and AP5 (50μM) with the fast perfusion of glutamate (100μM). Whole-cell current clamp recording of a PomcEGFP neuron with (B) DHPG (50μM) alone or (C) DHPG in the presence of CNQX (10μM), AP5 (50μM), and TTX (1μM). D, Bar graph summarizing the effect of DHPG alone, DHPG with blockers (CNQX, AP5, and TTX), and DCG-IV alone on POMC neurons. Cell numbers are indicated. E, Whole-cell current clamp recording of an ARC NpyGFP neuron in the presence of CNQX (10μM) and AP5 (50μM) with the fast perfusion of glutamate (100μM). Whole-cell current clamp recording of an ARC NpyGFP neuron with (F) DCG-IV (10μM) alone or (G) DCG-IV (10μM) in the presence of CNQX (10μM), AP5 (50μM), TTX (1μM), and picrotoxin (100μM). H, Bar graph summarizing the effect of DCG-IV alone, DCG-IV with blockers (CNQX, AP5, and TTX), and DHPG alone on AgRP neurons. Cell numbers are indicated. Using Newman-Keuls multiple comparison test; *, P < .05; **, P < .01.
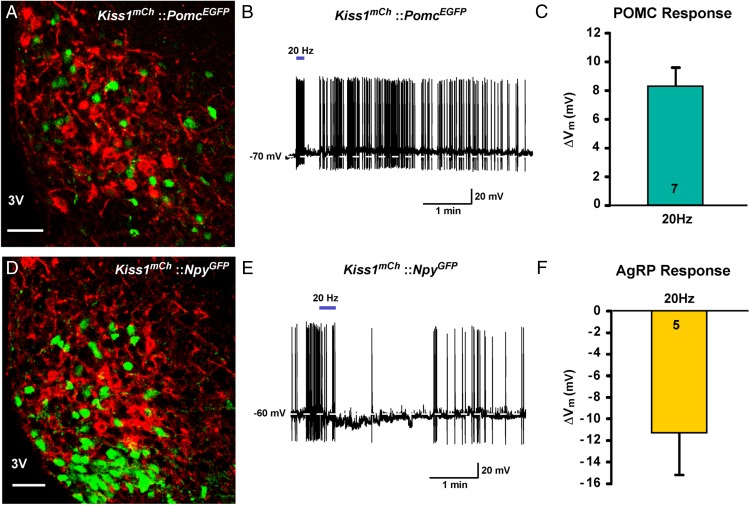
High frequency photo-stimulation of Kiss1ARC neurons evokes a slow depolarization in POMC but a slow hyperpolarization in AgRP neurons
Because glutamate signaling via group I mGluR agonists depolarizes POMC neurons and via group II mGluR agonists hyperpolarizes AgRP neurons, we sought to mimic these responses using a physiological stimulus, ie, photo-stimulation of Kiss1ARC neurons. Therefore, we used high frequency stimulation (20 Hz) in AAV-EF1α-DIO-ChR2:mCh injected Kiss1Cre:GFP::PomcEGFP castrated male mice (n = 5) and Kiss1Cre:GFP::NpyGFP castrated male mice (n = 3) to generate maximal glutamate release (45). Indeed, high frequency photo-stimulation (20 Hz) (39) evoked a slow depolarization (excitatory post-synaptic potential, EPSP) (8.3 ± 1.3 mV, n = 7) in POMC neurons but a slow hyperpolarization (inhibitory post-synaptic potential, IPSP) (−11.2 ± 4.0 mV, n = 4) in AgRP neurons (Figure 9). The EPSP response was slower developing in the POMC neurons indicative of a Gαq-coupled signaling pathway vs the faster membrane delimited Gαi.o-coupled IPSP response in AgRP neurons.
Figure 9.
High frequency photo-stimulation of ChR2 Kiss1ARC neurons stimulates POMC neurons and inhibits AgRP neurons. A, Representative confocal image of the ARC from a Kiss1Cre:GFP::PomcEGFP male mouse injected with AAV1-EF1α-DIO-ChR2:mCherry. Scale bar, 50 μm. 3V, third ventricle. B, High frequency photo-stimulation of ChR2 (20 Hz, solid blue bar) evoked a slow depolarization (EPSP) in a PomcEGFP neuron. C, Bar graph summarizing the effect of 20-Hz photo-stimulation in POMC neurons (n = 7). D, Representative confocal image of the ARC from a Kiss1Cre:GFP::NpyGFP male mouse injected with AAV1-EF1α-DIO-ChR2:mCherry. Scale bar, 50 μm. 3V, third ventricle. E, High frequency photo-stimulation of ChR2 (20 Hz, solid blue bar) evoked a slow hyperpolarization (IPSP) in an ARC NpyGFP neuron. F, Bar graph summarizing the effect of 20 Hz LED stimulation in AgRP neurons (n = 5). Note the hyperpolarization typical for activation of Gαi.o-coupled receptors.
