Abstract
Background
Identifying immune markers in blood that are informative for breast cancer patient survival would not only be useful for prognosis but might also provide mechanistic insights into processes facilitating survival.
Methods
We phenotyped circulating plasmacytoid dendritic cells (pDCs), myeloid-derived suppressor cells (MDSCs) and regulatory T-cells in relation to T-cell responses to Her-2 in vitro in 75 untreated breast cancer patients 28–87 years of age at diagnosis.
Results
Patients with later stage tumors had lower levels of circulating pDCs (p = 0.008). There was a positive association between 5-year survival and higher than median levels of circulating pDCs (p = 0.03). We confirmed that 5-year survival correlated with CD8+ but not CD4+ T-cell responsiveness to Her-2 peptides in this cohort of younger and older patients (p = 0.04). Including pDCs in the analysis of previously-established parameters revealed that patients who had a CD8+ T-cell response to Her-2 together with a low ratio of MDSCs:pDCs had 100 % 5-year survival. High levels of pDCs and the presence of a CD8+ T-cell response to Her-2 were independent positive survival indicators according to multivariate Cox analysis.
Conclusions
Our new results suggest that circulating pDCs could be a positive prognostic indicator in breast cancer patients of all ages, together with the previously established CD8+ T-cell reactivity to Her-2 antigens in older patients only. These two prognostic indicators were independent and emphasize the important role of immunity in ensuring breast cancer patient survival, even in those not undergoing immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1186/s12967-016-0905-x) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, T-cells, Plasmacytoid dendritic cells, Myeloid derived suppressor cells, Regulatory T-cells, Her-2
Background
Dendritic cells (DCs) play an important role in the presentation of antigens to T-cells, but also exert immunoregulatory activity [1]. There are two main subsets of DCs, monocytic DCs (mDCs) that are generally CD11c+, and plasmacytoid DCs (pDCs), also known as natural interferon-producing cells (IPCs), that are CD123+ (IL-α3R) [1, 2]. mDCs produce IL-12 and express Toll-like receptor (TLR)-1, -2, -3, -4, -5, -6, -7 and 8, whereas pDCs produce interferon-α and express TLR-7, -9 and 10 [3–6]. Many studies have used DCs to target cancer therapeutically [7, 8] but work on pDCs in the context of cancer immunity has focused more on their role in the tumor microenvironment than on whether their presence in the peripheral blood has any prognostic relevance. Increased levels of pDCs in breast cancer bone metastases and key roles in tumor growth have been reported in mice [9], and tumor-infiltrating pDCs have been negatively correlated with survival in some human cancers [10, 11] including breast cancer [12]. In melanoma, patients with smaller tumors have higher levels of blood pDCs [10] and numbers of circulating pDCs are reduced in cancer patients [13], suggesting that recruitment into the tumor may deplete these cells from peripheral blood. In melanoma, low levels of circulating pDCs have a negative correlation with survival [14]. On the other hand, high levels of circulating myeloid-derived suppressor cells (MDSCs), heterogeneous populations of immature dendritic cells, macrophages and granulocytes [15–17], have a negative impact on survival in different cancers [18, 19]. Together with regulatory T-cells (Tregs), these suppressive cells can form a formidable barrier preventing immune anti-tumor activity in cancer [20].
We have previously reported that peripheral T-cell reactivity to certain tumor-associated antigens (TAAs) in melanoma correlates with a survival benefit [21, 22]. Similarly, in breast cancer, the presence or absence of peripheral CD8+ T-cell responses to Her-2 peptides in vitro influences survival as shown in a cohort of elderly patients, whereas this was not the case for CD4+ T cell responses because these were present in almost all patients [23]. Compared to antibody therapy which is dependent on surface antigen expression, vaccination might induce better protection through the induction of T-cells recognizing cancer cells even with levels of surface Her-2 expression too low for antibody targeting and which are often designated “Her2-negative” in biopsy immunochemistry analyses [24]. An effective way to induce both TAA-reactive CD4+ and CD8+ T-cell responses is by using synthetic long peptides (SLPs) [25, 26]. Antigen presentation by pDCs could contribute to the induction of specific CD4+ and CD8+ T-cell responses [27, 28], but this would be contrary to the findings discussed above implying that high levels of pDCs in the tumor and low levels in the blood have a negative prognostic impact. Thus, the present study focuses on investigating the prognostic relevance of circulating antigen-presenting cells including total DCs, mDCs and pDCs separately, together with functional Her-2-reactive T-cells assayed in vitro, and an assessment of the impact of immunosuppressive cells on 5-year survival of breast cancer patients. This study goes beyond our previous work not only in examining pDCs but in extending the age range of the patients to include younger as well as elderly subjects.
Methods
Patients
Blood from 75 patients (28–87 years) from the University Hospital Tübingen was drawn between March and November 2009. Peripheral blood mononuclear cells (PBMCs) were isolated using standard Ficoll–Hypaque gradient centrifugation and cryopreserved because they were also intended to be used in multi-center studies requiring cell shipment. Patients were recruited at first diagnosis, prior to any treatment, and this was one of the main inclusion criteria. Also, in this study patients diagnosed with breast cancer from all age groups with any stage of the disease were included and no exclusion criteria were considered. Clinico-pathological data were available for almost all patients. Approval for the study was obtained from the Institutional Ethics Committee of University Clinic Tübingen (71/2009BO2) and a waiver of informed consent was granted.
