Abstract
Background
A variety of microbial communities exist throughout the human and animal body. Genetics, environmental factors and long-term dietary habit contribute to shaping the composition of the gut microbiota. For this reason the study of the gut microbiota of a mammal exhibiting an extraordinary life span is of great importance. The naked mole-rat (Heterocephalus glaber) is a eusocial mammal known for its longevity and cancer resistance.
Methods
Here we analyzed its gut microbiota by cultivating the bacteria under aerobic and anaerobic conditions and identifying their species by mass spectrometry.
Results
Altogether, 29 species of microbes were identified, predominantly belonging to Firmicutes, and Bacteroidetes. The most frequent species were Bacillus megaterium (45.2 %), followed by Bacteroides thetaiotaomicron (19.4 %), Bacteroides ovatus, Staphylococcus sciuri and Paenibacillus spp., each with a frequency of 16.1 %.
Conclusion
Overall, the gut of the naked mole-rat is colonized by diverse, but low numbers of cultivable microbes compared with humans and mice. The primary food plants of the rodents are rich in polyphenols and related compounds, possessing anti-microbial, anti-inflammatory, anti-oxidative as well as anti-cancer activity which may contribute to their exceptionally healthy life.
Electronic supplementary material
The online version of this article (doi:10.1186/s13099-016-0107-3) contains supplementary material, which is available to authorized users.
Keywords: Naked mole-rat, Microbiota, Diet, Polyphenols
Background
The microbiota is defined as the entity of microbial communities, which colonize different parts of the body of a host organism. These habitats include among others, the gut, oral cavity, skin, eyes and vagina [1]. Increasing evidence supports the notion that the microbiota has a strong influence on health and disease in humans [2, 3]. Recent studies have concluded that the composition and function of microbiota play a significant role in autoimmunity and immune regulation, development of cancer, Crohn’s disease, obesity, and type 1 and 2 diabetes [4–7]. This causal relationship came currently in the focus of scientific interest. Analyzing the composition, function, and distribution of the microbiota in relation to diet, environment, genetic factors, and host immunity will help to elucidate the pivotal role of the microbiota in health and pathogenesis of diseases. The naked mole-rat (Heterocephalus glaber) is a eusocial mammalian species that lives in colonies of up to 300 individuals. For this mouse-sized subterranean rodent a life span of about 30 years has been reported for both, reproductive and non-reproductive castes. Moreover, it is known to appear healthy over its life span, displays resistance to oxidative stress and is remarkably resistant to both spontaneous cancer and experimentally induced tumorigenesis [8]. However, the recent report of two cases of cancer in zoo-housed naked mole-rats may not alter the longstanding observation of cancer resistance of these animals [9]. A recent study has shown that captive naked mole-rats have higher levels of oxidative damage and decreased level of anti-oxidants compared to short-lived mice, indicating the involvement of further mechanisms known to counter high levels of oxidative damage [10]. Thus, naked mole-rats pose a challenge to the current theories that link aging, cancer and redox homeostasis. For these reasons as well as their close phylogenetic relation to humans, naked mole-rats are of special interest in the search for mechanisms leading to a particularly long and healthy life [11]. Recent publications demonstrate a strong link between different diseases and gut microbiota. This prompted us to investigate the gut microbiota of the naked mole-rat to obtain deeper insights into their astonishing longevity. Therefore, for the first time, we analyzed and characterized cultivable gut microbes obtained from the intestine and feces of wild naked mole-rats. Furthermore, we assessed the main diet of the animal in the wild and discuss their constituents in relation to their medical significance.
Methods
Naked mole-rat sampling
Eleven wild naked mole-rats from the Rift Valley of Ethiopia were captured and detained. Intestinal and fecal samples of the animals were obtained from individuals captured in Ethiopia. Permission comprising both, field permit and ethics approval was granted by the Ethiopian Wildlife Conservation Authority (EWCA; ref. No. 31/394/07 dated 27 November 2014). Intestinal content and feces were collected and immediately frozen in liquid nitrogen until further analysis. Animal collection and sampling were performed in accordance with the approved guideline and regulation of the national wildlife authority of Ethiopia.
Microbial growth conditions
Following standard aseptic procedures, samples of the colon, cecum and feces from naked mole-rats were subjected to microbial identification. One gram of intestine and feces of each animal were weighed and thoroughly dispersed and homogenized by vortex in 3 mL of sterile saline (0.85 %) (bioMérieux, Marcy I’Etoile, France) for 1 min and tenfold serial dilutions were made subsequently. Next, from each dilution 0.1 mL aliquot was plated onto blood agar (Carl Roth GmbH, Karlsruhe, Germany) and Brucella blood agar (Becton, Dickinson, Sparks, MD, USA) and incubated at 37 °C for 24–72 h at ambient air or anaerobically, respectively. Following incubation, the growth of bacteria was recorded and the number of colonies was counted based on their morphology. The colonies were further sub-cultured individually to obtain pure cultures. Subsequently, each of the pure colonies was identified by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry and gram staining was performed for each pure culture as a cross check with the MALDI-TOF results.
MALDI-TOF-MS based microbe identification
The automated MALDI-TOF was performed following the standard protocol (bioMérieux, Marcy I’Etoile, France). Freshly grown pure microbial cells from a single colony and a control (Escherichia coli) were deposited onto the target slides and to each, 1 µL of the α-cyano-4-hydroxycinnamic acid (CHCA) matrix solution (VITEK® MS-CHCA REF 411071, bioMérieux, Marcy I’Etoile, France) was added. After solvent evaporation at room temperature, the slides were inserted into the device and analyzed (VITEK®-MS, Marcy I’Etoile, France). Finally, sample spectra were compared to an extensive database of known bacterial species by VITEK®-MS proofing, allowing us to accurately identify the microorganism in question.
