Abstract
Modulating immune inhibitory pathways has been a major recent breakthrough in cancer treatment. Checkpoint blockade antibodies targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programed cell-death protein 1 (PD-1) have demonstrated acceptable toxicity, promising clinical responses, durable disease control, and improved survival in some patients with advanced melanoma, non-small cell lung cancer (NSCLC), and other tumor types. About 20 % of advanced NSCLC patients and 30 % of advanced melanoma patients experience tumor responses from checkpoint blockade monotherapy, with better clinical responses seen with the combination of anti-PD-1 and anti-CTLA-4 antibodies. Given the power of these new therapies, it is important to understand the complex and dynamic nature of host immune responses and the regulation of additional molecules in the tumor microenvironment and normal organs in response to the checkpoint blockade therapies. In this era of precision oncology, there remains a largely unmet need to identify the patients who are most likely to benefit from immunotherapy, to optimize the monitoring assays for tumor-specific immune responses, to develop strategies to improve clinical efficacy, and to identify biomarkers so that immune-related adverse events can be avoided. At this time, PD-L1 immunohistochemistry (IHC) staining using 22C3 antibody is the only FDA-approved companion diagnostic for patients with NSCLC-treated pembrolizumab, but more are expected to come to market. We here summarize the current knowledge, clinical efficacy, potential immune biomarkers, and associated assays for immune checkpoint blockade therapies in advanced solid tumors.
Keywords: Cancer immunotherapy, Cytotoxic T cells, Immune checkpoint blockade antibodies, PD-1, PD-L1, Immune-related adverse events, Biomarker, Precision oncology
Background
Since 2011, when the US Food and Drug Administration (FDA) approved ipilimumab for advanced melanoma, there has been an explosion of research interest and drug development for harnessing the immune system to fight against cancer. A general search for “immune therapy and cancer” in the clinicaltrials.gov database from 2000 to 2010 shows 640 hits, while the same search on March 20, 2015 shows 7815 hits and December 23, 2015 shows 8941 hits. Active research in tumor immunology includes studies on adoptive T cell therapy and cancer vaccine, as well as clinical investigations into immune checkpoint blockade in monotherapy or combination therapies. First generation programed cell-death protein 1 (PD-1) blockade antibodies pembrolizumab and nivolumab obtained US FDA approval for advanced melanoma in 2014 and as second line therapy for non-small cell lung cancer (NSCLC) in 2015. We have seen a surge in the availability of immune checkpoint inhibitors for multiple cancer indications. Forecasts for 2013–2020 project that the market for immunotherapy will increase from approximately $1 billion (US dollars) in 2013 to in excess of $7 billion in 2020 (corresponding to 33 % annual growth), across the seven major markets (USA, France, Germany, Italy, Spain, UK, and Japan) [1].
Unlike chemotherapy and molecularly targeted therapy, the checkpoint blockade immunotherapies result in durable clinical responses through the induction, activation, and expansion of tumor-specific cytotoxic T cells. Immune checkpoints play an essential role in maintaining self-tolerance and regulating the amplitude and duration of T cell responses. Two cytotoxic T cell immune checkpoint receptors (cytotoxic T-lymphocyte antigen 4 (CTLA-4) and PD-1) deliver inhibitory signals when bound to their respective ligands CD80/86 and programed cell-death ligand 1 (PD-L1)/2. Immunotherapies with checkpoint blockade antibodies that block CTLA-4 and PD-1 (or its ligand PD-L1) can restore and augment cytotoxic T cell responses against chemotherapy-refractory tumors, leading to durable responses and prolonged overall survival with tolerable toxicity. In the lymphatic tissues, anti-CTLA-4 antibody induces non-tumor-specific immune activation that is largely de novo induction of new responses. In tumor microenvironment, anti-PD-1/PD-L1 antibody targets tumor-induced immune defects and repairing ongoing tumor immunity. In addition to these anti-CTLA-4 and anti-PD-1/PD-L1 antibodies, drugs targeting other immune checkpoints and/or co-stimulatory ligand-receptor inhibitors, such as lymphocyte activation gene 3 protein (LAG3) and T cell immunoglobulin and mucin-domain containing protein 3 (TIM-3), are currently being explored in many tumor types and will be discussed further in the “Principles of cancer immunotherapy” section.
Given the distinct and novel mechanisms of action of immunotherapy on effector immune cells, rather than tumor cells, there are emerging tumor response patterns that are not accurately assessed by Response Evaluation Criteria in Solid Tumors (RECIST). In 2009, a guideline for immune-related response criteria (irRC) was proposed for patients with advanced melanoma receiving ipilimumab [2]. This guideline has been used to evaluate the tumor response for ongoing immune checkpoint blockade therapies [3]. When assessed appropriately, approximately 20 % of advanced NSCLC patients and 30–40 % of advanced melanoma patients have objective tumor responses to PD-1 blockade monotherapy. The combination of anti-PD-1 and anti-CTLA-4 antibodies increased the clinical response in advanced melanoma patients with a recent FDA approval [4]. In an attempt to increase the therapeutic, in checkpoint inhibitors, a companion diagnostic of PD-L1 immunohistochemistry (IHC) had been approved by the US FDA for selecting NSCLC patients treated with pembrolizumab. Interestingly, overall tumor responses, progression-free survival (PFS) and overall survival (OS) were not consistently associated with PD-L1 expression in the patients receiving immune checkpoint inhibitors [5]. In this age of personalized medicine, finding and validating better immune biomarkers will enable us to select patients for cancer immunotherapies. It will also lead to a better understanding of the unique mechanisms of tumor response and resistance, to allow for the development of strategies to improve clinical efficacy, and to lead to better evaluation of response and monitoring assays for host immune function. Validating potential immune biomarkers will enable us to better select patients for cancer immunotherapies and to avoid immune-related adverse effects (irAEs). Many excellent review articles on cancer biomarkers and immune checkpoint inhibitors have been recently published [6–12]. In this review, we will focus on the current knowledge, clinical efficacy, available monitoring assays, and emerging novel technologies for identifying translational biomarkers for immune checkpoint blockade immunotherapies in advanced solid tumors.
Principles of cancer immunotherapy
Clinically apparent cancer can be thought of as a host’s inability to eliminate transformed cells. Cancer immunotherapy refers to a diverse range of therapeutic approaches that harness the immune system to induce or restore the capacity of cytotoxic T cells, and other immune effector cells, and to recognize and eliminate cancer [13, 14]. The field of cancer immunotherapy is undergoing a renaissance due to a greater understanding of the immune system and immunosurveillance. The immune system protects the host against tumor development but also promotes tumor growth by selecting for tumors of lower immunogenicity. This dual effect of the immune system on developing tumors creates a dynamic process termed cancer immunoediting, which is comprised of three phases: elimination, equilibrium, and escape [15]. Cancer cells are immunogenic through the generation of tumor-specific mutant antigens (TSMA, neoantigens) from somatic gene structural and/or epigenetic alterations [16]. During the elimination phase, immune effector cells (mainly T and natural killer (NK) lymphocytes) are activated by the inflammatory cytokines released by the growing tumor cells, macrophages, and stromal cells surrounding the tumor cells. The recruited tumor-infiltrating NK cells and macrophages produce interleukin 2 (IL-2), interleukin 12 (IL-12), and interferon gamma (IFNγ), which kill tumor cells by cytotoxic mechanisms such as perforin, tumor necrosis factors (TNF)-related apoptosis-inducing ligands (TRAILs), and reactive oxygen species. Due to heterogeneity, tumor cells with a less immunogenic phenotype are able to escape this elimination phase (i.e., immunosurveillance) and expand during the equilibrium phase. The equilibrium phase may occur over a period of many years when, under Darwinian selection, new tumor cell variants emerge with mutations that further increase overall resistance to immune attack. During the escape phase, tumor cell variants breach the host’s immune defenses conferring further resistance to immune detection [17].
Major mechanisms for escape include defective antigen presentation, tumor-induced inhibitory checkpoint pathways against effector T cell activity, infiltrating immunosuppressive immune cells including regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs), and secretion of immunosuppressive cytokines (transforming growth factor beta (TGF-β), IL-6, vascular endothelial growth factor (VEGF)) [7, 18, 19]. Full activation of T and NK lymphocytes requires the coordinated participation of several surface receptors that meet their cognate ligands through structured transient cell-to-cell interactions known as immune synapses. Current PD-1/PD-L1 blockade therapies aim to boost the patient’s effector T cells to specifically recognize and kill cancer cells. Table 1 summarizes the current list of major immune checkpoint inhibitors that have obtained FDA approval or are in the late phases of clinical development.
Table 1.