Discussion
In the present study, we have used a combination of molecular biology, optogenetics, and electrophysiology to study the neurophysiological connection between Kiss1ARC neurons and POMC and AgRP neurons. Using qPCR of pooled ARC Kiss1Cre:GFP neurons, we found that mRNA expression for vGluT2 was higher in castrated males compared with gonad-intact male mice. Through the use of a Cre-dependent viral vector (AAV1-EF1α-DIO-ChR2:YFP) in Kiss1Cre:GFP male mice, we found that all individual harvested fluorescent cells are Kiss1 neurons and that the vast majority express mRNA that encodes for vGluT2. Using whole-cell recordings we found that low frequency photo-stimulation of ARC Kiss1-ChR2:YFP neurons generated fast glutamatergic EPSCs in POMC and AgRP neurons and as predicted from the changes in vGluT2 mRNA expression after castration, there was enhanced glutamate release from Kiss1ARC neurons with paired-pulse photo-stimulation. Finally, we show that group I and group II mGluR agonists depolarize and hyperpolarize POMC and AgRP neurons, respectively, and that high-frequency photo-stimulation (20 Hz) mimicked these effects.
Kiss1ARC neuron specific expression of ChR2:YFP
Kiss1 perikarya reside in two main regions of the ventral forebrain, AVPV/PeN (rodents)/medial preoptic region (nonrodents) and the ARC, and send projections to various areas of the CNS (22). Anatomical identification of Kiss1ARC neuronal origin has been accomplished using Kiss1 coimmunoreactivtiy with neurokinin B and/or dynorphin (47–49). However, reports looking at Kiss1 innervation of downstream neurons typically use confocal images of Kiss1 immuno-positive contacts (23, 24, 50) and leave open the possibility that neural input could be from either Kiss1 neuronal population. We achieved ARC Kiss1-ChR2:YFP expression by using local delivery of a Cre-dependent viral vector, which resulted in region-specific fluorescence in ARC neurons and enabled us to characterize Kiss1ARC selective expression. Furthermore, it allowed for the visualization of ChR2:YFP fibers that originate from Kiss1ARC neurons (not from the AVPV/PeN population) in order to selectively activate only these cells and target potential downstream neurons. In addition, we observed, as others have (51, 52), that Kiss1ARC neurons project rostrally into the AVPV/PeN and although the functional role of this projection in males is unknown, it lends support to the idea that Kiss1ARC neurons have physiological actions outside of the ARC.
Steroid-sensitive glutamate release from Kiss1ARC neurons
Glutamate and GABA are considered to be the major excitatory and inhibitory neurotransmitters in the CNS, respectively, and have direct actions on ARC neurons (53–56). Evidence exists that the release of these amino acid transmitters from ARC neurons elicits local fast postsynaptic potentials in hypothalamic slice preparations (53, 57). Although it has been shown using EM that POMC and AgRP neurons are contacted by glutamatergic and GABAergic nerve terminals (54), thus far any efforts to identify the local origin of these inputs have largely focused on reciprocal connections between POMC and AgRP neurons. AgRP neurons form GABAergic synaptic contacts on POMC neurons (27) and functional release of GABA has been confirmed using optogenetics (29). Although POMC neurons have been shown to reciprocally innervate and self-regulate via glutamate and GABA release in hypothalamic cultures (58, 59), POMC to POMC neuronal communication has not been seen using optogenetic stimulation in hypothalamic slice preparations (29, 60). Optogenetic stimulation of POMC neurons does release both glutamate and GABA (60), which indicates that POMC neurons regulate other (non-POMC) ARC neurons. Here, we show that Kiss1ARC firing at low frequencies provide excitatory glutamatergic input to both POMC and AgRP neurons and through the use of 4-AP, which blocks K+ channels responsible for repolarization of the nerve terminals (41, 61, 62), we were able to rescue the TTX blockade of photo-stimulated PSC and confirm direct synaptic input. Krashes et al reported labeling of afferents from ARC neurons to AgRP neurons after rabies viral injections (retrograde tracer), but were unable to detect ARC-derived glutamatergic input to AgRP neurons (63) most likely due to limited ARC ChR2 expression. Indeed, our results clearly indicate monosynaptic inputs from Kiss1ARC neurons to POMC and AgRP neurons.
