Abstract
Better understanding the mechanisms underlying adipogenesis may provide novel therapeutic targets in the treatment of obesity. Most studies investigating the mechanisms underlying adipogenesis focus on highly regulated transcriptional pathways; little is known about the epigenetic mechanisms in this process. Here, we determined the role of DNA methylation in regulating 3T3-L1 adipogenesis in early and late stage of differentiation. We found that inhibiting DNA methylation pharmacologically by 5-aza-2′-deoxycytidine (5-aza-dC) at early stage of 3T3-L1 differentiation markedly suppressed adipogenesis. This inhibition of adipogenesis by 5-aza-dC was associated with up-regulation of Wnt10a, an antiadipogenic factor, and down-regulation of Wnt10a promoter methylation. In contrast, inhibiting DNA methylation by 5-aza-dC at late stage of differentiation enhanced the lipogenic program. The differential effects of 5-aza-dC on adipogenesis were confirmed by gain or loss of function of DNA methyltransferase 1 using genetic approaches. We further explored the molecular mechanism underlying the enhanced lipogenesis by inhibition of DNA methylation at late stage of differentiation. The Srebp1c promoter is enriched with CpG sites. Chromatin immunoprecipitation assays showed that DNA methyltransferase 1 bound to the methylation region at the Srebp1c promoter. Pyrosequencing analysis revealed that the DNA methylation at the key cis-elements of the Srebp1c promoter was down-regulated in adipogenesis. Further, luciferase reporter assays showed that the Srebp1c promoter activity was dramatically up-regulated by the unmethylated promoter compared with the fully methylated promoter. Thus DNA methylation appears to exert a biphasic regulatory role in adipogenesis, promoting differentiation at early stage while inhibiting lipogenesis at late stage of 3T3-L1 preadipocyte differentiation.
Obesity results from both increased adipocyte size (hypertrophy) and number (hyperplasia) (1, 2). Better understanding the mechanisms underlying adipogenesis may provide novel therapeutic targets in the prevention and treatment of obesity and its related disorders. Adipogenesis is highly regulated by a sequential cascade of transcriptional events (3). Key transcriptional factors in this transcriptional program controlling adipogenesis include several members of CCAAT/enhancer-binding protein (C/EBP) family, including C/EBPα, C/EBPβ, and C/EBPδ, and the nuclear receptor peroxisome proliferator-activated receptor (PPAR)γ (3). The initiation of adipogenesis is caused by early induction of C/EBPβ and C/EBPδ. These early transcriptional factors subsequently activate 2 key transcriptional factors PPARγ and C/EBPα, interaction of which leads to the late determination of the adipocyte phenotype by inducing a variety of adipocyte phenotypic genes such as aP2, glucose transporter 4 (Glut4), etc (3). In the late stage of differentiation, the helix-loop-helix transcription factor sterol regulatory element-binding protein 1C (Srebp1c) is an important proadipogenic factor that may serve to enrich the mature adipocyte phenotype through inducing the lipogenic program such as the expression of fatty acid synthase (Fas), contributing to adipocyte lipid filling (3). On the other hand, a number of transcriptional repressors have also been identified to counterregulate the proadipogenic factors in the process of adipogenesis, including Gata2/3, chicken ovalbumin upstream promoter transcription factor, interferon regulatory factors, and Wnt family proteins (3–7). The differentiation process that determines the adipocyte fate is highly regulated by a coordinated control of these positive and negative transcriptional factors.
Most studies investigating the mechanisms underlying adipogenesis focus on highly regulated transcriptional pathways; little is known about the epigenetic mechanisms in this process. Epigenetic regulation, including DNA methylation, is a molecular link between environmental factors (eg, diets) and complex diseases, including obesity and diabetes. DNA methylation of cytosines at primarily CpG dinucleotides is the most common epigenetic modification. CpGs are often enriched in the promoter and the first exon/5′-untranslated region of genes (8). De novo methylation is mediated by DNA methyltransferase (DNMT)3a and DNMT3b. Once established, DNA methylation is then maintained through mitosis primarily by the maintenance enzyme DNMT1 (9). Promoters of transcriptionally active genes are typically hypomethylated (10), whereas DNA hypermethylation can result in gene silencing by affecting the binding of methylation-sensitive DNA-binding proteins and/or by interacting with various histone modifications and corepressors that alter DNA accessibility to transcriptional factors (10). Evidence converges to suggest that epigenetic events figure prominently in adipogenesis (11, 12). This is a new emerging research area; however, much remains to be discovered on how epigenetic mechanisms regulate adipogenesis and metabolism in general.
During the course of our present study, Gentile et al published an elegant study showing that DNMT1 regulates adipogenesis through maintaining DNA methylation pattern (13). We reasoned that proadipogenic and antiadipogenic programs during adipogenesis are tightly regulated in a timing fashion at different differentiation stages and that turning on or off DNA methylation at different differentiation stages may therefore play a different role in regulating the proadipogenic and antiadipogenic programs during adipogenesis. Accordingly, the present study was designed to determine the role of DNA methylation in regulation of 3T3-L1 adipogenesis in both early and late stages of differentiation. Using gain or loss of function approaches pharmacologically or genetically, we activated or inactivated DNMTs in a timing window of either early or late stage of differentiation and then examined the expression of adipogenic markers. We then explored the molecular mechanisms underlying the changes of the adipogenic phenotypes by DNA methylation. We specifically examined the promoter methylation of key anti- and proadipogenic genes (Wnt10a and Srebp1c) in early and late stages of differentiation in 3T3-L1 preadipocytes.
Materials and Methods
Cell culture and differentiation
3T3-L1 preadipocytes were grown in a DMEM containing 10% new born calf serum and 5% penicillin. Two days after confluence, 3T3-L1 preadipocytes were induced to differentiate with DMEM containing 10% fetal bovine serum (FBS), 0.5mM 3-isobutyl-1-methylxanthine, 1μM dexamethasone, and 400nM insulin. Cells were incubated in this medium for 2 days and then cultured in growth medium containing 400nM insulin for another 2 days. Subsequently, cells were maintained in growth medium (without insulin) throughout the adipocyte stage and cultures were refed every 2 days. Cells were treated with either PBS or 5-aza-2′-deoxycytidine (5-aza-dC) (0.5μM) at different time points indicated in the figures and were harvested at day 8 of the differentiation. ST2 cells were provided by Dr Ormond MacDougald and were cultured and differentiated as described (14). Briefly, ST2 cells were grown in an α-MEM medium (low glucose with L-glutamine) containing 10% FBS. Two days after confluence, ST2 cells were induced to differentiate with the α-MEM medium containing 10% FBS, 0.5mM 3-isobutyl-1-methylxanthine, 1μM dexamethasone, 800nM insulin, and 5μM troglitazone. Cells were then fed with ST2 medium containing 800nM insulin and 5μM troglitazone on day 2 and subsequently fed with ST2 medium containing 5μM troglitazone on day 4 for another 2 days until day 6. Oil Red O staining was conducted to visualize the lipid accumulation in differentiated cells. Glycerol-3-phosphate dehydrogenase (GPDH) activity was determined spectrophotometrically in crude cytosolic extracts of adipocytes by measuring the oxidation rate of NADH as we previously described (15, 16).
