Abstract
The colony stimulating factor-1 receptor (CSF-1R) kinase regulates tissue macrophage homeostasis, osteoclastogenesis, and Paneth cell development. However, recent studies in mice have revealed that CSF-1R signaling directly controls the development and maintenance of microglia, and cell autonomously regulates neuronal differentiation and survival. While the CSF-1R-cognate ligands, CSF-1 and interleukin-34 (IL-34), compete for binding to the CSF-1R, they are expressed in a largely non-overlapping manner by mature neurons. The recent identification of a dominantly inherited, adult-onset, progressive dementia associated with inactivating mutations in the CSF-1R highlights the importance of CSF-1R signaling in the brain. We review the roles of the CSF-1R and its ligands in microglial and neural development and function, and their relevance to our understanding of neurodegenerative disease.
CSF-1R and CSF-1R Ligands and their Expression Patterns in Brain
CSF-1R is a class III receptor tyrosine kinase activated by two homodimeric glycoprotein ligands, CSF-1 [1] and IL-34) [2], that exhibit low primary sequence homology but share a short chain four α-helical bundle cytokine fold and interact with overlapping regions of the CSF-1R (reviewed in [3]). CSF-1 signals exclusively through the CSF-1R, while IL-34 interacts with at least one additional receptor, receptor protein tyrosine phosphatase-ζ (PTP-ζ), which is coexpressed with the CSF-1R on neural progenitor cells [4] (Box 1). In myeloid cells, activation of the CSF-1R by CSF-1 or IL-34 leads to comparable biological outcomes [2,5]. However, in vivo the two endogenous CSF-1R ligands exhibit different spatiotemporal patterns of expression, and play complementary roles in controlling the development, maintenance, and activity of target cell types [5–8].
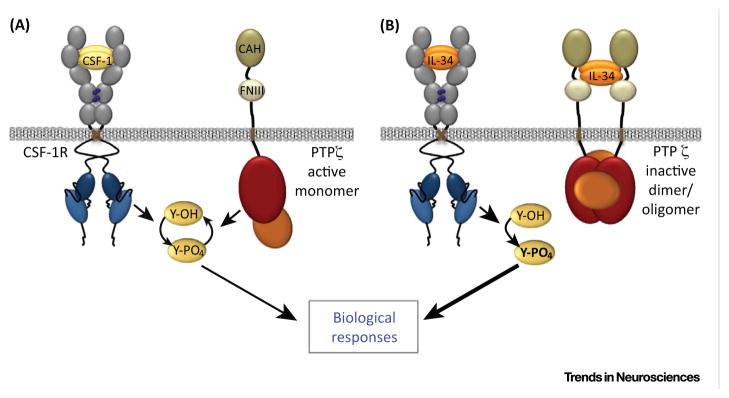
Box 1. PTP-ζ – A Second Receptor for IL-34.
The higher levels of brain IL-34 mRNA relative to those of CSF-1 and the CSF-1R, coupled with the greater efficacy of IL-34 over CSF-1 in regulating in vitro NPC self-renewal and differentiation [5,6], suggested that IL-34 might signal via an alternative receptor not recognized by CSF-1. Using an unbiased approach, the chondroitin sulfate proteoglycan receptor protein tyrosine phosphatase, PTP-ζ (also known as RPTP-β), was identified as a predominant IL-34-binding protein that does not bind CSF-1 [86]. Binding of IL-34 to PTP-ζ inhibits its phosphatase activity, leading to a rapid increase in the tyrosine phosphorylation of focal adhesion kinase and paxillin, and inhibits the proliferation, clonogenicity, and motility of the U251 human glioblastoma cell line in a PTP-ζ-dependent manner [86]. Because PTP-ζ and the CSF-1R are both expressed on NPCs [4,86], it is likely that IL-34 is more effective than CSF-1 in regulating NPC in vitro by simultaneously acting through both receptors (Figure I). IL-34 and PTP-ζ colocalize in cortical layer V, in midbrain, and in brainstem nuclei [86]. PTP-ζ signals via multiple ligands, including pleiotrophin, midkine, contactin, and tenascin-R [100], and plays important roles in the nervous system. PTP-ζ expression has not been reported in microglia. However, it is expressed in remyelinating oligodendrocytes, and PTP-ζ−/− mice exhibit faster recovery from experimental autoimmune encephalomyelitis (EAE)-induced loss of myelin than do wild-type mice, which is consistent with the action of the inhibitory ligand pleiotrophin in enhancing oligodendrogenesis [101]. In addition, the PTP-ζ gene is a schizophrenia-susceptibility gene [102], and PTP-ζ regulates tyrosine phosphorylation of voltage-gated sodium channels in neurons [103]. The identification of PTP-ζ as a second receptor for IL-34 necessitates its serious consideration in studies of IL-34 function in brain. Analogous to the PTP-ζ-mediated effects of IL-34 on NPCs, recent studies demonstrate that pleiotrophin suppresses hematopoietic stem cell self-renewal early after irradiation, and thus mitigates radiation injury to the hematopoietic system in a PTP-ζ-dependent manner [104], thus revealing a common effect of PTP-ζ in mediating stem cell quiescence.
Figure I.
Putative Mechanism for Differential Regulation by CSF-1 and IL-34 in Cells Coexpressing CSF-1R and PTPζ. (A) CSF-1 signaling: CSF-1 activates the CSF-1R tyrosine kinase leading to increased cellular tyrosine phosphorylation. Catalytically-active PTPζ decreases cellular tyrosine phosphorylation. (B) IL-34 signaling. Like CSF-1, IL-34 activates the CSF-1R, but also binds to PTPζ and inhibits its tyrosine phosphatase activity, further increasing tyrosine phosphorylation and downstream responses, such as NPC differentiation.
CSF-1R Expression in Brain
While the CSF-1R is expressed on all microglia [9,10], there are contradictory reports concerning its expression in the neuronal lineage. Initial studies, using several CSF-1R antibodies and in situ hybridization, reported that in adult brain CSF-1R was expressed in several neuronal subpopulations and that neuronal expression was increased following ischemic cerebral cortical injury [11,12]. However, in a recent study, none of six commercial anti-CSF-1R antibodies tested was specific, as demonstrated by their staining of Csf1r-deficient brain [13], calling into question the validity of the immunohistochemical data. Furthermore, several independent groups [14–16] were unable to detect neuronal expression using the same Csf1r–EGFP reporter mouse [17]. However, using a different Csf1r–EGFP reporter mouse [18] with GFP staining, corroborated by in situ hybridization, several of the findings of Wang et al. [11] were reproduced, including expression by a small fraction of hippocampal neurons and increased expression in injured neurons [13]. Further evidence for the neuronal expression of the CSF-1R comes from lineage-tracing studies with Csf1r–iCre;mTmG and Csf1r–iCre;ROSA–stopflox–CFP mice [13] and the detection of Csf1r mRNA in cultured primary neurons [11,13]. These studies emphasize the importance of selecting the appropriate CSF-1R detection system.
Reliable CSF-1R staining has been achieved using an in-house anti-CSF-1R antibody [19] shown to be specific by its failure to stain Csf1r-deficient brain [6]. Using this antibody, the authors showed that during development the CSF-1R is expressed by neural progenitor cells (NPCs), some cortical immature neurons, radial glia, and cerebellar Purkinje cells. Overall CSF-1R expression decreases by P60 [6]. In the young adult motor cortex, ~30% of neurons express the CSF-1R, increasing to ~50% by 10 months of age, with greatest expression in cortical layers V and VI [20].
CSF-1R Ligands in Brain
Immunohistochemical staining [6] and reporter mice [7,8,21] have shown that, in brain, CSF-1 and IL-34 are primarily expressed by neurons. Compared with CSF-1 mRNA, which is primarily expressed in the neocortex, corpus callosum, cerebellum, and spinal cord [6], IL-34 mRNA is primarily expressed in the forebrain (neocortex, olfactory bulb, and striatum) and at higher levels [5,6] (Figure 1). IL-34 is also expressed in ependymal cells and the choroid plexus [7]. Cellular colocalization of CSF-1 and IL-34 is rarely observed except in neurons of the CA3 region of the hippocampus [6] (Figure 1A). Furthermore, CSF-1 reporter expression decreases between postnatal day 20 (P20) and P60, whereas the expression of IL-34 is maintained [6].