Summary of clinical indication and ongoing evaluation of immune checkpoint inhibitors in major cancer types
| Target | Drug | Class | Company | Clinical indication and ongoing evaluation (status; approval date; trial identifier; country) |
|---|---|---|---|---|
| CTLA-4 | Ipilimumab (Yervoy®, MDX-010, MDX-101) | Human IgG1/kappa | Bristol-Myers Squibb | Metastatic melanoma (US FDA approved on March 25, 2011); metastatic NSCLC (phase I has been completed; NCT01165216; Japan; phase II reported, NCT00527735, USA; phase III ongoing, NCT01285609; USA; phase III ongoing NCT02279732; China) |
| Tremelimumab (ticilimumab, CP-675206) | Human anti-CTLA-4 IgG2 mab | MedImmune/AstraZeneca | Metastatic melanoma (phase I has been completed; NCT01103635; USA; phase II has been completed, NCT00471887, USA); advanced hepatocellular carcinoma (phase II has been completed; NCT01008358; Spain); Metastatic NSCLC (phase Ib has been reported, NCT02000947, USA; phase II has been reported, NCT02179671, USA; first line, phase III MYSTIC ongoing; NCT02453282; USA) | |
| PD-1 | Nivolumab (Opdivo®, ONO-4538, MDX-1106, BMS-936558) | Human IgG4/kappa | Bristol-Myers Squibb; Ono Pharmaceuticals | Metastatic melanoma (Japan approval on July 4, 2014; US FDA accelerated approval on December 22, 2014; US FDA approval of nivolumab in combination with ipilimumab for BRAF V600 wild-type tumor on September 30, 2015); Squamous NSCLC (US FDA approval on March 4, 2015; European Commission on July 20, 2015); expands to non-squamous NSCLC (US FDA approval on October 9, 2015); advanced (metastatic) renal cell carcinoma (US FDA approval on November 23, 2015); classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplantation and post-transplantation brentuximab vedotin (US FDA approval on May 17, 2016) |
| Pembrolizumab (Keytruda®, lambrolizumab, MK-3475) | Humanized IgG4 | Merck & Co. | Metastatic melanoma (USA accelerated approval on September 4, 2014 for patients with disease progression after ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor; US FDA expanded to initial treatment on December 18, 2015); metastatic NSCLC whose tumors express PD-L1 as determined by an FDA-approved test and who have disease progression on or after platinum-containing chemotherapy (US FDA approval on October 2, 2015) | |
| Pidilizumab (CT-011) | Humanized IgG1 | CureTech Ltd | Diffuse large-B cell lymphoma (phase II has been completed; NCT00532259; USA); metastatic melanoma (phase II has been completed; NCT01435369; USA) | |
| AMP-514 (MEDI0680) | Humanized IgG4 | MedImmune | Advanced malignancies (phase II is currently recruiting participants; NCT02013804; USA) | |
| AUNP-12 | Peptide antagonist | Aurigene, Pierre Fabre | Cancer (preclinical phase, Aurigene granted Pierre Fabre worldwide rights to develop AUNP12 for cancer indications; announced on February 11, 2014; India) | |
| PD-L1 | BMS936559 (MDX-1105) | Human IgG4 | Bristol-Myers Squibb | Advanced or recurrent solid tumors (phase II has been completed; NCT00729664; USA) |
| Atezolizumab (Tecentriq™, MPDL3280A, RG7446) | Human IgG1 | Roche & Genentech | Metastatic bladder cancer (phase III, US FDA granted breakthrough therapy designation on May 31, 2014; priority review on March 14, 2016; accelerated approval on May 18, 2016); metastatic NSCLC (phase III, US FDA grants breakthrough therapy designation on February 1, 2015) | |
| Durvalumab (MEDI4736) | Humanized IgG1 | AstraZeneca | Glioblastoma (phase II is currently recruiting participants; NCT02336165; USA); metastatic squamous cell carcinoma of the head and neck (phase II is currently recruiting participants; NCT02207530; USA); advanced or metastatic NSCLC (phase III is currently recruiting participants; NCT02352948; Global study); advanced colorectal cancer (phase II is currently recruiting participants; NCT02227667; USA); metastatic NSCLC (first line phase III MYSTIC is current recruiting participants; NCT02453282; USA; first line phase III ARCTIC is current recruiting participants; NCT02352948; Global); metastatic bladder cancer (US FDA granted breakthrough therapy designation for PD-L1-positive tumors in patients who progressed during or after one standard platinum-based regimen on February 17, 2016) | |
| Avelumab (MSB0010718C) | Fully humanized IgG1 | Merck KGaA, EMD Serono, Pfizer | Advanced solid tumors (phase I with consecutive parallel group expansion; currently recruiting participants in multiple tumor types and settings; NCT01772004; USA); metastatic NSCLC (phase III is currently recruiting participants after failure of a platinum-based doublet; NCT02395172; and first line versus platinum doublet; NCT02576574; USA) | |
| PD-L2 | AMP-224 | PD-L2-IgG2a fusion protein | Amplimmune | Advanced cancer (phase II has been completed; NCT01352884; USA) |
Last assessed information at ClinicalTrial.gov on December 28, 2015; updated FDA approvals on May 18, 2016. Italicized data highlights major cancer types in clinical evaluation
Abbreviations: CTLA-4 cytotoxic T-lymphocyte-associated protein 4, NSCLC non-small-cell lung cancer, PD1 programed cell-death protein 1, PDL1 programed cell-death ligand 1
Immune checkpoints normally maintain self-tolerance, regulate the amplitude and duration of T cell responses, and prevent tissue damage. PD-1 and PD-L1 immune checkpoint blockade can restore the function of exhausted CD8 T cells in chronic viral infection, augmenting the existing tumor-specific immunity [20, 21]. Nevertheless, immune checkpoint inhibitors can override immune self-tolerance, inducing a unique syndrome of autoimmune and autoinflammatory side effects (i.e., irAEs) [22]. Many of these irAEs resemble autoimmune diseases, such as autoinflammatory rheumatic disease, dermatologic disease, colitis, hepatitis, and endocrinopathies [23]. Therefore, almost all immunotherapy clinical trials have excluded individuals with preexisting autoimmune diseases, although a recent study demonstrates that the use of ipilimumab in cancer patients with autoimmune diseases is safe and effective [24]. However, a recent study showed the safety and efficacy of using anti-CTLA-4 antibody in patients with autoimmune disease [24]. Early detection and effective management of these irAEs are pivotal for a favorable clinical outcome. Theoretically, cytotoxic T cells against tumors and normal organs are different subclones that may not need to be activated in parallel. Further studies are warranted to specifically enhance tumor-specific cytotoxic T cell response and avoid unnecessary T cell response to normal tissues.
Biology and mechanisms of PD-1 and PD-L1 molecules
PD-1 (CD279) is expressed on many cell types including T cells, B cells, natural killer T cells, activated monocytes, and dendritic cells (DCs) in humans and mice [25]. In normal human lymphoid tissues, PD-1 is detected on germinal center-associated T cells [26]. PD-1 binds to the ligand PD-L1 (B7-H1, CD274) or PD-L2 (B7-DC, CD272). PD-L1 is typically expressed on the surface of tumor cells [27]. PD-L1 is also constitutively expressed on other cell types such as T and B cells, DCs, macrophages, mesenchymal stem cells, and bone marrow-derived mast cells [28]. Cell surface expression of PD-L1 can be upregulated on both tumor cells and other cell types after treatment with type I or type II interferons (IFNs) [29, 30], radiation [31–33], or chemotherapy [34–38]. Additionally, radiotherapy may induce direct tumor-cell killing and multiple immunomodulatory changes that can potentially influence the effectiveness of immunotherapy [31–33].
Engagement of PD-1 by its ligands, either PD-L1 or PD-L2, induces a negative control signal resulting in the inhibition of T cell proliferation, cytokine production, and cytotoxic activity. PD-L1 can provide inhibitory signals to activated T cells through interactions with the surface receptor PD-1 or CD80 [25, 39]. These signals enable PD-L1-expressing tumor cells to evade immune detection by NK cells or T cells [40–42]. PD-L2 is encoded by PDCD1LG2, which is essential for T cell proliferation and IFN production. PD-L2 can stimulate PD-1 receptor signaling, resulting in “T cell exhaustion,” a temporary inhibition of activation and proliferation that can be reversed by the removal of PD-1 signal [25].
Upregulation of PD-L1 on different tumor types inhibits the local antitumor T cell response. Figure 1 illustrates the expression and complex interaction of key molecules and co-stimulatory ligand receptors between tumor and immune cells in tumor microenvironment (TME), and the two potential mechanisms proposed to understand the biology and function of PD-L1 on tumor cells [43]. While constitutive oncogenic signals (such as protein kinase B (AKT), epidermal growth factor receptor (EGFR), KRAS pathways) upregulate PD-L1 expression on tumors as innate (tumor cell intrinsic) resistance (Fig. 1, left) [44–48], inflammatory signals generate cytokine-induced PD-L1 expression on either tumor cells or immune cells (myeloid suppressor cells, dendritic cell, macrophage, and lymphocytes) in the tumor microenvironment as adaptive resistance (Fig. 1, right) [49, 50]. Preclinical studies have demonstrated that blocking the PD-L1/PD-1 interaction increases the numbers of effector T cells, augments cytolytic activity of tumor-specific T cells, enhances the production of pro-inflammatory cytokines, brings effector T cells to the tumor sites, reduces numbers and suppression of Tregs at the tumor site, and downregulates the production of suppressive cytokines IL-10 [51–54]. Tumor-infiltrating T cells may also be functionally inert, due in part to the expression of PD-1 along with other inhibitory receptors [27, 55]. The hallmarks of the PD-1/PD-L1 blockage effect include immune modulation at tumor site, direct targeting of tumor-induced immune defects, and repairing ongoing tumor immunity. PD-1 deletion in mice can lead to autoimmunity, most notably when bred onto backgrounds of autoimmune-susceptible mouse strains [56, 57]. In multiple syngeneic mouse tumor models, blockade of PD-1 or its ligands promotes antitumor activity [58, 59]; anti-PD-1 activity in vivo can be enhanced by combination with antibodies to other T cell-negative regulators, such as CTLA-4 and LAG-3 [60–62]. A recent study suggests that PD-L1 is an independent prognostic marker in melanoma, defining a tumor subset with distinct genetic and morphophenotypic features [63].
Fig. 1.
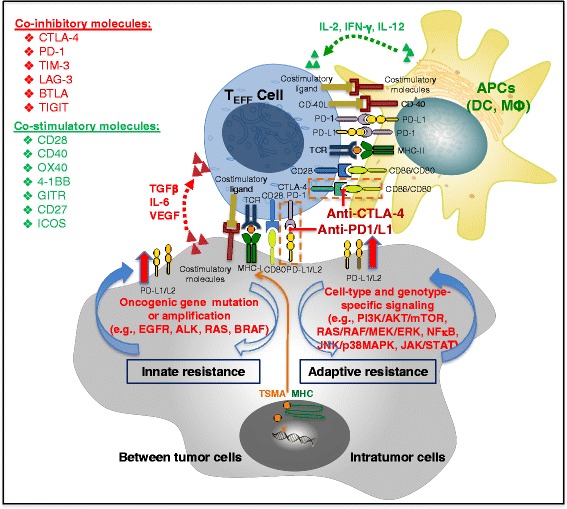
Schema interaction between tumor and immune cells. Full activation of T-lymphocytes requires the coordinated participation of several surface receptors on effector T cells and antigen-presenting cells (APCs) or tumor cells. The main route of T cell stimulation is driven by antigens recognized in the form of short polypeptides associated with MHC antigen-presenting molecules. However, the functional outcome of T cell stimulation towards clonal expansion and effector function acquisition is contingent on the contact of additional surface receptor-ligand pairs and on the actions of cytokines in the tumor microenvironment. While some of those interactions are inhibitory (in red), others are activating and are collectively termed co-stimulatory (in green) receptors. Communication between T cells and APCs is bidirectional. In some cases, this occurs when ligands themselves signal to the APC. In other cases, activated T cells upregulate ligands, such as CD40L, that engage cognate receptors on APCs. Tumor cells can upregulate PD-L1 expression via either the constitutionally activated oncogenic signaling (left, innate/intrinsic immune resistance) or the immune modulator-induced signaling pathways (right, adaptive immune resistance). Abbreviations: APC antigen-presenting cells, DC dendritic cell, IL-2R IL-2 receptor, MDSCs myeloid-derived suppressor cells, Teff effector T cell, Treg regulatory T cells, IDO indoleamin 2,3-dioxygenase, TIM-3 T cell immunoglobulin domain and mucin domain, LAG lymphocyte-activation gene, BTLA B- and T-lymphocyte attenuator, HVEM herpes virus entry mediator, TIGIT T cell immunoreceptor with Ig and ITIM domains, GITR glucocorticoid-induced tumor necrosis factor receptor, ICOS inducible costimulators, CEACAM carcinoembryonic antigen-related cell adhesion molecule, TSMA tumor-specific mutant antigens, JNK, c-Jun N-terminal kinase, MEK/ERK, mitogen/extracellular signal regulated kinase, PI3K, phosphatidylinositol 3-kinase, STAT, signal transducer and activator of transcription, NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells
Clinical efficacy of PD-1/PD-L1 blockade therapies and PD-L1 expression
Nivolumab and pembrolizumab are the first generation PD-1 immune checkpoint blockade antibodies. Nivolumab inhibits the interaction between PD-1 and its ligands, PD-L1, and PD-L2 with similar IC50 values (2.52 and 2.59 nmol/L, respectively). Nivolumab enhances T cell reactivity in the presence of a T cell receptor stimulus at the low concentration (~1.5 ng/mL) without nonspecific lymphocyte activation. Nivolumab also enhances the production of inflammatory cytokines in assays such as the human T cell/DC-mixed lymphocyte reaction (MLR) assays, and Staphylococcal enterotoxin B (SEB), and cytomegalovirus (CMV) recall response assays. In addition, nivolumab increases antigen-specific CD8+ T cell responses in patients with melanoma [64]. Nivolumab completely restored CD4+ T-responder cell proliferation and partially restored IFNγ production. Nivolumab also overcomes Treg suppression of CD8+ T cells by increasing resistance to Treg suppression and also by directly limiting suppressive capacity of Treg [65]. This was demonstrated in monkeys when increased numbers of CD8+ T-effector memory cells were detected in CMV-positive peripheral blood after repeated treatment at the highest dose of nivolumab for 3 months. Similarly, marked accumulation of CD8+ effector memory T cells in lymphoid organs and tissues of PD-1-deficient mice has been described [66].