Lentiviral shRNA knockdown and infection
The Dnmt1 and Dnmt3a lentiviral shRNA constructs were purchased from Open Biosystems. The DNMT1 shRNA lentivirus was generated as we previously described (17). Briefly, the DNMT1 or DNMT3a or control lentiviral vectors were con-transfected with the packaging plasmids pCMV-dR8.91 and the envelope plasmids VSV-G into 297T cells. Medium containing the lentivirus was harvested and filtered and used to infect 3T3-L1 preadipocytes. The infected cells were selected with puromycin (2 μg/mL) for 5 days, and the surviving cells were pooled and used for differentiation with the protocol described above.
siRNA knockdown
siRNA knockdown of DNMT1 or DNMT3a in 3T3-L1 cells at day 5 of the differentiation was performed as previously described (18). Briefly, 3T3-L1 cells after 5 days of differentiation were trypsinized from 100-mm dishes. DNMT1 siRNAs (SMART pool; Dharmacon, Thermo Scientific) were electroporated into 3T3-L1 cells (5 nmol siRNA/2.5 million cells) using a Bio-Rad gene pulser II system with electroporation parameters at 0.18 kV and 960 μF. Cells were then plated into 24-well plates for growing with growth medium described above. siRNA knockdown of DNMT1 in ST2 cells 2 days before adipogenic induction and at day 6 of the differentiation was conducted as 3T3-L1 cells described above.
Lentiviral constructs, infection, and inducible expression
The inducible expression of Dnmt1 and Dnmt3a was achieved using the Retro-X-Tet-on-3G system (Clontech) according to the instruction. Briefly, the Dnmt1 or Dnmt3a cDNA vector was purchased from Open Biosystems and was further subcloned into the pRetroX-TRE3G vectors (Clontech). The pRetroX-TRE3G Dnmt1 or Dnmt3a (response) and pRetroX-Tet3G (regulator) retrovirus were generated according to the instruction from Clontech. Medium containing the retrovirus was harvested and filtered and used to infect 3T3-L1 cells. Cells were first infected with only the pRetroX-Tet3G (regulator) retrovirus, followed by selection with G418. Surviving cells were pooled and further infected with pRetroX-TRE-3G Dnmt1 or Dnmt3a (response) retrovirus and followed by selection with puromycin. The doubly transduced cells were pooled for further differentiation. The inducible expression of Dnmt1 or Dnmt3a was achieved by addition of doxycycline (800 ng/mL) to the medium.
Total RNA extraction and quantitative RT-PCR
3T3-L1 total RNA was extracted using the Tri reagent kit (Molecular Research Center), according to the manufacturer's protocol. The expression of genes of interest was assessed by quantitative RT-PCR (ABI Universal PCR Master Mix; Applied Biosystems) using a Stratagene Mx3005p thermocycler (Stratagene), as we previously described (17, 19). The primer and probe pairs used in the assays were purchased from Applied Biosystems. A pooled mouse adipocyte total RNA was serially diluted in the range of 0.1–100 ng and was used to establish a standard curse for each gene expression of interest. The mRNA quantitation of gene of interest was further normalized by the corresponding cyclophilin mRNA measurement.
Immunoblotting
Immunoblotting was performed as we previously described (20). Briefly, cells were harvested and homogenized in a modified radioimmunoprecipitation assay lysis buffer containing 50mM Tris-HCl, 1mM EDTA, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150mM NaCl, 1mM phenylmethylsulfonyl fluoride, 200μM Na3VO3, 1% protease inhibitor mixture (Sigma), and 1% phosphatase inhibitor mixture (Sigma). Cell homogenates were incubated on ice for 45 minutes to solubilize all proteins, and insoluble portions were removed by centrifugation at 14 000g at 4°C for 15 minutes. Cell lysates were separated by SDS-PAGE. Proteins on the gels were transferred to nitrocellulose membrane (Bio-Rad). The transferred membranes were blocked, washed, and incubated with various primary antibodies, followed by Alexa Fluor 680-conjugated secondary antibodies (Life Science Techenologies). The blots were developed with a Li-COR Imager System (Li-COR Biosciences). Antibodies against DNMT1, DNMT3a, glyceraldehyde-3-phosphate dehydrogenase, and actin were purchased from Santa Cruz Biotechnology, Inc.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using a ChIP assay kit (Upstate) as we previously described (19). Briefly, cells were fixed with 1% of formaldehyde and then harvested in cell lysis buffer (5mM PIPES, 85mM KCl, and 0.5% NP-40, supplemented with protease inhibitors; pH 8.0). Lysates were sonicated to shear genomic DNA to an average fragment length of 200–1000 bp with a Diagenode Bioruptor (Diagenode). Lysates were centrifuged, and the supernatants were collected. Fifty microliters of each sample were removed as the input control. The supernatants underwent overnight immunoprecipitation, elution, reverse cross-linking, and protease K digestion, according to the manufacturer's manual. A mock immunoprecipitation without antibody was also included for each sample. The DNAs recovered from phenol/chloroform extraction were used for SYBR Green quantitative PCR (Applied Biosystems), and the DNA quantitation value of each sample was further normalized with the DNA quantitation of individual input control. The sequences of primers for Srebp1c were: forward, 5′-TTCAGAGCACCGGGAGAAAC-3′ and reverse, 5′-GATTTCACCTGTCAGGCCCC-3′.
Bisulfite conversion and pyrosequencing
Genomic DNA was prepared by phenol/chloroform extraction. Bisulfite conversion was performed using EpiTech Bisulfite kit (QIAGEN). The converted DNA was used as template to amplify DNA sequence covering the putative CpG sites at the Wnt10a or Srebp1c promoter regions. One microgram of bisulfite converted DNA was amplified by PCR pyrosequencing was performed by EpiGenDx. The amplification and pyrosequencing primers that were used to measure the DNA methylation on the Wnt10a (mainly on the first exon) or the Srebp1c promoter and 5′ regions covering CpG sites are described in Supplemental Table 1.
Srebp1c promoter cloning and luciferase reporter assays
A 550-bp fragment covering Srebp1c proximal promoter was PCR amplified from mouse gDNA with primers as follows. Srebp1c forward, 5′-TTCATGAGGGATCCAGAACT-3′ and reverse, 5′-ATGTGCAATCCATGGCTCCG-3′. The PCR fragments were ligated to the pGL3-Basic vector to generate the pGL3-Srebp1c constructs. The constructs were confirmed by sequencing. To obtain unmethylated promoter, the reporter constructs were transformed into the dam−/dcm− Escherichia coli strain (New England Biolabs). To obtain fully methylated reporter, constructs were incubated with 3-U/μg SssI methylase (New England Biolabs) in the presence of 160μM S-adenosylmethionine at 37°C for 3 hours (21, 22). Methylation was confirmed by checking the resistance of reporter constructs to HpyCH4IV digestion. The unmethylated or methylated Srebp1c reporter constructs were transfected into RAW cells using a SuperFect Transfection reagent kit (QIAGEN) and luciferase activity was measured using a Dual-Luciferase Reporter Assay kit (Promega) as we described (22).