Microbial inoculum preparation
Bacillus megaterium, Staphylococcus scuiri, Parabacteroides distasonis, Clostridium ramosum, Bacteroides ovatus from the naked mole-rat and two clinical human pathobionts, E. coli and Staphylococcus aureus were maintained on blood agar or Brucella blood agar at 37 °C for 24–48 h aerobically or anaerobically, respectively. Following incubation, isolates were adjusted to McFarland 0.5 (~108 cells/mL) in a 0.85 % saline solution. Subsequently, standardized microbial suspension at low (103 cells/mL) and high (106 cells/mL) concentrations were prepared in Roswell Park Memorial Institute (RPMI)-1640 medium.
Whole blood assay
Heparinized blood was obtained from healthy human volunteers and stimulated by lipopolysaccharide (LPS) E. coli 0111:B4 (10 ng/ml) (SigmaAldrich; Taufkirchen, Germany) as previously described [12]. Standardized live microbial suspension at low (103 cells/mL) or high (106 cells/mL) final concentrations were mixed with 200 µL heparinized human blood contained in a total volume of 800 µL RPMI-medium in 24-well culture plates (Cellstar® Greiner Bio-One, Frickenhausen, Germany) at 37 °C and 5 % CO2 for 8 h. Following incubation, the suspension was transferred into fresh sterile 1.5 mL micro centrifuge tubes and centrifuged at 13,000×g for 5 min. Finally, plasma supernatants were harvested and stored at −20 °C. The local ethics committee of the Faculty of Medicine of the University of Leipzig, Germany, approved this study in accordance to the ICH-GCP guidelines (Reference No.: 057-2010-08032010). Written informed consent was obtained from all subjects and the experiments were performed in accordance with the guidelines and regulation of the Medical Faculty of the University of Leipzig.
Cytometry bead array assay
Assessment of cytokine levels in plasma supernatants of human blood cell cultures was performed according to the manufacturer’s instruction using cytometry bead array (CBA) immunoassay as previously described [12]. The data were analyzed using a FACS Calibur and the CellQuest™ Software (BD Biosciences).
Feeding trials and analysis of biochemical constituents of the wild naked mole-rat’s diet
Different plant species that are distributed nearby the habitat of the animals were freshly collected. Feeding trials were performed on captured members of the colony temporarily managed near Hawassa, South Ethiopia. Within this trial the degree of consumption of the plant species offered to the animals was recorded and categorized as either main diet, or rarely consumed food source. In order to investigate the constituents and medical significance of the plant species consumed by the animals in the wild, a systematic review of the appropriate literature was performed. At first, the scientific name of all plant species was verified. To fulfill this special assignment, we used the dynamic checklist of the catalogue of life (www.catalogueoflife.org). For analyzing the chemical content of identified plants the database scifinder (www.cas.org) was used. Dependent on the subject of search, the number of the proven sources differed from nil to over 10,000, for instance the common crops Ipomoea batatas (sweet potato) and Arachis hypogaea (groundnut) are well-described whereas two plants, Aloe trichosantha and Endostemon tenuiflorus are rarely described. Therefore, for these species the chemical content and bioactive substances were suggested by comparing them with the higher-ranking genus and family only [13, 14].
Data analysis
The data obtained were analyzed in terms of descriptive statistics. Characteristics of each isolates, the constituent and medicinal values of plant species were systematically reviewed. One way ANOVA and Student’s t test were used to analyze the mean difference in cytokine levels for each group of microbes compared with negative control and LPS-stimulated samples using GraphPad Prism version 5 (San Diego, CA, USA) and plotted as mean ± SEM. p value <0.05 was considered statistically significant.
Results
Frequency of microbes isolated from wild naked mole-rats
In the present study, we identified the composition of gut microbes of the naked mole-rat (Table 1). Overall, 29 different species of microbes were identified from the colon, cecum and feces of wild naked mole-rats. The principal microorganisms of the naked mole-rats belong to the phylum Firmicutes (58.6 %), followed by Bacteroidetes (20.7 %). Less frequently identified phyla were, Proteobacteria (10.3 %), Actinobacteria (6.9 %) and Ascomycota (3.5 %). The amounts of cultivable microbes ranged from 102 to 5 × 105, 102–105, and 102–3 × 105 c.f.u./g in cecum, colon, and feces, respectively. In total, the most frequently occurring species were B. megaterium (45.2 %), followed in order by Bacteroides thetaiotaomicron (19.4 %), B. ovatus, Paenibacillus spp., Staphylococcus scuiri each with a frequency of 16.1 %, Staphylococcus gallinarum (12.9 %) and Enterobacter cloacae/asburiae (12.9 %). The remaining isolates were least identified with percentages ranging from 3.2 to 9.7 %.
Table 1.