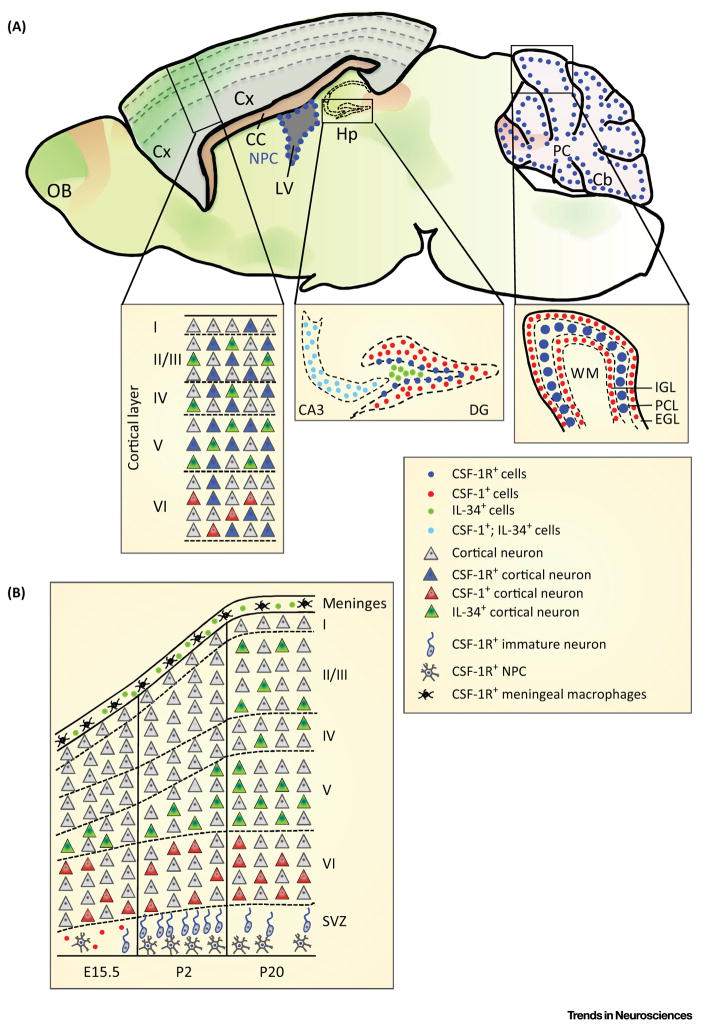
Figure 1.
Expression of Colony Stimulating Factor-1 Receptor (CSF-1R) and its Ligands in Brain. (A) Expression of CSF-1 (red shading) and interleukin-34 (IL-34) (green shading) in the adult brain (based on Allen Brain Atlas and [5–8]). Colocalization studies revealed that, apart from microglia (not shown), CSF-1R (blue) is expressed in ~30% of mature cortical neurons in the forebrain, in hippocampal cells, and in Purkinje neurons. CSF-1 expression (red) is low and restricted to the specific areas of the olfactory bulb, cortex, corpus callosum, hippocampus, and cerebellum. By contrast, IL-34 (green) is expressed throughout the telencephalon but is absent from the cerebellum. With the exception of the CA3 area of the hippocampus, there is no significant overlap in the cellular expression of CSF-1 and IL-34. (B) Dynamics of CSF-1R and ligand expression during cortical development. For clarity, CSF-1R+ microglia, which are present throughout the cortex and stain strongly in the SVZ, are not shown. Abbreviations: Cb, cerebellum; Cx, cortex; CC, corpus callosum; DG, dentate gyrus; EGL, external granular layer; Hp, hippocampus; IGL, internal granular layer; LV, lateral ventricle; NPCs, neural progenitor cells; OB, olfactory bulb; PC, Purkinje cells; PCL, PC layer; SVZ, subventricular zone; WM, white matter.
Complementary Expression of CSF-1 and IL-34 in the Developing Cortex
The complementary nature of CSF-1 and IL-34 expression in brain (Figure 1) is particularly evident during cortical development (Figure 1B) and of interest in relation to the cortical expression of the CSF-1R and of PTP-ζ (Box 1). At embryonic day 10.5 (E 10.5), microglia appear in the cephalic mesenchyme and neuroepithelium of the brain anlagen [22], and IL-34 is detected in the E11.5 telencephalon, before the appearance of CSF-1 reporter activity by E13.5 [5]. By E15.5, CSF-1R is detected throughout the dorsal telencephalon, including the ventricular zone (VZ)/subventricular zone (SVZ) [6]. At this stage, CSF-1 is expressed in the VZ/SVZ and IL-34 within the marginal zone and cortical plate. Within the P2 neocortex, CSF-1 is expressed solely in layer VI, colocalizing with Tbr1+ neurons, while IL-34 is expressed in layer V (colocalizing with CTIP2+ neurons) and in the meninges. At P2, the CSF-1R is strongly expressed in the SVZ (colocalizing with a subset of nestin+ NPCs, β-tubulin III+ neuronal progenitors and microglia) and at the meninges, where microglia are concentrated. CSF-1 is expressed between P7 and P14 by activated phagocytes present in the periventricular white matter [23], suggesting that autocrine CSF-1/CSF-1R signaling may contribute to the gradual de-activation of phagocytes at this stage. At P20, CSF-1 continues to be expressed in layer VI, whereas IL-34 expression has expanded into upper layers II–IV (colocalizing with Satb2 neurons) and remains expressed in the meninges. At this stage, the CSF-1R is expressed primarily in microglia, uniformly in the corpus callosum and cortical layers I–VI, and strongly in the meninges. Therefore, during development the CSF-1R ligands are expressed by different mature cortical neurons: CSF-1 in layer VI, where mostly excitatory neurons reside [24], and IL-34 in layers II–V (with both excitatory and inhibitory neurons and mature glia [24]) and in the meninges [6]. The CSF-1R is expressed early in NPCs of the SVZ and microglia within the meninges, and later assumes a broad distribution on microglia. The spatiotemporal regulations of CSF-1R and CSF-1R ligand expression suggest that they play key regulatory roles in cortical development.
Regulation of Microglial and Neuronal Lineages by the CSF-1R
Gross Abnormalities of Brain Development in CSF-1R- and CSF-1R Ligand-Deficient Mice
The relative importance of the CSF-1R and each of its ligands in development is reflected in the survival rates of mice bearing null mutations in each. C57BL/6 Il34−/− mice have a normal survival rate and their brains are grossly normal [7,8], suggesting that IL-34 does not play an essential role in development. By contrast, Csf1op/op and Csf1r−/− mice exhibit reduced survival rates (Table 1). The brains of both Csf1op/op and Csf1r−/− mice are smaller in size with a greater mass. In addition, Csf1r−/−, but not Csf1op/op brains, exhibit atrophy of the olfactory bulb, expansion of the lateral ventricle, and thinning of the neocortex [6,15] (Table 1). These gross anatomic abnormalities reflect important roles of CSF-1R signaling in brain development.
Table 1.