Both nivolumab and pembrolizumab have shown significant survival benefit in some patients with advanced melanoma, NSCLC, and renal cell carcinomas in early clinical trials (Table 1). These agents were generally well tolerated, even in elderly patients with prolonged dosing. Lower tumor response rates have been observed in never smokers and patients with EGFR mutations or ALK rearrangements when compared to heavy smokers. Table 2 summarizes this clinical efficacy data from four of the most studied PD-1 and PD-L1 inhibitors at various stages of clinical development. As only subsets of patients are benefiting from the PD-1/PD-L1 immune checkpoint blockade therapies, it is important to try to select for this population so that patients less likely to improve with therapy can be spared toxicities. An additional challenge is that response criteria used for standard therapy may not be applicable for immunotherapy. Unlike standard therapeutics in which tumor response is measured by a variety of radiographic and laboratory tests every 6–8 weeks, delayed tumor responses due to the time required for activation and function of effector T cells are frequently observed in cancer patients treated with immune checkpoint blockade therapies. In some patients, tumor regression persisted after discontinuation of PD-1 blockade therapy [67]. Evaluating responses appropriately and identifying patients who are most likely to respond are important aspects of immunotherapy that continue to be developed.
Table 2.
Summary of reported clinical efficacy of PD-1/PD-L1 inhibitors
| Agent | Clinical trials identifier | Phase of clinical trial | Sample size (no. Pt) | Patient population | Biomarker | Regimen | Tumor responses (ORR) | Median PFS (months) | OS (months; median unless otherwise specified) | Reference: author (year) |
|---|---|---|---|---|---|---|---|---|---|---|
| Nivolumab | NCT01642004 (CheckMate 017) | Phase III | 272 all: 135 nivolumab, 137 docetaxel | Advanced squamous NSCLC with disease progression during or after first-line chemotherapy | PD-L1-positive tumor cells | Nivolumab 3 mg/kg IV every 2 weeks | 20 % | 3.5 | 9.2 | Brahmer J (2015) [125] |
| Docetaxel 75 mg/m2 IV every 3 weeks | 9 % | 2.8 | 6 | |||||||
| NCT01673867 (CheckMate 057) | Phase III | 582 all: 292 nivolumab, 290 Docetaxel | Advanced non-squamous NSCLC after platinum-based doublet chemotherapy | PD-L1-positive tumor cells | Nivolumab 3 mg/kg IV every 2 weeks | 19 % | 2.3 | 12.2 | Borghaei H (2015) [126] | |
| Docetaxel 75 mg/m2 IV every 3 weeks | 12 % | 4.2 | 9.4 | |||||||
| NCT01668784 (CheckMate 025) | Phase III | 821 all: 406 nivolumab, 397 Everolimus | Advanced clear-cell renal-cell carcinoma with one or two regimens of anti-angiogenic therapy | PD-L1-positive tumor cells | Nivolumab 3 mg/kg IV every 2 weeks | 25 % | 4.6 | 25 | Motzer RJ (2015) [127] | |
| Everolimus 10 mg orally daily | 5 % | 4.4 | 19.6 | |||||||
| NCT01721746 (CheckMate 037) | Phase III | 631 all: 272 nivolumab,133 investigators choice of chemo | Unresectable or metastatic melanoma after ipilimumab or ipilimumab and BRAF inhibitor if BRAF positive | PD-L1-positive tumor cells | Nivolumab 3 mg/kg IV every 2 weeks | 32 % | 4.7 | NA | Weber JS (2015) [128] | |
| Chemo: either dacarbazine 1000 mg/m2 IV every 3 weeks or carboplatin AUC = 6 plus paclitaxel 185 mg/m2 IV every 3 weeks | 11 % | 4.2 | NA | |||||||
| NCT01927419 (CheckMate 069) | Phase III | 142 all: 95 nivolumab + ipilimumab, 47 ipilimumab | Unresectable or metastatic melanoma treatment naïve with measurable disease | Tissue available for PD-L1 biomarker analysis | Nivolumab 1 mg/kg IV every 3 weeks × 4 doses plus ipilimumab 3 mg/kg IV every 3 weeks × 4 doses, then maintenance nivolumab 3 mg/kg IV every 2 weeks | BRAF wild type: 61 %; BRAF mutation: 52 % | BRAF wild type: NR; BRAF mutation: 8.5 | NA | Postow MA (2015) [4] | |
| Same dose schedule with nivolumab placebo in both the combination and maintenance phase | BRAF wild type: 11 %; BRAF mutation: 22 % | BRAF wild type: 4.4; BRAF mutation: 2.7 | NA | |||||||
| NCT01721772 (CheckMate 066) | Phase III | 418 all: 210 nivolumab, 208 dacarbazine | Untreated metastatic melanoma without BRAF mutation | Tissue available for PD-L1 biomarker analysis | Nivolumab 3 mg/kg IV every 2 weeks plus placebo every 3 weeks | 40 % | 5.1 | 1-year OS: 72.9 % | Robert C (2015) [129] | |
| Dacarbazine 1000 mg/m2 IV every 3 weeks plus placebo every 2 weeks | 13.9 % | 2.2 | 1-year OS: 42.1 % | |||||||
| NCT01844505 (CheckMate 067) | Phase III | 945 all: 316 nivolumab, 314 combination, 315 ipilimumab | Untreated, unresectable stage III or IV melanoma | Tissue available for PD-L1 biomarker analysis | Nivolumab 3 mg/kg IV every 2 weeks (plus ipilimumab placebo) | 43.7 % | 6.9 | NA | Larkin J (2015) [130] | |
| Nivolumab 1 mg/kg IV every 3 weeks plus ipilimumab 3 mg/kg IV every 3 weeks × 4 doses; then maintenance nivolumab 3 mg/kg IV every 2 weeks | 58 % | 11.5 | ||||||||
| Ipilimumab 3 mg/kg IV every 3 weeks (plus nivolumab placebo) | 19 % | 2.9 | ||||||||
| NCT01721759 (CheckMate 063) | Phase II single arm trial | 117 | Advanced NSCLC | PD-L1-positive tumor cells | Nivolumab 3 mg/kg every 2 weeks until progression or unacceptable toxic effects | 14.5 % | 1.9 | 8.2 | Rizvi NA (2015) [131] | |
| NCT00730639 | Phase I with expansion cohorts | 107 | Advanced melanoma | Unselected | Nivolumab at 1, 3, or 10 mg/kg every 2 weeks for up to 96 weeks | 31 % | 3.7 | 16.8 | Topalian SL (2014) [67] | |
| Phase I with expansion cohorts | 34 | Previously treated advanced RCC | Unselected | Nivolumab at 1, 3, or 10 mg/kg every 2 weeks for up to 96 weeks | 29 % | 7.3 | 22.4 | McDermott DF (2015) [132] | ||
| Phase II with expansion cohorts | 129 | Heavily pretreated advanced NSCLC | Unselected | Nivolumab at 1, 3, or 10 mg/kg every 2 weeks for up to 96 weeks | 17 % | 2.6 | 9.9 | Gettinger SN (2015) [133] | ||
| Pembrolizumab | NCT01866319 (KEYNOTE-006) | Phase III | 834 all: 279 pembrolizumab, 277 pembrolizumab, 278 ipilimumab | Unresectable stage III or IV melanoma | PD-L1-positive tumor cells | Pembrolizumab 3 mg/kg IV every 2 weeks | 33.7 % | 5.5 | NA | Robert C (2015) [134] |
| Pembrolizumab 3 mg/kg IV every 3 weeks | 32.9 % | 4.1 | ||||||||
| Ipilimumab 3 mg/kg IV every 3 weeks | 11.9 % | 2.8 | ||||||||
| NCT01704287 (KEYNOTE-002) | Phase II | 540 all: 180 pembrolizumab, 181 pembrolizumab, 179 chemotherapy | Ipilimumab-refractory melanoma | Will be reported with the final overall survival analysis | Arm A 2 mg/kg (n = 180) | 21 % | 5.4 | NA | Ribas A (2015) [135] | |
| Arm B 10 mg/kg (n = 181) | 25 % | 5.8 | ||||||||
| Chemotherapy | 4 % | 3.6 | ||||||||
| NCT012958297 (KEYNOTE-001) | Phase I | 495 | Advanced NSCLC | PD-L1-positive tumor cells | Pembrolizumab 2 or 10 mg/kg IV every 3 weeks or 10 mg/kg every 2 weeks over a 30-min period | 19.4 % | 3.7 | 12 | Garon EB (2015) [5] | |
| Phase I | 655 | Melanoma | Unselected | Pembrolizumab 2 mg/kg every 3 weeks (Q3W), 10 mg/kg Q3W, or 10 mg/kg Q2W until unacceptable toxicity, disease progression, or investigator decision | 33 % | 12-month PFS 35 % | 23 | Adil Daud (2015) [136]; Ribas A (2016) [137] | ||
| Phase I with expansion cohort | 173 | Advanced melanoma after at least 2 ipilimumab doses | Unselected | Pembrolizumab 2 mg/kg IV every 3 week or 10 mg/kg IV every 3 weeks | 26 % | 2 | NA | Robert C (2014) [138] | ||
| NCT01848834 (KEYNOTE-012) | Phase IB | 32 | Metastatic triple-negative breast cancer | PDL-1-positive tumor cells | Pembrolizumab 10 mg/kg IV every 2 weeks | 19 % | 6-month PFS 23.3 % | NA | Nanda R (2014) [139] | |