Although our photo-stimulated glutamate release reflects the high degree of mRNA that encodes for vGluT2 expression in Kiss1ARC neurons, others have reported that 50% of Kiss1ARC neurons also express mRNA that encodes for glutamic acid decarboxylase 67 (17). However, Slc32a1 (vGAT) mRNA expression in Kiss1ARC neurons was essentially undetectable by two different methods, and we did not observe light-induced GABAergic PSCs from Kiss1-ChR2:YFP cells. Because postsynaptic recordings were conducted in ARC neurons that received PSCs with a decay time constant indicative of GABAergic input (64) and could be blocked with the GABAA receptor antagonist bicuculline, we are confident that these cells do receive GABAergic input, but this input is not from Kiss1ARC neurons. Moreover, given that the photo-stimulation protocol used here results in light-induced GABAergic PSCs from AgRP neurons (29, 65), we believe the absence of photo-stimulated GABA release from Kiss1ARC neurons is due to their inability to package GABA into secretory vesicles (66).
Castration dramatically increased the expression of vGluT2 in Kiss1ARC neurons. Furthermore, using a PPR paradigm, which has been extensively employed to measure release probability at central synapses in many brain structures (45), we found there was more glutamate released from Kiss1ARC neurons from castrated males.
Kiss1ARC neurons excite POMC neurons but inhibit AgRP neurons through mGluRs
With glutamatergic input to both POMC and AgRP neurons originating from Kiss1ARC neurons and the probability of release increased with castration, we were left with the question of how glutamate could differentially regulate these opposing neurons. One possibility was through activation of distinct mGluRs. Group I mGluRs are Gq/G11 coupled and activate phospholipase C, whereas group II mGluRs are Gi/Go coupled and inhibit adenylyl cyclase (46). Because group I and group II mGluRs are found in the ARC of female mice (67), we examined the effect of select activation of these receptors in POMC and AgRP neurons. Indeed a group I mGluR agonist (DHPG) depolarized POMC neurons, whereas a group II mGluR agonist (DCG-IV) hyperpolarized AgRP neurons. More importantly from a physiological perspective, we were able to generate with high-frequency optogenetic stimulation of Kiss1ARC neurons a slow EPSP in POMC but a slow IPSP in AgRP neurons. Therefore, Kiss1ARC neurons firing at high frequency can differentially excite POMC neurons and inhibit AgRP neurons via mGluRs. In addition, Kiss1 itself may augment the effects of the metabotropic glutamate response given that it depolarizes POMC neurons via its own Gq-coupled receptor (G protein-coupled receptor 54) (24).
Kiss1 neurons, energy balance, and other homeostatic functions
Androgens act to stimulate food intake in several species (68–73), but the central mechanisms underlying this effect remain largely unknown. Here, we have discovered a testosterone-regulated glutamatergic input from Kiss1ARC neurons to AgRP and POMC neurons within the ARC that can differentially regulate the activity of these two neuronal groups depending on the Kiss1 neuronal firing frequency. Although, AgRP and POMC neurons are primarily known for their role in stimulating or inhibiting feeding, respectively, energy homeostasis is a delicate balance between the consumption of food and the expenditure of energy. The ARC is an important area for metabolic control within the CNS given that two opposing neuronal populations, POMC neurons and AgRP neurons, reside there, which together integrate multiple physiological cues to ultimately regulate energy balance (21, 27, 74–77). In addition, these two neuronal groups have been implicated in the control of other homeostatic functions such as hormone secretion, sex behavior and temperature regulation (42, 78–82).
In summary, these data provide compelling evidence for glutamatergic synaptic input to POMC and AgRP neurons that originates from Kiss1ARC neurons. As such, they provide a neurophysiological framework whereby Kiss1 neurons could integrate changes in reproductive status with changes in energy status and/or other homeostatic functions. Further work is needed to define the importance of Kiss1 neurons in the control of global homeostasis.
Acknowledgments
We thank Uyen-Vy Navarro for excellent technical assistance, Dr Charles Meshul for providing the vGluT2 antiserum, and Dr Kristy Heppner for providing the GFP antiserum. Confocal microscopy was carried out at the Advanced Light Microscopy Core in the Oregon Health & Science University Jungers Center. We also thank Molly Radany and Maria Borisovska for skilled technical assistance with the electron microscopy analysis.