Neurological Phenotypes of Csf1op/op, Csf1r−/−, Nes–Cre/+;Csf1rfl/fl, and Csf1r/+/− Micea
| Phenotype | Mouse Mutant | Refs | |||
|---|---|---|---|---|---|
| Csf1op/op bd | Csf1r−/− bd | Nes–Cre/+; Csf1rfl/fl c | Csf1r+/− c | ||
| Postnatal Survival at 4 Weeks | [105,106] | ||||
| C3H/B6/SvJ outbred | 80% | 40% | |||
| FVB/NJ | 40% | 0%e | |||
| C57BL/6 | 0% | 0.8%f | 67% | 100% | |
| Neurological Phenotypes | |||||
| Hearing | Impaired | Impaired | [105,107] | ||
| Vision | Impaired | [107] | |||
| Olfaction | Impaired | Impaired | [15,20] | ||
| Spatial memory | Impaired | [20] | |||
| Motor coordination | Impaired | [20] | |||
| Depression, anxiety | Impairedg | [20] | |||
| GABAergic responses | Abnormal | [107] | |||
| Hypothalamic sex steroid hormone feed-back | Reduced | [108,109] | |||
| Brain Anatomy and Histology | |||||
| Size | Reduced | Reduced | Reduced | Normal | [6,20] |
| Mass | Increased | Increased | Normal | Normal | [6,20] |
| Olfactory bulb | Normal | Atrophic | Normal | Normal | [6,20] |
| Lateral ventricle size | Normal | Increased | Normal | Increased | [6,20] |
| Cortical thickness | Increased | Reduced | Variable | Reduced | [6,20] |
| Corpus callosum axonal crossing | 78% | 20% | 100% | 100% | [6,20] |
| Callosal thickness | Reduced | [20] | |||
| Cerebellar size | Reduced | Reduced | [6] | ||
| Myelination | Abnormal | [20] | |||
| Microglia | Reduced | Absenth | Normal | Increased | [6,15,20,22, 27,28,30] |
| Excitatory neuronal differentiation | Reduced | Reduced | Variable | [6] | |
| Subcortical OL differentiation | Reduced | Reduced | Normal | Increased | [6,20] |
| Cortical cellular apoptosis | Normal | Increased | Increased | [6] | |
| Cortical NPC proliferation | Increased | Increased | Increased | [6] | |
Csf1op/op, Csf1-deficient osteopetrotic mouse; Nes–Cre/+;Csf1rfl/fl, mice with neural progenitor-specific deletion of Csf1r; OL, oligodendrocytes; NPC, neural progenitor cells.
Phenotypes analyzed on the FVB/NJ background
Phenotypes analyzed on the C57BL/6 background
Toothless mice fed powdered chow on weaning (E.R.S., unpublished observations).
Die by 3 weeks of age.
Die perinatally (E18–P2).
Only in males.
6% microglia versus wild type (range 1–34%, depending on region) reported in 3-week-old C57BL/6 Csf1r−/− mice.
Central Role of the CSF-1R in the Development and Maintenance of Microglia
Genetic ablation and pharmacological studies have shown that the CSF-1R is required for mouse microglial development [22] and steady-state maintenance [25]. Treatment of adult mice with a small-molecule ATP binding site inhibitor of the CSF-1R causes rapid depletion of brain microglia, and its removal leads to their rapid regeneration from brain-resident progenitor cells [25]. At 3 weeks of age, microglial numbers in Csf1r-null brains are reduced by more than 94% [15,22]. Lineage-tracing experiments demonstrate that microglia are exclusively derived from primitive yolk sac progenitors that arise before E8 [22]. While a role of the CSF-1R in microglial lineage commitment has not been demonstrated, evidence indicates that the CSF-1R provides a crucial survival/proliferation signal. Like Csf1r+/+ precursors, Csf1r−/− yolk sac-derived microglial precursors seed the brain rudiment by E10.5 (Figure 2). However, they are greatly reduced by E12.5 compared to their wild-type counterparts which increase in numbers [22,26]. CSF-1R signaling also plays a central role in the establishment of microglial processes [27,28] and migration [29] in the developing brain.
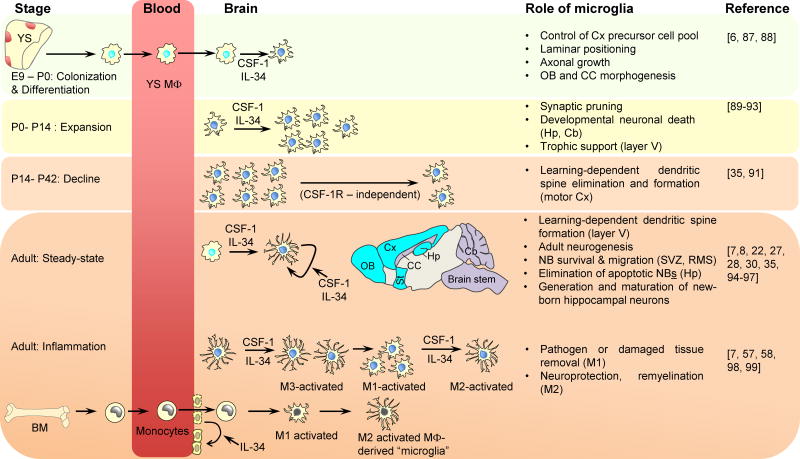
Figure 2.
Contribution of CSF-1R Ligands to the Development, Maintenance, and Activation of Microglia. Primitive microglia progenitors (macrophages) generated in the yolk sac blood islands are detectable in the blood circulation at E8.5 and enter the brain anlagen at E9.0, where they give rise to embryonic microglia that colonize the developing brain [22]. Between E12.5 and E18.5 the microglial distribution undergoes dynamic changes, with clustering along specific axonal tracts and at the generative zones [88]. Microglial density increases during the first two postnatal weeks and then declines, reaching stable levels by the 6th week of life [91]. During this period, microglia are important for synaptic pruning and have an amoeboid morphology that correlates with high phagocytic activity [89–93]. This converts to a highly ramified ‘resting’ morphology in the adult.The CSF-1R is required for the survival and proliferation of adult microglia [25]. Regional dominance of IL-34-(turquoise shaded areas) and CSF-1-(purple) mediated signals is indicated [7,8,28,30]. CSF-1 levels are upregulated in response to tissue injury and could drive the rapid expansion of microglia (M3 activation) [58,98]. Similarly,IL-34 promotes microglial expansion and neuroprotective microglial responses to viral infection [7]. Damage to the blood–brain barrier (BBB) allows the recruitment of bone marrow (BM)-derived progenitors that transiently supplement the microglial population. Independently of its actions on microglia, IL-34 can also activatetheCSF-1R expressed on capillary endothelial cells and restore BBB integrity by upregulating tight junction proteins [99]. The roles of microglia in neuronal development are based on [35,57,87–90,92,94–97]. Abbreviations: Cb, cerebellum; CC, corpuscallosum; Cx, cortex; Hp, hippocampus; MΦ, macrophage; M1–M3 denote microglial activation states with M1 representing inflammatory microglia; M2, alternatively activated, trophic microglia; and M3 rapidly proliferating microglia that are not M1-or M2- polarized; NB, neuroblast; OB, olfactorybulb; RMS, rostral migratory stream; St, striatum; SVZ, subventricular zone; YS, yolk sac.
Both CSF-1R ligands are expressed in the embryonic brain [6] and contribute to the development of microglia in a region-specific manner [7,8,30]. Microglia are reduced by ~30% in Csf1-null brains [22] and by ~70% in Il34-null brains [7,8]. CSF-1 is highly expressed in the yolk sac [31], where it may contribute to the expansion of CSF-1R+ microglial precursors. IL-34 contributes to the development and homeostasis of microglia in forebrain structures, but not in the cerebellum or brainstem where CSF-1 is more highly expressed during development [6–8]. CSF-1 contributes to microglial development and maintenance in the corpus callosum, pons, and spinal dorsal column, and also to a lesser extent in the cerebral cortex and adult cerebellum [28,30]. Because microglia regulate the development, maturation, and maintenance of neurons (Figure 2), their dependence on CSF-1R emphasizes the broad role played by CSF-1R signaling in the brain.
Altered Neuronal Lineage Differentiation in the Developing Brains of CSF-1R-Deficient Mice
Apart from the absence of microglia, immunohistological analysis of developing Csf1r−/− dorsal forebrain reveals significant changes in the number of NPCs and neuronal cells compared with wild-type forebrains [6]. Within the generative zone, the increase in proliferating neural progenitors at P20 suggests that CSF-1R signaling suppresses NPC self-renewal. Between E13.5 and E15.5, Tbr2+ basal progenitors are decreased in the generative zone [32] and increased in the cortex [6,32], where Pax6+ radial glia are also increased [6], suggesting that excess radial glia differentiate more efficiently into Tbr2+ basal progenitors and/or enhance their migration from the generative zone [33]. Furthermore, the decrease in neocortical lower layer (CTIP2+) and upper layer (Cux1+) neurons at both E15.5 and P20, together with transient changes in Tbr1+ and Satb2+ neurons in the Csf1r−/− neocortex, indicates that CSF-1R signaling regulates neuronal differentiation within the cortical laminae [6]. These alterations are associated with an increase in the number of apoptotic neural progenitors in the SVZ as well as an increase in cortical neuronal apoptosis. Apoptosis is also apparent at sites of adult neurogenesis in the granule cell layers of the olfactory bulb and in the subgranular zone of the dentate gyrus of Csf1r−/− mice [6]. Thus, CSF-1R signaling suppresses the expansion of forebrain NPCs, regulates their differentiation, and promotes the survival of NPCs and their early progeny (Figure 3).