Distribution of the identified microbiota from the wild naked mole-rats (n = 11)
| Sites | Species | Phylum | (c.f.u./g) in range | Charactersticsa |
|---|---|---|---|---|
| Colon | Staphylococcus sciuri | Firmicutes | 1 × 102–103 | Normal flora |
| Staphylococcus cohnii spp. urealyticus | Firmicutes | 1–2 × 103 | Normal flora | |
| Staphylococcus arlettae | Firmicutes | 3 × 102 | Normal flora | |
| Staphylococcus gallinarum | Firmicutes | 2 × 102–3 × 103 | Normal flora | |
| Staphylococcus hyicus | Firmicutes | 3 × 102 | Normal flora | |
| Staphylococcus xylosus | Firmicutes | 3 × 102 | Normal flora | |
| Bacillus megaterium | Firmicutes | 0.2–1 × 104 | Normal flora | |
| Bacillus subtilus/amyloliquefaciens | Firmicutes | 1 × 102 | Normal flora | |
| Bacillus pumilus | Firmicutes | 1 × 103 | Normal flora | |
| Bacillus simplex | Firmicutes | 2 × 103 | Normal flora | |
| Paenibacillus spp. | Firmicutes | 2–3 × 102 | Normal flora | |
| Paenibacillus pabuli | Firmicutes | 1 × 102 | Normal flora | |
| Bacteroides ovatus | Bacteroidetes | 1–2 × 103 | Normal flora | |
| Bacteroides thetaiotaomicron | Bacteroidetes | 1–4 × 105 | Normal flora | |
| Parabacteroides distasonis | Bacteroidetes | 1 × 103 | Normal flora | |
| Bacteroides vulgatus | Bacteroidetes | 5 × 105 | Normal flora | |
| Kluyvera ascorbata | Proteobacteria | 3 × 103 | Normal flora | |
| Klebsiella pneumoniae | Proteobacteria | 2 × 102 | Normal flora | |
| Enterobacter cloacae/asburiae | Proteobacteria | 5 × 103–105 | Normal flora | |
| Actinomyces viscosus | Actinobacteria | 1 × 103 | Normal flora | |
| Cecum | Bacillus megaterium | Firmicutes | 2–4 × 103 | Normal flora |
| Staphylococcus gallinarum | Firmicutes | 0.4–1 × 103 | Normal flora | |
| Staphylococcus xylosus | Firmicutes | 3 × 102 | Normal flora | |
| Staphylococcus sciuri | Firmicutes | 1 × 103 | Normal flora | |
| Paenibacillus spp. | Firmicutes | 0.1–1 × 104 | Normal flora | |
| Staphylococcus warneri | Firmicutes | 1 × 103 | Normal flora | |
| Brevibacillus spp. | Firmicutes | 4 × 102 | Normal flora | |
| Lysinibacillus fusiformis | Firmicutes | 2 × 102 | Normal flora | |
| Streptococcus mitis/oralis | Firmicutes | 2 × 102 | Normal flora | |
| Enterococcus casseliflavus | Firmicutes | 1 × 103 | Normal flora | |
| Bacteroides ovatus | Bacteroidetes | 0.1–1.2 × 104 | Normal flora | |
| Bacteroides thetaiotaomicron | Bacteroidetes | 0.2–1 × 105 | Normal flora | |
| Bacteroides fragilis | Bacteroidetes | 2 × 103 | Normal flora | |
| Bacteroides vulgatus | Bacteroidetes | 1 × 105 | Normal flora | |
| Enterobacter cloacae/asburiae | Proteobacteria | 0.7–2 × 103 | Normal flora | |
| Actinomyces viscosus | Actinobacteria | 1 × 102 | Normal flora | |
| Rhodococcus rhodochrous | Actinobacteria | 1 × 102 | Normal flora | |
| Candida tropicalis | Ascomycota | 1 × 103 | Normal flora | |
| Feces | Lysinibacillus fusiformis | Firmicutes | 1 × 102 | Normal flora |
| Bacillus simplex | Firmicutes | 1 × 103 | Normal flora | |
| Bacillus megaterium | Firmicutes | 0.2–3 × 105 | Normal flora | |
| Clostridium ramosum | Firmicutes | 2 × 102 | Normal flora | |
| Staphylococcus sciuri | Firmicutes | 1–2 × 103 | Normal flora | |
| Staphylococcus cohnii spp. urealyticus | Firmicutes | 1 × 103 | Normal flora | |
| Staphylococcus arlettae | Firmicutes | 1 × 102 | Normal flora | |
| Bacteriodes unifromis | Bacteroidetes | 3 × 102 | Normal flora | |
| Bacteroides thetaiotaomicron | Bacteroidetes | 2 × 103 | Normal flora | |
| Bacteriodes ovatus | Bacteroidetes | 1 × 103 | Normal flora | |
| Parabacteroides distasonis | Bacteroidetes | 1–3 × 103 | Normal flora |
The intestinal content particularly colon, cecum and feces of the naked mole-rat obtained from the wild were cultivated under aerobic and anaerobic conditions. The table illustrates the species name, phylum name, cultivable amount of microbial cells and characteristics of the isolated microbiota referring to their host. Normal flora is described as a relatively stable microbial community that routinely inhabits in and on the body of a wide range of animal species. Colony-forming unit (c.f.u.).
Occurrence of microbes within different intestinal compartments of wild naked mole-rats
The most frequently isolated species in all intestinal specimens were B. megaterium followed in descending prevalence by Bacteroides thetaiotaomicron, B. ovatus, and Staphylococcus sciuri in colon, cecum and feces. Paenibacillus spp., Enterobacter cloacae/asburiae, S. gallinarum, Actinomyces viscosus, Bacteroides vulgatus, Staphylococcus xylosus were identified from colon and cecum. Whereas P. distasonis, Bacillus simplex, Staphylococcus cohnii spp. urealyticus, and Staphylococcus arlettae were isolated from colon and feces. Except Lysinibacillus fusiformis, which was identified from cecum and feces, the remaining species were mono-isolates with a frequency of 3.2 % (Table 2).
Table 2.