| NCT1905657 (KEYNOTE-010) | Phase II/III | 1034 all: 339 pembrolizumab, 343 pembrolizumab, 309 docetaxel | Previously treated PD-L1-positive advanced NSCLC | PDL-1-positive tumor cells | Pembrolizumab 2 mg/kg, IV every 3 weeks | 18 % | 3.9 months | 14.9 | Herbst RS [2015] [140] | |
| Pembrolizumab 10 mg/kg, IV every 3 weeks | 18.5 % | 4.0 month | 17.3 | |||||||
| Docetaxel, 75 mg/m2 every 3 weeks | 9.3 % | 4.0 month | 8.2 | |||||||
| NCT01953692 (KEYNOTE-013) | Phase IB | 15 | Hodgkin lymphoma | Unselected | Pembrolizumab 10 mg/kg IV every 2 weeks up to 2 years | 53 % | NA | NA | Moskowitz C (2014) [141] | |
| Atezolizumab (MPDL3280A) | NCT01846416 | Phase II | 205 | NSCLC | PD-L1-positive tumor cells | Atezolizumab 1200 mg IV every 3 weeks | The highest ORR was seen in pts with PD-L1 TC3 or IC3 tumors | NA | NA | Spigel DR (2015) [142] |
| NCT01903993 (POPLAR) | Phase II | 287 | Previously treated NSCLC patients (pts) were stratified by PD-L1 IC status | PD-L1-positive tumor cells | Atezolizumab 1200 mg IV every 3 weeks | 57 % | 2.7 | 12.6 | Spira AI (2015) [143]; Fehrenbacher L (2016) | |
| Docetaxel 75 mg/m2 IV every 3 weeks | 24 % | 3.0 | 9.7 | |||||||
| NCT01375842 | Phase I | 35 | Metastatic melanoma | PD-L1-positive tumor cells | Atezolizumab IV every 3 weeks for up to 1 year | 26 % | 24-week PFS 35 % | NA | Omid Hamid (2013) [144] | |
| Phase I | 277 | Multiple cancer types | PD-L1-positive tumor cells | Atezolizumab intravenously every 3 weeks doses >1 ml/kg | 18 % | 2.6 | NA | Herbst RS (2014) [145] | ||
| NCT01633970 | Phase Ib | 37 | Untreated NSCLC | PD-L1-positive tumor cells | Atezolizumab 15 mg/kg IV every 3 weeks with standard chemo dosing for 4–6 cycles followed by MPDL3280A maintenance therapy until progression | 67 % | NA | NA | Stephen V (2015) [146] | |
| Phase Ib | 14 | Arm A: refractory metastatic colorectal cancer; arm B: oxaliplatin-naive mCRC | Not mentioned | Arm A: MPDL3280A 20 mg/kg every 3 weeks and bevacizumab (bev) 15 mg/kg every 3 weeks | 8 % (1/13) in arm A | NA | NA | Bendell, J.C. (2015) [147] | ||
| Arm B: MPDL3280A 14 mg/kg every 2 weeks, bev 10 mg/kg every 2 weeks and mFOLFOX6 at standard doses | 36 % (9/25) in Arm B | NA | NA | |||||||
| Phase Ib | 12 | Metastatic renal cell carcinoma | Not selected | Atezolizumab 15 mg/kg given alone on cycle 1 day 1 and concurrently with 20 mg/kg every 2 weeks thereafter | 40 % | NA | NA | Sznol M (2015) [148] | ||
| Durvalumab (MEDI4736) | NCT01693562 | Phase I/II | 198 | NSCLC | Tissue available for PD-L1 biomarker analysis | Durvalumab 10 mg/kg IV every 2 weeks until unacceptable toxicity, disease progression, or for up to 12 months | 14 % (23 % in PD-L1+ tumors) | NA | NA | Rizvi NA (2015) [149] |
| Phase I | 13 | NSCLC | Tissue available for PD-L1 biomarker analysis | Durvalumab 7 doses (1–25) across 6 cohorts (0.1–10 mg/kg every 2 weeks; 15 mg/kg every 3 weeks) | NA | NA | NA | Brahmer JR (2014) [150] | ||
| Multi-arm expansion study | 62 | A squamous cell carcinoma of the head and neck expansion cohort | Tissue available for PD-L1 biomarker analysis | Durvalumab IV every 2 weeks at 10 mg/kg for 12 months | 12 % (25 % in PD-L1+ pts) | NA | NA | Segal NH (2015) [151] | ||
| Phase I | 26 | Advanced solid tumors | Durvalumab IV every 2 (q2w) or 3 weeks (q3w) in a 3 + 3 dose escalation with a 28-day (q2w) or 42-day (q3w) DLT window | NA | NA | NA | Lutzky J (2014) [152] | |||
| NCT02088112 | Phase I | 10 | NSCLC | Unselect | Durvalumab cohort A received 3 mg/kg (starting dose) every 2 weeks plus gefitinib 250 mg QD | NA | NA | NA | Creelan BC (2015) [153] | |
| NCT02000947 | Phase Ib | 118 (102 eligible) | NSCLC | Tissue available for PD-L1 biomarker analysis | Durvalumab 10–20 mg/kg every 2 or 4 weeks plus tremelimumab 1 mg/kg (N = 56) | 23 % (6/26): 22 % (2/9) in PD-L1+ versus 29 % (4/14) in PD-L1- | NA | NA | Antonia SJ (2015) [154]; Updated in Antonia SJ (2016) [155] | |
| Durvalumab 10–20 mg/kg every 2 weeks plus tremelimumab 3 mg/kg (N = 34) | 20 % (5/25) | |||||||||
| Durvalumab 15 mg/kg every 4 weeks plus tremelimumab 10 mg/kg (N = 9) | 0 % (0/9) |
Abbreviations: DLT dose-limiting toxicity, q2w every 2 weeks, q3w every 3 weeks, q4w every 4 weeks, QD once daily, BRAF B-raf and v-raf murine sarcoma viral oncogene homologue B1, NA not available
Biomarkers for PD-1/PD-L1 blockade therapies
Translational biomarker research is critical for the future of drug development for clinical immune checkpoint blockade therapy. Only one PD-L1 IHC has been approved as a companion diagnostic by the US FDA for patients with advanced NSCLC for pembrolizumab [68]. The challenges in developing immuno-oncology biomarkers for clinical use have been recognized by regulatory agencies and professional societies such as the Society for Immunotherapy of Cancer (SITC) [69]. Over the past several years, the SITC Immune Biomarkers Task force has provided the roadmap and regular updates on immune biomarker development [70–72]. Immunological changes in peripheral blood and tumor could potentially reflect tumor response in patients and serve as immune biomarkers. The immune monitoring assays had been developed to evaluate these potential biomarkers before and after immune checkpoint blockade therapies and investigate their correlation with clinical outcome (Fig. 2). These biomarkers could be classified into two groups: tumor-derived immune biomarkers and immune cell-derived biomarkers as discussed in the following sections. Table 3 summarizes currently available quantitative biomarker assays for immune checkpoint inhibitors.
Fig. 2.
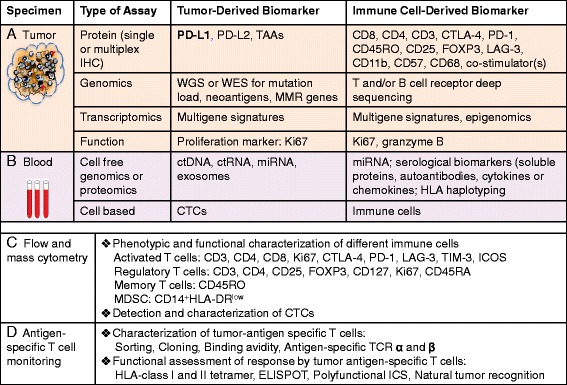
Immune monitoring strategies for patients receiving checkpoint blockage therapy. Technologies that are currently used to assess the potential immune biomarkers. a Tumor and immune cells in tumor specimens could be evaluated by immunohistochemical stain (IHC) or immunofluorescence assays, molecular or genetic profiling analysis, and cellular functional assays. The tumor microenvironment can be dissected histopathologically to characterize spatial relationships between tumor and immune infiltrates. Transcriptional profiling assays can evaluate changes in gene expression in both the tumor cells and lymphocytes. Deep sequencing techniques enable quantification of changes in individual T/B cell clonotypes. b Peripheral blood provides a minimally invasive way to allow serial monitoring of dynamic changes of immune biomarkers during cancer immunotherapy. The analysis of changes in cell counts with therapy, changes in cytokine levels, circulating tumor cells, tumor-derived nucleotides, and immune cells. c Flow cytometric analysis of TILs anPBMCs for quantitating the effect of therapy on immune subsets such as activated CD8 + PD1+ T cells, CD4 + FOXP3 + CD25hi Tregs, or myeloid-derived suppressor cells. Using polychromatic flow cytometry, multiple surface and intracellular markers can be detected, allowing in-depth characterization of T cell phenotype and activation state. d Multifunctional flow T cell assay, MHC tetramer staining and ELISPOT can be used to analyze the presence and function of tumor-specific T cell subpopulations. Abbreviations: PD-L1 programed death-1, IHC immunohistochemistry, ELISPOT enzyme-linked immunospot assay, CTCs circulating tumor cells, WES whole exome sequencing, NGS next-generation sequencing, TIM-3 T cell immunoglobulin domain and mucin domain, LAG lymphocyte-activation gene, ICOS inducible costimulators, MDSC myeloid-derived suppressor cells, HLA human leukocyte antigen
Table 3.