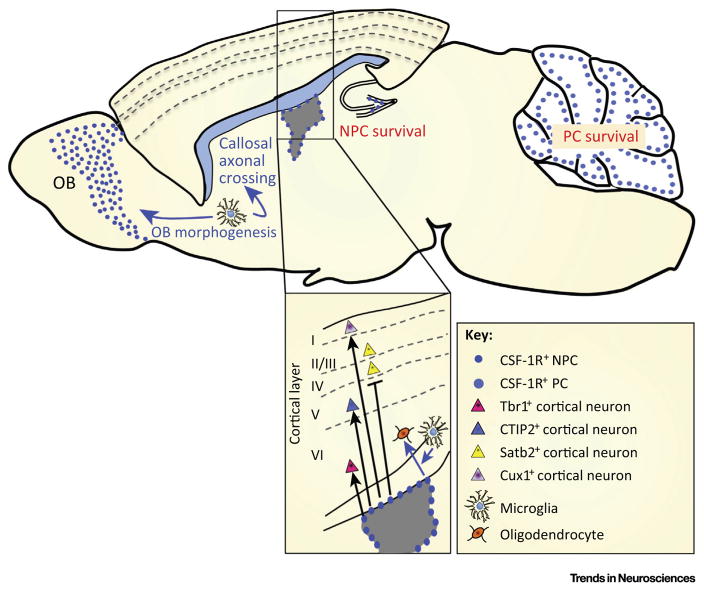
Figure 3.
Regulation of Neural Cells by the CSF-1R. Studies with purified neural progenitor cells (NPCs) show that the CSF-1R promotes NPC survival, proliferation, and differentiation towards the neuronal lineage in a cell-autonomous manner. The expanded panel illustrates CSF-1R support of the development of deep-layer and layer I neurons and suppression of layer II–IV (Satb2+) neurons. In the cerebellum, CSF-1 promotes the survival of Purkinje cells (PC) [12]. CSF-1R signaling in microglia, but not in purified NPCs, promotes oligodendrocyte differentiation (expanded panel). The lack of callosal axonal crossing defects and olfactory bulb (OB) atrophy in mice with NPC-specific ablation of CSF-1R expression suggests that microglia also mediate the effects of the CSF-1R in callosal and olfactory bulb development.
Direct and Indirect Regulation of Neuronal Lineage Cells by the CSF-1R
The CSF-1R Directly Regulates Neural Progenitor Self-Renewal, Differentiation, and Survival
Given the expression of the CSF-1R on cells of the neuronal lineage, and the dysregulation of NPC survival, proliferation, and differentiation in Csf1r−/− brains, it was important to determine the functions of neuronal expression of CSF-1R [6]. In vitro studies utilizing purified, microglia-depleted NPCs revealed that either CSF-1 or IL-34 suppresses NPC self-renewal, but not their proliferation. Furthermore, in clonal differentiation assays, CSF-1 or IL-34 each increased the percentage of pure neuronal clones, without affecting the percentage of astrocyte- or oligodendrocyte- containing clones. In these cultures, NPCs, protoplasmic astrocytes, and neurons, but not oligodendrocytes, expressed the CSF-1R. These observations indicate direct effects of both ligands in suppressing self-renewal and enhancing differentiation of the CSF-1R-expressing NPCs. Further support for such direct regulation was obtained by conditional deletion of Csf1r in NPCs using nestin (Nes)-Cre/+;Csf1rfl/fl mice. These mice have normal cortical microglial densities at E18.5 and P20, but resemble Csf1r−/− mice in their high perinatal lethality, smaller brain size and enhanced forebrain progenitor cell proliferation and apoptosis (Table 1). Thus the self-renewal, differentiation, and survival phenotypes of Csf1r−/− NPCs result from the absence of direct CSF-1R regulation of the neuronal lineage (Figure 3).
Regulation of Neuronal Cells via the CSF-1R Expressed in Other Cell Types
Several Csf1r−/− phenotypes, namely atrophy of the olfactory bulb, reduction of mature cortical forebrain oligodendrocyte numbers, increase in lateral ventricle size, and failure of midline crossing of callosal axons, are not recapitulated in the Nes-Cre/+;Csf1rfl/fl mice, indicating that CSF-1R signaling in non-neural lineage cells is involved (Table 1). Oligodendrocytes do not express the CSF-1R [6] and, because their decreased number in Csf1r−/− mice is not mimicked in Nes-Cre/+; Csf1rfl/fl mice, their regulation by the CSF-1R must be indirect. CSF-1 enhances oligodendrocyte differentiation in cultures of unpurified NPCs containing microglia, but there is no effect of CSF-1 in cultures prepared from purified, microglia-free NPCs [6]. Thus, CSF-1R-expressing microglia appear to mediate non cell-autonomous effects of the CSF-1R on neural lineage cells (Figure 3).
The physiological role of microglia in adult brain function has been explored by inducible microglial ablation (reviewed in [34]). Using an inducible diphtheria toxin receptor (DTR)-mediated microglial ablation system, Parkhurst et al. [35] showed that, although loss of microglia did not alter the overall densities of neurons or synapses in the motor cortex or the hippocampal CA1 region, it had profound effects on learning-induced synapse formation and produced deficits in multiple behavioral tasks, including performance improvement after motor learning, auditory-cued fear conditioning, and novel object recognition. These data suggest that microglia play important roles in learning and memory that involve multiple brain regions. By contrast, in studies utilizing CSF-1R inhibitors, mice depleted of microglia for up to 2 months did not develop anxiety or deficits in memory or motor function, but showed some evidence of enhanced learning [25,36]. Several differences may contribute to this discrepancy. First, there were different readouts for the behavioral tests. Second, there were different tissue responses to the microglial ablation strategies used. While both DTR- and CSF-1R inhibitor-mediated microglial ablation were expected to cause astrogliosis [25,37], this was not observed by Parkhurst et al. Third, differential effects on the peripheral monocyte compartment could contribute indirectly. DTR-mediated ablation causes acute loss of peripheral monocytes, which subsequently recover, while CSF-1R inhibition does not cause blood monocyte loss [38]. Fourth, because the host microbiota regulates microglia in the CNS [39], differences in housing environments may have influenced the results.
CSF1R Mutations Cause Adult-Onset Leukoencephalopathy with Axonal Spheroids and Pigmented Glia (ASLP)
Genome-wide linkage analysis and exome sequencing revealed that mutations in the CSF1R gene cause a rare, autosomal dominant, neurodegenerative disorder characterized by adult-onset dementia with motor impairments and epilepsy [40,41]. ALSP encompasses two similar diseases previously known as hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) [42] and familial pigmentary orthochromatic leukodystrophy (POLD) [43]. The median age of onset of ALSP is 42 ± 13 years (range 8–78) with disease duration of 5 ± 7 years (range 1–34) that is unrelated to the time of onset [44,45]. Symptoms may vary according to gender [45]. Patients often present with neuropsychiatric symptoms, including depression, behavioral changes, spastic paraplegia, dementia, and seizures, leading to variable clinical diagnoses. By magnetic resonance imaging (MRI), ALSP is characterized by asymmetric patchy cerebral white matter lesions that become confluent and symmetrical with disease progression [46,47]. The changes predominantly involve the frontal and parietal white matter, with thinning of the corpus callosum being an early feature [47,48], as well as evolving cortical atrophy affecting the frontal and parietal lobes.
