Abstract
Recent data demonstrate that chronic exposure of Caenorhabditis elegans (C. elegans) to a high-use glyphosate-containing herbicide, Touchdown (TD), potentially damages the adult nervous system. It is unknown, however, whether unhatched worms exposed to TD during the egg stage show abnormal neurodevelopment post-hatching. Therefore, we investigated whether early treatment with TD leads to aberrant neuronal or neurite development in C. elegans. Studies were completed in three different worm strains with green fluorescent protein (GFP)-tagged neurons to facilitate visual neuronal assessment. Initially, eggs from C. elegans with all neurons tagged with GFP were chronically exposed to TD. Visual inspection suggested decreased neurite projections associated with ventral nerve cord neurons. Data analysis showed a statistically significant decrease in overall green pixel numbers at the fourth larval (L4) stage (*p < 0.05). We further investigated whether specific neuronal populations were preferentially vulnerable to TD by treating eggs from worms that had all dopaminergic (DAergic) or γ-aminobutyric acid (GABAergic) neurons tagged with GFP. As before, green pixel number associated with these discrete neuronal populations was analyzed at multiple larval stages. Data analysis indicated statistically significant decreases in pixel number associated with DAergic, but not GABAergic, neurons (***p< 0.001) at all larval stages. Finally, statistically significant decreases (at the first larval stage, L1) or increases (at the fourth larval stage, L4) in superoxide levels, a developmental signaling molecule, were detected (*p < 0.05). These data suggest that early exposure to TD may impair neuronal development, perhaps through superoxide perturbation. Since toxic insults during development may late render individuals more vulnerable to neurodegenerative diseases in adulthood, these studies provide some of the first evidence in this model organism that early exposure to TD may adversely affect the developing nervous system.
Keywords: Glyphosate-containing herbicides, neurodevelopment, Touchdown, DAergic neurons, C. elegans strain EG1285, C. elegans strain BZ555
1.0 Introduction
Concomitant with an increase in the use of genetically-modified agricultural crops (e.g., cotton, corn, and soy beans), data suggest a parallel increase in pesticide use, particularly glyphosate-containing herbicides [27]. Touchdown® (TD; Syngenta) and Roundup® (Monsanto) are the two most commonly applied herbicides in this category [63]. Early studies evaluating the toxicity of glyphosate [58] indicated a relatively low acute toxicity for rats (oral LD50 = 2000 mg/kg) and mice (oral LD50 = 10,000 mg/kg). These experiments, however, seldom, if ever, investigated the toxicity of the whole commercial herbicide formulation (active compound with adjuvants). In the few comparison studies that have been published, results typically indicate that the toxicity of the formulation is greater than that of the active ingredient [5,50,59] or the putative surfactants (adjuvants) alone [41]. Specifically, studies have demonstrated mitochondrial inhibition in isolated liver mitochondria by Roundup®, but not by glyphosate alone [48]. Others also have shown differential genotoxicity between glyphosate and the formulation [50], although some question these findings [42,62]. Likewise, data from our lab suggest that Caenorhabditis elegans (C. elegans) exposed to various concentrations of a commercially available formation of TD results in apparent neurodegeneration, particularly of DAergic neurons [46]. As the use of this herbicide group has increased, so has the interest in the verifying whether differential toxicity actually exists between the commercial formulation and active ingredient [14,51].
Given recent research regarding the role that early (including pre- and perinatal) exposure to environmental toxicants may play in regulating gene expression [9,19,40], it is important to determine whether exposure to high-use pesticides might alter development. Furthermore, early toxic insults leading to subsequent disease phenotype expression is consistent with a “multiple-hit hypothesis” that may explain the “idiopathic” onset of various neurodegenerative diseases later in life [11]. For example, data already indicate that epigenetic changes following toxicant exposure early in life can lead to pathophysiology related to Alzheimer’s, Parkinson’s, or Hungtinton’s disease [25], as well as autism and Rett syndrome [64]. Other epidemiological data strongly suggest a correlation between children diagnosed with attention deficit/hyperactivity disorder (ADHD) or attention deficit disorder (ADD), and exposure to pesticides [7,52]. Therefore, it is likely that early xenobiotic exposures may render individuals more susceptible to future toxic exposures.