Electrophoretic mobility shift assays
The nuclear extracts from day-0 preadipocytes and day-8 3T3-L1 adipocytes were prepared as we previously described (19, 23). The sequence of the oligonucleotide DNAs containing the SRE box (24) and 3 CpG sites is 5′-tgcgctcacccgaggggcggg-3′. The double-strand complementary oligonucleotide DNAs were annealed, in vitro methylated (see above) and end-labeled using a Biotin 3′ End DNA Labeling kit (Pierce Biotechnology), and EMSAs were performed using a LightShift Chemiluminescent EMSA kit according to the company's instruction (Pierce Biotechnology).
Statistics
All data are expressed as mean ± SEM. Data were evaluated for statistical significance by one-way ANOVA, and statistical significance for comparison of means of different groups was calculated by the Bonferroni test using the SPSS software package version 11.5. P < .05 was considered significant.
Results
Inhibition of DNA methylation by 5-aza-dC at the early and the late stage of differentiation differentially regulates 3T3-L1 adipogenesis and lipogenesis
To determine the potential relevance of DNA methylation in regulating adipogenesis, we first measured the expression of DNMTs, including Dnmt1, Dnmt3a, and Dnmt3b, during the course of 3T3-L1 preadipocyte differentiation. We found that the Dnmt1 mRNA levels were rapidly induced at the very early stage of differentiation at 6 hours, reaching peak levels at 16 hours and declining within 48 hours (Supplemental Figure 1). Instead, Dnmt3a expression showed no change at the early stage of differentiation but displayed a slight increase only at the mature adipocyte stage after 8 days of differentiation, whereas Dnmt3b expression remained at very low levels throughout the differentiation process (Supplemental Figure 1). These data suggest that among all the DNMTs, DNMT1 may play a major role in regulation of adipogenesis.
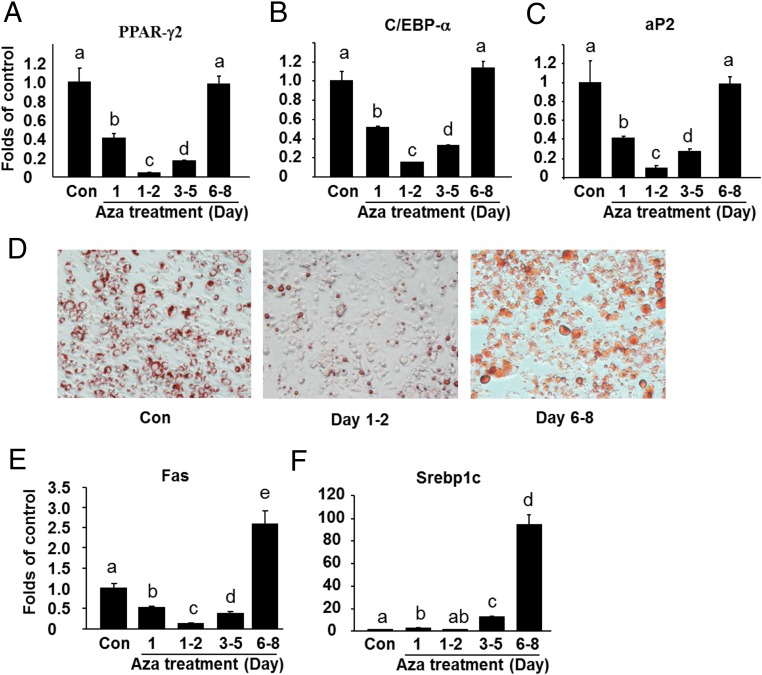
Due to the unique expression pattern of Dnmt1 with an early transient induction and rapid decline during the differentiation process, we reasoned that DNMT1 may play a differential role in regulating adipogenesis at the early and the late stage of differentiation. We first employed a pharmacological approach to inhibit DNA methylation at different time points of differentiation. 5-aza-dC is a nucleoside-based DNMT inhibitor widely used to study the role of DNA methylation in regulation of cell development and cancer (25, 26). 3T3-L1 preadipocytes were induced to differentiate with a differentiation medium and were concurrently treated with either PBS or 5-aza-dC at day 1, 1–2, 3–5, and 6–8, and cells were harvested at day 8 of the differentiation. We found that inhibiting DNA methylation by 5-aza-dC at the early differentiation period from day 1–5 inhibited adipogenesis, evident by decreased expression of adipogenic markers and adipocyte phenotypic genes such as PPARγ2, C/ebpα, aP2, Fas (Figure 1, A–C). Oil Red O staining confirmed that 5-aza-dC treatment at day 1–2 inhibited lipid accumulation, whereas 5-aza-dC treatment at day 6–8 had no effect on differentiation (Figure 1D). In contrast, treatment of 3T3-L1 cells with 5-aza-dC at the late stage of differentiation at day 6–8 markedly enhanced the expression of adipocyte lipogenic markers, such as Fas and Srebp1c (Figure 1, E and F), while having no effects on the expression of adipogenic markers such as PPARγ2, C/EBPα, and aP2 (Figure 1, A–C). These data suggest that inhibition of DNA methylation at the early and the late stage of differentiation plays a differential role in regulating adipogenesis and lipogenesis, respectively.
Figure 1.
Inhibition of DNA methylation by 5-aza-dC at the early and late stage of differentiation differentially regulates 3T3-L1 preadipocyte differentiation and lipogenesis. Inhibition of DNA methylation by 5-aza-dC at the early stage (d 1–5) of differentiation suppressed the expression of adipogenic markers such as PPARγ2 (A), Cebpα (B), aP2 (C), and Fas (E). D, Oil Red O staining reveals that 5-aza-dC treatment at day 1–2 inhibited lipid accumulation, whereas 5-aza-dC treatment at day 6–8 had no effect on differentiation. E and F, Inhibition of DNA methylation by 5-aza-dC at the late stage (d 6–8) of differentiation promotes the expression of lipogenic genes such as Fas and Srebp1c. 3T3-L1 preadipocyte were induced to differentiate with a differentiation medium as described in Materials and Methods and were concurrently treated with either PBS or 5-aza-dC at day 1, 1–2, 3–5, and 6–8 as indicated in the figure, and cells were harvested at day 8 of the differentiation for the measurement of gene expression. Gene expression was measured by real-time RT-PCR and normalized to cyclophilin. All data are expressed as mean ± SEM, n = 4–6; groups labeled with different letters are statistically different from each other.