CSF1R mutations associated with ALSP involve either missense mutations affecting highly conserved kinase domain residues or splice-site mutations leading to in-frame deletions (reviewed in [49]). Ligand-stimulated CSF-1R kinase activity was abolished for homodimeric receptors bearing each of 15 different missense mutations or each of four aberrant splice variants so far tested [40,41,47,50,51]. However, cotransfection experiments indicate that expression of the mutant chain does not suppress phosphorylation of the wild-type chain [47]. Therefore, in ALSP patients only 25% of ligand-bound cell-surface CSF-1R dimers are expected to be enzymatically inactive, and the remainder will be active, with 50% containing one active chain and 25% containing 2 wild-type chains. ALSP mutations may also alter the trafficking of hybrid CSF-1Rs because expression of a large majority of mutant receptors singly in human 293T cells has shown a decrease in the cell surface complement of mature CSF-1Rs and an increase in Golgi-associated immature, high-mannose CSF-1Rs [50]. Interestingly, loss-of-function mutations of either TREM2 or DAP12, components of the DAP12-TREM2 signaling complex that mediates CSF-1R signaling in macrophages [52] and microglia [53], lead to Nasu-Hakola disease (NHD) that has striking similarities to ALSP [54,55]. In the brain, TREM2 and DAP12 are expressed primarily by microglia, and knockdown of TREM2 in microglia inhibited phagocytosis of apoptotic neurons and increased proinflammatory responses, suggesting that NHD is primarily a microglial disorder [56]. It remains to be established whether ALSP is also a primary microgliopathy that leads to secondary myelin and axonal damage [41,44], or whether defects in neurogenesis and neuronal survival arising from impaired CSF-1R signaling in NPCs and mature neurons contribute to the disease phenotype.
Mouse Model of ALSP
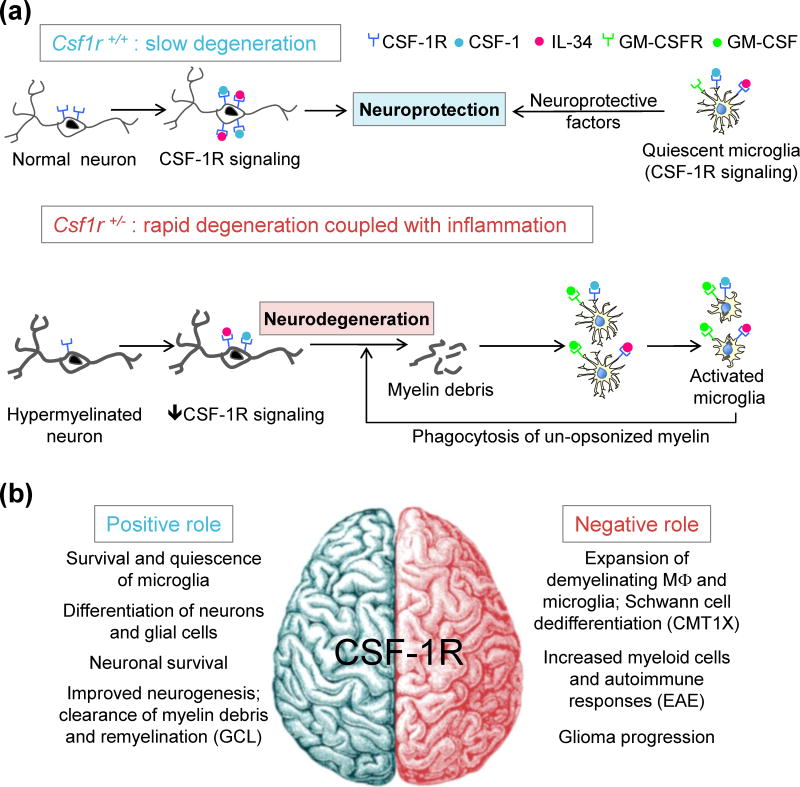
Recently, CSF1R haploinsufficiency was demonstrated in one ALSP patient [47], and Csf1r+/− mice were validated as a model of the human disease [20]. Older Csf1r+/− mice develop behavioral and histopathological deficits similar to those of ALSP patients. MRI of the brains of Csf1r+/− mice exhibiting sensorimotor deficits revealed enlargement of the lateral ventricle and thinning of the corpus callosum, in which neurodegeneration and the presence of axonal spheroids were uncovered by ultrastructural analysis. Preliminary analysis of the mechanisms involved in mouse ALSP showed that microglial densities were increased throughout the brain as early as 11 weeks of age. In addition, Csf1r+/− mice exhibited increased neuronal cell density in cortical layer V that normalized by 10 months of age. This was associated with a less-efficient upregulation of neuronal CSF-1R expression in layer V, consistent with the neuroprotective role of the CSF-1R. In Csf1r+/− mice, deep layer neurons and callosal axons were dysmyelinated and the number of oligodendrocyte precursor cells was increased in cortical layers II–III and V. Depending on their activation status, microglia can either actively demyelinate neurons or produce factors that stimulate oligodendrocyte precursor cell proliferation, differentiation, and oligodendrocyte myelination [57]. Gene expression analysis revealed early elevation of granulocyte-macrophage CSF (GM-CSF) mRNA and an age-dependent activation of the GM-CSF pathway in microglia (Figure 4A). Thus it appears that CSF-1R signaling deficits in neuronal and microglial lineages, plus high GM-CSF levels, play a central role in both the developmental and the degenerative phenotypes. By using mouse genetic and pharmacological approaches, many relevant questions can now be addressed using this model.
Figure 4.
Role of CSF-1R Signaling in Homeostasis and Diseases of the Nervous System. (A) Model of cellular interactions contributing to neurodegeneration in aging Csf1r+/− mice. In Csf1r+/+ mice, the upregulation of CSF-1R on aging neurons is neuroprotective, and CSF-1R signaling promotes a trophic phenotype in microglia. Thus the balance between age-related neurodegeneration and survival is maintained in favor of survival. Insufficient CSF1R signaling in Csf1r+/− neurons leads to more rapid neurodegeneration. These neurons are hypermyelinated and, upon their death, increase the autoantigenic load leading to inflammation and possibly to autoimmunity. Stimulation of Csf1r+/− microglia by neuronal debris in the presence of increased GM-CSF and decreased CSF-1R signaling induces an activated dendritic cell-like state, with the production of neurotoxic factors, and promotes the phagocytosis of un-opsonized myelin. This establishes a feedback loop that enhances neurodegeneration. (B) Studies in CSF-1R-deficient mice suggest that CSF-1R signaling is important for neuronal and glial cell differentiation. CSF-1R signaling in neurons limits neuronal cell death and inflammation under excitotoxic conditions. CSF-1R is also essential for the survival of microglia and promotes their quiescence. In disease states, upregulation of CSF-1 and CSF-1R expression leads to the expansion of microglia and macrophages. The final outcome (i.e., amelioration or worsening of pathology) will depend on whether the local micro-environment promotes trophic or inflammatory responses in phagocytes. Abbreviations: CMT1X, Charcot-Marie Tooth disease type 1X; EAE, Experimental autoimmune encephalomyelitis; GCL, globoid cell leukodystrophy; MΦ, macrophage.
CSF-1R as a Possible Target in Neurological Disease
The CSF-1R and its ligands have also been shown to play important roles in demyelinating diseases, neurodegeneration including Alzheimer’s disease (AD), and brain tumors (Figure 4B). Because increased levels of CSF-1, microgliosis, and microglial activation are found in many different CNS pathologies, it is important to understand the consequences of elevation of CSF-1R ligands. Mice engineered to overexpress CSF-1 in astrocytes exhibit increased microglial proliferation and decreased microglial responses to lipopolysaccharide [58]. These studies, together with the demonstration that CSF-1 increases DAP12 expression, decreases the expression of antigen-presenting proteins in human microglia [59,60] and decreases the inflammatory phenotype in mouse macrophages [61], suggest that, at steady-state, CSF-1 promotes a quiescent phenotype in microglia that may prevent their inappropriate activation and neurotoxicity. Furthermore, CSF-1R upregulation in neurons has been reported in different pathological contexts, and several studies suggest that it may promote neuronal survival [11,13,20,62]. However, in disease states in which microglia are effectors of tissue damage, CSF-1R signaling is detrimental through its promotion of microglial survival and proliferation [63–68].