Phenotypic analysis of DCs, MDSCs and Tregs
PBMCs were thawed, washed and incubated with Gamunex and EMA, then stained with a cocktail of lineage (Lin) markers (CD3, CD19, CD56)-Brilliant Violet 605 (BioLegend, BD-Biosciences), CD14-Brilliant Violet711, CD11c-PE-Cy7 (BioLegend), CD45-V500, CD123-BV421, HLA-DR-PerCP-Cy5.5, CD15-FITC, CD11b-APC-Cy7, CD33-Alexa Fluor 700 (BD-Biosciences), and CD124-APC (R&D Systems) using EMA to identify dead cells.
To characterize Tregs, we used the same panel as before [23], staining PBMCs forCD3 (OKT3 supernatant) with Pacific Orange-conjugated secondary antibody (Invitrogen) followed by staining for CD4-Pacific Blue, CD45RA-Alexa Fluor-700, CD8-Peridinin-chlorophyll protein (PerCP), CD279-PerCP-Cy5.5, CD127-Alexa Fluor-647 (Bio legend), CD25-APC-Cy7 (BD Biosciences) and intracellular staining for FoxP3-PE (Bio legend). All samples were measured using a BD LSRII (BD Biosciences) immediately after staining.
Detection of TAA-reactive T-Cells
PBMCs were thawed, washed, counted and re-suspended in X-Vivo 15 medium supplemented with IL-4 (5 ng/ml) and IL-7 (5 ng/ml) on day 0. On d1, pooled 15-mer Her-2 peptides (PepMix, JPT Technologies, Berlin) were added at 1 µg/ml to 1 × 106 cells per culture. IL-2 (40U/ml) was added on d3. T-cells were harvested on d12 and re-stimulated (0.4–0.5 × 106 cells/well) with 1 µg/ml Her2 peptides or left un-stimulated as a negative control for 12 h. Pepmixes of influenza nucleoprotein and matrix protein were used as positive controls. Golgi-plug (BD-Biosciences) was added at 1 µl/ml to prevent cytokine secretion. The cells were harvested, washed, incubated with Gamunex and EMA, fixed and permeabilized with Cytofix/Cytoperm (BD-Biosciences) before staining with CD3-Pacific Orange (Invitrogen), CD4-Pacific Blue, TNF-FITC, IL-2-Alexa-Fluor-700, IL-5-PE (BioLegend), CD8-APC-Cy7, IFN-γ-PE-Cy7 (BD-Biosciences), IL-10-APC (Miltenyi-Biotec) and IL-17-PerCP-Cy5.5 (eBioscience). After washing, the cells were immediately measured using a BD-LSR-II flow cytometer with FACS-Diva software (BD-Biosciences).
Data analysis
Data were analyzed using FlowJo software (Tree-Star Inc.) after exclusion of duplicates using an FSC-area-versus-FSC-height/width plot for all flow cytometry datasets. To analyze DCs, CD45+ cells were gated on live cells, followed by gating the Lin(−) and CD14(−) cells. DCs were gated as HLA-DR(+) and then plasmacytoid [CD45(+)CD14(−)Lin(−)HLA-DR(+)CD123(+)] and myeloid [CD45(+)CD14(−)Lin(-)HLA-DR(+)CD11c (+)] DCs were gated from the HLA-DR(+) population. Further, MDSC populations were defined as Lin(−)CD14(+)HLA-DR(−) (MDSC-1) and Lin(−)CD14(+)CD124(+) (MDSC-2) as before [23]. CD45(+) cells were considered as the parental population for calculating the percentage of different subsets (see FACS plots and gating strategy in Additional file 1: Figure S1). Her-2-reactive T-cells producing cytokines in un-stimulated (negative control) samples compared to the stimulated samples were also gated as before using the same response criteria [23].
Statistics
Chi square and Mann–Whitney U tests were performed to compare independent groups and Kaplan–Meier analysis (log-rank test) for survival, using GraphPad Prism 6. SPSS software was used to perform multivariate Cox analysis. A value of p < 0.05 was taken as statistically significant.
Results
Patients’ characteristics
The study comprised 75 (28–87 year-old) patients with a median age of 69 years at the time of blood draw. The clinico-pathological characteristics of the patients are summarized in Additional file 2: Table S1.
DCs, MDSCs, Tregs and tumor characteristics
Neither the frequencies of total DCs nor mDCs in the peripheral blood were found to differ between patients at tumor stage 0, 1-versus-2, 3, 4 (data not shown). In contrast, patients at tumor stage 0 and 1 had significantly higher frequencies of pDCs than later stage patients (Fig. 1a, p = 0.008). Furthermore, the ratio of cells with the MDSC-1 phenotype to pDCs (Fig. 1b, p = 0.03), and the ratio of MDSC-2:pDCs (Fig. 1c, p = 0.02) were significantly higher in tumor stage 2, 3, 4. The other MDSC phenotypes were not informative (data not shown). The ratios of Tregs:pDCs (Fig. 1d, p = 0.02), activated Tregs:pDCs (Fig. 1e, p = 0.01) and FoxP3+ CD4+ T-cells:pDCs (Fig. 1f, p = 0.01) were also higher in tumor stage 2, 3, 4. Importantly, whether tumors were oestrogen receptor-positive or -negative, progesterone receptor-positive or -negative, or triple-negative did not influence the distribution of DCs or immunosuppressive cell types (data not shown).
