Abstract
Hypothesis
Gene expression changes occur in conjunction with hearing threshold changes after cochlear implantation.
Background
Between 30–50% of individuals who receive electro-acoustic stimulation (EAS) cochlear implants lose residual hearing after cochlear implantation, reducing the benefits of EAS. The mechanism underlying this hearing loss is unknown; potential pathways include mechanical damage, inflammation, or tissue remodeling changes.
Methods
Guinea pigs were implanted in one ear with cochlear implant electrode arrays, with non-implanted ears serving as controls, and allowed to recover for 1, 3, 7, or 14 days. Hearing threshold changes were measured over time. Cochlear ribonucleic acid was analyzed using real-time quantitative reverse transcription-polymerase chain reaction from the following gene families: cytokines, tight junction claudins, ion and water (aquaporin) transport channels, gap junction connexins, and tissue remodeling genes.
Results
Significant increases in expression were observed for cochlear inflammatory genes (Cxcl1, IL-1b, TNFα and Tnfrsf1a/b) and ion homeostasis genes (Scnn1γ, Aqp3 and Gjb3). Upregulation of tissue remodeling genes (TGF-β, MMP2, MMP9) as well as a paracrine gene (CTGF) was also observed. Hearing loss occurred rapidly, peaking at 3 days with some recovery at 7 and 14 days after implantation. MM9 exhibited extreme upregulation of expression and was qualitatively associated with changes in hearing thresholds.
Conclusion
Cochlear implantation induces similar changes as middle ear inflammation for genes involved in inflammation and ion and water transport function, whereas tissue remodeling changes differ markedly. The upregulation of MMP9 with hearing loss is consistent with previous findings linking stria vascularis vessel changes with cochlear implant-induced hearing loss.
Keywords: Cochlear implants, Gene expression, Hearing loss, Ion homeostasis, Tissue remodeling, Inflammation response, Guinea pig model, Electro-acoustic stimulation
1. Introduction
The Hybrid or Electro-Acoustic Stimulation (EAS) cochlear implant (CI ), is shorter and thinner than a traditional CI and enables preservation of low-frequency residual hearing for combined acoustic and electric stimulation in the same ear (1,2). Compared to traditional, full-insertion CIs, EAS CIs dramatically improve speech perception in noise, voice recognition, and musical melody recognition (3–5). In addition, hearing preservation leads to superior speech perception outcomes even when the CI is used without the acoustic hearing, further indicating the importance of minimizing damage to neurosensory structures for effective electrical stimulation (6,7). However, between 30–55% of patients lose more than 30 dB of residual hearing within days to months after implantation, indicating a pressing need to improve hearing preservation rates and allow full usage of these benefits (8–10).
The exact mechanism of implantation-induced hearing loss is not yet clear. Proposed mechanisms include direct mechanical trauma to the basilar membrane or osseous spiral lamina (11–13), or an inflammatory or immune response leading to hair cell death (14). However, significant hearing loss can occur after cochlear implantation without evident mechanical trauma or hair cell loss (15,16). Fibrosis or osteogenesis after implantation can also theoretically cause hearing loss by attenuating the traveling wave (17); however, correlations between fibrosis/ossification and hearing loss are weak and require large numbers of animals to show significance (15,16). The lateral wall may also be vulnerable to damage as due to its location in the path of the electrode insertion (18). Recent studies showed a correlation of reduced stria vascular blood vessel density with hearing loss after cochlear implantation, suggesting that a reduced ability to maintain the endocochlear potential may cause hearing loss (15). Another possibility is that electrical stimulation and excessive current levels may also damage residual hearing via excitotoxic damage to afferent nerve terminals (19–20), especially with delayed hearing loss occurring months after implantation.
Gene expression changes have not been previously investigated in a cochlear implant animal model, and may help to clarify whether the following factors and pathways – inflammation and hair cell loss, fibrosis and other tissue remodeling changes, or damage to ion homeostasis mechanisms - are responsible for the hearing loss.
Genes associated with inflammatory reactions in response to surgical trauma include pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β). When added to cochlear explants, TNF- α leads to hair cell death in vitro (21–22); its effects can be reversed by dexamethasone administration (23). Interestingly, TNF-α is localized not just in the organ of Corti, but also to Type I fibrocytes and root cells in the spiral ligament where it mediates capillary constriction and reduction of cochlear blood flow (24–25). TNF-α and IL-1β are up-regulated with middle ear inflammation (26–27); TNF-α is also upregulated after noise exposure (28). Other inflammation related genes include chemokine (C-X-C motif) ligand 1 (CxCl1), tumor necrosis factor receptor superfamily member 1a and 1b (Tnfrf1a, Tnfrf1b), and TNF receptor-associated factor-2 and -4 (Traf2, Traf4). Cxcl1 is up-regulated with middle ear inflammation (26–27); Tnfr1a, Tnfr1b, and Traf4 are up-regulated after noise-exposure induced apoptosis (28).
Genes associated with fibrosis and osteogenesis responses as part of the wound healing process include growth factors and tissue remodeling genes. Transforming growth factor β (TGF-β) is upregulated in fibrotic disease (29) and regulates fibroblast growth factors (FGFs) and fibroblast growth factor receptors (FGFRs). FGFs are involved in extracellular matrix remodeling, i.e. regulate bone resorption and formation by osteoblasts (30), and in turn stimulate interleukins (IL-6) and matrix metalloproteinases (MMPs; 31); FGF-2 has an additional role in modulating vascular tone (30). Connective tissue growth factor (CTGF) is an autocrine/paracrine growth factor localized to the Type IV fibrocytes of the spiral ligament (32). Vascular endothelial growth factor (VEGF) is upregulated with shear stress on vascular endothelial cells and may contribute to tissue remodeling and angiogenesis (33).
