Abstract
The cell surface molecules used by Epstein-Barr virus (EBV) to attach to epithelial cells are not well-defined, although when CD21, the B cell receptor for EBV is expressed epithelial cell infection increases disproportionately to the increase in virus bound. Many herpesviruses use low affinity charge interactions with molecules such as heparan sulfate to attach to cells. We report here that the EBV glycoprotein gp150 binds to heparan sulfate proteoglycans, but that attachment via this glycoprotein is not productive of infection. We also report that only the aminoterminal two short consensus repeats of CD21 are required for efficient infection, This supports the hypothesis that, when expressed on an epithelial cell CD21 serves primarily to cluster the major attachment protein gp350 in the virus membrane and enhance access of other important glycoproteins to the epithelial cell surface.
Keywords: Epstein-Barr virus, glycoprotein 150, heparan-binding, CD21, epithelial cell infection
INTRODUCTION
Human herpesvirus 4 or Epstein-Barr virus (EBV) is one of eight human herpesviruses and is carried by more than 95% of the global adult population. Primary infections are often asymptomatic, but can be accompanied by infectious mononucleosis. The virus establishes latency in memory B lymphocytes and is modeled as replicating productively in epithelial cells of the oropharynx. Persistently infected carriers typically suffer no long term consequences, but the virus is associated, as a causal or contributing factor, with several lymphoid and epithelial malignancies, reflecting its tropism for cells of these lineages (Longnecker et al., 2013).
Tropism is in part determined by the virus glycoproteins, of which there are eleven in the virion envelope (Gore and Hutt-Fletcher, 2008). Virus attachment to B cells results from an interaction between the most abundant virus glycoprotein, gp350 (Nemerow et al., 1987; Tanner et al., 1987), and one of two complement receptors, CR2 or CD21 (Fingeroth et al., 1984) and CR1 or CD35 (Ogembo et al., 2013). Entry is mediated by glycoproteins gB and a complex of three glycoproteins gHgL and gp42 (Hutt-Fletcher, 2007). Glycoprotein gp42 interacts with HLA class II (Haan et al., 2000; Li et al., 1997) and this essential interaction (Wang and Hutt-Fletcher, 1998) is thought to initiate a cascade of events which ultimately leads to fusion, probably executed as gB, which is modeled as a class III fusion protein (Backovic and Jardetzky, 2009; Backovic et al., 2009), inserts into the cell membrane and undergoes a conformational change (Chesnokova et al., 2014).
Fusion with an epithelial cell is mediated by three rather than four glycoproteins (Wang et al., 1998). Epithelial cells do not constitutively express HLA class II and, instead of the interaction between gp42 and HLA class II, a direct interaction between gHgL, which carries a KGD motif, and one of three αv integrins, αvβ5, αvβ6 or αvβ8, provides the initiating event (Chesnokova and Hutt-Fletcher, 2011; Chesnokova et al., 2009). The presence of gp42 blocks access of gHgL to integrins (Chen et al., 2012; Wang et al., 1998). Thus the virus carries both two part gHgL complexes and three part gHgLgp42 to infect both cell types, with gp42 acting as a rheostat to switch tropism as its levels are modulated by the cell type in which virus is produced (Borza and Hutt-Fletcher, 2002).
Although the molecules involved in B cell attachment and B cell and epithelial cell fusion are generally agreed on, there remains uncertainty concerning productive epithelial cell attachment. Several glycoproteins and cell receptors have been proposed as being involved. Some, cultured epithelial cells express CD21 (Fingeroth et al., 1999), but CD21 expression in the oropharynx in vivo appears to be limited to tonsil and adenoid epithelium (Jiang et al., 2012; Jiang et al., 2008) and epithelial cells at other sites can also be infected (Temple et al., 2014; Vincent-Bugnas et al., 2013; Walling et al., 2001). In the absence of CD21 virus can attach to αv integrins via gHgL, but infection, when virus uses gHgL for attachment as well as fusion, is very inefficient (Borza et al., 2004), much less so that when virus is bound via CD21, for unknown reasons. Binding of virus cross-links CD21 and in an epithelial cell results in colocalization with the formin FHOS/FHOD1, which binds to the cytoplasmic domain of CD21 (Gill et al., 2004). Formins directly nucleate actin (Goode and Eck, 2007) and since actin dynamics seemed to be uniquely important to virus transport in epithelial cells, the possibility that CD21 might provide a relevant link to cytoskeletal reorganization was considered. Deletion of the cytoplasmic tail, however had no effect, although the possible interaction of the transmembrane domain or ectodomain with other epithelial molecules was not explored (Valencia and Hutt-Fletcher, 2012). The BMRF2 protein, which complexes with the BDLF2 protein (Gore and Hutt-Fletcher, 2008), can bind to α3β1, α5β1 and αvβ1 integrins via an RGD motif, and is important for infection of polarized epithelial cells (Tugizov et al., 2003), but whether its primary role is in attachment, signaling or lateral spread of virus, as it is for the homologous protein complex in the murine gammaherpesvirus 68 (Gill et al., 2008), is not entirely clear.