Fig. 1.
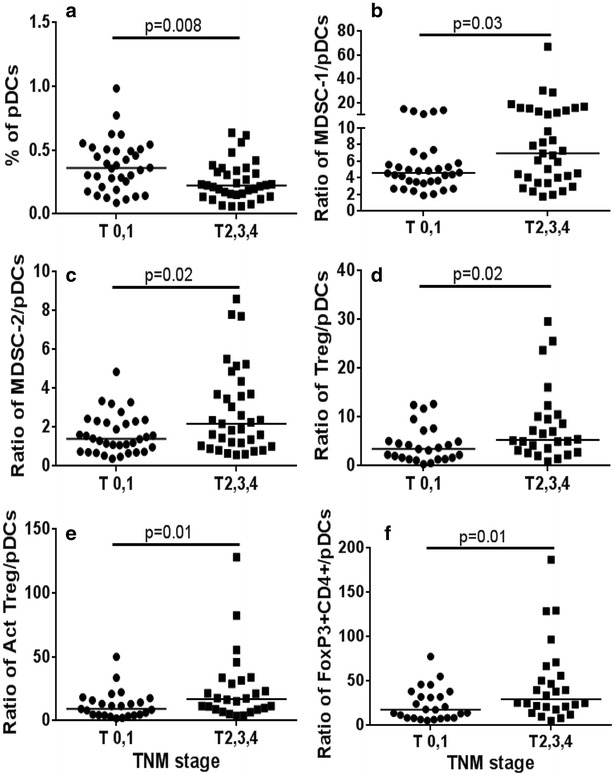
Distribution of DCs and ratio of DCs to immunosuppressive cells according to tumor stage. p values by Mann–Whitney U testfor (a) pDCs (b) MDSC/pDC (c) MDSC-2/pDCs (d) Treg/pDCs (e) aTreg/pDCs (f) FoxP3+ CD4+/pDCs
Level of DCs, immunosuppressive subsets and overall survival
Kaplan–Meier analysis performed after stratifying patients according to their median frequencies of total DCs, mDCs or pDCs indicated significant differences in 5-year survival. For non-metastatic patients, there were no significant correlations between higher than median levels of total DCs or mDCs at baseline and survival (data not shown), butpatients with higher levels of pDCs did have significantly better survival (Fig. 2a, p = 0.03). Overall 5-year survival was as high as 97 % for patients with high levels of pDCs, whereas it was only 77 % for those with low levels. This survival advantage was also observed when all patients (metastatic and non-metastatic) were considered together, although no longer reaching significance (Fig. 2b, p = 0.07).
Fig. 2.
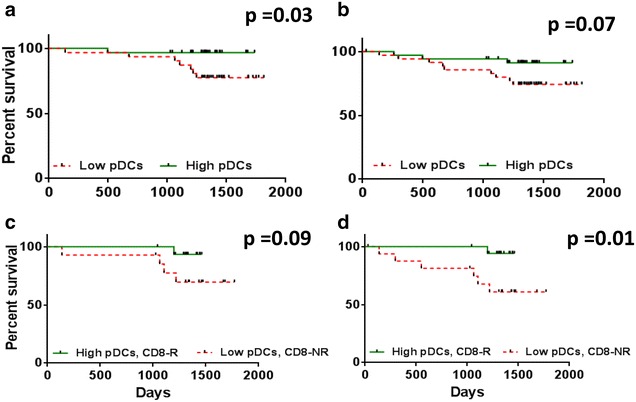
Kaplan–Meier survival analysis of non-metastatic (left) and all (right) patients according to the level of pDCs and for patients according to CD8+ T-cell response to Her-2 and level of DC subsets. Analysis of non-metastatic (a) and all patients (b) with low (≤median) versus high (>median) levels of DCs. Patients with CD8+ T-cell responses to Her-2 and high levels of pDCs compared to low levels of pDCs with no CD8+ T-cell response to Her-2, in c non-metastatic and d all patients
Although we did not observe any survival differences individually for the different immunosuppressive cells, there was some survival benefit when they were analysed together with the DCs. Thus, patients with a low ratio of MDSC-1:pDCs showed a trend towards better survival than those with a high ratio (p = 0.06) (Additional file 1: Figure S2A). This trend was also observed for the ratio of Tregs:DCs, where patients with low ratios of activated Tregs:total DCs (p = 0.07) (data not shown)and activated Tregs:mDCs (p = 0.07) (data not shown) tended to have better survival.