Currently available translational biomarker assays for immune checkpoint inhibitors
| Biospecimen | Method | Tissue/cell types | Pros | Cons | References and recommended reading |
|---|---|---|---|---|---|
| FFPE | IHC | Tumor cells or tumor infiltrating immune cells | Direct detection; accurately pinpoint cancer cells; highly sensitive; simplicity; low cost | Requirement of trained pathologists; inconsistency for criteria used to score tumors such as PD-L1-positive or negative | Herbst R (2014) [145]; Loughlin PA (2007) [164] |
| Multicolor IHC | Tumor cells or tumor infiltrating immune cells | Broad dynamic range; capability for multiplexing using different fluorescence channels; >10 protein targets are identified in the same sample; amenability for co-localization studies | Absence of rigorous quantitative tests; limitation in some biomarker-driven clinical trials; user must select combinations of dyes | Carvajal-Hausdorf DE (2014) [165] | |
| T cell receptor deep sequencing | TILs | T cell count information; T cell clonality in tumor |
Heterogeneous expression of TIL | Robbins HS (2013) [100] | |
| Whole exome sequencing (WES) | Tumor cells | Characterization of tumor mutation load including nucleotide substitutions; structural rearrangements and copy number alterations; identification of the neoantigens and neoepitopes; affordable cost | Require high-performance deep sequencing, computational bioinformatics support; The pipelines are still at early developmental phase |
Snyder A (2014) [104]; Rizvi NA (2015) [105]; Bouffet E (2016) [107]; Chen K-H (2016) [166] Hugo W (2016) [106] |
|
| Blood | ELISPOT assays (IFNγ and granzyme B) | T cells in PBMCs | Detection of tumor antigen-specific CD4+ and CD8+ T cell response with good assay sensitivity; Relatively well validated assay |
A poor correlation with clinically relevant immune responses | Shafer-Weaver K (2006) [167]; Janetzki S (2008) [95] Malyguine A (2012) [94]; Janetzki S (2015) [93]; |
| Flow cytometry (tetramer, polyfunctional analysis) | T cells in PBMCs | Assessment of tumor antigen-specific CD4+ and CD8+ T cells response; measure multiple functions; detection of neoantigen-specific CD8 + PD-1+ T cells; minimally invasive | Merely in lab research, not as routine clinical monitoring yet | Yuan J (2008) [84]; Attic S (2011) [85]; McNeil LK (2013) [86]; Barrera L (2015) [168]; Gros A (2016) [118] | |
| Flow cytometry phenotype staining | Whole blood immune phenotype | Analyses of the frequency and proliferation of different subsets of immune cells; routine operation | Dedicated resource and staff to perform the analyses | Streitz M (2013) [169]; van Dongen JJ (2012) [170] | |
| RNA-Seq (NGS) | T cells in PBMCs | Identification of genetic variants; a broader dynamic range; detection of more differentially expressed genes; fast and high efficiency | More expensive than microarray; more complex for analysis; bulk signature, not single cell signals; need more validation | Zhao S (2010) [171] | |
| qPCR assay | T cells in PBMCs | High specificity; able to detect the reactivity of low-frequency T cells in the peripheral blood of metastatic cancer patients | Bulk signature, not single cell signals; need more validation | Kammula US (2008) [172] | |
| Flow cytometry | CTCs | Qualitative analysis at the single cell level in a relatively short period of time; decrease the amount of blood needed; provide valuable information regarding the frequency, phenotype and/or the functionality of T cells | Expensive; need more validation | Zaritskaya L (2010) [83] | |
| Cell sieve microfiltration assay and QUASR technique | CTCs | PD-L1 levels from CTCs or CAMLs serves as a surrogate for PD-L1 expression in tumor; as a marker for immunotherapy response | Limited in lab research; need more validation | Steven HL (2015) [173]; Adams DL (2014) [174] |
Abbreviations: FFPE formalin-fixed paraffin-embedded, PD-L1 program death-1, TIL tumor-infiltrating lymphocytes, IHC immunohistochemistry, CAMLs cancer-associated macrophage-like cells, ELISPOT enzyme-linked immunospot assay, CTL cytotoxic T-lymphocytes, CTCs circulating tumor cells, PBMC peripheral blood mononuclear cell, WES whole exome sequencing, NGS next-generation sequencing
Current immune monitoring assays
IHC staining
PD-L1 is the best studied immuno-oncology biomarker to date. Currently, PD-L1 IHC using 22C3 antibody is the only FDA-approved companion diagnostic for selecting NSCLC patients for pembrolizumab [68]. Table 4 summarizes the IHC assays used for PD-L1 expression using baseline archival tumor specimens in these clinical studies [73]. There are many variables in these IHC assays. First, the time between sample collection and treatment with a PD-1 or PD-L1 inhibitor is not controlled. Second, tumor cells, immune cells, as well as stroma cells can express PD-L1 with considerably heterogeneity within the tumor microenvironment. Third, PD-L1 expression is induced by IFNγ during disease progression and treatment. Fourth, different PD-L1 antibodies were used for different immune checkpoint blockade clinical studies. Currently, there is no validated antibody for IHC staining for this class of immune checkpoint inhibitors—each sponsor uses different antibodies. Antibodies staining tumor cells or stroma cells may have different PD-L1 epitopes (Table 4). An additional challenge is that none of the commercial PD-L1 IHC test has been validated in terms of clinical outcomes. Head-to-head comparisons among three different commercially available PD-L1 assays (Dako 22C3, Dako 28-8, and Ventana SP263 as the reference) using 500 formalin-fixed, paraffin-embedded archival samples of NSCLC tissue obtained from commercial sources found 91 to 95 % correlation, which is comparable to typical within assay agreement for IHC across the dynamic range at multiple cutoffs [74]. A recent meta-analysis demonstrates that PD-L1 expression is significantly associated with clinical response to anti-PD-1/PD-L1 antibodies in patients with malignant melanoma (MM) or non-squamous NSCLC. However, a proportion of PD-L1-negative patients also benefits from anti-PD-1 therapy in MM and squamous-NSCLC. Thus, expression of PD-L1 in tumor tissues cannot be used as a predictive biomarker of eligibility for treatment with anti-PD-1/PD-L1 antibodies. It may represent a correlation marker for non-squamous NSCLC and melanoma [75].
Table 4.
Immunohistochemistry assays for PD-L1 expression
| Drug | Antibody (marker) | Rx line | Tumor type | Targeted cells | Tumor specimen | Cutoff point (%) | ORR % in IHC+ cases (95 % CI) | ORR % in IHC− cases (95 % CI) | Predictive role | P value | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nivolumab | 28-8 rabbit (Dako) | 1 L | Melanoma | TCs | Archival FFPE or new biopsy | 5 | 58 (46,69) | 41 (35,48) | Yes | 0.001 | Larkin J (2015) [130] |
| Nivolumab + ipilimumab | 72 (60,82) | 55 (48,62) | Yes | 0.001 | |||||||
| Nivolumab | ≥2 L | 5 | 44 (30,58) | 20 (11,32) | Yes | NA | Weber JS (2015) [128] | ||||
| Nivolumab + ipilimumab | 1 L | 5 | 58 (37,78) | 55 (42,69) | No | >0.05 | Postow MA (2015) [4] | ||||
| Nivolumab | 1 L | 5 | 53 (41,64) | 33 (25,42) | No | >0.05 | Robert C (2015) [129] | ||||
| ≥2 L | NSCLC | TCs | Archival FFPE or new biopsy | 1 | 31 (23,40) | 9 (5,16) | Yes | 0.002 | Paz-Ares L (2015) [156]; Updated in Borghaei H (2015) [126] | ||
| 5 | 36 (26,46) | 10 (6,17) | Yes | 0.002 | |||||||
| 10 | 37 (27,48) | 11 (6,17) | Yes | 0.002 | |||||||
| ≥2 L | Archival FFPE | 1 | 17 (9,29) | 17 (8,29) | No | 0.9364 | Brahmer JR (2015) [125] | ||||
| 5 | 21 (10,37) | 15 (8,25) | No | 0.2908 | |||||||
| 10 | 19 (8,36) | 16 (9,26) | No | 0.6411 | |||||||
| ≥2 L | Archival FFPE | 1 | 20 (5,35) | 13 (2,28) | No | >0.05 | Rizvi NA (2015) [131] | ||||
| 5 | 24 (5,43) | 14 (3,25) | No | ||||||||
| 1 L | Archival FFPE | 5 | 31 (NA) | 10 (NA) | No | >0.05 | Gettinger SN (2015) [157] | ||||
| Nivolumab + ipilimumab | 1 L | Archival FFPE | 5 | 19 (NA) | 14 (NA) | No | >0.05 | Antonia SJ (2014) [158] | |||
| Nivolumab | 5H1 and anti-PD-1 monoclonal M3 | ≥2 L | Archival FFPE | 5 | 39 (34,44) | 6 (1,12) | Yes | 0.025 | Taube JM (2014) [113] | ||
| Pembrolizumab | 22C3 mouse (Dako) | ≥1 L | NSCLC | TCs and ICs | New biopsy | 50 | 45 (33,57) | 17 (10,25) | Yes | 0.001 | Garon EB (2015) [5] |
| 1 L | New biopsy | 50 | 47 (23,72) | 19 (8,38) | Yes | NA | Rival NA (2015) [159] | ||||
| Any | Archival FFPE | 1 | 25 (NA) | 13 (NA) | Yes | NA | Garon EB (2014) [160] | ||||
| ≥1 L | Archival FFPE | 50 | 30 (23,39) | 9.8 (NA) | Yes | NA | Herbst RS (2015) [140] | ||||
| 29 (22,37) | 10.7 (NA) | ||||||||||
| 1 L | Archival FFPE | 50 | 16 (NA) | 10 (NA) | Yes | NA | Garon EB (2014) [161] | ||||
| Atezolizumab (MPDL3280A) | SP142 rabbit (Roche Ventana) | ≥2 L | NSCLC | TCs and ICs | Archival FFPE and new biopsy | 50 | 45 (23,68) | 14 (6,25) | Yes | NA | Leora H (2015) [162] |
| ≥2 L | NSCLC | 1+ | 31 (25,37) | 20 (14,26) | Yes | 0.015 | Herbst RS (2014) [145] | ||||
| Solid Tumor | 1+ | 29 (27,31) | 13 (10,16) | Yes | 0.007 | ||||||
| ≥2 L | NSCLC | 2+ | 18 (NA) | 8 (NA) | Yes | NA | Spira AI (2015) [143] | ||||
| Durvalumab (MEDI-4736) | SP263 rabbit (Roche Ventana) | ≥2 L | NSCLC | TCs | Archival FFPE and new biopsy | 25 | 39 (NA) | 5 (NA) | Yes | NA | Segal NH (2014) [163] |
| 33 (13,59) | 30 (16,47) | No for combo | NA | Antonia SJ (2016) [155]; | |||||||
| 22 (3, 60) | 29 (8, 58) | No for monotherapy | NA |
NA not available
The presence of tumor-infiltrating lymphocytes (TILs) at tumor microenvironment has been associated with an improved clinical outcome [76–78], regardless of cancer treatment. TIL counts are primarily measured by IHC [79]. Recent development of multicolored immunohistochemistry staining resulted in a multiplex of up to seven fluorescent dyes effectively interrogated in microscopes equipped with a multispectral camera [80]. The multispectral fluorescent IHC with a panel including CD3, CD8, FoxP3, CD163, and PD-L1 was used to quantify the density of TIL subpopulation in advanced melanoma patients.
Flow cytometry staining
Flow cytometry traditionally uses fluorochrome-labeled antibodies to identify cellular protein expression of the target cells. Flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) and TILs can quantitate the effect of therapy on low-frequency immune subsets such as Treg, MDSC, and activated CD8+ T cells [81]. The biggest advantage of flow cytometry is the combination of multiparameter measurements in a high-throughput manner. With recent advances in laser and fluorochrome technology, commercially available systems allow for detection of up to 18 colors at analysis rates of more than 20,000 events per second. This tremendous power allows for the analysis of multiple characteristics of rare cells such as tumor antigen-specific T-lymphocytes, including their phenotype and functional activity [82]. Flow cytometric assays are currently widely used for the enumeration and phenotypic characterization of lymphocytes, measuring cytokines, secreted molecules, intracellular signaling, function, and cell proliferation [83]. Using polychromatic flow cytometry, multiple surface and intracellular markers can be detected, allowing in-depth characterization of T cell phenotype and activation state [84]. In addition to flow cytometry, antigen-loaded soluble major histocompatibility complex (MHC) tetramer stains can be used to analyze tumor-specific T cell subpopulations [82]. This tetramer/pentamer staining has been used for phenotypic and quantitative analysis of antigen-specific CD8 T cells [85, 86]. The human leukocyte antigen (HLA) system is a gene complex encoding the MHC proteins in humans. HLA-peptide complexes binding to T cell receptors could further define the specificity for epitopes and alleles [87]. Recently, mass cytometry or CyTOF which replace the fluorescent labels with heavy metal ions is under investigation for its application in immune biomarker research [88].