Many herpesviruses, including the human herpesviruses herpes simplex virus (Spear, 2004), Kaposi’s sarcoma associated herpesvirus (Veettil et al., 2104), human cytomegalovirus (Compton et al., 1993) and human herpesvirus 7 (Secchiero et al., 1997), use low affinity charge interactions with molecules like heparan sulfate proteoglycans for initial attachment to cells. In some cases more than one virion glycoprotein is used for the purpose. There is a small amount of residual virus binding seen when a gH-null EBV is added to a CD21-negative epithelial cell where virus also does not use BMRF2 for attachment (Molesworth et al., 2000; Oda et al., 2000). We therefore considered the possibility that this residual binding might represent an interaction with heparan sulfate or another proteoglycan. A potential role for the transmembrane and ectodomains of CD21 beyond that of attachment was also explored.
RESULTS AND DISCUSSION
Binding of a gH-null virus to CD21-negative epithelial cells is low but saturable
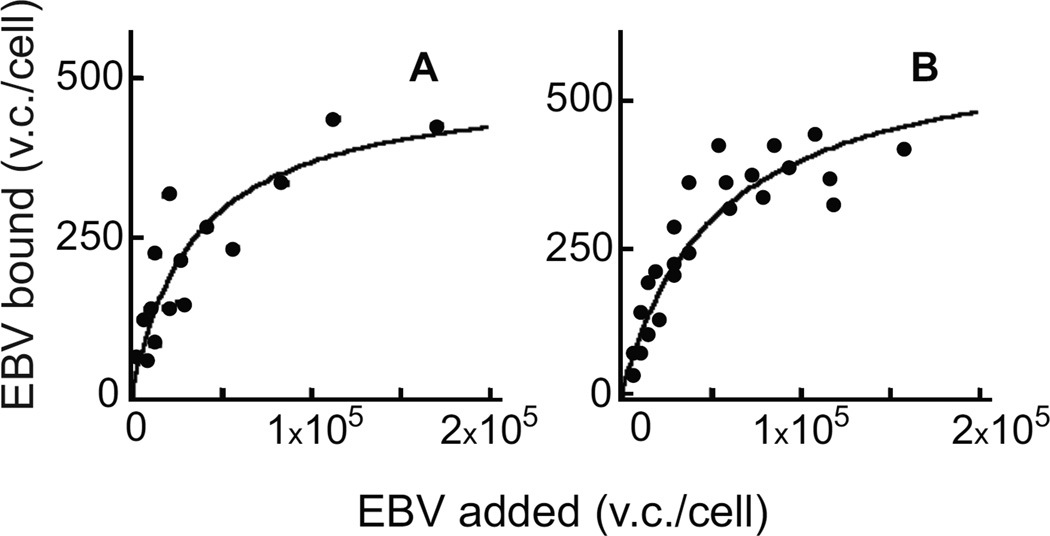
To determine the number and avidity of binding sites for a gH-null virus on CD21-negative epithelial cells, AGS gastric carcinoma cells and hTERT immortalized normal oral keratinocytes (NOK) were incubated with increasing copy numbers of virus for 1h on ice. Virus was removed, cells were washed and the amount of virus DNA remaining associated with cells was measured by quantitative real-time PCR (QPCR). The number of binding sites on the two cells was similar and low, 590±80 per cell on AGS and 640±50 on NOK, although saturation could be reached at high virus dose (Figure 1). The avidity, determined as the number of virus particles needed to achieve 50% saturation, was also low, 36,000±13,000 for AGS and 40,000±800 for NOK.
Figure 1.
Scatchard analysis of binding of gH-null virus. Different copy numbers of virus were added to AGS (A) or NOK (B) cells for 1h on ice, cells were washed and the amount of virus remaining bound per cell was determined by QPCR. Virus copies (v.c.).
Binding is heparan sulfate dependent