Tissue remodeling genes include bone morphogenetic proteins (BMPs) and the MMPs, zinc-dependent proteolytic enzymes that degrade extracellular matrix molecules and initiate the wound repair process. MMP2 and MMP9 are upregulated along with pro-inflammatory cytokines (TNF-β, IL-1β) in the heart during intrauterine hypoxia (hypoxic stress) (34–35), and have a role in blood vessel/basement membrane degradation/remodeling in the strial capillary basement membrane (36).
Genes associated with ion homeostasis include genes involved with ion channel regulation, water homeostasis, and gap junction proteins involved in potassium recycling and transport. Amiloride-sensitive sodium channel subunit alpha (Scnn1a) regulates sodium channel gene expression and is upregulated with inflammation in the middle ear (26). The aquaporin family of genes forms membrane pores involved in active water transport to maintain osmotic equilibrium in epithelial cells. Aquaporins 1 and 3 (aqp1, aqp3) are localized to the stria vascularis, spiral ligament, organ of Corti, and spiral ganglion (37), and aqp1 is expressed specifically by Type III fibrocytes (38). With middle ear inflammation, aqp1 and aqp5 are downregulated while aqp3 is upregulated 26). Claudins (e.g. cldn3) maintain tight junctions and prevent intercellular leakage of solutes and ions, and gap junction proteins are conduits for K+ movement in the recycling pathway (39). Gap junction protein Cx31 (Gjb3) is reduced with aging in the spiral ligament Type I and II fibrocytes and basal and intermediate cells in stria vascularis (40).
These genes are all part of an interconnected gene network, with expression in one part of the network potentially influencing the other parts. For instance, inflammatory cytokines also regulate the tissue remodeling and gap junction proteins (41). In order to clarify which pathways might be involved in hearing loss after cochlear implantation, we measured changes in gene expression of these various pathways in conjunction with hearing thresholds over time in a guinea pig cochlear implant model.
2. Methods
2.1. Subjects
Twelve male albino Dunkin-Hartley guinea pigs (body weight 300–350 grams) were divided into 4 groups (n=3 per group), in which gene expression was examined at various times after surgery: 1 day, 3 days, 7 days, and 14 days. Baseline auditory brainstem response (ABR) measurements were conducted in all animals. At 5 weeks of age, all groups underwent cochlear implantation surgeries in the left ear. The 1-day and 3-day groups underwent final ABR testing at 1 and 3 days post-surgery, respectively, immediately before tissues were collected. The 7-day and 14-day groups underwent ABR testing at multiple time points: the 7-day group was tested at 1, 3, and 7 days post-surgery, and the 14-day group was tested at 1, 3, 7, and 14 days post-surgery. After final ABR testing, animals in each group were sacrificed and cochlear tissues harvested for gene expression analysis. All animal procedures in the study were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee (Protocol MS15_IS00000672).
2.2. Auditory Brainstem Response (ABR) Testing
All ABR testing was performed in a soundproof booth. Prior to the testing, guinea pigs were anesthetized with ketamine (30mg/kg) and xylazine (5 mg/kg) administrated via intramuscular injection. Subcutaneous needle electrodes were placed at the vertex (noninverting), below the ipsilateral pinna near the cheek (inverting), and below the contralateral pinna near cheek (ground). Test stimuli consisted of alternating phase tone bursts at frequencies of 1, 2, 4, 8, 16, and 32 kHz. Signals were presented using an RX6 D/A converter and PA5 programmable attenuator (Tucker-Davis Technologies, Alachua, FL), amplifier (Crown D35, Elhart, IN), and speaker (Vifa, Madisound, Middleton, WI). The speaker was placed 10 cm from the ipsilateral external auditory meatus, and the contralateral ear was plugged with a silicon earplug. The animals’ evoked responses were amplified with a gain of 5000 and bandpass filtered from 100 to 3000 Hz using a Model 5113 preamplifier (Signal Recovery, Wokingham, UK). Responses to 300 sweeps were averaged at each stimulus level. Threshold was determined by decreasing the test signal level in 5-dB steps from 90 dB sound pressure level (SPL) to the lowest level that evoked a detectable and repeatable Wave III response.
2.3. Surgical Procedures
Each animal was anesthetized with ketamine (60 mg/kg) and xylazine (5 mg/kg) by intramuscular injection, with supplemental doses given as needed. Rectal temperature was monitored and controlled with a heating pad and feedback loop temperature controller. Electrocardiograms, breath rate and SPO2 were recorded by a veterinary monitor. The surgery area was shaved and aseptically prepared with Betadine. Local anesthesia (lidocaine 2 mg/kg) was injected subcutaneously along the intended incisions.
Through a left post-auricular incision, the left bulla was exposed and opened to gain access to the round window niche. A cochleostomy was made just inferior to the round window by a 0.5 mm diamond burr at a slow rotation speed (800 rpm) to limit the acoustic trauma. An 8-electrode CI electrode array designed for guinea pigs (HL8, Cochlear Inc., Australia) was inserted into the scala tympani until the white mark reached the edge of the cochleostomy, corresponding to an insertion depth of 7 mm. The electrode materials are similar to those in human electrode arrays. The cochleostomy was sealed with a small piece of muscle fascia and the skin incision was closed with 5–0 sutures.
2.4. Tissue collection and RNA extraction
At 1 day, 3 days, 7 days, and 14 days after cochlear implantation surgery for the 1-day, 3-day, 7-day, and 14-day groups, respectively, animals were deeply anesthetized by excess xylazine (10 mg/kg) and decapitated. The cochleae were quickly removed and perfused through the round window with RNAlater (Qiagen, Valencia, CA, USA) to preserve RNA. The cochleae were carefully dissected in RNAlater. The cochlear sensory epithelium (organ of Corti, OC) and lateral wall (stria vascularis together with spiral ligament, SV+SL) were collected. Cochlear tissue from the surgery side (left) or control side (right) were put into liquid nitrogen separately as one sample and then stored at −80°C until RNA was extracted.