Frequency of microbes in colon, cecum and feces of the naked mole-rats
| Species | Number of positive isolates (% isolation frequency) | |||
|---|---|---|---|---|
| Colon (n = 10) | Cecum (n = 11) | Feces (n = 10) | Total | |
| Bacillus megaterium | 5 (50) | 5 (45.5) | 4 (40) | 14 (45.2) |
| Staphylococcus sciuri | 2 (20) | 1 (9.1) | 2 (20) | 5 (16.1) |
| Bacteroides thetaiotaomicron | 2 (20) | 3 (27.3) | 1 (10) | 6 (19.4) |
| Bacillus simplex | 1 (10) | 0 (0) | 1 (10) | 2 (6.5) |
| Staphylococcus gallinarum | 2 (20) | 2 (18.2) | 0 (0) | 4 (12.9) |
| Lysinibacillus fusiformis | 0 (0) | 1 (9.1) | 1 (10) | 2 (6.5) |
| Paenibacillus spp. | 3 (30) | 2 (18.2) | 0 (0) | 5 (16.1) |
| Staphylococcus cohnii spp. urealyticus | 2 (20) | 0 (0) | 1 (10) | 3 (9.7) |
| Enterobacter cloacae/asburiae | 2 (20) | 2 (18.2) | 0 (0) | 4 (12.9) |
| Bacteroides ovatus | 1 (10) | 2 (18.2) | 2 (20) | 5 (16.1) |
| Actinomyces viscosus | 1 (10) | 1 (9.1) | 0 (0) | 2 (6.5) |
| Parabacteroides distasonis | 1 (10) | 0 (0) | 2 (20) | 3 (9.7) |
| Bacteroides vulgatus | 1 (10) | 1 (9.1) | 0 (0) | 2 (6.5) |
| Staphylococcus xylosus | 1 (10) | 1 (9.1) | 0 (0) | 2 (6.5) |
| Clostridium ramosum | 0 (0) | 0 (0) | 1 (10) | 1 (3.2) |
| Bacteroides fragilis | 0 (0) | 1 (9.1) | 0 (0) | 1 (3.2) |
| Staphylococcus arlettae | 1 (10) | 0 (0) | 1 (10) | 2 (6.5) |
| Brevibacillus spp. | 0 (0) | 1 (9.1) | 0 (0) | 1 (3.2) |
| Staphylococcus hyicus | 1 (10) | 0 (0) | 0 (0) | 1 (3.2) |
| Rhodococcus rhodochrous | 0 (0) | 1 (9.1) | 0 (0) | 1 (3.2) |
| Bacteroides unifromis | 0 (0) | 0 (0) | 1 (10) | 1 (3.2) |
| Staphylococcus warneri | 0 (0) | 1 (9.1) | 0 (0) | 1 (3.2) |
| Enterococcus casseliflavus | 0 (0) | 1 (9.1) | 0 (0) | 1 (3.2) |
| Bacillus subtilus/amyloliquefaciens | 1 (10) | 0 (0) | 0 (0) | 1 (3.2) |
| Bacillus pumilus | 1 (10) | 0 (0) | 0 (0) | 1 (3.2) |
| Klebsiella pneumoniae | 1 (10) | 0 (0) | 0 (0) | 1 (3.2) |
| Kluyvera ascorbata | 1 (10) | 0 (0) | 0 (0) | 1 (3.2) |
| Streptococcus mitis/oralis | 0 (0) | 1 (9.1) | 0 (0) | 1 (3.2) |
| Candida tropicalis | 0 (0) | 1 (9.1) | 0 (0) | 1 (3.2) |
Habitat and diet of wild naked mole-rats
According to continuous field observations, the main habitat of the naked mole-rat is characterized by arid vegetation such as shrubs and desert bush covered areas, which is located in arid zones of the Eastern and Southern Ethiopia. Naked mole-rat colonies dig extensive underground burrow systems, which they rarely leave. The type of diet consumed by the rodents was assessed. Naturally occurring plant species were found to be rich in flavonoids, essential fatty acid, solanine alkaloids, carotenoids, tannins, starch, fiber, vitamins, cafeic acid derivatives and others [20–32] (Additional file 1: Table S1).
Innate immune cytokine profiles upon stimulation of human whole blood by microbes of the naked mole-rat
Microbes are known to cross the gut barrier and possibly induce inflammatory responses in different organs [4]. Therefore, we asked to what extent microbes of the naked mole-rat are capable to stimulate the innate immune system of humans in an ex vivo setting in comparison to bacteria present in the human gut. For that heparinized human blood was mixed with a defined number of microbial cells, specifically B. megaterium, S. sciuri, P. distasonis, C. ramosum and B. ovatus at low (103 cells/mL) and high (106 cells/mL), concentrations respectively, and incubated at 37 °C for 8 h. Stimulation by 10 ng LPS was carried on to verify the viability and responsiveness of the white blood cells. Accordingly, at a low concentration of naked mole-rat bacterial cells no release of cytokines could be observed as exemplified for interleukin-1ß (IL-1ß) (Fig. 1). In contrast, using equivalent numbers of the pathobionts E. coli and S. aureus, an augmented release of IL-1β versus control was observed (p < 0.05). Likewise, a similar trend was seen with respect to tumor necrosis factor-α (TNF-α) and low concentrations of gut microbes. As expected, stimulation by E. coli and S. aureus induced a remarkable release of TNF-α from human white blood cells versus control (p < 0.05). In contrast, a low number of microbes was found to significantly induce IL-6, except B. megaterium. Again, this contrasts the strong effect of E. coli on this inflammatory cytokine (p < 0.05).
Fig. 1.