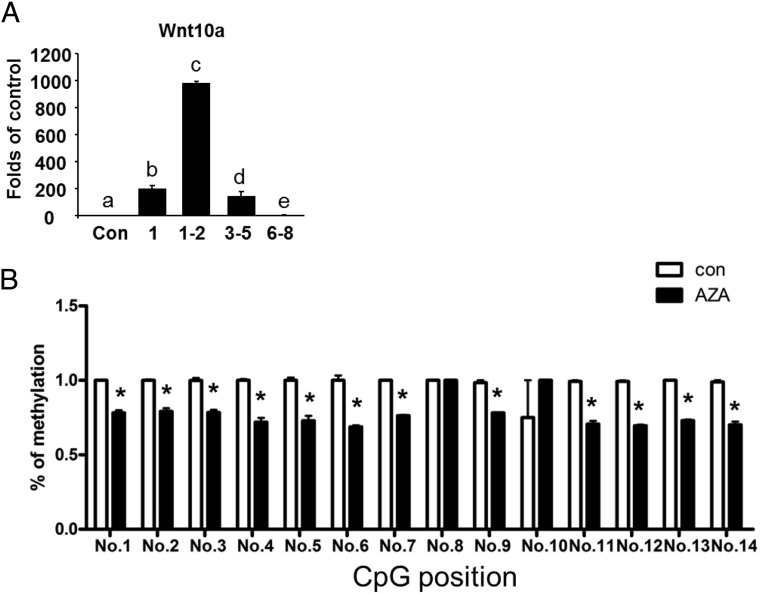
To further explore the mechanism underlying the suppression of adipogenesis by the early inhibition of DNA methylation with 5-aza-dC, we surveyed the expression of a number of key transcriptional factors that control the adipogenic program. Because demethylation of gene promoters by 5-aza-dC activates gene expression and the early treatment of 5-aza-dC led to inhibition of adipogenesis in our study, we reckoned that the antiadipogenic transcriptional factors might be potentially important sites of demethylation, activation of which may be responsible for the suppression of adipogenesis by the treatment of 5-aza-dC during the early differentiation. Among the adipogenic repressors we measured (data will be reported elsewhere), Wnt10a mRNA expression was dramatically induced by early treatment of 5-aza-dC between days 1 and 5 of differentiation, with a peak level reached at day 1–2 (Figure 2A), mirroring the timing of the lowest expression of the adipogenic markers. The promoter and 5′ region including part of the first exon of the Wnt10a gene is enriched with CpG sites, raising the possibility that Wnt10a is subjected to demethylation due to 5-aza-dC treatment. Among approximately 46 CpG sites in the promoter and 5′ region, we chose to measure the methylation status of the 14 CpG sites located on the first exon region of the Wnt10a gene (other CpG sites in the promoter region will be reported elsewhere) (Supplemental Figure 2). Using pyrosequencing analysis, we found that 5-aza-dC treatment significantly reduced the DNA methylation levels on 12 out of 14 CpG sites measured (Figure 2B). These data suggest that demethylation of the Wnt10a promoter due to 5-aza-dC treatment may cause Wnt10a gene overexpression, which is associated with suppression of adipogenesis during the early stage of differentiation.
Figure 2.
5-aza-dC demethylates the Wnt10a promoter. A, 5-aza-dC induces Wnt10a expression at the early stage of differentiation. B, Methylation at the CpG sites of the Wnt10a promoter is reduced by 5-aza-dC. A, 3T3-L1 preadipocytes were induced to differentiate with a differentiation medium as described in Materials and Methods and were concurrently treated with either PBS or 5-aza-dC at day 1, 1–2, 3–5, and 6–8 as indicated in the figure, and cells were harvested at day 8 of the differentiation for the measurement of gene expression. Gene expression was measured by real-time RT-PCR and normalized to cyclophilin. All data are expressed as mean ± SEM, n = 6; groups labeled with different letters are statistically different from each other. B, 3T3-L1 preadipocytes were induced to differentiate and were treated with either PBS or 5-aza-dC (0.5μM) at day 0–2 of differentiation for 2 days. CpG methylation was measured by pyrosequencing as described in Materials and Methods. All data are expressed as mean ± SEM, n = 4; *, P < .05 vs control. Con, control; AZA, 5-aza-dC.
DNA methylation positively regulates 3T3-L1 adipogenesis at early stage of differentiation
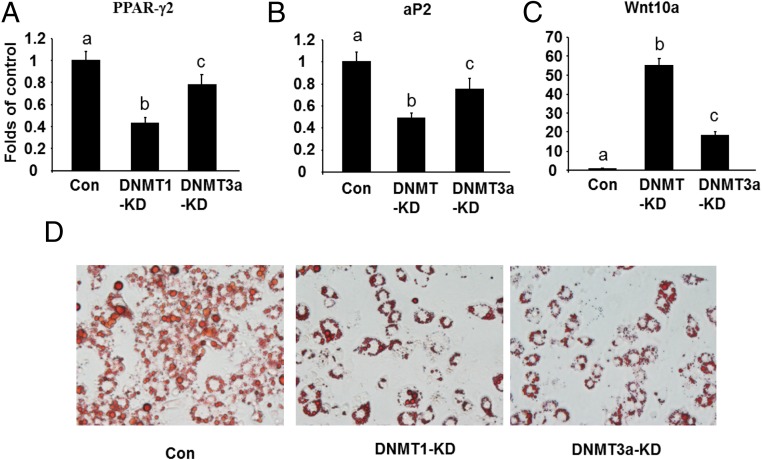
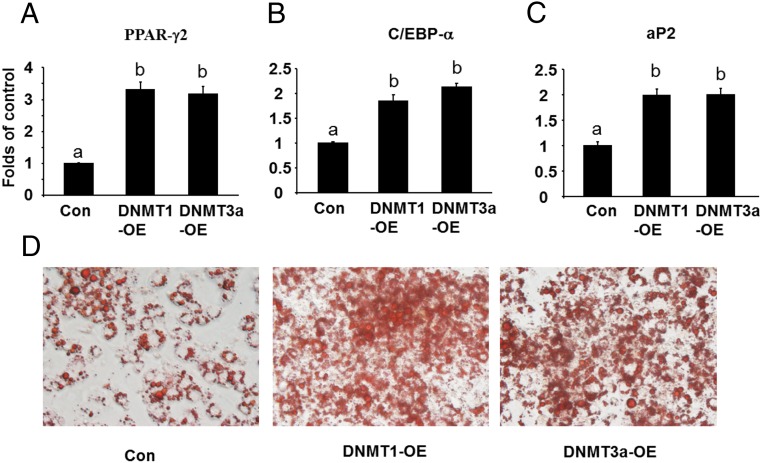
We also targeted DNA methylation using genetic approaches with gain- or loss-of-function experiments. Because our data on the expression level and pattern of Dnmts during the differentiation process suggest that DNMT1 may play a major, whereas DNMT3a may play a minor, role in regulation of 3T3-L1 adipogenesis, we focused on these 2 DNMTs. To determine the role of DNMT1 or DNMT3a in regulation of 3T3-L1adipogenesis, we first established 3T3-L1 preadipocytes with reduced Dnmt1 or Dnmt3a expression by lentiviral shRNA knockdown. The Dnmt1 mRNA and protein levels in knockdown L1 cells were suppressed by more than 75% (Supplemental Figure 3A). Similar reduction of Dnmt3a at both mRNA and protein levels were also achieved in Dnmt3a knockdown cells (Supplemental Figure 3B). The 3T3-L1 preadipocytes with Dnmt1 or Dnmt3a knockdown were then induced to differentiate with the differentiation protocol so that these preadipocytes were deficient in Dnmts at the beginning of the adipogenesis. Similar to the observations on the inhibition of adipogenesis by the early 5-aza-dC treatment, lack of DNMT1 at the early stage of differentiation decreased the expression of PPARγ2 and its downstream target gene aP2 (Figure 3, A and B). This inhibition of adipogenesis was associated with up-regulation of Wnt10a expression (Figure 3C). Oil Red O staining confirmed the inhibition of adipogenesis by early knockdown of DNMT1 in 3T3-L1 preadipocytes (Figure 3D). Similar results were observed in the L1 cells with DNMT3a knockdown, albeit to a much lesser extent (Figure 3, A–C). We also determined the role of DNMT1 in regulation of ST2 adipogenesis, a mesenchymal stem cell line. Consistent with 3T3-L1 cells, we found that early knockdown of DNMT1 in ST2 cells (Supplmental Figure 4) similarly inhibited adipogenesis, evident by decreased expression of PPARγ2 and aP2 and up-regulation of Wnt10a as well as less Oil Red O staining of lipid accumulation (Supplmental Figure 5). We further employed a gain- of function approach to overexpress DNMT1 or DNMT3a in 3T3-L1 preadipocytes. We established L1 cell lines with an inducible expression system (Retro-X-Tet-on-3G, Clontech) where DNMT1 or DNMT3a can be induced in a timing manner upon treatment of doxycycline (cells were treated with doxycycline at d 0 and lasted for 2 d) (Supplmental Figure 6). Overexpression of DNMT1 or DNMT3a at the beginning of differentiation (at d 2) promoted the expression of adipogenic markers such as PPARγ2, aP2, and CEBPα (Figure 4, A–C). This was further confirmed by Oil Red O staining of lipid accumulation (Figure 4D). These data suggest that DNMT1 and DNMT3a are positive regulators of 3T3-L1 adipogenesis at early stage of differentiation possibly through down-regulation of Wnt10a.