Survival advantage of patients with a CD8+ T-cell response to Her-2
The great majority of patients possessed T-cells responding to Her-2 peptides in vitro (97 %). Memory CD4+ T-cell responses to Her-2 were detected in 89 % (65/73), whereas CD8+ T-cell responses were detected in only 39/73 (53 %) of patients. Patients were stratified according to whether they mounted a CD8+ T-cell response to Her-2 (irrespective of whether they had a CD4+ T-cell response) for survival analysis. Patients with a CD8+ T-cell response to Her-2had significantly better survival (p = 0.04) (data not shown) validating our earlier data in a smaller cohort [23].
T-cell responses and tumor stage
We determined whether the proportion of patients possessing CD8+ T-cells responding to Her-2 differed depending on tumor stage. A significant decrease in the percentage of CD8+ T-cell responders to Her-2 was observed with increasing tumor stage, by Chi square testing (p = 0.02 for trend, Additional file 1: Figure S2B).
Survival advantage of patients with a CD8+ T-cell response to Her-2, according to their frequency of DCs
Patients were grouped according to their T-cell responses to Her-2 and their level of DCs. Kaplan–Meier analysis showed that there was no longer a significant survival benefit for those with a CD8+ T-cell response to Her-2 if they also had high levels of total DCs, both in the case of metastatic and non-metastatic patients (data not shown). Taking mDCs separately, however, non-metastatic patients with a CD8+ T-cell response to Her-2 tended to have better survival even when they also had high levels of these cells (Additional file 1: Figure S2C, p = 0.06), whereas those with a high level of mDCs but no CD8+ T-cell response to Her-2 had the worst survival. This difference was significant when all patients were included in the survival analysis (p = 0.02). For pDCs, there was a tendency for non-metastatic patients with high levels of pDCs together with a CD8+ T-cell response to Her-2 to have better survival than those with no CD8+ T-cell response to Her-2 and low levels of pDCs (Fig. 2c, p = 0.09). The survival rate was 93 % in patients with a high level of pDCs together with a CD8+ T-cell response to Her-2 compared to 70 % in patients with no CD8+ T-cell response to Her-2 and low levels of pDCs. This survival advantage was also observed when all patients, both metastatic and non-metastatic, were considered together (Fig. 2d, p = 0.01), where patients with high pDCs and CD8+ T-cell responses to Her-2 had a 94 % 5-year survival compared to only 61 % for patients with no CD8+ T-cell response to Her-2 and low levels of pDCs. Further, similar advantages were observed for non-metastatic (Additional file 1: Figure S3A, p = 0.07) and all patients (Additional file 1: Figure S3B, p = 0.03) for the low ratio of mDC:pDC together with a CD8+ T-cell response to Her-2.
Kaplan–Meier analysis showed that metastatic patients had poorer survival, as did those not receiving radiotherapy and chemotherapy, as expected (Table 1). Multivariate Cox analysis showed that lack of CD8+ T-cell response to Her-2 had an independent impact on survival, in addition to no radiotherapy (Table 2, Model-1). As shown earlier, non-metastatic patients with high levels of pDCs had significantly better survival than those with low levels, in Model-2 (Table 2). When metastatic patients were not included in the multivariate Cox analysis and only four factors (T-cell response to Her-2, pDCs, chemotherapy and radiotherapy) were considered, there was an independent impact on survival for patients with high levels of pDCs in addition to a CD8+ T-cell response to Her-2.
Table 1.
Kaplan–Meier analysis
| Factor | N | % Dead (5 years) | p |
|---|---|---|---|
| Triple negative | 0.57 | ||
| Yes | 13 | 23 | |
| No | 60 | 17 | |
| Her-2 status | 0.7 | ||
| Neg | 63 | 17 | |
| Pos | 9 | 22 | |
| Metastasis | <0.0001 | ||
| Yes | 7 | 57 | |
| No | 67 | 15 | |
| Radiotherapy | <0.0001 | ||
| Yes | 57 | 11 | |
| No | 17 | 47 | |
| Hormonal therapy | 0.08 | ||
| Yes | 60 | 17 | |
| No | 12 | 33 | |
| Chemotherapy | 0.0006 | ||
| Yes | 30 | 3 | |
| No | 42 | 31 | |
| CD8 responseto Her-2 | 0.04 | ||
| Yes | 39 | 13 | |
| No | 34 | 26 | |
| DCs | 0.2 | ||
| <Median | 36 | 22 | |
| ≥Median | 35 | 11 | |
| mDCs | 0.8 | ||
| <Median | 36 | 17 | |
| ≥Median | 35 | 17 | |
| pDCs | 0.07 | ||
| <Median | 36 | 25 | |
| ≥Median | 35 | 9 |
Model 1 & 2 show the two different Multivariate analysis considering different significant factors, with and without metastasis
Table 2.