Tissue total RNA was extracted using the RNeasy Mini Kit (Qiagen) after tissue homogenization as per manufacturer’s protocols. Total RNA of OC and SV+SL tissue from one cochlea was pooled for one sample each. RNase-Free DNase kit was used to remove DNA contamination. RNA quantity and quality were evaluated with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). All samples demonstrated good quality RNA (A260/A280 > 1.8).
2.5. Quantitative RT-PCR Analyses
After total RNA extraction, first-strand cDNA was synthesized using 500 ng total RNA from each sample and Oligo-dT primers synthesized by reverse transcription (RT2 First Strand Kit, SABiosciences Corp., Frederick, MD, USA). Then, cDNA product was prepared for real-time PCR using the RT2 SYBR Green/ROX qPCR Master Mix (Qiagen). Primers were designed using OLIGO Primer Analysis Software, version 7 (42; Molecular Biology Insights, Inc., Cascade, CO, USA; Table 1). The gene sequences of each target were downloaded from Genebank. Primers were then tested by reverse-transcription PCR and electrophoresis. PCR primers were custom-synthesized by Integrated DNA Technologies (Coralville, USA).
Table 1.
Primers used in the RT-PCR.
| Target | Accession No. | Sequences (5′-3′) (S: Sense, A: Anti-sense) | Product length(bp) |
|---|---|---|---|
| TGFβ1 | NM_001172968.1 | S: CTCCACGACATACAGCATA A: CGGGCATTTTGTTATCACT |
145 |
| CTGF | XM_003468462.1 | S: GAGCGCCTGTTCCAAGACCT A: AGCCAGACAGCTCGAACTTGACA |
200 |
| FGF2 | XM_003476776.1 | S: GCGAACCGTTACCTTGCT A: CGTTTCAGTGCCACATACCAA |
152 |
| BMP3 | XM_003477672.1 | S: AATTGTGCCAGGAGATACCTT A: CACCGCTCTCACTATGCTCT |
174 |
| VEGF | M84230 | S: AGTATATCTTCAAGCCGTCCTG A: GGAGGAAACTCATCTCTCCGA |
162 |
| HIF1a | XM_003472501.1 | S: GCCACTTCCCCACAATGTGA A: GTCACCATCATCAGTAAGCACC |
193 |
| MMP2 | XM_003477541.1 | S: GATGCCTGGAATGCCATCCCT A: ATGCTTCCAAACTTCACGCTCT |
137 |
| MMP9 | NM_001173023.1 | S: TGTGCCTGACCCTCTGTTGCT A: ACAACCACTCTGGGGTATCACT |
161 |
| Cxcl1 | NM_001172938.1 | S: CCAAGAACATCCAGAGCGTA A: GACTTTCTGCACCATGGGA |
128 |
| IL-1b | NM_001172968 | S: CTCCACGACATACAGCATA A: CGGGCATTTTGTTATCACT |
145 |
| TNFα | NM_001173025.1 | S: TGGCCCAGACGCTCACAC A: ATGAGGTACAGCCCATCCGAA |
190 |
| Tnfrsf1a | XM_003463287.1 | S: CGCCCTGACCCTGTAATTAAGCC A: GATGCCTTCAAGCTCGCCCTC |
198 |
| Tnfrsf1b | XM_003471198.1 | S: GGTCAATGTCACCTGCATCGT A: TGCTCATCCTTCGAGGCACT |
129 |
| Traf2 | XM_003473106.1 | S: AGCCTTCTACACAAGCCGGTA A: TCGAGCAGCATCAGTGTCACC |
171 |
| Traf4 | XM_003469720.1 | S: GCTATGATGTGTGCCCTGGTG A: AGCACTCCAGGTTAGGCTT |
163 |
| Scnn1γ | NM_001173064.1 | S: GCCCAAGTTCCTCAACACGAT A: TTCCCACTGACTTTCCGCTTC |
102 |
| Aqp1 | XM_003467867.1 | S: CGAGTTCTTGGCTATGACCCT A: TGACACCTTCACGTTGTCCT |
118 |
| Aqp3 | XM_003470832.1 | S: CATCTTTGCCACCTACCCCTC A: GTGCCGATGACAAGGACCAC |
177 |
| Aqp5 | XM_003475791.1 | S: CTGGCTGCTCCATGAACCC A: CTCATATGTGCCCTTGACGAT |
146 |
| Cldn3 | XM_003470014.1 | S: ACGGCCTTCATCGGGAGCAAC A: CGACAGCGCCAGCATCGAGT |
126 |
| Gja1 | NM_001172748.1 | S: TGCTCTACCTGGCACACGTCT A: ACCTTGCCATGCTCCTCGATG |
166 |
| Gjb3 | XM_003471487.1 | S: CAATCTCCAACATCCGCCTCT A: CCACAGGCCACCATGCTTT |
176 |
| β-Actin | NM_001172909.1 | S: GCGTGACATCAAGGAGAA A: GCCACAGGATTCCATACC |
193 |
| GAPDH | NM_001172951.1 | S: TGCTGATGCCCCTATGTTCGT A: GTCCCTCCACAATGCCGAAG |
146 |
A StepOnePlus Real-Time PCR System (SABiosciences Corp.) was used to run each sample in triplicate. Negative controls without a template were included in every PCR run. Thermal cycle conditions were set as follows: 95°C 10 min, then 40 cycles: 95°C 15 sec, 60°C 1 min followed by a melt curve. The amplification efficiency was estimated. Two house-keeping genes, β-actin and glyceraldehyde-3-phosphate dehydrogenase were chosen as the endogenous reference set for all samples. The relative gene-expression of every target gene was calculated using the ΔΔCt method (43–44). The results were expressed as a fold change in gene expression in the surgery side relative to the control side.