ELISA and protein microarray
Biomarkers in the blood have been pursued because specimens are easy to collect and assays are available for serial monitoring and quantitative measurements [89]. Enzyme-linked immunoabsorbent assay (ELISA) is widely used to assess the production of soluble mediators such as cytokines, chemokines, and tumor antigen-specific antibodies in peripheral blood before and after treatment. Novel protein microarray technologies such as ProtoArray® are available to analyze the serological response of up to 9000 proteins simultaneously [90–92].
ELISPOT
Enzyme-linked immunospot (ELISPOT) assays are robust and standardized tests that quantify both the frequency and function of T or B cells at the single cell level. Both PBMCs and isolated CD4+ and CD8+ T cell subsets could be stimulated by antigens and cultured in a 96-well plate with a nitrocellulose membrane coated with antibodies for cytokines of interest. These assays, such as the IFNγ ELISPOT, have gained increasing popularity for monitoring clinical trials and in basic research [93–95]. Results from various clinical trials, including peptide and whole tumor cell vaccination and cytokine treatment, showed the suitability of the IFNγ ELISPOT assay for monitoring T cell response [96]. ELISPOT assays revealed that 75 % of melanoma patients vaccinated with antigenic peptide demonstrated specific immune responses [97, 98]. Besides IFNγ, other cytokines such as IL-2, IL-5, IL-10, IL-17, granzyme B, TNF, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are commonly measured by ELISPOT assays. It has been expanded to more than 2~3 parameters with the introduction of fluorescent dyes [99].
T cell or B cell receptor deep sequencing
To develop a robust, quantitative method with high reproducibility, Robins et al. [100] uses a digital droplet PCR technique call a QuanTILfy assay. High-throughput quantitative sequencing of the rearranged TCR β genes uses the ImmunoSeq assay. The TCR loci undergo somatic gene rearrangements in the 52 variable (V), diversity (D), and 13 joining (J) regions of the β chain and variable (V) and joining (J) regions of α chain. There are 52 Vβ segments and 13 Jβ segments that can rearrange. With the right TaqMan probes-DNA from T cells can be easily identified with reference to total DNA, as was shown when purified human T cells were obtained from a healthy donor and then diluted and mixed with human lung fibroblasts at different ratios. This technique can also identify clonal expansion (the mark of the adaptive immune response). TCR deep sequencing has been used to show the correlation between TCR repertoire and clinical response to cancer immunotherapy in patients with melanoma or prostate cancer treated with ipilimumab [101]. Creation of a responder library for TCR repertoire will greatly advance the field, especially for genetically modified TCR cell transfer therapy.
WES for mutation load
The cancers that respond best to PD-1 inhibitors, such as advanced melanoma and NSCLC, are the tumor types that are genetically very complex with a high nonsynchronous mutation load. This discovery led to an interest in neoantigens (i.e., tumor-specific mutant antigens (TSMAs)) that are immunogenic [102]. The advances in both affordable next-generation sequencing (NGS) technology and bioinformatics make it feasible to assess the full mutation load in individual tumors and to identify neoantigens by comparing WGS of tumor and normal tissues [103]. Computation prediction algorithms and the tandem mini-gene library allow for the evaluation of the immunogenicity of both CD4+ and CD8+ T cell neoepitopes. Mutation load and neoepitopes have been explored for their correlation with clinical outcomes in cancer patients treated with the PD-1/PD-L1 immune checkpoint blockade [104–107]. However, whole exome sequencing (WES) currently is not used in routine clinical practice. The role of tumor load assessed in targeted exome sequencing (TES) assays in predicting response to immune checkpoint inhibitors remains to be determined.
Genetic susceptibility
Genetic factors are known to modulate the immune response to a therapeutic protein product. In particular, some HLA haplotypes may predispose patients to developing either exceptional or undesirable antibody responses to immune checkpoint blockage therapies [108]. If both are appropriate and feasible, HLA mapping studies may help define a subset of patients who are at increased risk. Moreover, genetic polymorphisms in cytokine genes may upregulate or downregulate immune responses [109], which is why the FDA recommends that researchers evaluate the genetic factors that may modulate the immune response to a therapeutic protein. For example, the subset of patients who generate neutralizing antibodies to IFNβ products are more likely to possess distinct HLA haplotypes [108]. Thus, knowledge of the heightened susceptibility of patients with such HLA haplotypes may guide us to prevent such responses or pursuit other treatment options (http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm338856.pdf).
Tumor-derived immune biomarkers
PD-L1 expression
Upregulation of PD-L1 expression level has been reported in many different cancer types (e.g., melanoma (40–100 %), NSCLC (35–95 %), and multiple myeloma (93 %)). High levels of PD-L1 expression have been linked to poor clinical outcomes [42, 110, 111]. Until now, anti-PD-L1 IHC on tumor specimens is the most commonly used biomarker for selecting patients who are likely to respond to treatments [89]. Constitutively overexpressed PD-L1 in melanoma is associated with poor clinical outcome [112]. However, the PD-L1 overexpression in the context of CD8-positive cells is associated with a better prognosis [113]. Although PD-L1 IHC using the 22C3 antibody has been approved by the US FDA as the only predictive companion diagnostic for selecting NSCLC patients for pembrolizumab, tumor responses have been seen in low PD-L1-expressing tumors. Conflicting results have been reported with other PD-L1 antibodies and drugs. Table 4 summarizes the reported data of PD-L1 IHC in current clinical trials.
Several tumor characteristics, such as a high mutation load, smoking-related tumors, and tumors with mismatch repair genes are associated with improved objective tumor response rate (ORR), durable clinical benefit (DCB), and PFS to immune checkpoint blockade antibodies. Notably, whole exome sequencing has revealed that a nonsynonymous mutation load of >200 per tumor correlates with clinical responses to PD-1 mAb in NSCLC, melanoma, and colorectal cancer with microsatellite instability (MSI; i.e., mutations in DNA mismatch repair genes) [114–116]. Rizvi and his colleagues showed that mutation load correlates with improved ORR (63 versus 0 %), DCB (73 versus 13 %), and PFS (14.5 versus 3.7 months) [105]. Moreover, the efficacy was associated with a molecular smoking signature. The ORR was 56 and 17 % in transversion-high tumors and transversion-low tumors, respectively; the rate of DCB was 77 versus 22 %, respectively; and the PFS was also significantly longer in transversion-high tumors compared to transversion-low tumors. Efficacy was also connected with higher neoantigen burden, but additional genetic features are likely to play a role in determining the tumor response [116, 117]. Neoantigen-specific CD8+ T cell responses paralleled tumor regression in one responder, implying that anti-PD-1 therapy enhanced neoantigen-specific T cell reactivity [118].
Immune cell-derived biomarkers
TIL cells have been shown to express significantly higher levels of PD-1 than T cells in normal tissue [119]. The tumor microenvironment may secrete pro-inflammatory cytokines to upregulate the expression of PD-1 on TIL cells to ensure that they can respond to the high levels of PD-L1 expressed on the tumor [120]. Preexisting CD8+ T cells distinctly located at the invasive tumor margin are associated with expression of the PD-1/PD-L1 immune inhibitory axis [121]. The proliferation of intratumoral CD8+ T cells directly correlated with radiographic reduction in tumor size in metastatic melanoma patients treated with pembrolizumab. Pretreatment samples obtained from responding patients showed higher numbers of CD8+ T cells, PD-1, and PD-L1-expressing cells at the invasive tumor margin and inside tumors, with a more clonal TCR repertoire. Using multivariate analysis, researchers established a predictive model based on CD8 expression at the invasive margin and validated the model in an independent cohort of 15 patients. Their findings indicate that tumor regression after PD-1 blockade requires preexisting CD8+ T cells that are negatively regulated by PD-1/PD-L1-mediated adaptive immune resistance. In addition, the tumor microenvironment can be dissected histopathologically in order to characterize spatial relationships between tumor and immune infiltrate, and transcriptional profiling assays can evaluate changes in gene expression in both the tumor and in lymphocytes [122].
Given that TIL in the tumor microenvironment affects the prognosis of the tumor, a new TNM Immune system (TNM-I) system has been proposed and is currently being validated by an international consortium [123]. Early studies suggest that activated T cells are not pharmacodynamic markers of nivolumab treatment [124]. It remains to be determined whether nivolumab treatment increases CD8 effector memory cells in cancer patients. With technical advances, detection of CD8-positive, PD-1-positive T cells in the peripheral blood of melanoma patients receiving PD-1 inhibitors is feasible. Furthermore, the tumor-antigen specificities and TCR repertoires of the circulating and tumor-infiltrating CD8+PD-1+ cells appeared similar [118].
Conclusions
The era of cancer immunotherapy has arrived. This major breakthrough in cancer treatment holds great promise for increasing cure rates for patients with various cancer types in multiple disease settings. Checkpoint blockade antibodies targeting CTLA-4, PD-1, and PD-L1 axis have demonstrated impressive and durable disease control and promising responses in patients with multiple tumor types. Despite these advances, only a subset of patients benefits from immune checkpoint blockade therapies, with some patients experiencing mechanism-based irAEs. Translational biomarker research is one way to overcome these limitations of therapy. Biomarkers play a critical role in understanding potential mechanisms of action in patients, identifying patients who are likely to respond to the growing list of cancer therapeutics and avoiding the irAEs. Several tumor-derived biomarkers have been reported from recent correlative studies including PD-L1 expression on tumor cells, high tumor mutational load, and neoantigens. In addition, several immune cell-derived biomarkers showed better correlation with the clinical outcomes. The presence of TILs in tumor microenvironments, increased PD-L1 expression on immune cells, and the ratio of effector CD8+ T cells to FoxP3+ regulatory T cells in tumors are some examples of markers for clinical outcomes. Future studies are warranted to harmonize companion diagnostics (such as PD-L1 IHC) for accurate clinical assessment and application of the class of PD-1/PD-L1 inhibitors. It is of importance to further evaluate mutation load as a potential biomarker and develop effective tumor antigen-specific T cell assays to differentiate immunogenic neoepitopes from putative ones. The advances in translational immune biomarker research are essential for personalized cancer immunotherapy as either monotherapy or combinational therapy.