Excitotoxic Injury
Apart from their regulation of microglia, the CSF-1R ligands have been shown to enhance neuronal survival following treatment with kainic acid (KA), an excitotoxic agent that induces neurodegeneration and seizures in mice [13]. Systemic administration of CSF-1 or IL-34, given before or during the acute phase of injury, is neuroprotective and inhibits microgliosis, without causing the infiltration of peripheral monocytes. Both CSF-1 and IL-34 activate cAMP responsive element-binding protein (CREB) signaling, which plays a key role in neuronal survival [69]. In vivo, systemic treatment with CSF-1 prevents loss of KA-induced phosphorylated CREB (pCREB) neuronal immunoreactivity [13]. Neuronal deletion of CSF-1R expression was sufficient to increase KA-induced neurodegeneration and lethality, indicating that CSF-1R ligands directly suppress neurodegeneration by promoting neuronal survival [13].
Demyelinating Diseases
In mouse models of demyelinating diseases, CSF-1R signaling can have destructive or reparative functions depending on the underlying cellular mechanisms. Studies in mouse models indicate that mononuclear phagocytes play major roles, both in the onset and the progression of multiple sclerosis (MS) [70,71]. In experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, administration of CSF-1R inhibitors either therapeutically, or prophylactically, reduced disease severity [66,67]. Amelioration of the disease was associated with a reduction in the number of microglia/macrophages, inhibition of myelin oligodendrocyte glycoprotein-specific T cell responses, and reduced circulating levels of tumor necrosis factor. These data suggest that CSF-1R pathways play a pivotal role in EAE. How these findings relate to the observation that CSF-1R expression is lower in MS lesions than in normal control white matter [72] is not clear.
In connexin 32-deficient mice, a model of the demyelinating peripheral neuropathy, Charcot–Marie–Tooth disease type 1X (CMT1X), both MCP-1 produced by Schwann cells and CSF-1 from endoneurial cells support the expansion of monocyte-derived macrophages and microglia that cause myelin damage [63–65]. By contrast, in the β-galactocerebrosidase-deficient twitcher mouse, a model of globoid cell leukodystrophy (GCL), progressive demyelination was exacerbated by removal of CSF-1, which decreased the number of microglia/macrophages, increased myelin debris, and decreased the recruitment of oligodendrocyte precursor cells, suggesting that clearance of myelin debris by CSF-1-activated phagocytes is crucial for remyelination [73]. In addition, CSF-1 has been shown to enhance the proliferation and survival of β-galactocere-brosidase-deficient NPCs [74]. These studies indicate that the outcome of pharmacological inhibition of the CSF-1R in demyelinating diseases may be beneficial in some cases, but not in others, depending on the cellular mechanisms involved.
Alzheimer’s Disease
The robust activation of microglia [75] and the beneficial effects of non-steroidal anti-inflammatory drugs [76] suggest that inflammation plays a central role in AD. In mouse models of AD, microglia proliferate and accumulate around senile plaques concomitantly with plaque appearance [77]. Microglial expression of CSF-1R is elevated in lesions of both AD [9] and of the amyloid β precursor protein (AβPP)V717F transgenic mouse disease model [78], and CSF-1 stimulates phagocytosis of Aβ1–42 peptide by primary human microglia in vitro [59]. Unexpectedly, approaches aimed at either activating or inhibiting CSF-1R signaling have both been able to improve cognitive function in mouse models of AD.
In the early stages of AD, tau protein ‘spreads’ from the entorhinal cortex to the hippocampus. Microglia contribute to tau propagation via exosomal secretion and, in a mouse model, microglial depletion using a CSF-1R inhibitor suppresses tau spreading [79]. Continuous inhibition of CSF-1R signaling for 3 months in two similar mouse AD models, APPSwe;PSEN1dE9; APP/PS1 [16] and βAPPSwe;PS1M146V;tauP301L [36] triple transgenic mice improves performance in memory and behavioral tasks, without decreasing the number of plaques. In both studies, the treatment did not eliminate microglia but reduced their numbers by approximately 30%. Microglial association with the plaques was decreased, and one study [36] showed that that Aβ oligomer-stimulated microglia produce factors that are chemotactic to microglia and that microglial chemotaxis towards these factors depends on CSF-1R signaling. These studies suggest that inhibition of CSF-1R signaling alters the response of microglia to the plaques.
Administration of IL-34 promotes the clearance of soluble oligomeric Aβ (oAβ), which mediates synaptic dysfunction and neuronal damage in AD. Interestingly, levels of circulating CSF-1 are reduced in AD patients [80], and intraperitoneal injection of CSF-1 in APPSwe/PS1 [81] or human amyloid precursor protein (hAPP) transgenic mouse models of AD [13] improves cognitive function with [81], or without [13], a decrease of Aβ levels in the cortex and hippocampus. In other experiments, intracerebroventricular administration of IL-34 ameliorated impairments of associative learning and reduced oAβ levels in the APP/PS1 transgenic mouse model of AD [82]. These effects were associated with upregulation of insulin-degrading enzyme, which aids in the clearance of oAβ, and with induction of the anti-oxidant enzyme heme oxygenase-1 in microglia. Together, these studies suggest that CSF-1R acts by promoting a neuroprotective phenotype in microglia. However, the observation that systemic treatment of hAPP mice with CSF-1 increases the neuronal expression of pCREB, an essential step in the survival pathway [13], is consistent with the possibility that CSF-1R ligands also directly promote neuronal survival in AD.
While at first glance the similar outcomes of inhibiting or enhancing CSF-1R signaling in AD appear contradictory, they may both be beneficial for different reasons. Depending on the treatment regimen, inhibition of CSF-1R eliminates microglia, or prevents their association with and activation by plaques. On the other hand, enhancing CSF-1R signaling may have the twofold benefit of promoting a trophic state in microglia and triggering survival in CSF-1R-expressing neurons.
Glioblastoma
CSF-1R signaling in the tumor microenvironment plays an important role in the progression of glioblastomas that secrete CSF-1. CSF-1R inhibitor treatment in mouse transgenic and human xenograft glioblastoma models has been shown to suppress tumor growth and improve survival. While tumor-associated macrophages were not depleted, owing to stimulation of their survival by glioma-secreted GM-CSFand interferon-γ, their tumor-promoting functions were inhibited by their conversion to an inflammatory M1 phenotype [83]. In addition, blockade of CSF-1R signaling suppressed EGF production by CSF-1-stimulated macrophages, thus reducing their ability to enhance glioblastoma invasion [84].
Considerations in Targeting the CSF-1R in Neurodegenerative Disease
The Yin and Yang of CSF-1R targeting in neurodegeneration (Figure 4) is illustrated by the study of Rice et al. [85], who examined the effects of inhibition of CSF-1R signaling on recovery from hippocampal lesions. Inhibition of CSF-1R signaling post-lesioning improved neuronal survival and functional recovery, whereas inhibition of CSF-1R signaling during the lesioning period increased neuronal loss. This requirement for CSF-1R signaling during the lesioning period is consistent with the findings of Luo et al. [13], who showed a direct, protective role of CSF-1R signaling in injured neurons. Thus the beneficial and detrimental roles of microglia, as well as direct effects of the CSF-1R ligands on neuronal survival, should be considered in planning the nature and timing of CSF-1R-based therapies.