2.6. Statistical analysis
A 2-way analysis of variance (ANOVA) was employed to assess differences in ABR thresholds (group × frequency) using SPSS software (Version 19.0, IBM). Relative expression software, REST 2009 (Qiagen) was used to calculate the relative expression ratios on the basis of group means for target genes versus the housekeeping gene set(45). REST software also tested the group ratio results for significance using the randomization test (46). Statistical significance was based on p values < 0.05.
3. Results
3.1. ABR threshold shifts over time after cochlear implantation surgery
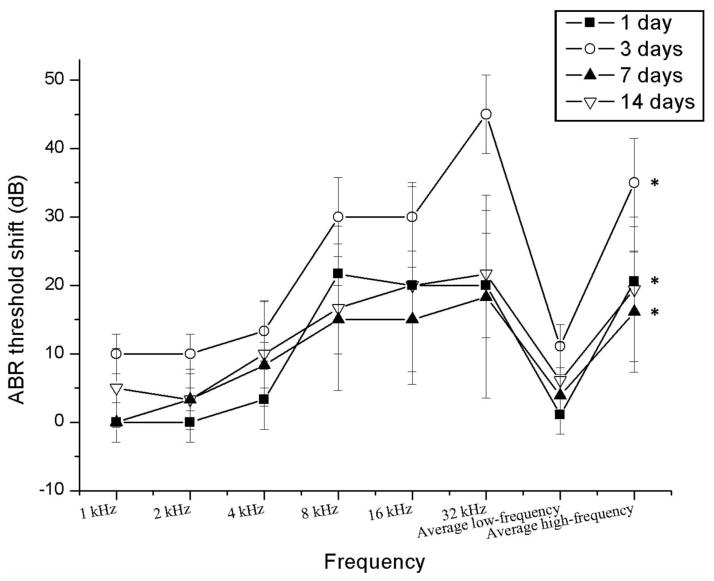
Average post-surgery ABR threshold shifts relative to baseline ABRs are shown for each frequency and group in Figure 1. All groups experienced significant increases in hearing threshold at high frequencies in the implanted ear after surgery at 1, 3 and 7 days. Also note that there was a peak threshold shift at 3 days post-surgery with a slight recovery at 7 and 14 days post-surgery. No threshold shifts were observed for the control/non-implanted ear.
Figure 1.
Average ABR threshold shifts for each group for low frequencies (pooled across 1, 2 and 4 KHz) and high frequencies (pooled across 8, 16 and 32 KHz) (mean ± SEM, * P<0.05) 1 day: t=−6.417, p=0.000; 3 days: t=−5.759, p=0.000; 7 days: t=−2.275, p=0.046; 14 days: t=−1.937, p=0.076.
3.2. RT-PCR expression changes over time after surgery
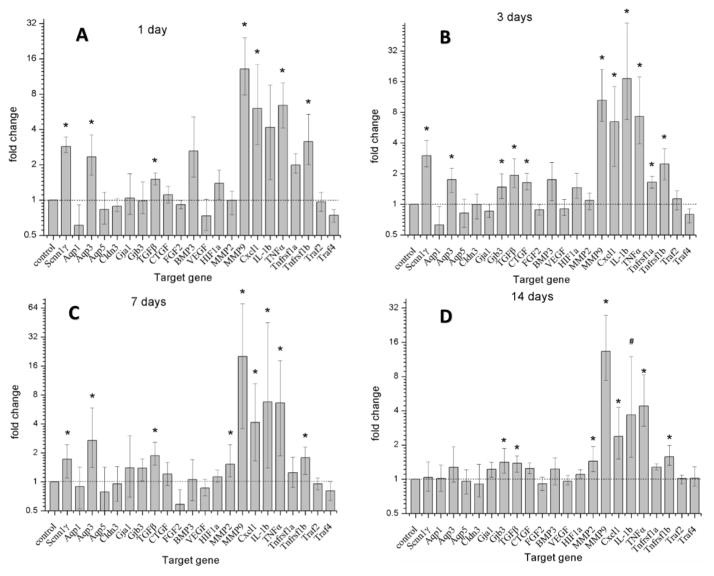
Information for primers is shown in Table 1. Implantation led to significant up-regulation of genes involved in all three pathways: ion homeostasis, tissue remodeling, and inflammation, as shown in Figure 2.
Figure 2.
Changes in target gene expression following cochlear implantation as compared to non-surgery control ears. Error bars depict SEM. Asterisks indicate statistically significant values as compared to control ears. The gene expression changes are ordered as follows: housekeeping gene (column 1), ion homeostasis genes (columns 2–8), tissue remodeling genes (columns 9–16), and inflammation-related genes (columns 17–23).
Statistically significant up-regulation of several ion homeostasis function genes was seen at multiple time points. Scnn1γ was up-regulated 2.8 fold in the cochleae 1 day after electrode array insertion relative to control non-implanted contralateral cochleae (Fig. 2A). Scnn1γ was also up-regulated to 3 and 1.7 fold at 3 days (Fig. 2B) and 7 days (Fig. 2C) post-surgery, respectively, but the small increase in mRNA expression in the 14 days post-surgery group implanted cochleae (Fig. 2D) was not significant. Aqp3 was up-regulated to 2.3, 1.7 and 2.7 fold at 1 day, 3 days and 7 days (Fig. 2A–C), but not at 14 days post-surgery (Fig. 2D). Gjb3 was up-regulated to 1.47 and 1.41 fold at 3 days and 14 days (Fig. 2B–D).