Multivariate Cox analysis
| Prognostic factor | N | Dead over 5 years (%) | Hazard ratio (95 % CI) | p |
|---|---|---|---|---|
| Model-1 | ||||
| CD8 response to Her-2 | 0.198 (0.05–1.17) | 0.018 | ||
| Yes | 37 | 11 | ||
| No | 33 | 24 | ||
| Metastasis | 3.647 (0.89–14.88) | 0.071 | ||
| No | 32 | 15 | ||
| Yes | 42 | 57 | ||
| pDCs | 0.292 (0.07–1.17) | 0.083 | ||
| ≤Median | 36 | 25 | ||
| >Median | 34 | 9 | ||
| Radiotherapy | 0.107 (0.027–0.41) | 0.001 | ||
| Yes | 57 | 11 | ||
| No | 18 | 44 | ||
| Chemotherapy | <0.001 (<0.001–1.67e+130) | 0.941 | ||
| Yes | 30 | 3 | ||
| No | 42 | 31 | ||
| Model-2 | ||||
| CD8 response to Her-2 | 0.145 (0.02–0.81) | 0.029 | ||
| Yes | 34 | 12 | ||
| No | 28 | 21 | ||
| pDCs | 0.093 (0.011–0.809) | 0.031 | ||
| ≤Median | 31 | 23 | ||
| >Median | 31 | 3 | ||
| Radiotherapy | 0.091 (0.017–0.492) | 0.005 | ||
| Yes | 53 | 9 | ||
| No | 13 | 38 | ||
| Chemotherapy | <0.001 (<0.001–1.9e+157) | 0.949 | ||
| Yes | 30 | 3 | ||
| No | 34 | 26 | ||
Model 1 & 2 show the two different Multivariate analysis considering different significant factors, with and without metastasis
DCs, immunosuppressive subsets and cellular responses to Her-2
To investigate the influence of the presence of cells with a suppressive phenotype on the ability of patients’ PBMCs to mount a CD8+ T-cell response to Her-2, the frequencies of DCs, MDSCs and Tregs were grouped according to the presence or absence of the T-cell response. Patients without CD8+ T-cell responses to Her-2 had significantly higher levels of MDSC-1 (p = 0.01), activated Tregs (p = 0.03) and FoxP3+/CD4+ T-cells (p = 0.03) as well as a trend for MDSC-2 (p = 0.08) (data not shown). Patients with no CD8+ T-cell response to Her-2 had significantly higher ratios of MDSC-1:DCs (Fig. 3a, p = 0.02) and MDSC-1:mDCs (Fig. 3b, p = 0.02) again with a trend for MDSC-2:mDCs (Fig. 3c, p = 0.05). No differences were observed for ratios of MDSC-1:pDCs and MDSC-2:pDCs (Fig. 3d, e). Similar trends were observed for the ratio of Tregs:DCs (data not shown).
Fig. 3.
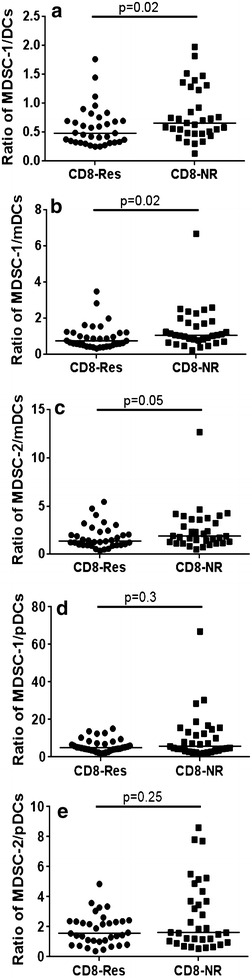
Percentage of immune subtypes in CD8-Res and CD8-NR. Patients with CD8+ T-cell responses to Her-2 (CD8-Res) compared to patients with no CD8+ T-cell responses (CD8-NR) with the ratio of a MDSC-1 to DCs (p = 0.02), b MDSC-1 to mDCs (p = 0.02), c MDSC-2 to mDCs, d MDSC-1 to pDCs and e MDSC-2 to pDCs
Prognostic relevance of DCs together with MDSCs, regulatory T-cells and CD8+ T-cell responses to Her-2
Because pDCs emerged in this analysis as having a significant impact on survival, we next analyzed associations between DCs and different immunosuppressive cells, in relation to T-cell responses to Her-2 peptides. After calculating the ratio of MDSCs:pDCs, we grouped the patients according to whether or not they mounted a CD8+ T-cell response to Her-2. Kaplan–Meier analysis showed that patients with a CD8+ T-cell response to Her-2 together with a low ratio of MDSC-1:pDCs had a very significantly better survival (Fig. 4a, log rank test: p = 0.009) and that there was an early impact on survival (Breslow test: p = 0.009) with 100 % survival compared to those with a high ratio of MDSC-1:pDCs and no CD8+ T-cell responses to Her-2. This strong survival advantage was still present when only non-metastatic patients were considered (Fig. 4b, p = 0.04). In contrast, no survival association was observed for levels of MDSC-2 and total DCs. Finally, all patients with a CD8+ T-cell response to Her-2 and with a low ratio of Tregs:pDCs (Additional file 1: Figure S4A, p = 0.03), activated Tregs:pDCs (Additional file 1: Figure S4B, p = 0.03) or FoxP3+ CD4+ T-cells:pDCs (Additional file 1: Figure S4C, p = 0.03) survived for the 5 year follow-up. Similar survival benefits were observed for total DCs and mDCs as well.
Fig. 4.