Abbreviations
AKT, protein kinase B; APCs, antigen-presenting cells; BRAF, B-raf and v-raf murine sarcoma viral oncogene homologue B1; BTLA, B- and T-lymphocyte attenuator; CAMLs, cancer-associated macrophage-like cells; CEACAM, carcinoembryonic antigen-related cell adhesion molecule; CTCs, circulating tumor cells; CTL, cytotoxic T-lymphocytes; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DC, dendritic cell; DLT, dose-limiting toxicity; EGFR, epidermal growth factor receptor; ELISPOT, enzyme-linked immunospot assay; FFPE, formalin-fixed paraffin-embedded; GITR, glucocorticoid-induced tumor necrosis factor receptor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA, human leukocyte antigen; HVEM, herpes virus entry mediator; ICOS, inducible costimulators; IDO, indoleamin 2,3-dioxygenase; IHC, immunohistochemistry; IL-2R, IL-2 receptor; irAEs, immune-related adverse effects; JNK, c-Jun N-terminal kinase; LAG, lymphocyte-activation gene; MDSCs, myeloid-derived suppressor cells; MEK/ERK, mitogen/extracellular signal regulated kinase; MHC, major histocompatibility complex; NGS, next-generation sequencing; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NSCLC, non-small-cell lung cancer; PBMCs, peripheral blood mononuclear cells; PD-1, programed death-1; PD-L1, programed cell-death ligand 1; PI3K, phosphatidylinositol 3-kinase; q2w, every 2 weeks; q3w, every 3 weeks; q4w, every 4 weeks; QD, once daily; STAT, signal transducer and activator of transcription; Teff, effector T cell; TGF-β, transforming growth factor beta; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TIL, tumor-infiltrating lymphocytes; TIM-3, T cell immunoglobulin domain and mucin domain; TNF, tumor necrosis factors; Treg, regulatory T cells; TSMA, tumor-specific mutant antigen; VEGF, vascular endothelial growth factor; WES, whole exome sequencing
ᅟ
ᅟ
Acknowledgements
None.
Funding
None.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Authors’ contributions
WM, JY, and TL contributed to the conception and design of the study. WM, JY, and TL carried out the development of methodology. WM, JY, and TL analyzed and interpreted the data. WM, BMG, JY, and TL wrote, reviewed, revised, and approved the final submission of the study. All authors read and approved the final manuscript.
Competing interests
JY owns a TCR sequencing patent with Adaptive Biotechnologies.
Consent for publication
Not applicable.
References
- 1.Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov. 2014;13(12):883–4. doi: 10.1038/nrd4476. [DOI] [PubMed] [Google Scholar]
- 2.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–7. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Smith AD, Roda D, Yap TA. Strategies for modern biomarker and drug development in oncology. J Hematol Oncol. 2014;7:70. doi: 10.1186/s13045-014-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pico de Coana Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med. 2015;21(8):482–91. doi: 10.1016/j.molmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffelt SB, de Visser KE. Immune-mediated mechanisms influencing the efficacy of anticancer therapies. Trends Immunol. 2015;36(4):198–216. doi: 10.1016/j.it.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015;36(4):250–6. doi: 10.1016/j.it.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goel G, Sun W. Advances in the management of gastrointestinal cancers—an upcoming role of immune checkpoint blockade. J Hematol Oncol. 2015;8:86. doi: 10.1186/s13045-015-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–7. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 14.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29(36):4828–36. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 16.Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1(1):11–5. doi: 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27(45):5894–903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- 19.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–49. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 20.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 21.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22(2):223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361–75. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2(2):234–40. doi: 10.1001/jamaoncol.2015.4368. [DOI] [PubMed] [Google Scholar]
- 25.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30(7):802–10. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169(10):5538–45. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 29.Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9(2):133–45. doi: 10.1080/713774061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, et al. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155(1-2):172–82. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daly ME, Monjazeb AM, Kelly K. Clinical trials integrating immunotherapy and radiation for non-small-cell lung cancer. J Thorac Oncol. 2015;10(12):1685–93. doi: 10.1097/JTO.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 34.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120(4):1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–41. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 38.Kanterman J, Sade-Feldman M, Biton M, Ish-Shalom E, Lasry A, Goldshtein A, et al. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014;74(21):6022–35. doi: 10.1158/0008-5472.CAN-14-0657. [DOI] [PubMed] [Google Scholar]
- 39.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110(1):296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 43.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5(8):860–77. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–23. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 46.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25(10):1935–40. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 47.Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–21. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 48.Lastwika KJ, Wilson W, 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–38. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 49.Lou Y, Diao L, Parra Cuentas ER, Denning WL, Chen L, Fan YH, et al. Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016. doi:10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed]
- 50.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915–9. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–70. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 53.Okiyama N, Katz SI. Programmed cell death 1 (PD-1) regulates the effector function of CD8 T cells via PD-L1 expressed on target keratinocytes. J Autoimmun. 2014;53:1–9. doi: 10.1016/j.jaut.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–66. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 55.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 57.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 58.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 59.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–96. [PubMed] [Google Scholar]
- 60.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai KK, Daud AI. Nivolumab plus ipilimumab in the treatment of advanced melanoma. J Hematol Oncol. 2015;8(1):123. doi: 10.1186/s13045-015-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol. 2014;25(12):2433–42. doi: 10.1093/annonc/mdu452. [DOI] [PubMed] [Google Scholar]
- 64.Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19(10):1223–34. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21(9):1065–77. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charlton JJ, Chatzidakis I, Tsoukatou D, Boumpas DT, Garinis GA, Mamalaki C. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol. 2013;190(12):6104–14. doi: 10.4049/jimmunol.1201617. [DOI] [PubMed] [Google Scholar]
- 67.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MERCK . FDA approves KEYTRUDA® (pembrolizumab) for the treatment of patients with metastatic non-small cell lung cancer whose tumors express PD-L1 with disease progression on or after platinum-containing chemotherapy. 2015. [Google Scholar]
- 69.Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, Ascierto PA, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9:214. doi: 10.1186/1479-5876-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Butterfield LH, Disis ML, Khleif SN, Balwit JM, Marincola FM. Immuno-oncology biomarkers 2010 and beyond: perspectives from the iSBTc/SITC biomarker task force. J Transl Med. 2010;8:130. doi: 10.1186/1479-5876-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bedognetti D, Balwit JM, Wang E, Disis ML, Britten CM, Delogu LG, et al. SITC/iSBTc cancer immunotherapy biomarkers resource document: online resources and useful tools—a compass in the land of biomarker discovery. J Transl Med. 2011;9:155. doi: 10.1186/1479-5876-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3. doi: 10.1186/s40425-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hansen AR, Siu LL. PD-L1 testing in cancer: challenges in companion diagnostic development. JAMA Oncol. 2016;2(1):15–6. doi: 10.1001/jamaoncol.2015.4685. [DOI] [PubMed] [Google Scholar]
- 74.Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scorer P, Walker J. Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16-20. New Orleans, LA Philadelphia (PA): AACR; 2016. A comparative study of PD-L1 diagnostic assays and the classification of patients at PD-L1 positive and PD-L1 negative [abstract] [Google Scholar]
- 75.Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58(3):449–59. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–8. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng Z, Puri S, Moudgil T, Wood W, Hoyt CC, Wang C, et al. Multispectral imaging of formalin-fixed tissue predicts ability to generate tumor-infiltrating lymphocytes from melanoma. J Immunother Cancer. 2015;3:47. doi: 10.1186/s40425-015-0091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 81.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9(12):4404–8. [PubMed] [Google Scholar]
- 82.Basu S, Campbell HM, Dittel BN, Ray A. Purification of specific cell population by fluorescence activated cell sorting (FACS). J Vis Exp. 2010(41): e1546. doi:10.3791/1546. [DOI] [PMC free article] [PubMed]
- 83.Zaritskaya L, Shurin MR, Sayers TJ, Malyguine AM. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev Vaccines. 2010;9(6):601–16. doi: 10.1586/erv.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105(51):20410–5. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Attig S, Price L, Janetzki S, Kalos M, Pride M, McNeil L, et al. A critical assessment for the value of markers to gate-out undesired events in HLA-peptide multimer staining protocols. J Transl Med. 2011;9:108. doi: 10.1186/1479-5876-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNeil LK, Price L, Britten CM, Jaimes M, Maecker H, Odunsi K, et al. A harmonized approach to intracellular cytokine staining gating: results from an international multiconsortia proficiency panel conducted by the cancer immunotherapy consortium (CIC/CRI) Cytometry A. 2013;83(8):728–38. doi: 10.1002/cyto.a.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vales-Gomez M, Reyburn HT, Erskine RA, Lopez-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999;18(15):4250–60. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maecker HT, Harari A. Immune monitoring technology primer: flow and mass cytometry. J Immunother Cancer. 2015;3:44. doi: 10.1186/s40425-015-0085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR, et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol. 2015;10(7):985–9. doi: 10.1097/JTO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 90.Gnjatic S, Ritter E, Buchler MW, Giese NA, Brors B, Frei C, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107(11):5088–93. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yuan J, Wang E, Fox BA. Immune monitoring technology primer: protein microarray (‘seromics’) J Immunother Cancer. 2016;4:2. doi: 10.1186/s40425-016-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inc. TFS . General information, technology overview, and applications using the ProtoArray® Human Protein Microarray. ProtoArray® Applications Guide. 2015. [Google Scholar]
- 93.Janetzki S, Price L, Schroeder H, Britten CM, Welters MJ, Hoos A. Guidelines for the automated evaluation of Elispot assays. Nat Protoc. 2015;10(7):1098–115. doi: 10.1038/nprot.2015.068. [DOI] [PubMed] [Google Scholar]
- 94.Malyguine AM, Strobl S, Dunham K, Shurin MR, Sayers TJ. ELISPOT assay for monitoring cytotoxic T lymphocytes (CTL) activity in cancer vaccine clinical trials. Cells. 2012;1(2):111–26. doi: 10.3390/cells1020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the cancer vaccine consortium (CVC/SVI) Cancer Immunol Immunother. 2008;57(3):303–15. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Butterfield LH, Buffo MJ. Immunologic monitoring of cancer vaccine trials using the ELISPOT assay. Methods Mol Biol. 2014;1102:71–82. doi: 10.1007/978-1-62703-727-3_5. [DOI] [PubMed] [Google Scholar]
- 97.Gabrielsson S, Brichard V, Dhellin O, Dorval T, Bonnerot C. IFN-gamma responses in peptide-treated melanoma patients measured by an ELISPOT assay using allogeneic dendritic cells. Anticancer Res. 2004;24(1):171–7. [PubMed] [Google Scholar]
- 98.Scheibenbogen C, Lee KH, Stevanovic S, Witzens M, Willhauck M, Waldmann V, et al. Analysis of the T cell response to tumor and viral peptide antigens by an IFNgamma-ELISPOT assay. Int J Cancer. 1997;71(6):932–6. doi: 10.1002/(SICI)1097-0215(19970611)71:6<932::AID-IJC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 99.Janetzki S. Immune monitoring technology primer: the enzyme-linked immunospot (Elispot) and fluorospot assay. J Immunother Cancer. 2015;3:30. doi: 10.1186/s40425-015-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robins HS, Ericson NG, Guenthoer J, O'Briant KC, Tewari M, Drescher CW, et al. Digital genomic quantification of tumor-infiltrating lymphocytes. Sci Transl Med. 2013;5(214):214ra169. doi: 10.1126/scitranslmed.3007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6(238):238ra270. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 103.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125(9):3413–21. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed]
- 108.Hoffmann S, Cepok S, Grummel V, Lehmann-Horn K, Hackermuller J, Stadler PF, et al. HLA-DRB1*0401 and HLA-DRB1*0408 are strongly associated with the development of antibodies against interferon-beta therapy in multiple sclerosis. Am J Hum Genet. 2008;83(2):219–27. doi: 10.1016/j.ajhg.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.International Multiple Sclerosis Genetics C, Wellcome Trust Case Control C, Sawcer S, Hellenthal G, Pirinen M, Spencer CC, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–9. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 111.Chen YY, Wang LB, Zhu HL, Li XY, Zhu YP, Yin YL, et al. Relationship between programmed death-ligand 1 and clinicopathological characteristics in non-small cell lung cancer patients. Chin Med Sci J. 2013;28(3):147–51. doi: 10.1016/S1001-9294(13)60040-1. [DOI] [PubMed] [Google Scholar]
- 112.Massi D, Brusa D, Merelli B, Falcone C, Xue G, Carobbio A, et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann Oncol. 2015;26(9):1980–7. doi: 10.1093/annonc/mdv255. [DOI] [PubMed] [Google Scholar]
- 113.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350(6266):1387–90. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin AY, Lin E. Programmed death 1 blockade, an Achilles heel for MMR-deficient tumors? J Hematol Oncol. 2015;8(1):124. doi: 10.1186/s13045-015-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19(6):747–52. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22(4):433–8. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–16. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yung S, Ledran M, Moreno-Gimeno I, Conesa A, Montaner D, Dopazo J, et al. Large-scale transcriptional profiling and functional assays reveal important roles for Rho-GTPase signalling and SCL during haematopoietic differentiation of human embryonic stem cells. Hum Mol Genet. 2011;20(24):4932–46. doi: 10.1093/hmg/ddr431. [DOI] [PubMed] [Google Scholar]
- 123.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci U S A. 2013;110(8):2946–51. doi: 10.1073/pnas.1222738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 129.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 130.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33(18):2013–20. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 135.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Daud A, Ribas A, Robert C, Hodi FS, Wolchok JD, Joshua AM, et al. Long-term efficacy of pembrolizumab (pembro; MK-3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE-001. ASCO Meeting Abstr. 2015;33(15_suppl):9005. [Google Scholar]
- 137.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–9. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 138.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 139.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Abstract S1-09: a phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Cancer Res. 2015;75(9 Supplement):S1–09. doi: 10.1158/1538-7445.SABCS14-S1-09. [DOI] [Google Scholar]
- 140.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2015;387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 141.Moskowitz C, Ribrag V, Michot J-M, Martinelli G, Zinzani PL, Gutierrez M, et al. PD-1 Blockade with the monoclonal antibody Pembrolizumab (M-3475) in patients with classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary results from a phase 1b study [abstract]. Blood. 2014;124:290. abstract.