Several chemokine and tissue remodeling genes exhibited significant up-regulation at various time points. TGFβ1 was up-regulated 1.38 to 1.92 fold at 1 day, 3 days, 7 days and 14 days (Fig. 2A–D). CTGF was significantly up-regulated 1.63 fold at 3 days (Fig. 2B). MMP2 was up-regulated 1.53 and 1.44 fold at 7 days and 14 days (Fig. 2C–D). MMP9 was up-regulated by a factor of 12 at 1 day, 9.5 at 3 days, 19 at 7 days and 12.3 at 14 days (Fig. 2A–D). FGF-2 was down-regulated, most notably at 7 days, but this was not statistically significant.
Several inflammation-related genes showed up-regulation at all time points. Cxcl1 was up-regulated 6.04, 6.4, 4.15 and 2.37 fold at 1 day, 3 days, 7 days and 14 days (Fig. 2A–D). IL-1b was up-regulated 4.16, 17.1, 6.7 and 3.7 fold at 1 day, 3 days, 7 days and 14 days (Fig. 2A–D). TNFα was up-regulated 6.4, 7.2, 6.6 and 4.4 fold at 1 day, 3 days, 7 days and 14 days (Fig. 2A–D). Tnfrsf1a was up-regulated 1.65 at 3 days (Fig. 2B). Tnfrsf1b was up-regulated 3.1, 2.5, 1.8 and 1.6 fold at 1 day, 3 days, 7 days and 14 days. (Fig. 2A–D).
3.3 Individual ABR threshold shift and gene expression results within groups
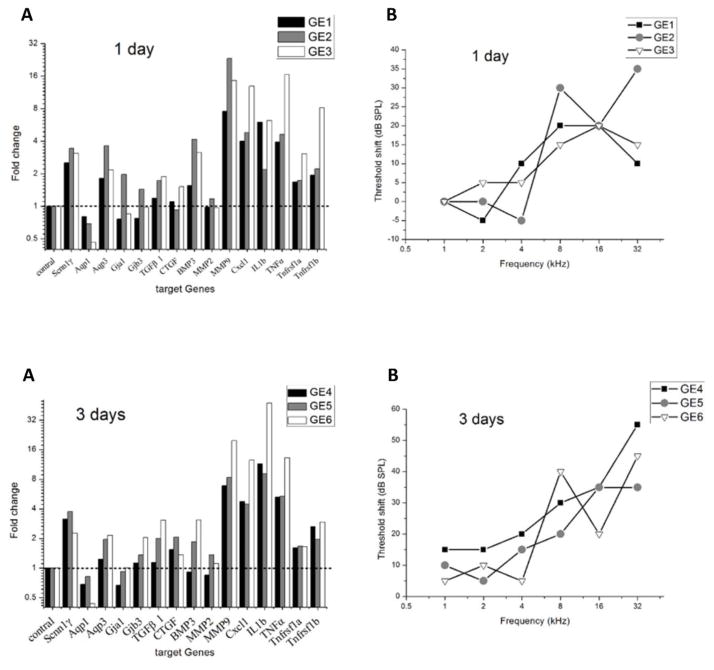
ABR threshold shifts post-surgery at different time points and associated gene expression changes are shown for individual animals in the 1-day and 3-day groups in Figure 3, the 7-day group in Figure 4, and the 14-day group in Figure 5. Only genes with significant expression changes are shown.
Figure 3.
Changes in target gene expression and ABR threshold shifts in the implanted ear following cochlear implantation for each animal from the 1-day and 3-day groups. The gene expression changes are again grouped in each subplot from left to right as follows: housekeeping gene (column 1), ion homeostasis genes (columns 2–6), tissue remodeling genes (columns 7–11), and inflammation-related genes (columns 12–16).
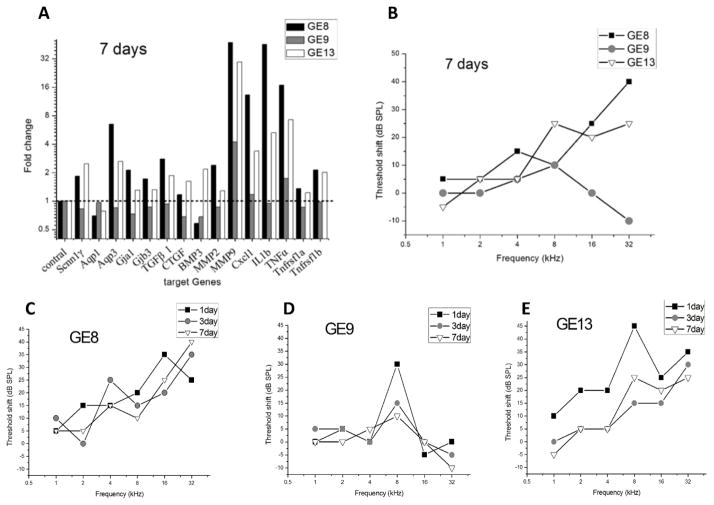
Figure 4.
Changes in target gene expression and ABR threshold shifts for each animal following cochlear implantation in the 7-day group. Gene expression changes are shown at top left and plotted as in Fig. 3. Final ABR threshold shifts are shown at top right. Detailed changes over time for each animal are shown in the bottom row.
Figure 5.
Change in target gene expression and ABR threshold shifts for each animal following cochlear implantation in the 14-day group. Gene expression changes are shown at top left and plotted as in Fig. 3. Final ABR threshold shifts are shown at top right. Detailed changes over time for each animal are shown in the bottom row.