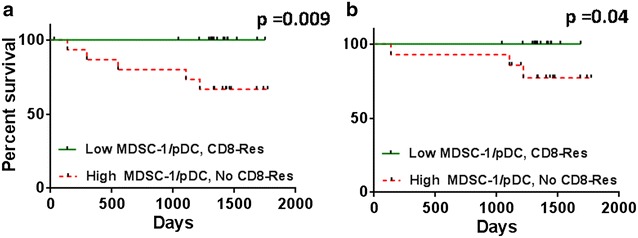
Kaplan–Meier survival analysis of all (left) and non-metastatic (right) patients according to CD8+ T-cell responses to Her-2 and ratios of MDSC-1 to pDCs. Kaplan–Meier analysis for patients with CD8+ T-cell responses to Her-2 and low ratios of MDSCs to pDCs compared to high ratios of MDSCs to pDCs with no CD8+ T-cell response to Her-2, in a all and b non-metastatic patients
Discussion
DCs are recognized as important players in cancer immunotherapy and pDCs have emerged as potential vectors for immunotherapy [27, 29]. So far, to the best of our knowledge, there have been no studies correlating the levels of circulating DCs with clinical outcome in breast cancer. In addition, no study has investigated the relationships between DCs and immunosuppressive cell subsets and their influence on T-cell responses to TAA in breast cancer. We previously showed that older patients having low levels of MDSCs and CD8+ T-cell responses to Her-2 survived longer than those with high levels of MDSCs and no CD8+ T-cell responses to Her-2. Here, we extend and validate these results in a larger cohort also including younger patients and for the first time report a leading role of circulating pDCs in survival correlates.
Recent studies in mice have shown that pDCs can effectively induce anti-tumor CD8+ T-cell responses [30], and that pDCs efficiently cross-present TAA to trigger T-cell responses [28]. In the present study, we asked if there was any correlation between survival, patients’ in vitro T-cell responses to Her-2 peptides, and frequencies of circulating DCs. We found that 97 % of patients mounted CD4+ T-cell responses to Her-2, but only half had CD8+ responses, which associated positively with 5-year overall survival. Here, we sought clinicopathological parameters distinguishing those patients with CD8+ T-cell responses from those without, as well as correlates with the impact of DCs. Increasing tumor stage was identified as negatively associating with the frequency of anti-Her-2 CD8+ T-cell responses. Although no differences were observed in the levels of total DCs and mDCs between tumor stage 0, 1 and 2, 3, 4, the frequencies of peripheral pDCs were lower in patients with larger tumors. This may suggest sequestration of pDCs at the tumor site. Similar findings have been reported by others in an earlier study on melanoma, where the migratory profile of pDCs together with their frequency suggested their capture at the tumor site and draining lymph node, resulting in depletion of circulating pDCs [10]. We found that the ratios of different immunosuppressive subsets to pDCs were higher in tumor stage 2, 3, 4 patients, reflecting low pDC frequencies at larger tumor stage and indicating a possible indirect role played by the immunosuppressive cell subsets on DCs. Kaplan–Meir analysis showed that there was a survival advantage for patients with high levels of pDCs and CD8+ T-cell responses to Her-2 compared to patients with low levels of pDCs and no CD8+ T-cell response to Her-2 peptides. Similar results were observed for patients with a low ratio of mDCs:pDCs and a CD8+ T-cell response to Her-2. From the multivariate Cox analysis, it was concluded that high levels of pDCs and the presence of CD8+ T-cell responses to Her-2 had an independent effect on survival.
Although there are many studies showing the impact of Tregs and MDSCs in cancer, their relationship with total DCs, pDCs and mDCs, TAA-specific T-cell responses, and clinical outcome has been largely unexplored. In our study, a trend towards better survival was observed in patients with a low ratio of immunosuppressive cells to DC. Previous studies have shown that Tregs can suppress pDCs by forming aggregates [31] and that mDCs are also sensitive to Tregs [32]. In the present study, consistent with this notion, patients with CD8+ T-cell responses to Her-2 and low Tregs:pDCs ratios had better survival compared to those with no CD8+ T-cell response to Her-2 and a higher ratio of Tregs:pDCs, suggesting a clinically relevant suppressive role of Tregs. Also, we observed that patients with no CD8+ T-cell response to Her-2 had significantly higher ratios of MDSC-1:total DCs, MDSC-1:mDCs and MDSC-2:mDCs, indicating that those with no in vitro CD8+ T-cell response to Her-2 probably had low levels of APCs. Importantly, every patient exhibiting a CD8+ T-cell response to Her-2 together with low ratios of MDSC-1:pDCs was still alive at 5 years. Furthermore, we observed a high ratio of MDSCs:pDCs in patients with larger tumor burden, also indicating a possible negative impact on the maturation of myeloid cells with increasing disease stage. MDSCs not only impair T-cell and NK cell function, but also DC-vaccine quality as reported in one study, where it was shown that high levels of MDSCs in DC cultures could affect the co-stimulatory molecules CD80 and CD86, and important molecules like CD1a and DC-sign [33]. In another study by other investigators, patients with a high ratio of DCs:MDSCs responded more favorably to high-dose IL-2 [34], indicating the potential general importance of high levels of DCs and low levels of MDSCs.