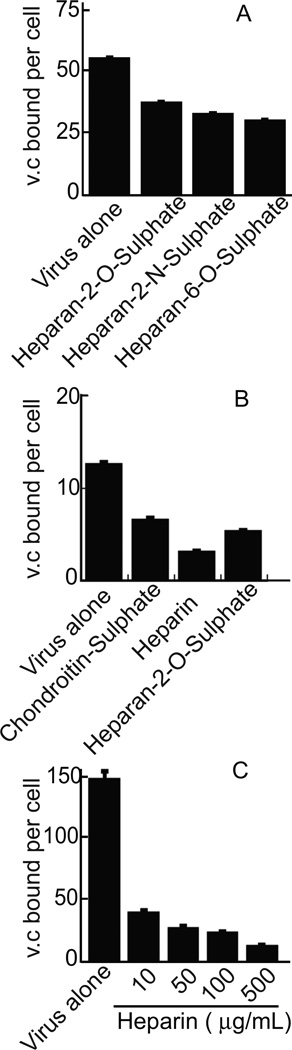
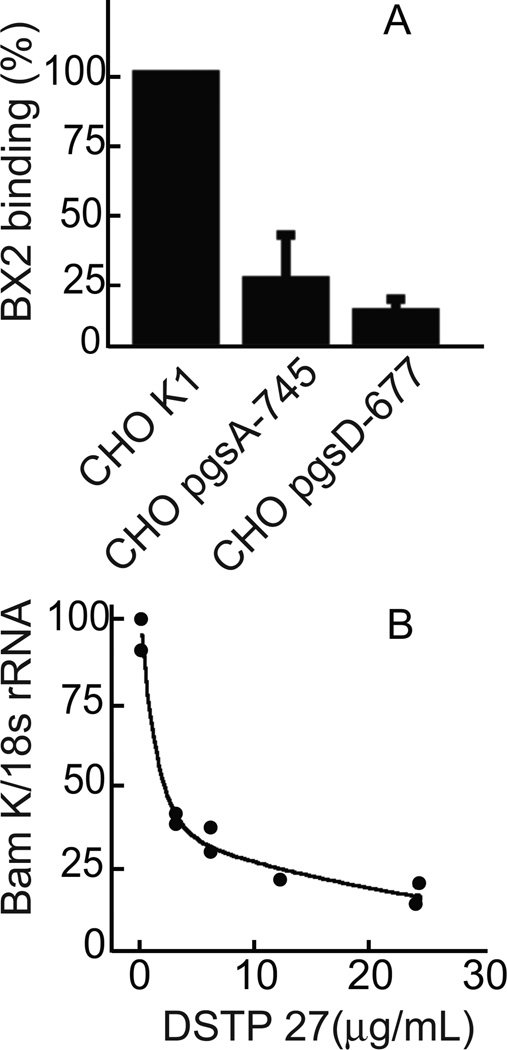
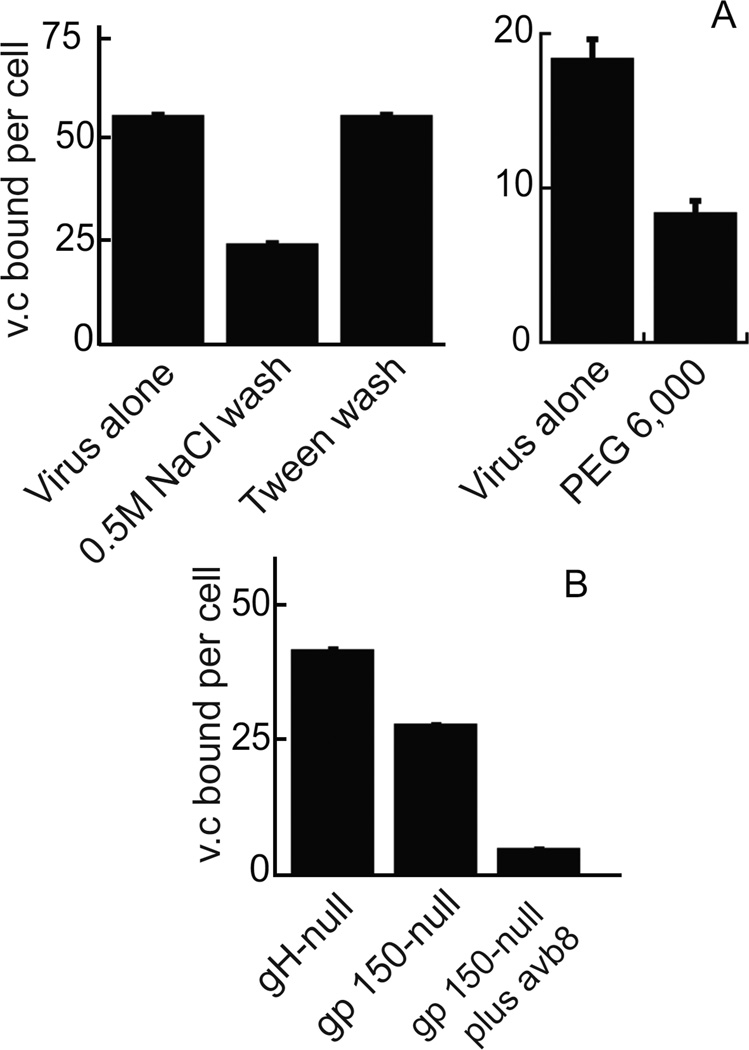
Attachment of herpesviruses that use heparan sulfate proteoglycans for initial binding to cells can be blocked by the presence of soluble heparins. Binding of gH-null EBV to NOK or AGS cells could be inhibited by preincubation of virus with different derivatives of heparan sulfate or chondroitin sulfate (Figure 2). Heparin was the most effective and, in the presence of increasing concentrations, virus binding was reduced in a dose dependent manner. Since heparin was able to reduce binding, the ability of virus to bind to CHO K1 cells, CHO pgsA-745 cells, which lack glycosoaminoglycans, and CHO pgsD-677 cells, which cannot synthesize heparan sulfate, was compared. gH-null virus bound well to CHO K1 cells, but poorly to both CHO pgsA-754 and CHO pgsD-677 cells (Figure 3). Virus binding was also reduced in a dose dependent manner by preincubation of CHO K1 cells with the N-N’-(bis-5-nitropyrimidyl) dispirotripoperazine derivate 27 (DSTP 27), a compound that binds to heparan sulfate residues and can block infection of viruses that use heparan sulfate proteoglycans for attachment (Schmidtke et al., 2003; Selinka et al., 2007). gH-null virus binding to NOK cells was reduced by a high salt wash, but not by washing in 0.1% Tween 20, 25mM HEPES pH 7.0–0.15M NaCl, suggesting an ionic and not a hydrophobic interaction (Figure 4A). Polyethylene glycol, which alters the hydration shell of a protein (Rawat et al., 2010) and masks charge, also inhibited binding by a little over 50%.
Figure 2.
Reduction of virus binding by soluble proteoglycans. gH-null virus was preincubated on ice with 100 µg/ml of the indicated proteoglycans for 1h and then added to NOK (A and C) or AGS (B) cells for 1h on ice. Cells were washed and virus (BamK) and cell (CRP) DNA were measured by QPCR. Virus copies (v.c.). Error bars are calculated as the standard quotient error. Experiments in panel A were repeated 4 times and those in panels B and C were repeated 3 times.