This work was supported by National Institutes of Health Grants R01-NS38809 (to M.J.K.), R01-NS43330 (to O.K.R.), R01-DK68098 (to M.J.K. and O.K.R.), R01-DA24908 (to R.D.P.), R01-DK62179 (to W.F.), T32-DK07680 (to C.CN.), T32-HD07133 (to C.CN.), F32-DK103446 (to C.CN.), and P30-NS061800 (to S.A.A.) and an instrumentation grant from the Murdock Charitable Trust (S.A.A.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAV
- adeno-associated viral
- AgRP
- agouti-related peptide
- 4-AP
- 4-aminopyridine
- AP5
- DL-amino-5-phosphonovaleric acid
- ARC
- arcuate nucleus
- AVPV/PeN
- anteroventral periventricular/periventricular preoptic nucleus
- ChR2
- channelrhodopsin 2
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- CNS
- central nervous system
- DCG-IV
- 2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- DHPG
- 3,5-dihydroxyphenylglycine
- EF1
- elongation factor 1
- EM
- electron microscopy
- EPSP
- excitatory post-synaptic potential
- GABA
- gamma aminobutyric acid
- GFP
- green fluorescent protein
- IPSP
- inhibitory post-synaptic potential
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Kiss1
- kisspeptin
- Kiss1ARC
- ARC Kiss1
- LED
- light-emitting diode
- mGluR
- metabotropic glutamate receptor
- NPY
- neuropeptide Y
- POMC
- proopiomelanocortin
- PSC
- postsynaptic current
- qPCR
- quantitative polymerase chain reaction
- scRT-PCR
- single cell reverse transcription polymerase chain reaction
- TTX
- tetrodotoxin
- vGAT
- vesicular GABA transporter
- vGluT2
- vesicular glutamate transporter 2
- YFP
- yellow fluorescent protein.
References
- 1. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 2. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. [DOI] [PubMed] [Google Scholar]
- 4. de Bond JA, Smith JT. Kisspeptin and energy balance in reproduction. Reproduction. 2014;147:R53–R63. [DOI] [PubMed] [Google Scholar]
- 5. Roa J, Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014;397:4–14. [DOI] [PubMed] [Google Scholar]
- 6. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. [DOI] [PubMed] [Google Scholar]
- 7. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 8. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang C, Tonsfeldt KJ, Qiu J, et al. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endocrinol Metab. 2013;305:E1384–E1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol. 1999;276:R1366–R1373. [DOI] [PubMed] [Google Scholar]
- 13. Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intake in adolescent and adult rats. J Nutr. 2001;131:2351–2357. [DOI] [PubMed] [Google Scholar]
- 14. Gropp E, Shanabrough M, Borok E, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. [DOI] [PubMed] [Google Scholar]
- 15. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. [DOI] [PubMed] [Google Scholar]
- 16. Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. [DOI] [PubMed] [Google Scholar]
- 17. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011;152:1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donato J, Jr, Cravo RM, Frazão R, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cravo RM, Frazao R, Perello M, et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One. 2013;8:e58698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu J, Zhang C, Borgquist A, et al. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–2243. [DOI] [PubMed] [Google Scholar]
- 24. Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madisen L, Mao T, Koch H, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. [DOI] [PubMed] [Google Scholar]
- 28. van den Pol AN, Yao Y, Fu LY, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gore BB, Soden ME, Zweifel LS. Manipulating gene expression in projection-specific neuronal populations using combinatorial viral approaches. Curr Protoc Neurosci. 2013;65:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saunders A, Johnson CA, Sabatini BL. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front Neural Circuits. 2012;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bosch MA, Tonsfeldt KJ, Rønnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol. Mol Cell Endocrinol. 2013;367:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Δ Δ C(T)) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 34. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29:2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silverman MB, Hermes SM, Zadina JE, Aicher SA. μ-Opioid receptor is present in dendritic targets of endomorphin-2 axon terminals in the nuclei of the solitary tract. Neuroscience. 2005;135:887–896. [DOI] [PubMed] [Google Scholar]
- 36. Todd AJ, Hughes DI, Polgár E, et al. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2016;17:13–27. [DOI] [PubMed] [Google Scholar]
- 37. Bulloch K, Miller MM, Gal-Toth J, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508:687–710. [DOI] [PubMed] [Google Scholar]
- 38. Qiu J, Bosch MA, Tobias SC, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qiu J, Nestor CC, Padilla SL, Palmiter RD, Rønnekleiv OK, Kelly MJ. High frequency-induced peptide release governs the synchronization of the arcuate kisspeptin neurons. Annual Meeting of the Society for Neuroscience, Chicago, IL 2015 (Program 613.24/Poster S19). [Google Scholar]