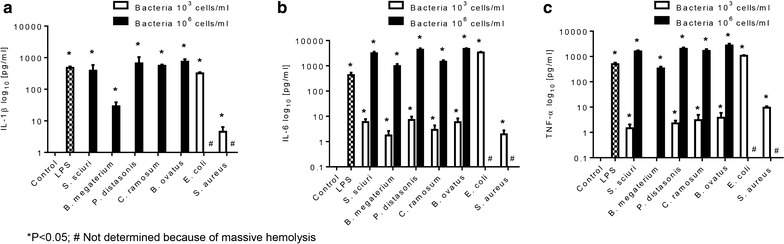
Stimulation of inflammatory cytokines by naked mole-rat microbes. Heparinized blood from healthy volunteers was stimulated by medium alone (negative control), LPS (positive control), S. sciuri, B. megaterium, P. distasonis, C. ramosum, B. ovatus and two human pathobionts E. coli and S. aureus at 37 °C and 5 % CO2 for 8 h. The levels of the cytokines IL-1β (a), TNF-α (b), IL-6 (c) were measured by cytometric bead array assays. The experiment was done in triplicates and data are expressed as mean ± SEM. *p < 0.05; #strong hemolysis
Higher number of microbes causes an enhanced release of inflammatory cytokines as manifested for IL-1β, TNF-α, and IL-6 (p < 0.05) respectively, irrespective of the type of isolates (Fig. 1a–c). With exception of B. megaterium (p < 0.05) all other isolates caused an elevated response of IL-1β and TNF-α. Moreover, B. megaterium was found to induce less IL-6 release compared to other microbes. Unfortunately, high concentration of E. coli and S. aureus could not be tested as they caused massive hemolysis of the blood that resulted in the incapability of the cells to secret cytokines.
Discussion
The human intestine is believed to contain approximately 100 trillion intestinal (gut) microbiota comprising about 500–1000 different species [33]. These intestinal microbiota exist in a symbiotic relationship with their host, by metabolizing compounds that the host is unable to utilize and controlling the immune balance of the host’s body. However, the composition of the intestinal microbiota is known to vary, depending on diet, nutritional status, and other factors. It has been recognized that the intestinal microbiota is involved in the pathogenesis of diverse diseases not only in the intestine but also in organs distant from the intestine. Thus, the intestinal microbiota might contribute to the onset of diseases such as cancer by the pro-carcinogenic activities of specific pathogens and by synthesis of bacterial metabolites circulating in the host’s body, as well. For instance, mice supplemented orally with certain bacteria in early life were resistant to oncogene-associated mammary carcinogenesis later in life [34].
Resistance to cancer and extraordinary life span are hallmarks of the naked mole-rat [35]. Therefore, it was our aim to investigate the composition of the cultivable microbiota of these animals. Our results show that in wild naked mole-rats, Firmicutes and Bacteroidetes were consistently found in highest number. Similarly, previous studies in humans suggested that Firmicutes and Bacteroides are among the dominant enterotypes of the gut microbiota of most mammals across a wide range of species [36]. For the naked mole-rat we found a ratio of Firmicutes to Bacteroidetes of 8/1, which is similar to one found in healthy human adults of 10/1 [15]. For comparison, in obese adults the ratio rises to 100/1 and drops to 1/1 in people with chronic inflammatory bowel disease [37]. Interestingly, the phylum Proteobacteria, which comprises a wide range of potential pathogens [38] was among the less identified microbiota of the naked mole-rat. Moreover, most microbes identified were found to be the normal flora, suggesting that the animals harbor healthy-like microbiota.
The link between microbiota and aging is still poorly understood. However, it has been reported that age-related differences in the microbiota composition could be associated with immunosenescence [39] and increased frailty [40]. A recent study has shown increased microbial loads in aged Drosophila, which was associated with age-related dysplasia [41]. In contrast to human and mice that harbor 108–1012 and 106–109 c.f.u./g, respectively [42, 43], the quantity of the microbiota of the naked mole-rat was lower, ranging from 102 to 105 c.f.u./g when verified by cultivation. However, diet intervention could potentially have effect in a general population of microorganism of the rodent [44]. We may also expect a considerable higher diversity of microbes when using more sensitive approaches such as 16S rRNA sequencing.
Early in life, exposure to microbes is known to shape the immune system [45]. The gastrointestinal tract immune network includes neutrophils and regulatory T-cells which communicate with the commensal microbiota. Recent data suggest that commensal bacteria–host crosstalk is continuous and reciprocal throughout life [46]. Enrichment of facultative anaerobes, notably pathobionts, is associated with an enhanced inflammatory status, as determined by inflammatory markers such as IL-6 and IL-8 in blood. Especially, chronic inflammation is known to be associated with metastasizing of tumor cells [47].
These finding forced us to analyze the microbes of the naked mole-rats’ intestine with respect to their capability of stimulating inflammatory cytokines in blood. Overall, naked mole-rat microbes were found to be less stimulatory of inflammatory cytokines like IL-1β, IL-6 and TNF-α compared with pathobionts from human gut (Fig. 1). Notably, B. megaterium which constitutes the most abundant species (45.2 %) in the naked mole-rat intestine seems to induce the least inflammatory response. Whether this has biological implications for the animal’s health needs further investigation. Harboring of abundant B. megaterium in the gut of naked mole-rats might not be surprising as they are very likely to acquire it from the soil due to their subterranean lifestyle. This bacterium is a common gram-positive, mainly aerobic, spore forming, soil bacterium, which is currently widely used in the field of biotechnology for recombinant protein production [48]. B. megaterium is used to produces penicillin amidase (essential for the synthesis of β-lactam antibiotics), various amylases, pyruvate, vitamin B12, as well as further unusual enzymes and components, which provide various health benefits such as playing a key role in several metabolic pathways, as endogenous scavenger of certain reactive oxygen species, being involved in DNA repair and synthesis, epigenetic gene regulation, and possessing antifungal and antiviral properties [49–51]. In addition, Paenibacillus spp. was among frequently identified isolates from the gut of naked mole-rats which are known to produce a polymyxin-like antibiotic that is effective against most gram-negative bacteria [52]. This might suggest that B. megaterium and Paenibacillus spp. are beneficial gut symbionts of naked mole-rat. Thus, the presence of these bacteria may probably contribute to the naked mole-rat’s resistance to various diseases, which however, needs to be proved yet.