Figure 3.
Virus binding in the absence of heparan sulfate proteoglycans or in the presence of an inhibitory ligand for heparan sulfate. (A) gH-null virus was incubated for 1h on ice with CHO K1, CHO pgs-745 or CHO pgsD-677 cells as indicated, cells were washed and virus (BamK) and cell (18s rRNA) DNA were determined by QPCR. Binding is expressed as a percent of binding to CHO K1. The experiment was repeated 3 times and the difference between binding to CHO K1 versus the other two CHO mutant lines has a p value of <0.001 as calculated by an unpaired T-test. (B) CHO K1 cells were incubated with indicated concentrations of DSTP 27 for 30 min and then gH-null virus was added for 1h on ice. Cells were washed and virus (BamK) and cell (18s rRNA) DNA were measured by QPCR.
Figure 4.
Virus binding is reduced by high salt or polyethylene glycol and is lost if binding via gH and gp150 is abrogated. (A) gH-null virus was either added to NOK cells for 1h on ice and then cells were washed with salt or Tween 20 as indicated (left panel) or virus was preincubated with polyethylene 6,000 (PEG 6,000) before adding to cells (right panel). Virus (BamK) and cell (CRP) DNA were measured by QPCR. (B) Virus that was gH-null, gp150-null virus or gp150-null and preincubated with soluble αvβ8, as indicated, was added to NOK cells for 1 h on ice. Cells were washed and virus (Bam K) and cell (CRP) DNA were measured by QPCR. Virus copies (v.c.). Error bars are calculated as the standard quotient error. Each experiment was repeated 3 times.
Binding is mediated by virus glycoprotein gp150
The envelope proteins of EBV, like those of most viruses, are glycosylated, some carrying both N- and O-linked sugars which like, heparan sulfate, would have a negative charge (Bishop et al., 2007). However, many of the protein backbones themselves are theoretically cationic. The protein with the highest predicted pI is gp150, the product of the BDLF3 gene. Several years ago we made a virus in which the BDLF3 gene was interrupted by a neomycin resistance cassette (Borza and Hutt-Fletcher, 1998). This gp150-null virus bound slightly less well to NOK cells than an equal copy number of gH-null virus and the small amount of virus that bound could be blocked almost completely by pre-incubation with soluble integrin αvβ8 which competes with cellular integrins for binding to gH (Figure 4B).
The observation that, like many herpesviruses, EBV can bind to heparan sulfate proteoglycans is perhaps not surprising. What is more surprising is that this binding, at least on epithelial cells, is apparently non-productive. We previously found that the virus that lacks gp150, although it has no phenotype for B cell infection, infects epithelial cells very slightly better than does wild type virus (Borza and Hutt-Fletcher, 1998). Virus lacking gp350, the ligand of CD21, has been reported to be able to infect B cells, including B cell lines (Janz et al., 2000). However, B cell lines often lack heparan sulfate (Jarousse et al., 2008) and normal B cells also express only very low levels (Bala Chandran, personal communication) so it also seems unlikely that gp150 and heparan sulfate proteoglycans can account for B cell infection by a gp350-null virus.
The transmembrane domain and most of the ectodomain of CD21 do not influence infection
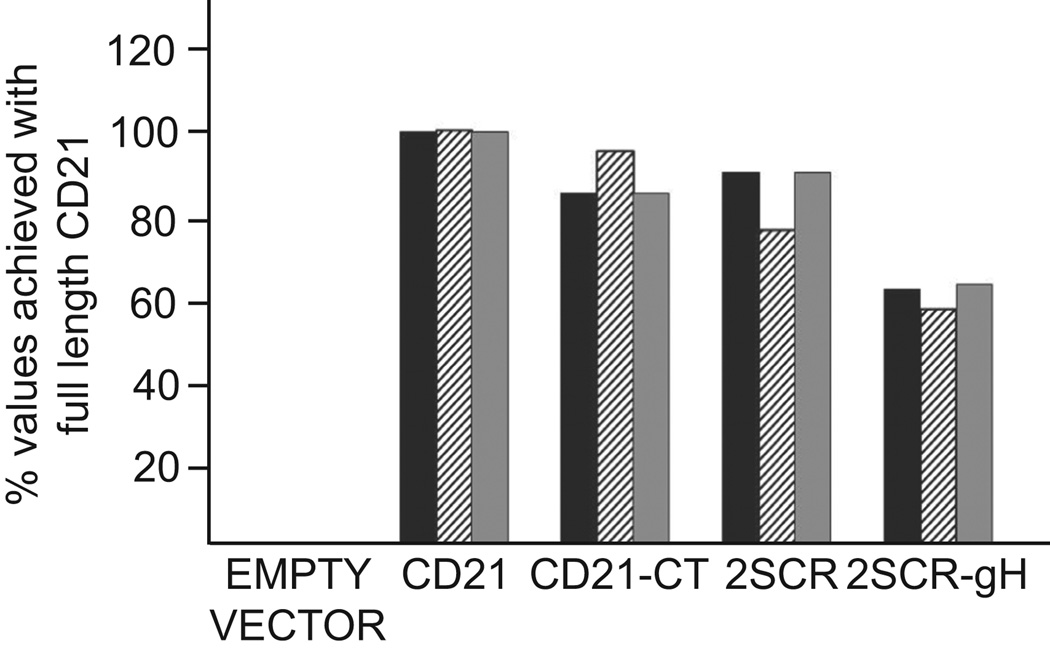
The robust levels of virus binding to an epithelial that can be achieved as a result of the interaction between gHgL and an integrin, do not translate into the robust levels of infection that can be achieved when CD21 is used. The cytoplasmic tail of CD21 plays no role in this robust level of infection (Valencia and Hutt-Fletcher, 2012), but to explore if other domains of the protein are important, perhaps mediating interactions with additional cell proteins, two new CD21 constructs were made, one in which 13 of the 15 short consensus repeats (SCR) in the ectodomain (Ahearn and Fearon, 1989) were deleted, putting the two aminoterminal SCRs containing the gp350 binding site (Martin et al., 1991) immediately adjacent to the transmembrane domain (CD21-2SCR) and one in which the two aminoterminal SCRs were placed adjacent to the cytoplasmic tail and transmembrane domain of EBV glycoprotein gH (CD21-2SCR-gH). Each of four constructs, full length CD21, CD21-CT, missing the cytoplasmic tail, CD21-2SCR and CD21-2SCR-gH were nucleoporated into AGS cells. Binding of gH-null virus, which cannot interact with