In our previous studies, we showed that worms exposed to TD during the first and second larval stages (L1 and L2) showed DAergic neuron abnormalities as adults [45,46]. It is unknown, however, if C. elegans hatched from fertilized eggs that were exposed to TD would show similar neuronal abnormalities during the various developmental periods. Similar to other nematodes, C. elegans lay eggs that hatch and go through four larval stages (L1, L2, L3, and L4) that are partially designated by the end of a molt (L1 designates the larval stage after hatching; L2 is marked by the end of the first molt; L3 is marked by the end of the second molt; L4 is marked by the end of the third molt; young adult designation is used for the stage following the fourth molt). Neuronal development can be monitored at each different larval stage using various C. elegans strains with green fluorescent protein (GFP)-tagged neuronal populations. Thus, to test our hypothesis that early exposure of fertilized eggs to low concentrations of TD used for weed control [43,56] would abnormally affect neurodevelopment, we used fluorescence microscopy to follow neuronal maturation through all four larval stages to assess whether differences would be observed post-exposure. Finally, we also assayed superoxide levels at the various larval stages to see if changes in this signaling molecule could explain abnormalities in neuron development or growth.
2.0 Materials and Methods
2.1. Worm and Escherichia coli strains
N2 (wild-type), NW1229, BZ555, and EG1285 nematodes (Table 1), as well as NA22 Escherichia coli (E. coli) and OP50-1 E. coli were provided by the Caenorhabditis Genetics Center (CGC). NW1229 worms allowed assessment of all neurons in the worms, while BZ555 and EG1285 worms permitted visualization of DAergic or GABAergic neurons, respectively. In NW1229 worms (evIs111 [F25B3.3::gfp + dpy-20(+)]V), F25B3.3 (rgef-1), a Ca2+-regulated Ras nucleotide exchange factor, is fused with a green fluorescent protein gene (gfp). Reporter fusions of rgef-1 are expressed in post-mitotic neurons (pan-neuronally) beginning during late embryogenesis [36]. BZ555 worms (egIs1 [dat-1p::gfp]), are genetically modified such that dat-1p, the plasma membrane dopamine transporter (DAT) promoter, is fused with gfp, which results in the green fluorescence of all DAergic neurons [24]. Finally, EG1285 worms (oxIs12 [unc-47p::gfp + lin-15(+)]) are genetically modified such that unc-47p, a promoter for the transmembrane vesicular γ-aminobutyric acid (GABA) transporter (GAT), is fused with gfp, leading to expression of green fluorescent protein in all GABAergic neurons [37].
Table 1.
| Strain Designation | Tagged Neuronal Population | Gene Promoter | Protein |
|---|---|---|---|
| N2 | None | None—Wild-type | None—Wild-type |
| NW1229 | All neurons | regf-1 | Ca2+-regulated Ras nucleotide exchange factor |
| BZ555 | Dopamine | dat-1p | Dopamine transporter |
| EG1285 | GABA | unc-47p | Vesicular GABA transporter |
2.2 Synchronization
In order to isolate a sufficient number of worms, adult C. elegans were grown on 8P plates (51.3 mM NaCl, 25.0 g bactoagar/L, 20.0 g bactopeptone/L, 1 mM CaCl2, 0.5 mM potassium phosphate buffer [pH 6], 0.013 mM cholesterol [in 95% ethanol], 1 mM MgSO4) with a lawn of NA22 E coli (grown in 16 g tryptone/L, 10 g yeast extract/L, 85.5 mM NaCl) until gravid. Per standard protocols, NA22 bacteria was used on these plates because their rapid growth and reproduction can sustain large numbers of worms [55]. Worms were then washed off of the plates and into tubes to facilitate exposure to a bleaching solution (0.55% NaOCl, 0.5 mM NaOH), which allows or promotes the release eggs from the gravid adult. The egg/bleach mixture was diluted and washed with M9 solution (20 mM KH2PO4, 40 mM Na2HPO4, 68 mM NaCl), exposed to 30% sucrose, and then centrifuged to separate eggs from debris.
2.3 Treatment of Isolated C. elegans’ Eggs
Eggs obtained from synchronization were treated with the indicated concentrations of glyphosate as the commercial formulation of TD one hour post-synchronization. Eggs in the respective treatment solution were then placed on fresh NGM/OP50-1 plates at 20°C for an additional 25 hours, providing a chronic exposure prior to hatching. Once the worms from the treated eggs had hatched, they were removed from plates and washed an additional 3X with dH2O to remove residual TD. Pictures were taken at varying times post-incubation of 3–5 worms from each treatment group using a digital camera attached to a fluorescence microscope: one hour (L1), nine hours (L2), 21 hours (L3), or 46 hours (L4). Since the focus of this study was to determine if treatment of C. elegans’ eggs could lead to neuronal changes during development, time points after L4 (young adult and adult) were not assessed.