- 142.Spigel DR, Chaft JE, Gettinger SN, Chao BH, Dirix LY, Schmid P, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1-selected patients with non-small cell lung cancer (NSCLC) ASCO Meeting Abstr. 2015;33(15_suppl):8028. [Google Scholar]
- 143.Spira AI, Park K, Mazieres J, Vansteenkiste JF, Rittmeyer A, Ballinger M, et al. Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR) ASCO Meeting Abstr. 2015;33(15_suppl):8010. [Google Scholar]
- 144.Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) ASCO Meeting Abstr. 2013;31(15_suppl):9010. [Google Scholar]
- 145.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu SV, Powderly JD, Camidge DR, Ready N, Heist RS, Hodi FS, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with platinum-based doublet chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstr. 2015;33(15_suppl):8030. [Google Scholar]
- 147.Bendell JC, Powderly JD, Lieu CH, Eckhardt SG, Hurwitz H, Hochster HS, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC) ASCO Meeting Abstr. 2015;33(3_suppl):704. [Google Scholar]
- 148.Sznol M, McDermott DF, Jones SF, Mier JW, Waterkamp D, Rossi C, et al. Phase Ib evaluation of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) in patients (pts) with metastatic renal cell carcinoma (mRCC) ASCO Meeting Abstr. 2015;33(7_suppl):410. [Google Scholar]
- 149.Rizvi NA, Brahmer JR, Ou S-HI, Segal NH, Khleif S, Hwu W-J, et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC) ASCO Meeting Abstr. 2015;33(15_suppl):8032. [Google Scholar]
- 150.Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A, Li X, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. ASCO Meeting Abstr. 2014;32(15_suppl):8021. [Google Scholar]
- 151.Segal NH, Ou S-HI, Balmanoukian AS, Fury MG, Massarelli E, Brahmer JR, et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. ASCO Meeting Abstr. 2015;33(15_suppl):3011. [Google Scholar]
- 152.Lutzky J, Antonia SJ, Blake-Haskins A, Li X, Robbins PB, Shalabi AM, et al. A phase 1 study of MEDI4736, an anti-PD-L1 antibody, in patients with advanced solid tumors. ASCO Meeting Abstr. 2014;32(15_suppl):3001. [Google Scholar]
- 153.Creelan BC, Chow LQ, Kim D-W, Kim S-W, Yeh T, Karakunnel JJ, et al. Safety and tolerability results from a phase I study of MEDI4736, a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib in patients (pts) with non-small-cell lung cancer (NSCLC) ASCO Meeting Abstr. 2015;33(15_suppl):3047. [Google Scholar]
- 154.Antonia SJ, Goldberg SB, Balmanoukian AS, Sanborn RE, Steele K, Narwal R, et al. Phase Ib study of MEDI4736, a programmed cell death ligand-1 (PD-L1) antibody, in combination with tremelimumab, a cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibody, in patients (pts) with advanced NSCLC. ASCO Meeting Abstr. 2015;33(15_suppl):3014. [Google Scholar]
- 155.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paz-Ares L, Horn L, Borghaei H, Spigel DR, Steins M, Ready N, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC) ASCO Meeting Abstr. 2015;33(18_suppl):LBA109. [Google Scholar]
- 157.Gettinger SN, Hellmann MD, Shepherd FA, Antonia SJ, Brahmer JR, Chow LQM, et al. First-line monotherapy with nivolumab (NIVO; anti-programmed death-1 [PD-1]) in advanced non-small cell lung cancer (NSCLC): safety, efficacy and correlation of outcomes with PD-1 ligand (PD-L1) expression. ASCO Meeting Abstr. 2015;33(15_suppl):8025. [Google Scholar]
- 158.Antonia SJ, Gettinger SN, Chow LQM, Juergens RA, Borghaei H, Shen Y, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: interim phase I results. ASCO Meeting Abstr. 2014;32(15_suppl):8023. [Google Scholar]
- 159.Rizvi NA, Garon EB, Leighl N, Hellmann MD, Patnaik A, Gandhi L, et al. Optimizing PD-L1 as a biomarker of response with pembrolizumab (pembro; MK-3475) as first-line therapy for PD-L1-positive metastatic non-small cell lung cancer (NSCLC): Updated data from KEYNOTE-001. ASCO Meeting Abstr. 2015;33(15_suppl):8026. [Google Scholar]
- 160.Garon EB, Gandhi L, Rizvi N, Hui R, Balmanoukian AS, Patnaik A, et al. Antitumor activity of pembrolizumab (pembro; mk-3475) and correlation with programmed death ligand 1 (pd-l1) expression in a pooled analysis of patients (pts) with advanced non–small cell lung carcinoma (NSCLC) Ann Oncol. 2014;25(suppl 4):LBA43. [Google Scholar]
- 161.Garon EB, Leighl NB, Rizvi NA, Blumenschein GR, Balmanoukian AS, Eder JP, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) ASCO Meeting Abstr. 2014;32(15_suppl):8020. [Google Scholar]
- 162.Horn L, Spigel DR, Gettinger SN, Antonia SJ, Gordon MS, Herbst RS, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study. ASCO Meeting Abstr. 2015;33(15_suppl):8029. [Google Scholar]
- 163.Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. ASCO Meeting Abstr. 2014;32(15_suppl):3002. [Google Scholar]
- 164.Loughlin PM, Cooke TG, George WD, Gray AJ, Stott DI, Going JJ. Quantifying tumour-infiltrating lymphocyte subsets: a practical immuno-histochemical method. J Immunol Methods. 2007;321(1-2):32–40. doi: 10.1016/j.jim.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 165.Carvajal-Hausdorf DE, Schalper KA, Neumeister VM, Rimm DL. Quantitative measurement of cancer tissue biomarkers in the lab and in the clinic. Lab Invest. 2015;95(4):385–96. doi: 10.1038/labinvest.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen KH, Yuan CT, Tseng LH, Shun CT, Yeh KH. Case report: mismatch repair proficiency and microsatellite stability in gastric cancer may not predict programmed death-1 blockade resistance. J Hematol Oncol. 2016;9(1):29. doi: 10.1186/s13045-016-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shafer-Weaver K, Rosenberg S, Strobl S, Gregory Alvord W, Baseler M, Malyguine A. Application of the granzyme B ELISPOT assay for monitoring cancer vaccine trials. J Immunother. 2006;29(3):328–35. doi: 10.1097/01.cji.0000203079.35612.c8. [DOI] [PubMed] [Google Scholar]
- 168.Barrera L, Rodriguez OA, Morales-Flores R, Garcia-Vicente A, Servín EM, Salinas-Parra F, et al. MINI 02.03 over expression of CD47, decrease of apoptosis and phagocytosis of neutrophils in advanced non-small cell lung cancer patients. J Thoracic Oncol. 2015;10(9_suppl 2):S266. [Google Scholar]
- 169.Streitz M, Miloud T, Kapinsky M, Reed MR, Magari R, Geissler EK, et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplant Res. 2013;2(1):17. doi: 10.1186/2047-1440-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Dongen JJ, Lhermitte L, Bottcher S, Almeida J, van der Velden VH, Flores-Montero J, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–75. doi: 10.1038/leu.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One. 2014;9(1):e78644. doi: 10.1371/journal.pone.0078644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kammula US, Serrano OK. Use of high throughput qPCR screening to rapidly clone low frequency tumour specific T-cells from peripheral blood for adoptive immunotherapy. J Transl Med. 2008;6:60. doi: 10.1186/1479-5876-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin SH, He J, Edelman M, Xu T, Gao H, Reuben J, et al. MINI 02.04 sequential assessment of DNA damage response and PD-L1 expression in circulating cells of lung cancer patients during treatment with radiotherapy. J Thoracic Oncol. 2015;10(9_suppl 2):S266–7. [Google Scholar]
- 174.Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, Bergan RC, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci U S A. 2014;111(9):3514–9. doi: 10.1073/pnas.1320198111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.