Concluding Remarks
The discovery of high expression of the newly discovered CSF-1R ligand, IL-34, in brain [5], the drastic reduction of microglia in CSF-1R-deficient mice [22], together with earlier reports of neuronal expression of the CSF-1R [12,62], prompted a detailed analysis of both CSF-1R and ligand expression and the effects of CSF-1R deficiency in the developing brain [6]. This study demonstrated direct CSF-1R regulation of the neuronal lineage and revealed complementary expression patterns of the CSF-1R ligands. Analysis of IL-34- [7,8] and CSF-1- [22,27,28,30] deficient mice showed that these complementary patterns of expression were reflected in the regional regulation of microglial development and maintenance by IL-34 and CSF-1. The identification of PTPζ as a second receptor for IL-34, but not for CSF-1 [86], might explain the greater effect of IL-34 over CSF-1 on NPC differentiation [6]. These studies indicate important roles for the CSF-1R and its ligands in the direct regulation of both microglial and neuronal lineages in brain. The discovery that ALSP is caused by dominant inherited mutations in the CSF1R gene [40,41], or by CSF1R haploinsufficiency [47], provides further evidence of a central role of the CSF-1R in the brain.
These discoveries provide us with a firm footing for future work (see Outstanding Questions). An important priority is to further define the CSF-1R-regulated functions of microglia in brain development and brain function in the adult. IL-34-deficient mice can now be used to analyze the regulation of neuronal network development and function by IL-34 and be combined with CSF-1-deficiency to formally establish that these are the only activating ligands for the CSF-1R. The synergistic actions of CSF-1R and PTPζ signaling in NPCs have been identified and the underlying mechanisms involved can now be studied. The identification of a mouse model of ALSP [20] permits the assessment of how developmental and adult changes contribute to disease development and the initiation of new approaches to therapy.
Outstanding Questions.
What are the consequences of microglial deletion of the CSF-1R in development?
Can simultaneous deletion of CSF-1 and IL-34 completely recapitulate CSF-1R deficiency?
What are the consequences of IL-34 deficiency on neuronal network organization and function?
Can engagement of CSF-1R alone by CSF-1, versus engagement of both CSF-1R and PTPζ by IL-34, explain the increased efficacy of IL-34 in promoting NPC differentiation?
What is the contribution to ALSP of reduced CSF-1R signaling in the neuronal versus the microglial lineages?
What are the relative contributions of developmental deficits and post-developmental dysregulation to ALSP?
What is the optimal window for therapeutic intervention in ALSP (e.g., early postnatal prodromal phase vs post-disease onset)?
Trends.
CSF-1R is a receptor tyrosine kinase with two cognate ligands, CSF-1 and IL-34, that are expressed in largely non-overlapping areas of the CNS and that regulate microglial proliferation, and survival.
The CSF-1R is also expressed in neural progenitor cells and regulates their survival, proliferation and neuronal differentiation.
Upregulation of CSF-1R expression in injured neurons promotes survival.
Mutations in the CSF1R gene lead to an autosomal dominant, neurodegenerative disorder known as adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP).
The Csf1r+/− mouse is a validated model for testing therapeutic strategies for ALSP.
CSF-1R ligands and inhibitors are potential modulators of several neurological diseases including Alzheimer’s disease, globoid cell leukodystrophy (Krabbe’s disease), Charcot–Marie–Tooth disease, multiple sclerosis, and glioma.
Acknowledgments
This work was supported by National Institutes of Health grants R01NS071571 and R01NS096144 (to M.F.M.) and R01NS091519 and PO1 CA100324 (to E.R.S.).
Footnotes
References
- 1.Stanley ER, Heard PM. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977;252:4305–4312. [PubMed] [Google Scholar]
- 2.Lin H, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 3.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6:1–21. doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Holst A, et al. The unique 473HD-chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci. 2006;26:4082–4094. doi: 10.1523/JNEUROSCI.0422-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei S, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandi S, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greter M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama H, et al. Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis. Brain Res. 1994;639:171–174. doi: 10.1016/0006-8993(94)91779-5. [DOI] [PubMed] [Google Scholar]
- 10.Raivich G, et al. Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: a quantitative immunofluorescence study using confocal laser microscopy. J Comp Neurol. 1998;395:342–358. doi: 10.1002/(sici)1096-9861(19980808)395:3<342::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Expression of colony stimulating factor-1 receptor (CSF-1R) by CNS neurons in mice. J Neurosci Res. 1999;57:616–632. [PubMed] [Google Scholar]
- 12.Murase S, Hayashi Y. Expression pattern and neurotrophic role of the c-fms proto-oncogene M-CSF receptor in rodent Purkinje cells. J Neurosci. 1998;18:10481–10492. doi: 10.1523/JNEUROSCI.18-24-10481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo J, et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med. 2013;210:157–172. doi: 10.1084/jem.20120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sierra A, et al. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 15.Erblich B, et al. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olmos-Alonso A, et al. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain. 2016;139:891–907. doi: 10.1093/brain/awv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasmono RT, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 18.Burnett SH, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J Cell Biochem. 1999;72:119–134. [PubMed] [Google Scholar]
- 20.Chitu V, et al. Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP) Neurobiol Dis. 2015;74:219–228. doi: 10.1016/j.nbd.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan GR, et al. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- 22.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hristova M, et al. Activation and deactivation of periventricular white matter phagocytes during postnatal mouse development. Glia. 2010;58:11–28. doi: 10.1002/glia.20896. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elmore MR, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wegiel J, et al. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res. 1998;804:135–139. doi: 10.1016/s0006-8993(98)00618-0. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki A, et al. Effects of macrophage-colony-stimulating factor deficiency on the maturation of microglia and brain macrophages and on their expression of scavenger receptor. Neuropathology. 2000;20:134–142. doi: 10.1046/j.1440-1789.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 29.Lelli A, et al. The NADPH oxidase Nox2 regulates VEGFR1/CSF-1R-mediated microglial chemotaxis and promotes early postnatal infiltration of phagocytes in the subventricular zone of the mouse cerebral cortex. Glia. 2013;61:1542–1555. doi: 10.1002/glia.22540. [DOI] [PubMed] [Google Scholar]
- 30.Kondo Y, Duncan ID. Selective reduction in microglia density and function in the white matter of colony-stimulating factor-1-deficient mice. J Neurosci Res. 2009;87:2686–2695. doi: 10.1002/jnr.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson GR, Burgess AW. Molecular and biological properties of a macrophage colony-stimulating factor from mouse yolk sacs. J Cell Biol. 1978;77:35–47. doi: 10.1083/jcb.77.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arno B, et al. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 2014;5:13. doi: 10.1038/ncomms6611. [DOI] [PubMed] [Google Scholar]
- 33.Englund C, et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waisman A, et al. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol. 2015;36:625–636. doi: 10.1016/j.it.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dagher NN, et al. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3×Tg-AD mice. J Neuroinflammation. 2015;12:139. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruttger J, et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Chitu V, et al. PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts. Blood. 2012;120:3126–3135. doi: 10.1182/blood-2012-04-425595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rademakers R, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet. 2011;44:200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson AM, et al. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology. 2013;80:1033–1040. doi: 10.1212/WNL.0b013e31828726a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Axelsson R, et al. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl. 1984;314:1–65. [PubMed] [Google Scholar]
- 43.Van Bogaert L, Nyssen R. Le type tardif de la leukodystrophie progressive familiale. Rev Neurol (Paris) 1936;65:21–45. [Google Scholar]
- 44.Wider C, et al. Leukoencephalopathy with spheroids (HDLS) and pigmentary leukodystrophy (POLD): a single entity? Neurology. 2009;72:1953–1959. doi: 10.1212/WNL.0b013e3181a826c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann S, et al. Enlarging the nosological spectrum of hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) Brain Pathol. 2014;24:452–458. doi: 10.1111/bpa.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundal C, et al. MRI characteristics and scoring in HDLS due to CSF1R gene mutations. Neurology. 2012;79:566–574. doi: 10.1212/WNL.0b013e318263575a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konno T, et al. Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS. Neurology. 2014;82:139–148. doi: 10.1212/WNL.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondo Y, et al. Early involvement of the corpus callosum in a patient with hereditary diffuse leukoencephalopathy with spheroids carrying the de novo K793T mutation of CSF1R. Intern Med. 2013;52:503–506. doi: 10.2169/internalmedicine.52.8879. [DOI] [PubMed] [Google Scholar]