2.4 Viability and Fecundity Studies
Eggs from wild-type (N2) worms were initially treated with varying concentrations of glyphosate (as TD) for 30 min in microcentrifuge tubes. Following this 30 min incubation, the number of eggs/μL solution was determined so that a total of 50 eggs would be consistently added to seeded NGM/OP50-1 plates for each replication. Twenty-four hour post-hatching, the number of moving worms was counted on each plate to determine the number of live animals. For fecundity studies, 15 offspring were picked from the NGM/OP50-1 plates used for egg studies, and were transferred to fresh NGM plates (five worms/plate) with an OP50-1 lawn. These nematodes were allowed to grow (in the absence of additional TD) and reproduce. The number of live offspring laid within a 24-h period was counted and normalized to the number of live offspring from control hermaphrodites.
2.5 Microscopy
Photomicrographs were taken of three to five worms on 4% agarose pads with a fluorescence microscope (Leitz & Wetzlar, Halco Instruments, Inc) equipped with a 50W AC mercury source lamp (E Leitz, Rockleigh, NJ) and a 40X objective (Leitz & Wetzlar, Halco Instruments, Inc.). A digital camera (Micrometrics, Miles Co Scientific, Princeton, MN) operated by Micrometrics software (Micrometrics SE Premium) was attached to the microscope to obtain images. The images were standardized by programming software to ensure that pictures were obtained with identical levels of gamma, gain, exposure time, saturation, and color gain. Photomicrographs were opened with Adobe Photoshop and the total number of green pixels was recorded for further data analysis.
2.6 Dihydroethidium (DHE) Detection
Eggs obtained from synchronizations of N2 worms were treated with 5.5% glyphosate (as TD) for 30 min, and then placed on fresh NGM/OP50-1 plates and incubated at 20°C for 24 h. After incubation, worms were removed from plates, and then washed 3X with dH2O to remove residual TD. Worms at various life stages were subsequently exposed to 500 μM dihydroethidium (DHE; EMD Millipore, Billerica, MA), which forms a red fluorescent product when it reacts with superoxide, for three hours at 20°C. At the conclusion of the DHE incubation, control and treated worms from each life stage were visualized by a BenchTop 2 UV-Transilluminator light box (UVP, Upland, CA) at λ = 302 nm. Photomicrographs were analyzed using Adobe Photoshop to determine the number and intensity of red pixels, which is indicative of DHE activation.
2.7 Data Analysis
Data analysis was performed using GraphPad Prism (v5.03 for Windows, GraphPad Software, San Diego, CA). For each synchronization, experiments were completed a minimum of three times (n ≤ 3) with analysis of three to five worms, resulting in a minimum of twelve worms for each data point. TD concentrations are presented as percent active ingredient (% glyphosate) in order to facilitate comparisons with other glyphosate-containing herbicides [45,46]. Differences in pixel number were determined by one-way analysis of variance (ANOVA), followed by a post-hoc Bonferroni test. Data were considered to be statistically significant if *p < 0.05.