Few studies have focused on the importance of pDCs and their influence on mDCs and vice versa [35–37]. We observed that patients with low ratios of mDCs:pDCs, who also had a CD8+ T-cell response to Her-2, had better survival compared to those with high ratios of mDCs:pDCs and no CD8+ T-cell response to Her-2, again indicating the importance of pDCs. In our study, patients with high levels of mDCs and a CD8+ T-cell response to Her-2 had better survival compared to those with high levels of mDCs and no CD8+ T-cell response to Her-2. This might be due to defective mDCs lacking efficient antigen presentation capacity, which might in turn be due to the lack of sufficient pDCs in these patients that produce type I IFNs indirectly affecting the activation of mDCs. It is known that activated pDCs can stimulate adjacent mDCs by the production of type I IFNs that could enhance their ability to cross-prime CD8+ T-cells. This notion is supported by mouse studies showing that type I IFNs play an important role in the induction of anti-tumor responses [4, 38].
A previous clinical study on metastatic melanoma showed that activated pDCs injected into lymph nodes resulted in the induction of TAA-specific CD8+ T-cells, some of which had high functional activity. From the retrospective analysis in that study, it was observed that patients treated with autologous TLR-activated and tumor antigen-loaded pDCs had increased survival compared to patients treated with chemotherapy [27]. Also, I-IFNs derived from pDCs are known to regulate T cell function, which includes long term T-cell survival and memory Th1 polarization, CD8+ T-cell cytotoxicity and IFN-γ production [39]. pDCs exert their effect snot only on T-cells but on NK cells as well, and they are known to increase NK cell-mediated cytotoxicity and IFN-γ production [40]. Our findings in the current study indicate an important role of Her-2-specific T-cells in patients having higher levels of circulating pDCs. We observed either CD4+ T-cell responses to Her-2, or both CD4+ and CD8+ T-cell responses, in almost all patients, although the majority of tumors had low expression of this molecule according to routine pathology (Her-2-0, 1, 2). This is consistent with the benefit of Her-2 vaccination that patients may experience even when their resected tumors are classified as having low or no expression of Her-2, as reported in some studies [41, 42].
Conclusions
Our results from the present study emphasize the importance of the presence of high levels of circulating pDCs, low levels of immunosuppressive cells and the presence of CD8+ T-cell responses to Her-2 in predicting a favorable outcome in breast cancer patients both for all patients, and when only non-metastatic patients were considered. High levels of pDCs in blood, particularly in non-metastatic patients might reflect that they have not been sequestered by the tumor, hence there might be more pDCs available in the lymph node for TAA presentation, which in turn could enhance the induction of specific T-cells. On the other hand, the finding that high levels of pDCs and the presence of a CD8+ T-cell response to Her-2 were independent positive survival indicators according to multivariate Cox analysis suggests that mechanisms other than antigen presentation are responsible for the positive association of higher levels of circulating pDCs with survival in breast cancer. Regardless of the reason for these findings, the major observation from this study remains that circulating pDCs are positive prognostic indicators. Defining blood immune biomarkers informative for survival could not only enable prediction of an individual patient’s disease course without the need for biopsies, with all their limitations (limited access, unpleasant, potential triggering metastasis), but also provide information on the mechanisms mediating more successful cancer control by the immune system. Thus, measuring blood pDCs could represent part of a relatively simple blood test facilitating personalized interventions specifically tailored to the immune capacity of each individual patient.
Authors’ contributions
JKB performed assays, analyzed, interpreted data, and wrote the manuscript. BG collected the samples, obtained clinicopathological data, wrote and edited the manuscript. GP planned and coordinated the project, wrote and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Lilly Oettinger for technical help. This work was supported by the European Commission (FP7 259679 “IDEAL”) and the Bundesministerium für Bildung und Forschung (ISPE-BREAST, FKZ 01EI1401). We thank the Deutsche Forschungsgemeinschaft for supporting the Open Access Publishing Fund of the University of Tuebingen.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- Her-2
human epidermal growth factor receptor-2
- FoxP3
forkhead box P3
- IL
interleukin
- DCs
dendritic cells
- MDSC
myeloid derived suppressor cell
- TAAs
tumor associated antigens
- aTreg
activated regulatory T-cells
- MP
matrix protein
- NP
nucleoprotein
Additional files
10.1186/s12967-016-0905-x Representative FACS plots and additional results.
10.1186/s12967-016-0905-x Patients clinico-pathological parameters.
Contributor Information
Jithendra Kini Bailur, Phone: +49-7071-2981269, Email: jithendra.kini@gmail.com.
Brigitte Gueckel, Email: Brigitte.Gueckel@med.uni-tuebingen.de.
Graham Pawelec, Email: graham.pawelec@uni-tuebingen.de.