Within the 1-day group, GE2 had the most hearing loss (shaded circles in Fig. 3B), and gene expression changes of ion homeostasis genes (Aqp3, Gja1, Gja3) and tissue remodeling genes (BMP3, MMP9) were correspondingly largest for GE2 (gray shaded bars in Fig. 3A). Inflammation response genes, on the other hand, showed the largest increase in expression for GE3 (open bars in last 5 columns of Fig. 3A).
Within the 3-day group, there was a mix of hearing loss patterns versus frequency. GE6 had more up-regulated genes, especially inflammation-related genes (open bars in last 5 columns in Fig. 3C), than the other two animals in the group, but did not show more overall hearing loss than the other animals (open triangles compared to other symbols in Fig. 3D).
Within the 7-day group, ABR thresholds over multiple time points indicate some recovery of thresholds over time after surgery. GE13 had a peak hearing loss of 45 dB threshold shift at 8 kHz at 1-day post-surgery, which recovered over time to a 15 dB threshold shift by 7-days post-surgery (Fig 4E). GE9 also showed a recovery after 1-day post-surgery, ultimately ending with the lowest threshold shifts at 7-days post-surgery (Fig. 4D; gray shaded circles in Fig. 4B). GE8 showed the most post-surgery hearing loss at 7-days post-surgery (Fig. 4C; black squares in Fig. 4B) as well as the greatest up-regulation of cochlear mRNA for MMP9, MMP2, Cxcl1, TNFα, IL1b, TGFb1, Aqp3 and Gjb3 (black bars in Fig. 4A). GE13, which had the second highest amount of threshold shift at 7-days post-surgery (open triangles in Fig. 4B), also showed more up-regulation of these genes (open bars compared to other bars in Fig. 4A) compared to GE9 which had the smallest threshold shift, but generally less than GE8 which had the largest threshold shift.
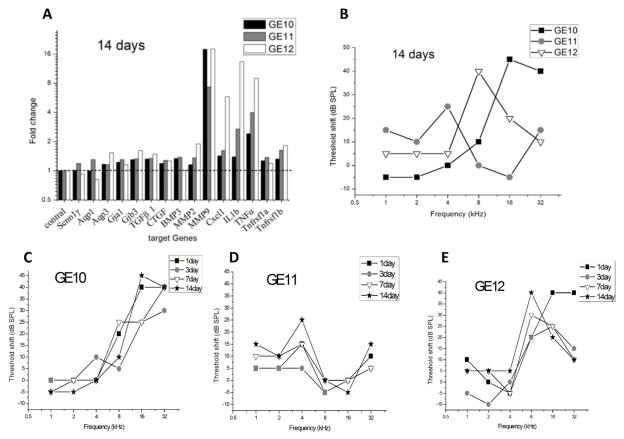
Within the 14-day group, more hearing loss was observed for GE10 and GE12 than for GE11 (black squares and open triangles compared to gray shaded circles in Fig. 5B). Accordingly higher gene level up-regulation was observed for GE10 and GE12 in MMP9 (black and open bars compared to gray bars in Fig. 5A), and for GE12 only in MMP2, Cxcl1, IL1b and TNFα (open bars in Fig. 5A).
3.4 Tissue remodeling post-surgery
Cochleae of all animals were dissected under a light microscope and the micro-structure checked before cochlear tissue harvesting. No fracture of osseous spiral lamina or tear of basilar membrane was observed in any of the implanted cochleae. Osteogenesis in the cochleostomy area was seen in GE13, but no evidence of fibroplasia or osteogenesis as observed for any other cochleae. Note that GE13 did not have the largest threshold shift in the 7-day group.
4. Discussion
Consistent with previous studies, significantly greater hearing loss was induced at high frequencies than at low frequencies after implantation, as expected due to the shallow electrode insertion and limitation of surgical trauma to the basal, high frequency region. Hearing loss peaked at 3 days post-surgery, with thresholds showing some recovery at 7 and 14 days post-surgery. The temporarily higher threshold shifts may reflect temporary fluid accumulation, or threshold recovery due to upregulated genes involved in inflammation and ion homeostasis.
There was statistically significant upregulation of nearly all inflammatory cytokines studied (TNF-α, CxCl1, IL-1β, Tnfr1a, and Tnfr1b). Some ion homeostasis genes were also upregulated (Scnn1a, Aqp3, and Gjb3/Cx31), while others were downregulated (Aqp1). The upregulation of inflammation genes TNF-α, CxCl1, and IL-1β, upregulation of the ion homeostasis genes Scnn1a, Aqp3, and Gjb3/Cx31, and downregulation of Aqp1 qualitatively resemble the trends seen with middle ear inflammation (26–27), suggesting generic gene expression changes associated with an inflammatory response. The upregulation of apoptosis-related genes Tnfr1a and Tnfr1b is consistent with upregulation seen after noise exposure; however, Traf4 was downregulated instead of upregulated, suggesting activation of slightly different pathways (28).
Tissue remodeling genes, on the other hand, showed different gene expression patterns after cochlear implantation, compared to those seen with middle ear inflammation. MMP2 and MMP9 were significantly upregulated, and FGF2 was downregulated slightly, whereas middle ear inflammation does not lead to changes in expression of these genes (27). These genes have specific roles related to blood flow; FGF-2 regulates vascular tone (29), while MMP2 and MMP9 are involved in stria vascularis capillary basement membrane remodeling (36). In addition, when the hearing loss of individual animals was compared with gene expression, MMP9 was the single factor most associated with degree of high-frequency hearing loss. The upregulation of genes involved in blood flow is consistent with previous findings linking reduced stria vascularis blood vessel density with increased hearing loss after cochlear implantation (15). It is not clear, though, whether the MMP9 expression changes directly cause the hearing loss, or are a downstream result of electrode insertion trauma to blood vessels in the stria vascularis which could affect the ability to maintain the endocochlear potential. The continued elevated expression of MMP9 at 14 days post-implantation, long after the hearing loss has stabilized, suggest the latter interpretation.