3.0 Results
3.1 Treated Eggs Show Decreased Offspring Viability
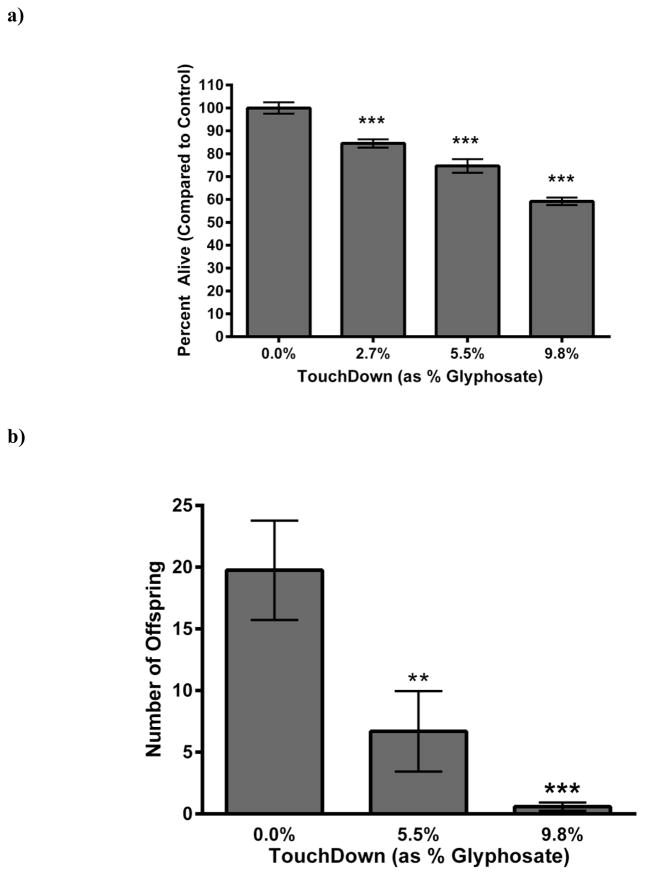
Eggs from wild-type (N2) worms were chronically (24 h) treated with varying concentrations of TD (normalized to percent glyphosate) and allowed to hatch. Twenty-four hours post- treatment, the number of hatched, live nematodes per plate was counted (Figure 1). Analysis of data, normalized to the number of eggs successfully hatched by control (CN) worms (Figure 1a), indicated a statistically significant, dose-dependent decrease in viability of larva (***p < 0.001). Furthermore, surviving hermaphrodites from eggs chronically treated with TD laid statistically significantly fewer offspring (**p < 0.01 for 5.5% glyphosate as TD, or ***p < 0.001 for 9.8% glyphosate as TD) than CN worms (Figure 1b). It is important to note that brood size, which refers to the total number of eggs a C. elegans lays in a lifespan, was not determined. The data presented here represents the number of eggs laid within a 24-h period by adult animals that were treated as embryos. This number is consistent with the results of others who have measured the number of eggs at the end of 24 h [28,29], but less than the average of 300 eggs often reported for brood size over a life-time [31]. Taken together, these initial data suggest that early-life exposure of C. elegans to TD may adversely affect common reproductive parameters in these worms.
Figure 1.
Decreased larval viability following chronic treatment with TD. (a) Eggs from wildtype (N2) worms treated chronically with varying concentrations of TD (as percent glyphosate) showed a statistically significant and dose-dependent decrease in viable offspring compared to CN worms. (b) Hermaphrodites that successfully hatched from treatment groups in Figure 1a were allowed to grow and reproduce. Total number of live offspring over a 24-h period was statistically significantly decreased in a dose-dependent manner compared to CN. Data are presented as mean ± SEM; **p < 0.01, ***p < 0.001.
3.2 Worms from Treated Pan-Neuronal::GFP1 (NW1229) Eggs Show Neurodegeneration
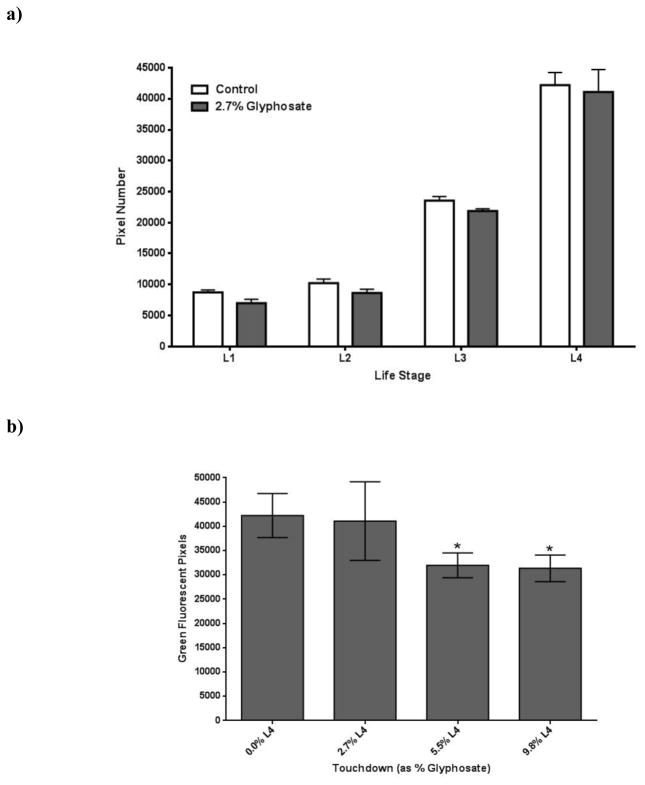
While a decrease in the number of viable offspring may provide important clues regarding teratogenicity or infant death, it provides little evidence that early toxicant exposure could lead to greater vulnerability to environmental insults later in life. Thus, we extended these studies to include C. elegans with GFP reporter constructs to follow neuron morphology throughout each larval stage. In order to determine whether exposure of nematode eggs to TD resulted in morphologic changes to neuronal populations, we initially treated fertilized eggs from the pan-neuronal tagged NW1229 strain (Table 1) with this herbicide. A statistically significant decrease (***p < 0.001) in the total number of pixels throughout the entire worm was observed only at the L4 stage (Figure 2a).