- 40. Cousin MA, Robinson PJ. Ca(2+) influx inhibits dynamin and arrests synaptic vesicle endocytosis at the active zone. J Neurosci. 2000;20:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab. 2013;305:E632–E640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qiu J, Bosch MA, Tobias SC, et al. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herman MA, Ackermann F, Trimbuch T, Rosenmund C. Vesicular glutamate transporter expression level affects synaptic vesicle release probability at hippocampal synapses in culture. J Neurosci. 2014;34:11781–11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Phys. 2002;64:355–405. [DOI] [PubMed] [Google Scholar]
- 46. Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 48. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wakabayashi Y, Nakada T, Murata K, et al. Nuerokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153:2756–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399. [DOI] [PubMed] [Google Scholar]
- 52. Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156:2582–2594. [DOI] [PubMed] [Google Scholar]
- 53. van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. [DOI] [PubMed] [Google Scholar]
- 54. Pinto S, Roseberry AG, Liu H, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. [DOI] [PubMed] [Google Scholar]
- 55. Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH-› arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. [DOI] [PubMed] [Google Scholar]
- 56. Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Belousov AB, Van den Pol AN. Local synaptic release of glutamate from neurons in the rat hypothalamic arcuate nucleus. J Physiol (Lond). 1997;499:747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ. GABA release from proopiomelanocortin neurons. J Neurosci. 2004;24:1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci. 2012;32:4042–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci USA. 2007;104:11453–11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Westphalen RI, Desai KM, Hemmings HC., Jr Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth. 2013;110:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krashes MJ, Shah BP, Madara JC, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524(pt 1):91–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Padilla SL, Qiu J, Soden ME, et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci. 2016;19:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chee MJ, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rønnekleiv OK, Fang Y, Zhang C, Nestor CC, Mao P, Kelly MJ. Research resources: gene profiling of G protein-coupled receptors in the arcuate nucleus of the female. Mol Endocrinol. 2014;28:1362–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nohara K, Zhang Y, Waraich RS, et al. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152:1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rana K, Fam BC, Clarke MV, Pang TPS, Zajac JD, MacLean HE. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol. 2011;301:E767–E778. [DOI] [PubMed] [Google Scholar]
- 70. Rowland DL, Perrings TS, Thommes JA. Comparison of androgenic effects on food intake and body weight in adult rats. Physiol Behav. 1980;24:205–209. [DOI] [PubMed] [Google Scholar]
- 71. Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol. 1999;276:R1366–R1373. [DOI] [PubMed] [Google Scholar]
- 72. Borgquist A, Meza C, Wagner EJ. The role of AMP-activated protein kinase in the androgenic potentiation of cannabinoid-induced changes in energy homeostasis. Am J Physiol Endocrinol Metab. 2015;308:E482–E495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anukulkitch C, Rao A, Dunshea FR, Blache D, Lincoln GA, Clarke IJ. Influence of photoperiod and gonadal status on food intake, adiposity and gene expression of hypothalamic appetite regulators in a seasonal mammal. Am J Physiol. 2006;292:R242–R252. [DOI] [PubMed] [Google Scholar]
- 74. Elias CF, Aschkenasi C, Lee C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. [DOI] [PubMed] [Google Scholar]
- 75. van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. [DOI] [PubMed] [Google Scholar]
- 76. Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Williams KW, Margatho LO, Lee CE, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bauer-Dantoin AC, McDonald JK, Levine JE. Neuropeptide Y potentiates luteinizing hormone (LH)-releasing hormone-stimulated LH surges in pentobarbital-blocked proestrous rats. Endocrinology. 1991;129:402–408. [DOI] [PubMed] [Google Scholar]
- 79. Leupen SM, Levine JE. Role of protein kinase C in facilitation of luteinizing hormone (LH)-releasing hormone-induced LH surges by neuropeptide Y. Endocrinology. 1999;140:3682–3687. [DOI] [PubMed] [Google Scholar]
- 80. Acosta-Martinez M, Horton T, Levine JE. Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol Metab. 2007;18:48–50. [DOI] [PubMed] [Google Scholar]
- 81. Mills RH, Sohn RK, Micevych PE. Estrogen-induced μ-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dodd GT, Decherf S, Loh K, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]