Figure 3.
DNMT1 or DNMT3a knockdown at beginning of the differentiation inhibits 3T3-L1 adipogenesis. DNMT1 or DNMT3a knockdown at the beginning of the differentiation inhibited the expression of adipogenic markers such as PPARγ2 (A) and aP2 (B) while inducing the antiadipogenic factor Wnt10a (C). D, Oil Red O staining revealed the inhibition of adipogenesis by early knockdown of DNMT1 and DNMT3a. 3T3-L1 preadipocytes were infected with the Dnmt1 or DNMT3a shRNA lentivirus, selected with puromycin for 5 days and differentiated as described in Materials and Methods. Cells were harvested at day 8 of the differentiation for the measurement of gene expression. Gene expression was measured by real-time RT-PCR and normalized to cyclophilin. All data are expressed as mean ± SEM, n = 6; groups labeled with different letters are statistically different from each other. KD, knockdown.
Figure 4.
DNMT1 or DNMT3a overexpression at beginning of the differentiation promotes 3T3-L1 adipogenesis. DNMT1 or DNMT3a overexpression at beginning of the differentiation enhanced the expression of adipogenic markers such as PPARγ2 (A), C/EBPα (B), and aP2 (C). D, Oil Red O staining revealed the enhanced adipogenesis by early DNMT overexpression in 3T3-L1 cells. 3T3-L1 preadipocytes were sequentially infected with the pRetroX-TRE3G DNMT1 or DNMT3a (response) and pRetroX-Tet3G (regulator) retrovirus to establish cells with a doxycycline-inducible expression system as described in Materials and Methods. The pooled cells were induced to differentiate and were concurrently treated with doxycycline at day 0 of differentiation to induce DNMT1 or DNMT3a expression. Cells were harvested at day 8 of the differentiation for the measurement of gene expression. Gene expression was measured by real-time RT-PCR and normalized to cyclophilin. All data are expressed as mean ± SEM, n = 6; groups labeled with different letters are statistically different from each other. OE, overexpression.
DNA methylation negatively regulates lipogenesis at late stage of 3T3-L1 preadipocyte differentiation
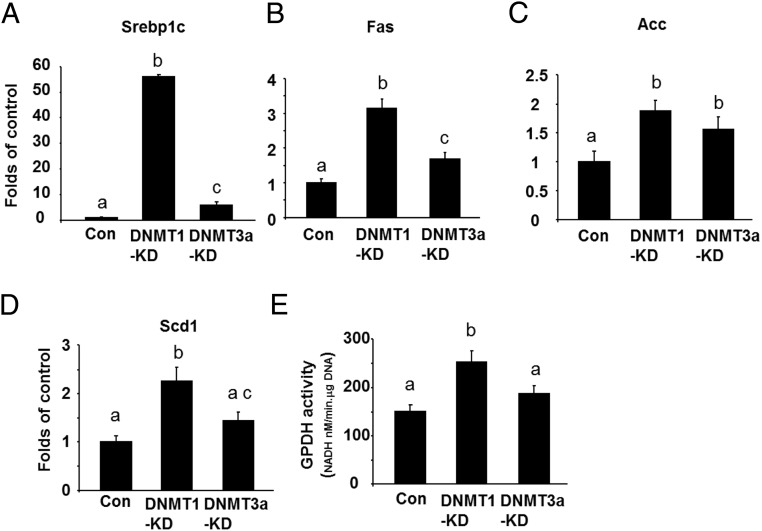
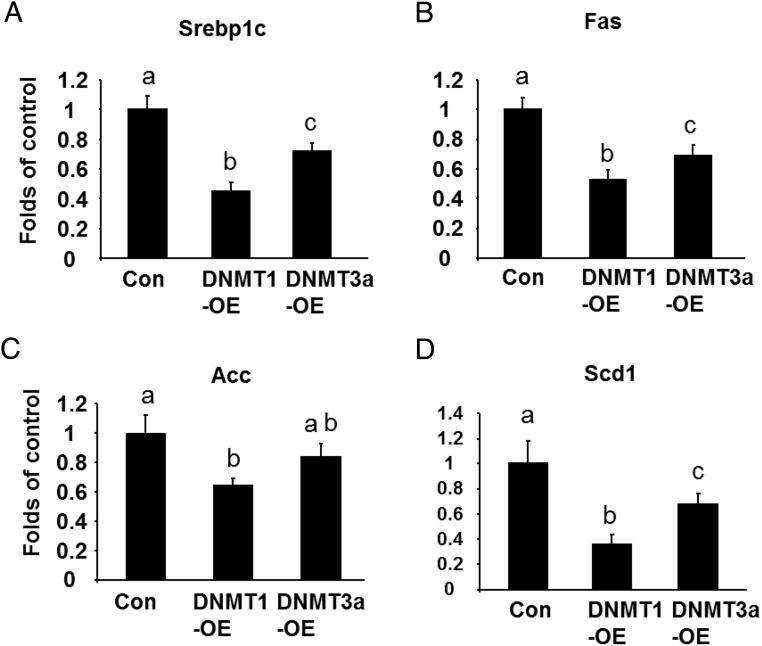
We further determined the role of DNMTs in regulation of the late stage of adipogenesis when most functional genes involved in glucose and lipid metabolism are expressed to render the adipocyte phenotype. We chose to knock down DNMT1 or DNMT3a after 5 days of differentiation using electroporation of the cells with siRNA (Supplmental Figure 7). We found that DNMT1 knockdown markedly enhanced the expression of lipogenic markers Srebp1c and its downstream target Fas, Acc, and Scd1 (Figure 5, A–D). Similar results were observed in the L1 cells with DNMT3a knockdown, albeit to a much lesser extent (Figure 5, A–D). Moreover, DNMT1 knockdown at late stage of differentiation also increased GPDH activity, an indicator of lipogenesis, whereas DNMT3a knockdown had a tendency to increase GPDH activity but had no statistical significance (Figure 5E). We also determined the role of DNMT1 in regulation of ST2 lipogenesis at late stage. Similar to 3T3-L1 cells, we found that late knockdown of DNMT1 in ST2 cells (Supplmental Figure 8) enhanced lipogenesis, evident by increased expression of Srebp1c and Fas (Supplmental Figure 9). In contrast, overexpression of DNMT1 or DNMT3a after 7 days of differentiation in the L1 cells with a doxycycline-inducible expression system (cells were treated with doxycycline at d 5 and lasted for 2 d) (Supplmental Figure 10) significantly reduced Srebp1c, Fas, Acc, and Scd1 expression (Figure 6, A–D). These data suggest that DNMT1 and DNMT3a are negative regulators of lipogenesis at late stage of differentiation in 3T3-L1 cells.