There was little upregulation of bone morphogenesis related genes such as BMP3, consistent with the lack of ossification observed around the electrode array in all but one animal. TGF-β and CTGF, which were not studied in (26), were also upregulated. TGF-β is involved with the fibrotic tissue response (29), so up-regulation of these genes is consistent with the typically observed fibrotic tissue (wound healing) response to surgical trauma; however, the down-regulation of FGF2 implies opposite or differing effects on multiple fibrosis related pathways. In addition, the time frame of the hearing loss, within 1–3 days after cochlear implantation, was too rapid to be explained by bone or fibrotic tissue growth effects on cochlear mechanics. Little is known about the role of CTGF, a growth factor expressed in the Type IV fibrocytes of the spiral ligament (32); it may be that direct damage to the spiral ligament region triggers a response in these fibrocytes.
In summary, genes affected by cochlear implantation include those involved in inflammation, ion homeostasis, and tissue remodeling, with tissue remodeling changes differing most from those seen with inflammation of the middle ear. Genes involved in tissue remodeling, particularly MMP2 and MMP9, were significantly upregulated and qualitatively associated with the degree of hearing loss. In this study, only a subset of genes has been ruled out as potential players in implantation-induced hearing loss. Further study with more animals and more genes, as well as examining the effects of electrical stimulation, is needed to clarify the mechanisms of implantation-induced hearing loss. Gene expression changes may also be used to measure the effects of clinically applicable interventions including various drugs (steroids, anti-oxidants) or new electrode designs in the future.
References
- 1.Kiefer J, Tillein J, von Ilberg C, Pfennigdorff T, Sturzebecher E, Klinke R, Gstottner W. Fundamental aspects and first clinical results of the clinical application of combined electric and acoustic stimulation of the auditory system. In: Kubo T, Takahashi Y, Iwaki T, editors. Advances in Cochlear Implants - An Update. Kugler Publications; The Hague: 2002. pp. 569–576. [Google Scholar]
- 2.Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–30. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Wilson BS, Lawson DT, Muller JM, Tyler RS, Kiefer J. Cochlear implants: some likely next steps. Annu Rev Biomed Eng. 2003;5:207–49. doi: 10.1146/annurev.bioeng.5.040202.121645. [DOI] [PubMed] [Google Scholar]
- 4.Turner CW, Gantz BJ, Vidal C, Behrens A, Henry BA. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115:1729–35. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- 5.Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11(Suppl 1):12–5. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- 6.Carlson ML, Driscoll CLW, Gifford RH, Service GJ, Tombers NM, Hughes-Borst BJ, Neff BA, Beatty CW. Implications of minimizing trauma during cochlear implantation. Otol Neurootol. 2011;32:962–968. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzpatrick DC, Campbell AT, Choudhury B, Dillon MP, Forgues M, Buchman CA, Adunka OF. Round window electrocochleography just before cochlear implantation: Relationship to word recognition outcomes in adults. Otol Neurootol. 2014;35:64–71. doi: 10.1097/MAO.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14(Suppl 1):32–8. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gstoettner W, Helbig S, Settevendemie C, Baumann U, Wagenblast J, Arnoldner C. A new electrode for residual hearing preservation in cochlear implantation: first clinical results. Acta Otolaryngol. 2009;129:372–9. doi: 10.1080/00016480802552568. [DOI] [PubMed] [Google Scholar]
- 10.Santa Maria PL, Domville-Lewis C, Sucher CM, Chester-Browne R, Atlas MD. Hearing preservation surgery for cochlear implantation--hearing and quality of life after 2 years. Otol Neurotol. 2013;34:526–31. doi: 10.1097/MAO.0b013e318281e0c9. [DOI] [PubMed] [Google Scholar]
- 11.Briggs RJ, Tykocinski M, Stidham K, Roberson JB. Cochleostomy site: implications for electrode placement and hearing preservation. Acta Otolaryngol. 2005;125:870–6. doi: 10.1080/00016480510031489. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary MJ, Fayad J, House WF, Linthicum FH., Jr Electrode insertion trauma in cochlear implantation. Ann Otol Rhinol Laryngol. 1991;100:695–9. doi: 10.1177/000348949110000901. [DOI] [PubMed] [Google Scholar]
- 13.Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- 14.Eshraghi AA, Gupta C, Van De Water TR, Bohorquez JE, Garnham C, Bas E, Talamo VM. Molecular mechanisms involved in cochlear implantation trauma and the protection of hearing and auditory sensory cells by inhibition of c-Jun-N-terminal kinase signaling. Laryngoscope. 2013;123(Suppl 1):S1–14. doi: 10.1002/lary.23902. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka C, Nguyen-Huynh A, Loera K, et al. Factors associated with hearing loss in a normal-hearing guinea pig model of hybrid cochlear implants. Hear Res. 2014;316C:82–93. doi: 10.1016/j.heares.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, Marovic P, O’Leary JS, Richardson R, Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Choi CH, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear Res. 2005;205:193–200. doi: 10.1016/j.heares.2005.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright CG, Roland PS. Vascular trauma during cochlear implantation: a contributor to residual hearing loss? Otol Neurotol. 2013;34:402–7. doi: 10.1097/MAO.0b013e318278509a. [DOI] [PubMed] [Google Scholar]