integrins, was measured by QPCR, infection with virus expressing GFP and levels of expression of the aminoterminal two SCRs of CD21 were evaluated by flow cytometry. The expression levels of CD21 lacking a cytoplasmic tail or containing only the aminoterminal SCRs were slightly lower than those of full length CD21 (set at 100%) and expression of CD21 containing the aminoterminal SCRs anchored by the cytoplasmic tail and transmembrane domain of gH was only about sixty percent of the expression levels of full length CD21. However, virus binding and virus infection in each case paralleled expression levels of the aminoterminal 2 SCRs suggesting that, unless the aminoterminal 2 SCRs themselves interact with other membrane components, which cannot be absolutely ruled out, interactions of CD21 with additional cell membrane components are unlikely to be important for infection. Instead, several observations support an alternative explanation for why CD21 expression makes such a difference to ease of infection of an epithelial cell. Binding of virus to CD21 on a B cell, which causes capping of the gp350/CD21 complex, followed by transfer of bound virus to an epithelial cell enhances infection and a gp350-null virus is better able to infect a CD21-negative epithelial cell than is wild type virus, implying that when not used, gp350 gets in the way (Shannon-Lowe et al., 2006). Further, antibodies to gp350 capable of cross-linking the protein enhance epithelial infection. Glycoprotein gp350 is not only the most abundant virus glycoprotein (Johannsen et al., 2004), it is also modeled as a long extended structure (Moore et al., 1989) which when bound to CD21 initially places the virus approximately 50 nm from the cell surface (Nemerow and Cooper, 1984). It seems possible then that CD21 serves not only to anchor virus to the cell surface, but also to cluster gp350 in the virus membrane, facilitating access of other virus glycoproteins critical to the entry process.
MATERIALS AND METHODS
Cells, virus, antibodies and reagents
Akata-GFP, a Burkitt lymphoma-derived cell carrying a recombinant EBV in which the thymidine kinase gene is interrupted with a double cassette expressing neomycin resistance and the green fluorescence protein, Akata-gH-null, carrying a recombinant EBV in which the BXLF2 open reading frame encoding gH is interrupted with the same cassette (Molesworth et al., 2000) and Akata-gp150-null, carrying a recombinant EBV in which the BDLF3 gene is interrupted by a neomycin resistance cassette (Borza and Hutt-Fletcher, 1998) were grown in RPMI 1640 (Sigma) supplemented with G-418 at 500µg/mL. AGS, human gastric carcinoma cells (American Type Culture Collection) that have been cured of parainfluenza type 5 infection by treatment with ribavirin, and CHO K1 cells, CHO pgsA-745 cells (Esko et al., 1985), which are deficient in glycosaminoglycans and CHO pgsA-677 cells (Lidholt et al., 1992), which are deficient in heparan sulfate biosynthesis, were grown in Ham’s F-12 medium (Sigma). SVK cells, SV40-transformed keratinocytes (Li et al., 1992) were grown in Joklik’s modified Eagle’s medium. NOK cells, hTert-immortalized normal oral keratinocytes (a gift of Karl Műnger, Harvard University) were grown in Keratinocyte SFM (Gibco). 293-B8 AVAP cells (Mu et al., 2002), which secrete truncated αvβ8 conjugated to alkaline phosphatase (AP) and were a gift of Stephen Nishimura (UCSF), were grown in DMEM (Sigma) supplemented with 1% nonessential amino acids. Media for all cells, except SVK cells, which was supplemented with 10% heat inactivated Cosmic calf serum (Hyclone), and NOK cells, were also supplemented with 10% heat-inactivated fetal bovine serum (Gibco). Akata virus made in B cells was harvested from the spent culture media of cells that had been induced by treatment with anti-human immunoglobulin as described (Molesworth et al., 2000). Truncated αvβ8 was purified from media of 293-B8 AVAP cells as previously described (Chesnokova and Hutt-Fletcher, 2011). DTSP 27 was a gift of Dr. Martin Sapp, LSU Health Sciences Center-Shreveport. Heparin, heparan sulfates and chondroitin sulfate and polyethylene glycol 6000 were obtained from Sigma and Tween 20 from Fisher Scientific. Monoclonal antibody 171 which reacts with SCR1 and SCR2 of CD21 was a gift of Dr. Michael Holers, University of Colorado. CD21 expression was visualized with antibody 171 and goat anti-mouse antibody conjugated to phycoerythrin (PE) (Jackson ImmunoResearch).