In humans first colonization of the gut occurs during birth. In naked mole rat there is an additional delivery of bacteria to the pups by coprophagy [8]. This may provide pups with endosymbiotic gut flora and transitional source of food. Against this background and the life in a very confined space it is hard to understand the microbial diversity found in the animals in our study. However, as deduced from human studies it is quite possible that difference in gut microbial composition may have impact on shaping the behavior of individuals in the colony [53].
Our field study of natural food sources shows that this long-lived rodent has adapted to consume a wide variety of different plant species, many of which contain large quantities of polyphenols. Furthermore, the content and medical importance of each plant were systematically reviewed and shows that the plant-derived diet of wild naked mole-rats is rich in various anti-oxidants, anti-inflammatory, anti-cancer and anti-microbial agents (Additional file 1: Table S1). Despite the fact that the underlying mechanisms are not yet fully understood, recent evidence exists that polyphenols may actively contribute to the prevention of certain illnesses such as cardiovascular and chronic intestinal diseases. Likewise, many studies emphasize the role of polyphenols in prevention of oxidative stress in the pathogenesis of age-related human diseases. Polyphenols have been shown to scavenge free radicals and protect cell constituents against oxidative damage [54]. Oral administration of polyphenols to rats limits DNA oxidative damage in caeca mucosal cells and can act as a pro-oxidant, thereby inducing apoptosis and reducing the tumor incidence and growth [55–57]. However, the relationship between phenolic compounds and microbiota in terms of health benefits is still poorly understood [58]. Nonetheless, polyphenols have been shown to inhibit the adherence of potentially pathogenic microbes to host cells, while enhancing the proliferation and adhesion of beneficial probiotic bacteria thereby contributing to sustain gut health [59]. Additionally, the consumption of polyphenols has a prebiotic effect on the gut microbiota [60]. In this line, the polyphenol-rich natural diet of wild naked mole-rats in combination with various other plant constituents might contribute to their astonishing resistance to various diseases and their healthy ageing. Therefore, it is not surprising, that as far as longevity is concerned, recent studies revealed that the microbiota composition in individual ‘centenarianas’ showed tenfold increase in Eubacteriumlimosum [39, 61] that is an anaerobic acetogenic bacterium producing acetate, butyrate, ethanol and vitamin B12.
Conclusion
In summary, the cultivable gut flora of the naked mole-rat is composed of a microbiota that is low in number, but diverse and dominated mainly by B. megaterium. Our finding that naked mole-rats consume polyphenol-rich plants suggests that it could exert a protective effect against different diseases and aging.
Authors’ contributions
TD, GB and BK designed the experiment and TD characterized the microbes. SH, TBH, MM, TD, AL and GB took part in the animal specimen collection. SR critically reviewed plant constituents and their medical importance. SY, DW and AL involved in the rats’ habitat and diet consumption assessment in the wild. RT, TBH, KH, MP took part in manuscript revision. TD and GB compiled the data and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge Angelika Schäfer and Heike Knaack for her excellent technical assistance and Senait Likasa for excellent animal keeping. We also thank Mohammed Shubo from Addis Ababa University for assisting us in the field. We thank the technicians of the Institute of Medical Microbiology, Faculty of Medicine, University of Leipzig for the assistance during microbial identification. We acknowledge support from the German Research Foundation (DFG) and University of Leipzig within the program of Open Access Publishing.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article and its additional file, and is available upon request to the corresponding author.
Funding
GB, RT were supported by Grants of the Europäischer Sozialfond ESF (100098250). TD was supported by Katholischer Akademischer Ausländer Dienst (KAAD). TBH, SH, and MM were supported by the Leibniz Association (SAW-2012-FLI-2). The funding bodies were not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Abbreviations
- LPS
lipopolysaccharide
- RPMI
Roswell Park Memorial Institute
- CBA
cytometry bead array
- c.f.u.
colony-forming unit
- spp.
species
- FACS
fluorescence-activated cell sorting
- MALDI-TOF
matrix-assisted laser desorption ionization time-of-flight
- CHCA
α-cyano-4-hydroxycinnamic acid
- IL
interleukin
- TNF
tumor necrosis factor
Additional file
10.1186/s13099-016-0107-3 Biochemical contents of food-plants consumed by naked mole-rats in the wild and their medical significance. The table demonstrates naturally occurring plant species consumed by the rodent in the wildlife and bioactive constituents of the plants including their medical significance. Naked mole-rats were obtained from wild and housed in a colony of 20 animals. Feeding trials were done by offering of different fresh plant species that are proximate to the habitat of the animal. The biochemical constituents and their medicinal importance of naturally occurring plant species consumed by the rodent in the wild were systematically reviewed. The main diet of the animal is assigned with double asterisk (**) and rarely consumed plant species are those with a single asterisk (*).
Contributor Information
Tewodros Debebe, Email: tewodrosdebebe.aklilu@medizin.uni-leipzig.de.