Figure 5.
DNMT1 or DNMT3a knockdown at late stage of the differentiation promotes lipogenesis in 3T3-L1 cells. DNMT1 or DNMT3a knockdown at the late stage of the differentiation promoted the expression of lipogenic markers such as Srebp1c (A) and its downstream target Fas (B), Acc (C) and Scd1 (D), and enhanced GPDG activity (E). 3T3-L1 cells at day 5 of the differentiation were electroporated with Dnmt1 or Dnmt3a siRNA and differentiated as described in Materials and Methods. Cells were harvested at day 8 of the differentiation for the measurement of gene expression. Gene expression was measured by real-time RT-PCR and normalized to cyclophilin. All data are expressed as mean ± SEM, n = 6; groups labeled with different letters are statistically different from each other. KD, knockdown.
Figure 6.
DNMT1 or DNMT3a overexpression at late stage of the differentiation inhibits lipogenesis in 3T3-L1 cells. DNMT1 or DNMT3a overexpression at the late stage of the differentiation inhibited the expression of lipogenic markers such as Srebp1c (A) and its downstream target Fas (B), Acc (C), and Scd1 (D). 3T3-L1 preadipocytes were sequentially infected with the pRetroX-TRE3G DNMT1 or DNMT3a (response) and pRetroX-Tet3G (regulator) retrovirus to establish cells with a doxycycline-inducible expression system as described in Materials and Methods. The pooled cells were induced to differentiate and were concurrently treated with doxycycline at day 5 of differentiation to induce DNMT1 or DNMT3a expression. Cells were harvested at day 8 of the differentiation for the measurement of gene expression. Gene expression was measured by real-time RT-PCR and normalized to cyclophilin. All data are expressed as mean ± SEM, n = 6; groups labeled with different letters are statistically different from each other.
The Srebp1c promoter is regulated by DNA methylation in 3T3-L1adipogenesis
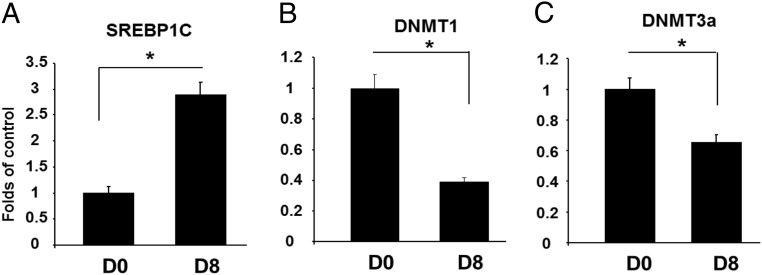
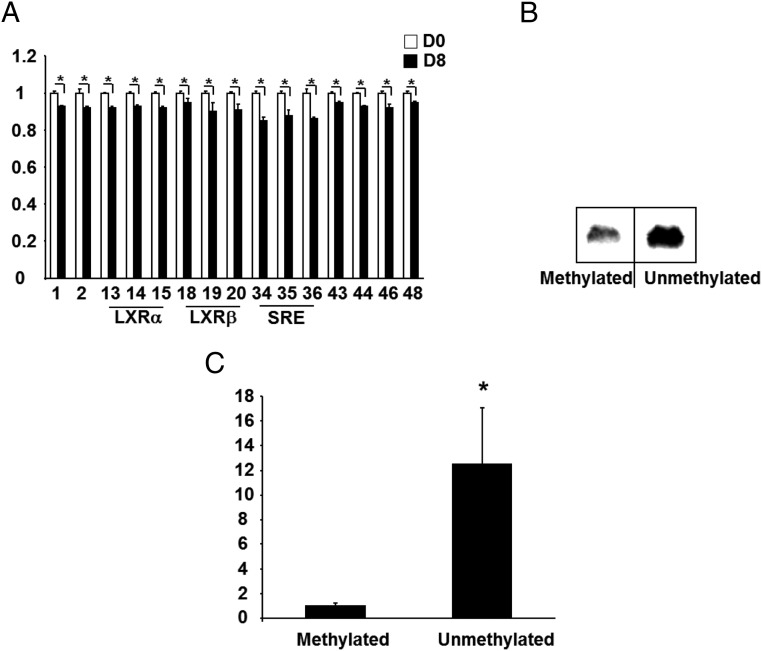
SREBP1C is a key transcriptional factor in regulation of lipogenesis. The Srebp1c promoter contains several cis-elements including an E-box, liver X receptor (LXR)α- and LXRβ-binding sites, and a SRE complex (for SREBP1C binding to increase its own transcription), which are important for its promoter activity (24). We found that the proximal promoter of Srebp1c is enriched with CpG sites, raising a possibility that Srebp1c is also subjected to epigenetic regulation by DNA methylation. Interestingly, the key elements such as the LXRα/β-binding sites and the SRE complex on the promoter contain multiple CpG sites (Supplmental Figure 11), suggesting that DNA methylation on these CpG sites may regulate binding of the transcriptional factors on these cis-elements. Because SREBP1C is induced during the course of adipogenesis and later regulates the expression of lipogenic genes, resulting in the features of mature adipocytes, we characterized the dynamic changes of the methylation status on the Srebp1c promoter during the course of adipogenesis. Indeed, our ChIP assays showed that as expected, the binding of SREBP1C to the SRE region of its own promoter was significantly increased in 3T3-L1 cells on day 8 of the differentiation compared with day 0, suggesting enhanced Srebp1c transcription and activation of the lipogenic program during adipocyte maturation (Figure 7A). This was associated with the down-regulation of DNMT1 or DNMT3a binding to the same SRE and proximate regions (Figure 7, B and C), suggesting that the decreased association of DNMTs with the promoter may reduce CpG methylation on these elements and therefore reciprocally increase the SREBP1C binding to the SRE. Indeed, our pyrosequencing analysis revealed that DNA methylation was decreased in a number of CpG sites of the Srebp1c promoter in 3T3-L1 cells differentiated at day 8 compared with day 0 (Figure 8A). More importantly, several methylation-decreased CpG sites are located within the key cis-elements such as LXRα (CpG sites 13, 14, and 15), LXRβ (CpG sites 18, 19, and 20), and the SRE complex (CpG sites 34, 35, and 36) (Figure 8A). We next tested whether the methylation of these CpG sites might be of importance in regulation of transcriptional factor binding to their corresponding elements. We used the synthetic oligonucleotides containing the SRE site with flanking sequences whose CpG sites were made fully methylated or unmethylated. EMSA analysis revealed a decreased binding of nuclear extracts to the fully methylated SRE oligonucleotides compared with the unmethylated SRE (Figure 8B). To further determine whether the Srebp1c promoter activity is indeed regulated by methylation, we cloned a 550-bp Srebp1c promoter fragment covering a TATA box, several key cis-elements including the SRE complex and LXRα/β-binding sequences with 50 CpG sites and then examined the fully methylated vs unmethylated Srebp1c promoter activity using luciferase assays. Figure 8C showed that the luciferase activity of the unmethylated promoter construct was 12-fold higher than that of the fully methylated one. Taken together, all these data suggest that the transcriptional activity of Srebp1c promoter is highly regulated by DNA methylation during adipogenesis and that the decreased methylation of the Srebp1c promoter may subsequently drive its gene expression and the lipogenic program during the late stage of differentiation.