- 49.Chitu V, et al. PDGF receptor family. In: Wheeler DL, Yarden Y, editors. The Receptor Tyrosine Kinases: Family and Subfamilies. 1. Springer Science; 2015. pp. 373–538. [Google Scholar]
- 50.Hiyoshi M, et al. M-CSF receptor mutations in hereditary diffuse leukoencephalopathy with spheroids impair not only kinase activity but also surface expression. Biochem Biophys Res Commun. 2013;440:589–593. doi: 10.1016/j.bbrc.2013.09.141. [DOI] [PubMed] [Google Scholar]
- 51.Pridans C, et al. CSF1R mutations in hereditary diffuse leukoencephalopathy with spheroids are loss of function. Sci Rep. 2013;3:3013. doi: 10.1038/srep03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otero K, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guan Z, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paloneva J, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 55.Paloneva J, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, et al. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De I, et al. CSF1 overexpression has pleiotropic effects on microglia in vivo. Glia. 2014;62:1955–1967. doi: 10.1002/glia.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AM, et al. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J Neuroinflammation. 2013;10:15. doi: 10.1186/1742-2094-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith AM, et al. Adult human glia, pericytes and meningeal fibroblasts respond similarly to IFNγ but not to TGFbeta1 or M-CSF. PLoS ONE. 2013;8:e80463. doi: 10.1371/journal.pone.0080463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caescu CI, et al. Colony stimulating factor-1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA-21. Blood. 2015;125:e1–e13. doi: 10.1182/blood-2014-10-608000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berezovskaya O, et al. Colony stimulating factor-1 potentiates neuronal survival in cerebral cortex ischemic lesion. Acta Neuropathol. 1996;92:479–486. doi: 10.1007/s004010050550. [DOI] [PubMed] [Google Scholar]
- 63.Groh J, et al. Colony-stimulating factor-1 mediates macrophage-related neural damage in a model for Charcot–Marie–Tooth disease type 1X. Brain. 2012;135:88–104. doi: 10.1093/brain/awr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groh J, et al. CSF-1-activated macrophages are target-directed and essential mediators of Schwann cell dedifferentiation and dysfunction in Cx32-deficient mice. Glia. 2015;63:977–986. doi: 10.1002/glia.22796. [DOI] [PubMed] [Google Scholar]
- 65.Klein D, et al. Targeting the colony stimulating factor 1 receptor alleviates two forms of Charcot–Marie–Tooth disease in mice. Brain. 2015;138:3193–3205. doi: 10.1093/brain/awv240. [DOI] [PubMed] [Google Scholar]
- 66.Uemura Y, et al. The selective M-CSF receptor tyrosine kinase inhibitor Ki20227 suppresses experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;195:73–80. doi: 10.1016/j.jneuroim.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 67.Crespo O, et al. Tyrosine kinase inhibitors ameliorate autoimmune encephalomyelitis in a mouse model of multiple sclerosis. J Clin Immunol. 2011;31:1010–1020. doi: 10.1007/s10875-011-9579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez-Nicola D, et al. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci. 2013;33:2481–2493. doi: 10.1523/JNEUROSCI.4440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- 70.McQualter JL, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hesske L, et al. Induction of inhibitory central nervous system-derived and stimulatory blood-derived dendritic cells suggests a dual role for granulocyte-macrophage colony-stimulating factor in central nervous system inflammation. Brain. 2010;133:1637–1654. doi: 10.1093/brain/awq081. [DOI] [PubMed] [Google Scholar]
- 72.Werner K, et al. The relative number of macrophages/microglia expressing macrophage colony-stimulating factor and its receptor decreases in multiple sclerosis lesions. Glia. 2002;40:121–129. doi: 10.1002/glia.10120. [DOI] [PubMed] [Google Scholar]
- 73.Kondo Y, et al. Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy. J Neurosci. 2011;31:3610–3624. doi: 10.1523/JNEUROSCI.6344-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee H, et al. Defective self-renewal and differentiation of GBA-deficient neural stem cells can be restored by macrophage colony-stimulating factor. Mol Cells. 2015;38:806–813. doi: 10.14348/molcells.2015.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoozemans JJ, et al. Soothing the inflamed brain: effect of non-steroidal anti-inflammatory drugs on Alzheimer’s disease pathology. CNS Neurol Disord Drug Targets. 2011;10:57–67. doi: 10.2174/187152711794488665. [DOI] [PubMed] [Google Scholar]
- 77.Kamphuis W, et al. Differential cell proliferation in the cortex of the APPswePS1dE9 Alzheimer’s disease mouse model. Glia. 2012;60:615–629. doi: 10.1002/glia.22295. [DOI] [PubMed] [Google Scholar]
- 78.Murphy GM, Jr, et al. Expression of macrophage colony-stimulating factor receptor is increased in the AbetaPP(V717F) transgenic mouse model of Alzheimer’s disease. Am J Pathol. 2000;157:895–904. doi: 10.1016/s0002-9440(10)64603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asai H, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray S, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 81.Boissonneault V, et al. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- 82.Mizuno T, et al. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-beta neurotoxicity. Am J Pathol. 2011;179:2016–2027. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coniglio SJ, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519–527. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rice RA, et al. Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. J Neurosci. 2015;35:9977–9989. doi: 10.1523/JNEUROSCI.0336-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nandi S, et al. Receptor-type protein-tyrosine phosphatase zeta is a functional receptor for interleukin-34. J Biol Chem. 2013;288:21972–21986. doi: 10.1074/jbc.M112.442731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cunningham CL, et al. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Squarzoni P, et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 89.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 90.Wu Y, et al. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nikodemova M, et al. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J Neuroimmunol. 2015;278:280–288. doi: 10.1016/j.jneuroim.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marin-Teva JL, et al. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 93.Wakselman S, et al. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato K. Effects of microglia on neurogenesis. Glia. 2015;63:1394–1405. doi: 10.1002/glia.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ribeiro Xavier AL, et al. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci. 2015;35:11848–11861. doi: 10.1523/JNEUROSCI.1217-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao F, et al. CX3 chemokine receptor 1 deficiency leads to reduced dendritic complexity and delayed maturation of newborn neurons in the adult mouse hippocampus. Neural Regen Res. 2015;10:772–777. doi: 10.4103/1673-5374.156979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walker DG, Lue LF. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7:9. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin S, et al. Interleukin-34 restores blood-brain barrier integrity by upregulating tight junction proteins in endothelial cells. PLoS ONE. 2014;9:e115981. doi: 10.1371/journal.pone.0115981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peles E, et al. Multi-ligand interactions with receptor-like protein tyrosine phosphatase beta: implications for intercellular signaling. Trends Biochem Sci. 1998;23:121–124. doi: 10.1016/s0968-0004(98)01195-5. [DOI] [PubMed] [Google Scholar]
- 101.Kuboyama K, et al. Protein tyrosine phosphatase receptor type z negatively regulates oligodendrocyte differentiation and myelination. PLoS ONE. 2012;7:e48797. doi: 10.1371/journal.pone.0048797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buxbaum JD, et al. Molecular dissection of NRG1–ERBB4 signaling implicates PTPRZ1 as a potential schizophrenia susceptibility gene. Mol Psychiatry. 2008;13:162–172. doi: 10.1038/sj.mp.4001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ratcliffe CF, et al. A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase beta. Nat Neurosci. 2000;3:437–444. doi: 10.1038/74805. [DOI] [PubMed] [Google Scholar]
- 104.Himburg HA, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dai XM, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 106.Marks SC, Jr, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976;67:11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- 107.Michaelson MD, et al. CSF-1 deficiency in mice results in abnormal brain development. Development. 1996;122:2661–2672. doi: 10.1242/dev.122.9.2661. [DOI] [PubMed] [Google Scholar]
- 108.Cohen PE, et al. Colony-stimulating factor-1 plays a major role in the development of reproductive function in male mice. Mol Endocrinol. 1997;11:1636–1650. doi: 10.1210/mend.11.11.0009. [DOI] [PubMed] [Google Scholar]
- 109.Cohen PE, et al. Colony-stimulating factor 1 regulation of neuroendocrine pathways that control gonadal function in mice. Endocrinology. 2002;143:1413–1422. doi: 10.1210/endo.143.4.8754. [DOI] [PubMed] [Google Scholar]