Virus binding and quantitative real time PCR (QPCR)
Cells were detached by trypsin and recovered in complete media for one hour. Virus was diluted in RPMI media containing 100 µg/ml bacitracin and bound to cells for one hour on ice. Unbound virus was removed by three washes with phosphate buffered saline (PBS) and DNA was isolated using QUIamp DNA Blood mini kit (Qiagen, Cat. #51104), according to the manufacturer’s protocol. A 76-bp region of the EBV EBNA1 gene in the BamHI K fragment was amplified together with a 101-bp region of the human C-reactive protein (CRP) gene. The EBV probe was labeled with 6-carboxyfluorescein (FAM) and the CRP probe was labeled with VIC (PE Applied Biosystems). Serial dilutions of DNA from IB4, a Burkitt’s lymphoma cell line containing five copies of EBV per cell, served as a standard (Valencia and Hutt-Fletcher, 2012). Binding to CHO K1 cells was determined using a SYBR Green assay with the same BamHI K primers as were used for the TaqMan analysis and primers that amplified an 83-bp region of the CHO K1 18s ribosomal RNA gene (Soltany-Rezaee-Rad et al., 2015). This is a multicopy gene and virus amplification was expressed relative to 18s ribosomal amplification and not as absolute values per cell.
CD21 plasmid constructs
Plasmids pCAGGS-CD21 and pCAGGS-CD21-CT were made as previously described (Valencia and Hutt-Fletcher, 2012). pCAGGS-CD21-2SCR was made by cutting pCAGGS-CD21 at an Xho 1 site at 454, 2 bp prior to the start of the sequence of the third SCR and at a Bgl II site in pCAGGS, 3’ to the stop codon of CD21. 209 bp including the sequence encoding the cytoplasmic tail and transmembrane domain of CD21 and 10 residues of the adjacent ectodomain were amplified with primers 5’-CGTATTCTCGAG GGGTGCCAGGTGTGCCAACTTG-3’ and 5’-CTGATT AGATCT TCAGCTGGCTGGGTTGTA TGGATC-3’, cut with Xho 1 and Bgl II and cloned into pCAGGS-CD21 cut with the same enzymes. pCAGGS-CD21-2SCR-gH was made from pCAGGS-CD21-2SCR by amplification with the forward primer 5’-AATTATGAATTCATGGGCGCCGCGGGC-3’ which included the initiation codon and the reverse primer 5'-CGATTAGCATGCCTAAAGGAAAAACATAACAATC TTGTGAACCAGAAAGATACCCAGAGCAAAAGCAATAAAGTACAGGATTATTGCCAAAACAA CGTGTGCTCTGCAAACCGCCAGGG-3' which included sequences encoding the cytoplasmic tail and transmembrane domain of EBV gH and 17 bp corresponding to the sequence of the ectodomain of CD21 immediately adjacent to its transmembrane domain.
Infection of cells expressing CD21 constructs
Three batches of 106 AGS cells were each nucleofected with 5 µg pCAGGS, pCAGGS-CD21, pCAGGS-CD21-2SCR or pCAGGS-2SCR-gH using an Amaxa Nucleofector 4DX, kit SF and program DS138. The three batches were pooled and incubated for 72 h. The pooled cells were again divided into three, one set was stained with monoclonal antibody 171 and goat anti-mouse antibody conjugated to phycoerythrin and examined by flow cytometry. Virus lacking gH was bound to a second set for 1 h and binding was measured by QPCR. The third set was infected with Akata GFP-virus and GFP expression was measured by flow cytometry 48 h later.
Figure 5.
Only the aminoterminal two short consensus repeats are needed to enhance infection. AGS cells were nucleoporated with empty vector, plasmids expressing full length CD21, CD21 lacking a cytoplasmic tail (CD21-CT), CD21 comprised of only the aminoterminal 2 SCRs (2SCR) or the aminoterminal 2SCRs anchored by the cytoplasmic tail and transmembrane domain of gH (2SCR-gH). At 24 h cells were stained with an antibody to the aminoterminal 2SCRs and examined by flow cytometry (black bar), the number of gH-null virus copies able to bind was measured by QPCR (hatched bar), and cells were infected with virus expressing GFP and measured for GFP expression by flow cytometry 48 h later (grey bar). Values are expressed as a percent of those achieved with full length CD21. The experiment was repeated with the same results.
Highlights.
EBV glycoprotein gp150 binds to heparan sulfate
EBV binding to heparan sulfate does not promote infection
Only the aminoterminal SCRs of CD21 are required to enhance epithelial infection
Acknowledgments
This work was supported by Public Health Service grants DE016669 (to LMH-F) and DE019599 (to SMV) from the National Institute of Dental and Craniofacial Research. We thank Karl Munger for the gift of the NOK cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Liudmila S. Chesnokova, Email: lchesn@lsuhsc.edu.
Sarah M. Valencia, Email: sarah.valencia@nih.gov.
Lindsey M. Hutt-Fletcher, Email: lhuttf@lsuhsc.edu.