Figure 7.
DNMT1 or DNMT3a binds to the Srebp1c promoter. A, The binding of SREBP1C to the SRE region of its own promoter was significantly increased along the adipogenic process. The binding of DNMT1 (B) or DNMT3a (C) to the SRE region of the Srebp1c promoter was down-regulated during the adipogenic process. 3T3-L1 preadipocyte were induced to differentiate and cells were harvested at day 0 or 8 of the differentiation for the ChIP assays, which were conducted as described in Materials and Methods. All data are expressed as mean ± SEM, n = 4; *, P < .05 vs D0. D0, day 0; D8, day 8.
Figure 8.
The Srebp1c promoter is regulated by DNA methylation during 3T3-L1 adipogenesis. A, DNA methylation levels were decreased at CpG sites on the key cis-elements of Srebp1c promoter during adipogenesis. 3T3-L1 preadipocyte were induced to differentiate and cells were harvested at day 0 or 8 of the differentiation for the pyrosequencing analysis, which was conducted as described in Materials and Methods. All data are expressed as mean ± SEM, n = 4; *, P < .05 vs D0. D0, day 0; D8, day 8. B, The binding of nuclear extracts to the fully methylated SRE oligonucleotides was decreased compared with the unmethylated SRE. The binding of nuclear extracts to SRE oligonucleotides was measured by EMSA, which was conducted as described in Materials and Methods. C, DNA methylation regulates Srebp1c promoter activity. A 550-bp Srebp1c promoter was cloned into the pGL3 luciferase reporter. Unmethylated vs fully methylated SREBP1C promoter activity was conducted as described in Materials and Methods. All data are expressed as mean ± SEM, n = 4; *, P < .05 vs methylated.
Discussion
In this study, we demonstrate that DNA methylation, catalyzed by DNMT1 or DNMT3a, plays a regulatory role in adipogenesis and lipogenesis. Our data indicate that DNMT1 or DNMT3a promotes adipogenesis at early stage of 3T3-L1 differentiation while inhibiting lipogenesis at the late stage of differentiation presumably through suppressing Srebp1c expression by methylating its promoter. Therefore DNA methylation appears to exert a biphasic role in regulation of adipogenesis by promoting early stage of differentiation while inhibiting the late stage of lipogenesis and the mature adipocyte phenotype.
We demonstrate in this study that DNA methylation orchestrates the adipogenic program in a timing manner through a coordinated control of both positive and negative transcriptional factors. We first observed a pulse of Dnmt1 expression during the early stage of differentiation, with a rapid peak reached at 16 hours and a rapid decline within 48 hours. Methylation of gene promoter by Dnmts typically results in gene silencing. Therefore, we reasoned that the surge of Dnmt1 expression at the beginning of the differentiation may silence the early transcriptional repressors that otherwise prohibit the initiation of adipogenic program, one of which is Wnt10a (14). Indeed, we found that 5-aza-dC causes demethylation on the Wnt10a promoter and may subsequently induce its expression, which is associated with suppression of the adipogenic program. Adipogenesis is a complex program with a sequential cascade of transcriptional events (3). The late stage of adipogenesis also requires the expression of adipocyte functional and phenotypic genes mostly involved in lipid and glucose metabolism. One of such genes is Srebp1c that directs the lipogenic program involving de novo synthesis of fatty acids from glucoses (3). Therefore, the decline of Dnmt1 expression after 48 hours of differentiation is equally important as the surge of its early expression. This may reduce the DNA methylation on the Srebp1c promoter and increase its expression, leading to activation of lipogenesis partially responsible for the mature adipocyte phenotype. The expression pattern of Dnmt1 is extremely interesting and dictates the differential role of DNMT1 in regulation of early adipogenesis and later lipogenesis. Further studies are warranted to explore the upstream signals inducing such expression pattern for DNMT1 during the differentiation process. Although DNMTs are responsible for maintaining the homeostasis of the global DNA methylation and gene expression, it is noteworthy that the regulation of gene transcription by DNA methylation can be very specific and accurate. We observed that methylation changes only occur in a number of CpG sites that lie within the key cis-elements of the Srebp1c promoter, which may affect the binding of the transcriptional factors such as SREBP1C to these sequences and therefore alter the gene transcription. The pinpoint action targeting the specific CpG sites at the Srebp1c promoter reflects the abilities of DNMTs to regulate specific pathways in timing and spatial manner, which is very important in the developmental biology where sequentially turning on and off genes that resemble the biological features of the specific developmental stages are tightly regulated. Therefore, additional studies are required to study the molecular mechanisms underlying the specific regulation of biological pathways by DNA methylation. On the other hand, it would be interesting to know whether DNMTs also act on other genes' promoters to regulate transcription during adipogenesis. Unbiased approaches such as ChIP sequencing with antibodies against DNMT1 or DNMT3a will be required to explore the genes Dnmts regulate during adipogenesis. Moreover, further studies involving genome-wide profiling of DNA methylation analysis will be warranted to address the significance and role of DNA methylation in adipogenesis.