References
- 1.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1:442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 2.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 4.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, de Vries IJ. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1573–1582. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Zaidi N, He LZ, Zhang L, Kuroiwa JM, Keler T, Steinman RM. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012;14:R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, Schlesinger SJ, Steinman RM. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012;271:183–192. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–4265. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1:402–415. doi: 10.1158/2326-6066.CIR-13-0114-T. [DOI] [PubMed] [Google Scholar]
- 11.Labidi-Galy SI, Treilleux I, Goddard-Leon S, Combes JD, Blay JY, Ray-Coquard I, Caux C, Bendriss-Vermare N. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology. 2012;1:380–382. doi: 10.4161/onci.18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A, Goddard S, Pin JJ, Barthelemy-Dubois C, Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 13.Sciarra A, Lichtner M, Autran GA, Mastroianni C, Rossi R, Mengoni F, Cristini C, Gentilucci A, Vullo V, Di Silverio F. Characterization of circulating blood dendritic cell subsets DC123+ (lymphoid) and DC11C+ (myeloid) in prostate adenocarcinoma patients. Prostate. 2007;67:1–7. doi: 10.1002/pros.20431. [DOI] [PubMed] [Google Scholar]
- 14.Chevolet I, Speeckaert R, Schreuer M, Neyns B, Krysko O, Bachert C, Van Gele M, van Geel N, Brochez L. Clinical significance of plasmacytoid dendritic cells and myeloid-derived suppressor cells in melanoma. J Transl Med. 2015;13:9. doi: 10.1186/s12967-014-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20:1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 20.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 21.Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries IJ, Sucker A, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol. 2012;30:1835–1841. doi: 10.1200/JCO.2011.40.2271. [DOI] [PubMed] [Google Scholar]
- 22.Zelba H, Weide B, Martens A, Derhovanessian E, Bailur JK, Kyzirakos C, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, et al. Circulating CD4+ T cells that produce IL4 or IL17 when stimulated by melan-A but not by NY-ESO-1 have negative impacts on survival of patients with stage IV melanoma. Clin Cancer Res. 2014;20:4390–4399. doi: 10.1158/1078-0432.CCR-14-1015. [DOI] [PubMed] [Google Scholar]
- 23.Bailur JK, Gueckel B, Derhovanessian E, Pawelec G. Presence of circulating Her2-reactive CD8+ T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. 2015;17:34. doi: 10.1186/s13058-015-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B. Targeting dendritic cells in situ for breast cancer immunotherapy. Oncoimmunology. 2012;1:1398–1400. doi: 10.4161/onci.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 26.Kovjazin R, Volovitz I, Kundel Y, Rosenbaum E, Medalia G, Horn G, Smorodinsky NI, Brenner B, Carmon L. ImMucin: a novel therapeutic vaccine with promiscuous MHC binding for the treatment of MUC1-expressing tumors. Vaccine. 2011;29:4676–4686. doi: 10.1016/j.vaccine.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 27.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 28.Aspord C, Leloup C, Reche S, Plumas J. pDCs efficiently process synthetic long peptides to induce functional virus- and tumour-specific T-cell responses. Eur J Immunol. 2014;44(40):2880–2892. doi: 10.1002/eji.201444588. [DOI] [PubMed] [Google Scholar]
- 29.Aspord C, Leccia MT, Salameire D, Laurin D, Chaperot L, Charles J, Plumas J. HLA-A(*)0201(+) plasmacytoid dendritic cells provide a cell-based immunotherapy for melanoma patients. J Invest Dermatol. 2012;132:2395–2406. doi: 10.1038/jid.2012.152. [DOI] [PubMed] [Google Scholar]
- 30.Schlecht G, Garcia S, Escriou N, Freitas AA, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–1815. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- 31.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+ CD25+ high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 33.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2012;61:827–838. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelstein SE, Carey T, Fricke I, Yu D, Goetz D, Gratz M, Dunn M, Urbas P, Daud A, DeConti R, et al. Changes in dendritic cell phenotype after a new high-dose weekly schedule of interleukin-2 therapy for kidney cancer and melanoma. J Immunother. 2010;33:817–827. doi: 10.1097/CJI.0b013e3181ecccad. [DOI] [PubMed] [Google Scholar]
- 35.Piccioli D, Sammicheli C, Tavarini S, Nuti S, Frigimelica E, Manetti AG, Nuccitelli A, Aprea S, Valentini S, Borgogni E, et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood. 2009;113:4232–4239. doi: 10.1182/blood-2008-10-186890. [DOI] [PubMed] [Google Scholar]
- 36.Lozza L, Farinacci M, Fae K, Bechtle M, Staber M, Dorhoi A, Bauer M, Ganoza C, Weber S, Kaufmann SH. Crosstalk between human DC subsets promotes antibacterial activity and CD8+ T-cell stimulation in response to bacille Calmette–Guerin. Eur J Immunol. 2014;44:80–92. doi: 10.1002/eji.201343797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178:1534–1541. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 38.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 41.Mittendorf EA, Clifton GT, Holmes JP, Clive KS, Patil R, Benavides LC, Gates JD, Sears AK, Stojadinovic A, Ponniah S, Peoples GE. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2012;118:2594–2602. doi: 10.1002/cncr.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneble EJ, Berry JS, Trappey FA, Clifton GT, Ponniah S, Mittendorf E, Peoples GE. The HER2 peptide nelipepimut-S (E75) vaccine (NeuVax) in breast cancer patients at risk for recurrence: correlation of immunologic data with clinical response. Immunotherapy. 2014;6:519–531. doi: 10.2217/imt.14.22. [DOI] [PubMed] [Google Scholar]