REFERENCES
- Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv. Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- Backovic M, Jardetzky TS. Class III viral membrane fusion proteins. Curr. Opin. Struct. Biol. 2009;19:189–196. doi: 10.1016/j.sbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc. Natl. Acad. Sci. USA. 2009;106:2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. Heparin sulfate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Borza C, Hutt-Fletcher LM. Epstein-Barr virus recombinant lacking expression of glycoprotein gp150 infects B cells normally but is enhanced for infection of the epithelial line SVKCR2. J. Virol. 1998;72:7577–7582. doi: 10.1128/jvi.72.9.7577-7582.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nature Med. 2002;8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- Borza CM, Morgan AJ, Turk SM, Hutt-Fletcher LM. Use of gHgL for attachment of Epstein-Barr virus to epithelial cells compromises infection. J. Virol. 2004;78:5007–5014. doi: 10.1128/JVI.78.10.5007-5014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rowe CL, Jardetzky TS, Longnecker R. The KGD motif of Epstein-Barr virus gH/gL is bifunctional, orchestrating infection of B cells and epithelial cells. mBio. 2012;3:e00290. doi: 10.1128/mBio.00290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, Ahuja MK, Hutt-Fletcher LM. Epstein-Barr virus glycoproteins gB and gHgL can mediate fusion and entry in trans; heat can act as a partial surrogate for gHgL and trigger a conformational change. J. Virol. 2014;88 doi: 10.1128/JVI.01597-14. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, Hutt-Fletcher LM. Fusion of EBV with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8 and integrin binding triggers a conformational change in gHgL. J. Virol. 2011;85:13214–13223. doi: 10.1128/JVI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, Nishimura S, Hutt-Fletcher L. Fusion of epithelial cells by Epstein- Barr virus proteins is triggered by binding of viral proteins gHgL to integrins avb6 or avb8. Proc. Natl. Acad. Sci. USA. 2009;106:20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Nowlin DM, Cooper NR. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosoaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth JD, Diamond ME, Sage DR, Hayman J, Yates JL. CD-21 dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 1999;73:2115–2125. doi: 10.1128/jvi.73.3.2115-2125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr virus receptor of human B lymphocytes is the C3d complement CR2. Proc. Natl. Acad. Sci. USA. 1984;81:4510–4516. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MB, Edgar R, May JS, Stevenson PG. A gamma-herpesvirus glycoprotein complex manipulates actin to promote viral spread. PLos ONE. 2008;3:e1808. doi: 10.1371/journal.pone.0001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MB, Roecklein-Canfield J, Sage DR, Zambela-Soediono M, Longtine N, Uknis M, Fingeroth JD. EBV attachment stimulates FHOS/FHOD1 redistribution and coaggregation with CD21:formin interactions with the cytoplasmic domain of human CD21. J. Cell. Sci. 2004;117:2709–2720. doi: 10.1242/jcs.01113. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Ann. Rev. Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Gore M, Hutt-Fletcher L. The BDLF2 protein of Epstein-Barr virus is a type II glycosylated envelope protein whose processing is dependent on coexpression with the BMRF2 protein. Virology. 2008;383:162–167. doi: 10.1016/j.virol.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan KM, Kwok WW, Longnecker R, Speck P. Epstein-Barr virus entry utilizing HLA-DP or HLA-DQ as a coreceptor. J. Virol. 2000;74:2451–2454. doi: 10.1128/jvi.74.5.2451-2454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher LM. Epstein-Barr virus entry. J. Virol. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz A, Oezel M, Kurzeder C, Mautner J, Pich D, Kost M, Hammerschmidt W, Delecluse HJ. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 2000;74:10142–10152. doi: 10.1128/jvi.74.21.10142-10152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarousse N, Chandran B, Coscoy L. Lack of heparan sulfate expression in B-cell lines: implications for Kaposi's Sarcoma-Associated Herpesvirus and murin gammaherpevirus 68 infections. J. Virol. 2008;82:12591–12597. doi: 10.1128/JVI.01167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Gu X, Moore-Medlin TN, Nathan C-A, Hutt-Fletcher LM. Oral dysplasia and squamous cell carcinoma: correlation between increased expression of CD21, Epstein-Barr virus and CK19. Oral Oncol. 2012;48:836–841. doi: 10.1016/j.oraloncology.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Gu X, Nathan C, Hutt-Fletcher L. Laser-capture microdissection of oropharyngeal epithelium indicates restriction of Epstein-Barr virus receptor/CD21 mRNA to tonsil epithelial cells. J. Oral Path. Med. 2008;37:626–633. doi: 10.1111/j.1600-0714.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen E, Luftig M, Chase MR, Weicksel S, Cahir-McFarland E, Illanes D, Sarracino D, Kieff E. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA. 2004;101:16286–16291. doi: 10.1073/pnas.0407320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QX, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, Hutt-Fletcher LM. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 1997;71:4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QX, Young LS, Niedobitek G, Dawson CW, Birkenbach M, Wang F, Rickinson AB. Epstein-Barr virus infection and replication in a human epithelial system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- Lidholt K, Weinke JL, Kiser CS, Lugemwa FN, Bame KJ, Cheifetz S, Massague J, Lindahl U, Esko JD. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. USA. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker RM, Kieff E, Cohen JI. Epstein-Barr Virus. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 1898–1959. [Google Scholar]