Most studies investigating the mechanisms underlying adipogenesis focus on highly regulated transcriptional pathways; little is known about the epigenetic mechanisms in this process. Epigenetic regulation, including DNA methylation, is a molecular link between environmental factors (eg, diets) and complex diseases, including obesity and diabetes. This is a new emerging research area; however, evidence converges to suggest that epigenetic events figure prominently in adipogenesis (11, 12). Mikkelsen et al have done fabulous epigenomic analysis of adipogenesis revealing dynamic changes of histone methylation/acetylation marks on thousands of putative cis-elements along the time course of differentiation (27). These changes on epigenetic marks may actively regulate adipogenesis through interaction with key transcriptional factors that turn on or off the adipogenic process. Indeed, the transcriptional active mark H3K4me3 levels are increased on the promoter of PPARγ during adipogenesis (11). Demethylation of H3K27me3 inhibits adipogenesis through derepressing the Wnt family proteins (11). Changes in DNA methylation have been shown to modulate histone methylation, which may act cooperatively to influence chromatin structure and thereby regulate gene expression (28). This raises another interesting question as to whether DNA methylation also interacts with histone marks to regulate adipogenesis. Future studies will be required to disclose the relationship between DNA methylation and histone modification in regulation of adipogenesis. It should be noted that during the course of our present study, Gentile et al published an elegant study showing that DNMT1 regulates adipogenesis through maintaining DNA methylation pattern during the stage of mitotic clonal expansion (13). They showed that DNMT1 deficiency promotes adipogenesis. It has been known that mitotic clonal expansion is required for terminal adipogenesis (2, 29). The transient induction of DNMT1 within 48 hours, during which mitotic clonal expansion occurs, implicates an important role of DNMT1 in this key event required for further differentiation. Besides, the early induction of DNMT1 expression appears to suggest DNMT1 as a proadipogenic factor at least at early stage of differentiation. The reasons for the discrepancy between the findings by Gentile et al (13) and ours are not clear. However, we noticed that the approach they used to knock down DNMT1 was siRNA transient transfection, which is different from our lentiviral shRNA approach that generated a pool of stable cells after the puromycin selection and enrichment. Nonetheless, their data are consistent with our findings that inhibition of DNMT1 at late stage of differentiation promotes lipogenesis, leading to potential lipid filling and the adipocyte phenotype.
In summary, our data indicate that DNMT1 is induced at early stage of differentiation in 3T3-L1 preadipocytes. Inhibition of DNA methylation at early stage of differentiation suppresses 3T3-L1 adipogenesis. This is associated with up-regulation of the antiadipogenic factor Wnt10a due to demethylation on its promoter. In contrast, inhibition of DNA methylation at late stage of differentiation promotes lipogenesis and adipocyte phenotype in 3T3-L1 cells. This is likely mediated by induction of the lipogenic transcriptional factor SREBP1C, whose promoter activity is regulated by DNA methylation during the adipogenic process. We conclude that DNA methylation appears to exert a biphasic regulatory role in regulation of 3T3-L1 adipogenesis, promoting differentiation at the early stage while inhibiting lipogenesis at the late stage of adipogenesis.
Acknowledgments
This work was supported by National Institutes of Health Grants R01HL107500 (to B.X.), R01DK084172 (to H.S.), and R01DK085176 (to L.Y.); American Heart Association Grants 10SDG3900046 (to B.X.) and 11GRNT7370080 and 15GRNT25710256 (to H.S.); and the American Diabetes Association Grant 7-13-BS-159 (to H.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 5-aza-dC
- 5-aza-2′-deoxycytidine
- aP2
- adipocyte protein 2
- C/EBP
- CCAAT/enhancer-binding protein
- ChIP
- chromatin immunoprecipitation
- DNMT
- DNA methyltransferase
- Fas
- fatty acid synthase
- FBS
- fetal bovine serum
- GPDH
- glycerol-3-phosphate dehydrogenase
- LXR
- liver X receptor
- PPAR
- peroxisome proliferator-activated receptor
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- Srebp1c
- sterol regulatory element-binding protein 1C.
References
- 1. Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. [DOI] [PubMed] [Google Scholar]
- 2. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. [DOI] [PubMed] [Google Scholar]
- 4. Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290:134–138. [DOI] [PubMed] [Google Scholar]
- 5. Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. [DOI] [PubMed] [Google Scholar]
- 6. Eguchi J, Yan QW, Schones DE, et al. Interferon regulatory factors are transcriptional regulators of adipogenesis. Cell Metab. 2008;7:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Z, Yu S, Hsu CH, Eguchi J, Rosen ED. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proc Natl Acad Sci USA. 2008;105:2421–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luczak MW, Jagodziski PP. The role of DNA methylation in cancer development. Folia Histochem Cytobiol. 2006;44:143–154. [PubMed] [Google Scholar]
- 9. Maunakea AK, Chepelev I, Zhao K. Epigenome mapping in normal and disease states. Circ Res. 2010;107:327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. [DOI] [PubMed] [Google Scholar]
- 11. Ge K. Epigenetic regulation of adipogenesis by histone methylation. Biochim Biophys Acta. 2012;1819:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musri MM, Gomis R, Párrizas M. Chromatin and chromatin-modifying proteins in adipogenesis. Biochem Cell Biol. 2007;85:397–410. [DOI] [PubMed] [Google Scholar]
- 13. Londoño Gentile T, Lu C, Lodato PM, et al. DNMT1 is regulated by ATP-citrate lyase and maintains methylation patterns during adipocyte differentiation. Mol Cell Biol. 2013;33:3864–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cawthorn WP, Bree AJ, Yao Y, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi H, Moustaid-Moussa N, Wilkison WO, Zemel MB. Role of the sulfonylurea receptor in regulating human adipocyte metabolism. FASEB J. 1999;13:1833–1838. [DOI] [PubMed] [Google Scholar]
- 16. Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1α,25-dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001;15:2751–2753. [DOI] [PubMed] [Google Scholar]
- 17. Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- 19. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zha L, Li F, Wu R, et al. The histone demethylase UTX promotes brown adipocyte thermogenic program via coordinated regulation of H3K27 demethylation and acetylation. J Biol Chem. 2015;290:25151–25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Wang X, Liu D, Yu L, Xue B, Shi H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol Endocrinol. 2014;28:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao Q, Wang X, Jia L, et al. Inhibiting DNA methylation by 5-aza-2′-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology. 2014;155:4925–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xue B, Yang Z, Wang X, Shi H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One. 2012;7:e45990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amemiya-Kudo M, Shimano H, Yoshikawa T, et al. Promoter analysis of the mouse sterol regulatory element-binding protein-1c gene. J Biol Chem. 2000;275:31078–31085. [DOI] [PubMed] [Google Scholar]
- 25. Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. [DOI] [PubMed] [Google Scholar]
- 26. Patra SK, Bettuzzi S. Epigenetic DNA-(cytosine-5-carbon) modifications: 5-aza-2′-deoxycytidine and DNA-demethylation. Biochemistry (Mosc). 2009;74:613–619. [DOI] [PubMed] [Google Scholar]
- 27. Mikkelsen TS, Xu Z, Zhang X, et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. [DOI] [PubMed] [Google Scholar]
- 29. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. [DOI] [PubMed] [Google Scholar]