Susanne Holtze, Email: holtze@izw-berlin.de.
Michaela Morhart, Email: morhar.morhart@izw.berlin.de.
Thomas Bernd Hildebrandt, Email: hildebrandt@izw-berlin.de.
Steffen Rodewald, Email: rodewald@rz.uni-leipzig.de.
Klaus Huse, Email: klaus.huse@leibniz-fli.de.
Matthias Platzer, Email: mplatzer@fli-leibniz.de.
Dereje Wyohannes, Email: wyohannesdereje@gmail.com.
Salomon Yirga, Email: solyirga@yahoo.com.
Alemayehu Lemma, Email: alemma2008@gmail.com.
Rene Thieme, Email: rene.thieme@medizin.uni-leipzig.de.
Brigitte König, Email: brigitte.koenig@medizin.uni-leipzig.de.
Gerd Birkenmeier, Email: gerd.birkenmeier@medizin.uni-leipzig.de.
References
- 1.Cenit MC, Matzaraki V, Tigchelaar EF, Zhernakova A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim Biophys Acta. 2014;1842:1981–1992. doi: 10.1016/j.bbadis.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, et al. The gut microbiome modulates colon tumorigenesis. mBio. 2013;4:e00692-13. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkanani AK, Hara N, Gottlieb PA, Ir D, Robertson CE, Wagner BD, et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes. 2015;64:3510–3520. doi: 10.2337/db14-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S, Denman SE, Morrison M, Yu ZT, Dore J, Leclerc M, et al. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis. 2010;16:2034–2042. doi: 10.1002/ibd.21319. [DOI] [PubMed] [Google Scholar]
- 8.Sherman PW, Jarvis JUM, Alexander RD. The biology of the naked mole-rat. Princeton, NJ: Princeton University Press; 1991. [Google Scholar]
- 9.Delaney MA, Ward JM, Walsh TF, Chinnadurai SK, Kerns K, Kinsel MJ, et al. Initial case reports of cancer in naked mole-rats (Heterocephalus glaber) Vet Pathol. 2016;53:691–696. doi: 10.1177/0300985816630796. [DOI] [PubMed] [Google Scholar]
- 10.Lewis KN, Andziak B, Yang T, Buffenstein R. The naked mole-rat response to oxidative stress: just deal with it. Antioxid Redox Signal. 2013;19:1388–1399. doi: 10.1089/ars.2012.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 12.Hollenbach M, Hintersdorf A, Huse K, Sack U, Bigl M, Groth M, et al. Ethyl pyruvate and ethyl lactate down-regulate the production of pro-inflammatory cytokines and modulate expression of immune receptors. Biochem Pharmacol. 2008;76:631–644. doi: 10.1016/j.bcp.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Paton A, Harley M, Harley R, Weeks S. A revision of Endostemon (Labiatae) Kew Bull. 1994;49:673–716. doi: 10.2307/4118065. [DOI] [Google Scholar]
- 14.Berger A. About the systematic classification of the genus Aloe. Bot Yearb Syst Plant Hist Plant Geogr. 1905;36:36–62. [Google Scholar]
- 15.Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 16.Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155:1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 17.Heyrman J, Logan NA, Rodriguez-Diaz M, Scheldeman P, Lebbe L, Swings J, et al. Study of mural painting isolates, leading to the transfer of ‘Bacillus maroccanus’ and ‘Bacillus carotarum’ to Bacillus simplex, emended description of Bacillus simplex, re-examination of the strains previously attributed to ‘Bacillus macroides’ and description of Bacillus muralis sp. nov. Int J Syst Evol Microbiol. 2005;55:119–131. doi: 10.1099/ijs.0.63221-0. [DOI] [PubMed] [Google Scholar]
- 18.Hong HA, Khaneja R, Tam NM, Cazzato A, Tan S, Urdaci M, et al. Bacillus subtilis isolated from the human gastrointestinal tract. Res Microbiol. 2009;160:134–143. doi: 10.1016/j.resmic.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Cornelison CT, Keel MK, Gabriel KT, Barlament CK, Tucker TA, Pierce GE, et al. A preliminary report on the contact-independent antagonism of Pseudogymnoascus destructans by Rhodococcus rhodochrous strain DAP96253. BMC Microbiol. 2014;14:246. doi: 10.1186/s12866-014-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyid A, Abebe D, Debella A, Makonnen Z, Aberra F, Teka F, et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J Ethnopharmacol. 2005;97:421–427. doi: 10.1016/j.jep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–525. doi: 10.1016/j.jep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Akor J, Anjorin T. Phytochemical and antimicrobial studies of Commiphora africana root extracts. Int J Agric Biol. 2009;11:795–797. [Google Scholar]
- 23.Ayoub S. Polyphenolic molluscicides from Acacia nilotica. Planta Med. 1984;50:532. doi: 10.1055/s-2007-969797. [DOI] [PubMed] [Google Scholar]
- 24.Debella A, Haslinger E, Kunert O, Michl G, Abebe D. Steroidal saponins from Asparagus africanus. Phytochemistry. 1999;51:1069–1075. doi: 10.1016/S0031-9422(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 25.Humphry CM, Clegg MS, Keen CL, Grivetti LE. Food diversity and drought survival. The Hausa example. Int J Food Sci Nutr. 1993;44:1–16. doi: 10.3109/09637489309017417. [DOI] [Google Scholar]
- 26.Ma J, Jones SH, Hecht SM. A dihydroflavonol glucoside from Commiphora africana that mediates DNA strand scission. J Nat Prod. 2005;68:115–117. doi: 10.1021/np0400510. [DOI] [PubMed] [Google Scholar]
- 27.Mueller-Harvey I, Hartley RD, Reed JD. Characterisation of phenolic compounds, including flavonoids and tannins, of ten Ethiopian browse species by high performance liquid chromatography. J Sci Food Agric. 1987;39:1–14. doi: 10.1002/jsfa.2740390102. [DOI] [Google Scholar]