- Martin DR, Yuryev A, Kalli KR, Fearon DT, Ahearn JM. Determination of the structural basis for selective binding of Epstein-Barr virus to human complement receptor type 2. J. Exp. Med. 1991;174:1299–1311. doi: 10.1084/jem.174.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesworth SJ, Lake CM, Borza CM, Turk SM, Hutt-Fletcher LM. Epstein-Barr virus gH is essential for penetration of B cell but also plays a role in attachment of virus to epithelial cells. J. Virol. 2000;74:6324–6332. doi: 10.1128/jvi.74.14.6324-6332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MD, DiScipio RG, Cooper NR, Nemerow GR. Hydrodynamic, electron microscopic and ligand binding analysis of the Epstein-Barr virus/C3dg receptor (CR2) J. Biol. Chem. 1989;34:20576–20582. [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Braoaddus VC, Nishimura S. The integrin avb8 mediates epithelial homeostasis through MT-1-MMP-dependent activation of TGF-b1. J. Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow GR, Cooper NR. Early events in the infection of human B lymphocytes by Epstein-Barr virus. Virology. 1984;132:186–198. doi: 10.1016/0042-6822(84)90102-8. [DOI] [PubMed] [Google Scholar]
- Nemerow GR, Mold C, Schwend VK, Tollefson V, Cooper NR. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J. Virol. 1987;61:1416–1420. doi: 10.1128/jvi.61.5.1416-1420.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Imai S, Chiba S, Takada K. Epstein-Barr virus lacking glycoprotein gp85 cannot infect B cells and epithelial cells. Virology. 2000;276:52–58. doi: 10.1006/viro.2000.0531. [DOI] [PubMed] [Google Scholar]
- Ogembo JG, Kannan L, Ghiran I, Nicholson-Weller A, Finberg R, Fingeroth JD. Human complement receptor type1/CD35 is an Epstein-Barr virus receptor. Cell Rep. 2013;3:1–15. doi: 10.1016/j.celrep.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat S, Suri CR, Sahoo DK. Molecular mechanism of pollyethylene glycol mediated stabililzation of protein. Biochem. Biophys. Res. Commun. 2010;392:561–566. doi: 10.1016/j.bbrc.2010.01.067. [DOI] [PubMed] [Google Scholar]
- Schmidtke M, Karger A, Meerback A, Egerer R, Stelzner A, Makarow V. Binding of a N,N'-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology. 2003;311:134–143. doi: 10.1016/s0042-6822(03)00166-1. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Sun D, De Vico A, Crowley RW, Reitz W, Jr, Zauli G, Lusso P, Gallo RC. Role of the extracellular domain of herpesvirus 7 glycoprotein B in virus binding to cell surface heparin sulfate proteoglycans. J. Virol. 1997;71:4751–4580. doi: 10.1128/jvi.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinka H-C, Florin L, Patel H, Freitag K, Schmidtke M, Makarov VA, Sapp M. Inhibition of transfer to decondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papilllomavirus. J. Virol. 2007;81:10970–10980. doi: 10.1128/JVI.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon-Lowe CD, Neuhierl B, Baldwin G, Rickinson AB, Delecluse H-J. Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc. Natl. Acad. Sci. USA. 2006;103:7065–7070. doi: 10.1073/pnas.0510512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltany-Rezaee-Rad M, Sepehrizadeh Z, Mottaghi-Dastjerdi N, Yadzi MT, Seyatesh N. Comparison of SYBR Green and TaqMan real-time PCR methods for quantitative detection of residual CHO host-cell DNA in biopharmaceuticals. Biologicals. 2015;43:130–135. doi: 10.1016/j.biologicals.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Spear PG. Herpes simplex virus receptors and ligands for cell entry. Cell. Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Tanner J, Weis J, Fearon D, Whang Y, Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping and endocytosis. Cell. 1987;50:203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- Temple RM, Zhu J, Budgeon LR, Christensen ND, Meyers C, Sample CE. Efficient replication of Epstein-Barr virus in stratified epithelium in vitro. Proc. Natl. Acad. Sci. USA. 2014;111:16544–16549. doi: 10.1073/pnas.1400818111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nature Med. 2003;9:307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- Valencia SM, Hutt-Fletcher LM. Important but differential roles for actin in trafficking of Epstein-Barr virus in B cells and epithelial cells. J. Virol. 2012;86:2–10. doi: 10.1128/JVI.05883-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veettil MV, Bandyopadhyay C, Dutta D, Chandran B. Interaction of KSHV with host cell surface receptors. Viruses. 2104;6:4024–4046. doi: 10.3390/v6104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent-Bugnas S, Vitale S, Mouline CC, Khaali W, Charbit Y, Mahler P, Precheur I, Hofman P, Maryanski ML, Doglio A. EBV infection is common in gingival epithelial cells of the periodontium and worsens during periodontitis. PLos ONE. 2013;8:e80336. doi: 10.1371/journal.pone.0080336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling DM, Flaitz CM, Nichols CM, Hudnall SD, Adler-Storthz K. Persistent productive Epstein-Barr virus replication in normal epithelial cells in vivo. J. Inf. Dis. 2001;184:1499–1507. doi: 10.1086/323992. [DOI] [PubMed] [Google Scholar]
- Wang X, Hutt-Fletcher LM. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 1998;72:158–163. doi: 10.1128/jvi.72.1.158-163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kenyon WJ, Li QX, Mullberg J, Hutt-Fletcher LM. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 1998;72:5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]