- 19.Kopelovich JC, Ieanyi C, Robinson B, Soken H, Goodman S, Hansen MR. Is high intensity electrical stimulation excitotoxic in hearing cochleae?. Evidence from the mouse model, vol. 36, Midwinter Research Meeting of the Association for Research in Otolaryngology; Baltimore, MD. 2013. [Google Scholar]
- 20.Stark G, Li H, Spear K, Zhang H, Tanaka C, Nguyen-Huynh A, Reiss L. Changes in Hearing Thresholds and Hair Cell Synapses After Chronic Electro-Acoustic Stimulation in Guinea Pigs With High-Frequency Hearing Loss. Midwinter Research Meeting of the Association for Research in Otolaryngology; San Diego, CA. 2014. [Google Scholar]
- 21.So H, Kim H, Lee J, Park C, Kim Y, Kim E, Kim J, Yun K, Lee K, Lee H, Moon S, Lim DJ, Park R. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-κβ. JARO. 2007;8:338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Water TR, Dinh CT, Vivero R, Hoosien G, Eshraghi AA, Balkany TJ. Mechanisms of hearing loss from trauma and inflammation: Otoprotective therapies from the laboratory to the clinic. Acta Oto-Laryngologica. 2010;130:308–311. doi: 10.1080/00016480903124655. [DOI] [PubMed] [Google Scholar]
- 23.Dinh CT, Haake S, Chen S, Hoang K, Nong E, Eshraghi AA, Balkany TJ, van de Water TR. Dexamethasone protects Organ of Corti explants against tumor necrosis factor-alpha- induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes. Neuroscience. 2008;157:405–413. doi: 10.1016/j.neuroscience.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Ihler F, Sharaf K, Bertlich M, Strieth S, Reichel CA, Berghaus A, Canis M. Etanercept prevents decrease of cochlear blood flow dose-dependently caused by tumor necrosis factor alpha. Ann Otol Rhinol Laryngol. 2013;122(7):468–73. doi: 10.1177/000348941312200711. [DOI] [PubMed] [Google Scholar]
- 25.Scherer EQ, Yang J, Canis M, Reimann K, Ivanov K, Diehl CD, Backx PH, Wier WG, Strieth S, Wangemann P, Voigtlaender-Bolz J, Lidington D, Bolz SS. Tumor necrosis factor-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41(11):2618–24. doi: 10.1161/STROKEAHA.110.593327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacArthur CJ, Hausman F, Kempton JB, Trune DR. Murine middle ear inflammation and ion homeostasis gene expression. Otology & Neurotology. 2011;32:508–515. doi: 10.1097/MAO.0b013e31820e6de4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacArthur CJ, Hausman F, Kempton JB, Sautter N, Trune DR. Inner ear tissue remodeling and ion homeostasis gene alteration in murine chronic otitis media. Otology & Neurotology. 2013;34:338–346. doi: 10.1097/MAO.0b013e31827b4d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu BH, Cai Q, Manohar Sm, Jiang H, Ding D, Coling DE, Zheng G, Salvi R. Differential expression of apoptosis-related genes in the cochlea of noise-exposed rats. Neuroscience. 2009;161(3):915–925. doi: 10.1016/j.neuroscience.2009.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radical Biology and Medicine. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beenken A, Mohammadi M. The FGF family: Biology, pathophysiology, and therapy. Nature Reviews: Drug Discovery. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobue T, Gravely T, Hand A, Min YK, Pilbeam C, Raisz LG, Zhang X, Larocca D, Florkiewicz R, Hurley MM. Regulation of fibroblast growth factor 2 and fibroblast growth factor receptors by transforming growth factor β in human osteoblastic MG-63 cells. J Bone Mineral Res. 2002;17(3):502. doi: 10.1359/jbmr.2002.17.3.502. [DOI] [PubMed] [Google Scholar]
- 32.Adams JC. Immunocytochemical traits of Type IV fibrocytes and their possible relations to cochlear function and pathology. J Assoc Res Otolaryngol. 2009;10:369–382. doi: 10.1007/s10162-009-0165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou J, Pykko I, Sutinen P, Toppila E. Vibration induced hearing loss in guinea pig cochlea: Expression of TNF-α and VEGF. Hear Res. 2005;202:13–20. doi: 10.1016/j.heares.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstetrics and Gynecology. 2008;199:78.e1–78.e6. doi: 10.1016/j.ajog.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Evans LC, Liu H, Pinkas GA, Thompson LP. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr Res. 2012;71(1):25–31. doi: 10.1038/pr.2011.10. [DOI] [PubMed] [Google Scholar]
- 36.Gratton MA, Rao VH, Meehan DT, Askew C, Cosgrove D. Matrix metalloproteinase dysregulation in the stria vascularis of mice with Alport syndrome: implications for capillary basement membrane pathology. Am J Pathol. 2005;166(5):1465–74. doi: 10.1016/S0002-9440(10)62363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong S, Liu Z. Expression of aquaporins in the cochlea and endolymphatic sac of guinea pig. ORL. 2003;65:284–289. doi: 10.1159/000075227. [DOI] [PubMed] [Google Scholar]
- 38.Kelly JJ, Forge A, Jagger DJ. Contractility in Type III cochlear fibrocytes is dependent on non-muscle myosin II and intercellular gap junctional coupling. JARO. 2012;13:473–484. doi: 10.1007/s10162-012-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 40.Xia A, Kikuchi T, Minowa O, Katori Y, Oshima T, Noda T, Ikeda K. Late-onset hearing loss in a mouse model of DFN3 non-syndromic deafness: morphologic and immunohistochemical analyses. Hear Res. 2002;166:150–158. doi: 10.1016/s0378-5955(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 41.Adams JC. Clinical implications of inflammatory cytokines in the cochlea: A technical note. Otology and Neurotology. 2002;23:316–322. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Rychlik W. OLIGO 7 primer analysis software. Springer; 2007. [DOI] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 45.Pfaffl M. Rest 2009 Software user guide. Qiagen, Hilden, Germany: 2009. [Google Scholar]
- 46.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]