- 28.Oketch-Rabah H, Dossaji S, Christensen SB, Frydenvang K, Lemmich E, Cornett C, et al. Antiprotozoal compounds from Asparagus africanus. J Nat Prod. 1997;60:1017–1022. doi: 10.1021/np970217f. [DOI] [PubMed] [Google Scholar]
- 29.Paton AJ, Springate D, Suddee S, Otieno D, Grayer RJ, Harley MM, et al. Phylogeny and evolution of basils and allies (Ocimeae, Labiatae) based on three plastid DNA regions. Mol Phylogenet Evol. 2004;31:277–299. doi: 10.1016/j.ympev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cushnie T, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Hanakahi LA, Bartlet-Jones M, Chappell C, Pappin D, West SC. Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell. 2000;102:721–729. doi: 10.1016/S0092-8674(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 33.Wu GD, Lewis JD. Analysis of the human gut microbiome and association with disease. Clin Gastroenterol Hepatol. 2013;11:774–777. doi: 10.1016/j.cgh.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, et al. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–540. doi: 10.1002/ijc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher GJ. Cancer resistance, high molecular weight hyaluronic acid, and longevity. J Cell Commun Signal. 2015;9:91–92. doi: 10.1007/s12079-015-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serban DE. Microbiota in inflammatory bowel disease pathogenesis and therapy: is it all about diet? Nutr Clin Pract. 2015;30:760–779. doi: 10.1177/0884533615606898. [DOI] [PubMed] [Google Scholar]
- 38.Sherman PW, Jarvis JUM, Alexander RD. The biology of the naked mole-rat. Princeton, NJ: Princeton University Press; 1991. [Google Scholar]
- 39.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 41.Guo L, Karpac J, Tran SL, Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Dubos R, Savage D, Schaedler R. The indigenous flora of the gastrointestinal tract. Dis Colon Rectum. 1967;10:23–34. doi: 10.1007/BF02617382. [DOI] [PubMed] [Google Scholar]
- 44.Buffenstein R, Yahav S. The effect of diet on microfaunal population and function in the caecum of a subterranean naked mole-rat, Heterocephalus glaber. Br J Nutr. 1991;65:249–258. doi: 10.1079/BJN19910084. [DOI] [PubMed] [Google Scholar]
- 45.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 47.Fischer-Fodor E, Miklasova N, Berindan-Neagoe I, Saha B. Iron, inflammation and invasion of cancer cells. Clujul Med. 2015;88:272–277. doi: 10.15386/cjmed-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vary PS, Biedendieck R, Fuerch T, Meinhardt F, Rohde M, Deckwer W-D, et al. Bacillus megaterium—from simple soil bacterium to industrial protein production host. Appl Microbiol Biotechnol. 2007;76:957–967. doi: 10.1007/s00253-007-1089-3. [DOI] [PubMed] [Google Scholar]
- 49.Bunk B, Schulz A, Stammen S, Münch R, Warren MJ, Rohde M, et al. A short story about a big magic bug. Bioeng Bugs. 2010;2:1–7. doi: 10.4161/bbug.1.2.11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer. 2002;2:694–704. doi: 10.1038/nrc886. [DOI] [PubMed] [Google Scholar]
- 51.Huang CY, Kuo WT, Huang YC, Lee TC, Yu LCH. Resistance to hypoxia-induced necroptosis is conferred by glycolytic pyruvate scavenging of mitochondrial superoxide in colorectal cancer cells. Cell Death Dis. 2013;4:e622. doi: 10.1038/cddis.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tambadou F, Caradec T, Gagez AL, Bonnet A, Sopena V, Bridiau N, et al. Characterization of the colistin (polymyxin E1 and E2) biosynthetic gene cluster. Arch Microbiol. 2015;197:521–532. doi: 10.1007/s00203-015-1084-5. [DOI] [PubMed] [Google Scholar]
- 53.DiazHeijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson G, Holst B. Dietary reference intake (DRI) value for dietary polyphenols: are we heading in the right direction? Br J Nutr. 2008;99:S55–S58. doi: 10.1017/S0007114508006867. [DOI] [PubMed] [Google Scholar]
- 55.Scalbert A, Morand C, Manach C, Rémésy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. 2002;56:276–282. doi: 10.1016/S0753-3322(02)00205-6. [DOI] [PubMed] [Google Scholar]
- 56.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 57.Caderni G, De Filippo C, Luceri C, Salvadori M, Giannini A, Biggeri A, et al. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis. 2000;21:1965–1969. doi: 10.1093/carcin/21.11.1965. [DOI] [PubMed] [Google Scholar]
- 58.Valdes L, Cuervo A, Salazar N, Ruas-Madiedo P, Gueimonde M, Gonzalez S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food Funct. 2015;6:2424–2439. doi: 10.1039/C5FO00322A. [DOI] [PubMed] [Google Scholar]
- 59.Parkar SG, Stevenson DE, Skinner MA. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int J Food Microbiol. 2008;124:295–298. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, et al. Flavanol monomer-induced changes to the human faecal microflora. Br J Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 61.Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2016;19:26–30. doi: 10.1097/MCO.0000000000000242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article and its additional file, and is available upon request to the corresponding author.