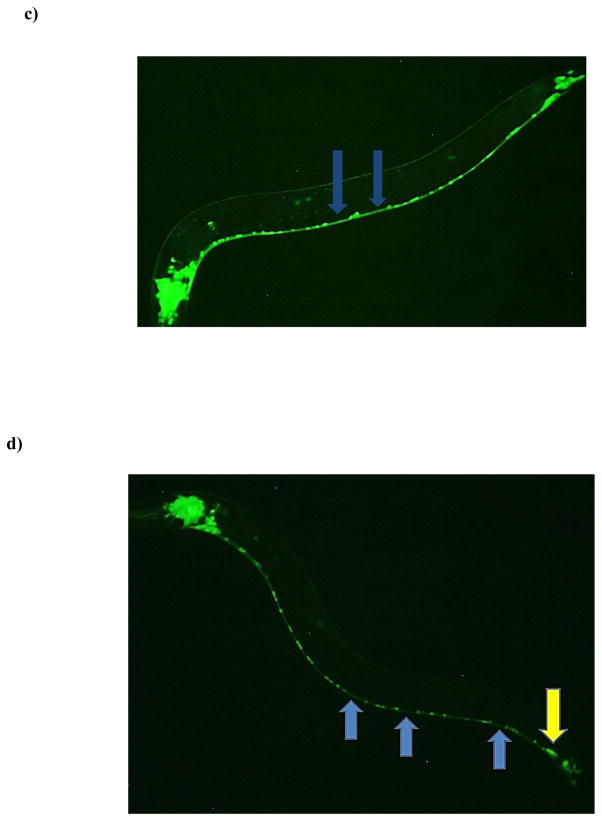
Figure 2.
Decreased neuron-associated green fluorescence following chronic TD exposure. Following treatment of eggs from the pan-neuronal::GFP (NW1229) strain with varying concentrations of TD (as percent glyphosate), number of green pixels was determined (see Section 2.5). Data from analyzing pixel number associated with either the entire worm (a) or only the ventral nerve cord (b) are presented as mean pixel number ± SD. *p < 0.05, ***p < 0.001 compared to CN. Photomicrographs of a representative control (c) or treated (d) worm at the L4 stage show abnormal regions in the nervous system. The photomicrograph of the CN worm demonstrates nerve ring neurons in close proximity to the cuticle, and nerve cord neurons with their associated neurites. The representative photomicrograph of an age-matched treated worm shows gaps or diminished connectivity of neurites (blue arrows) and apparent morphological changes in nerve cord neurons (yellow arrow).
In order to determine if a one general region contributed more (or less) to this decrease, the nerve ring was assessed separately from the ventral nerve cord. This separation also allowed for a gross assessment of specific neuronal populations since the ventral nerve cord is predominantly composed of cholinergic and GABAergic neurons [34], and the nerve ring is largely comprised of DAergic neurons [13]. When the ventral nerve cord was analyzed separately, a statistically significant decrease (*p < 0.05) in pixel number was observed at the L4 stage (Figure 2b). Visual inspection of the ventral nerve cord suggested the decreased pixel number resulted from a loss of discrete and defined expression of GFP, leading to an overall reduction of neuron size (yellow arrow), and an apparent loss of neuronal connectivity (blue arrows) when compared to CN worms (Figures 2c & 2d). Separate pixel analysis of the nerve ring indicated no statistically significant difference between CN and treated worms at any of the assessed larval stages (data not shown).
3.3 Worms from Treated vGAT::GFP (EG1285) Eggs Show Neurodegeneration
To better characterize the neuronal populations adversely affected in the ventral nerve cord, we next used the EG1285 strain, in which all GABAergic neurons are tagged with GFP. We determined that using this strain would allow for better assessment of this neuronal population since there are only 26 GABAergic neurons [34], as compared to the 120 cholinergic neurons [49], in C. elegans. When photomicrographs of CN worms were compared to those from treated worms, no statistically significant change in pixel number was detected across development from L1–L4 (Figure 3a). Visual inspection supported the quantitative analysis. These data strongly suggest that the decreased pixel number observed in the pan-neuronal-GFP worms (Figure 2) was due changes associated with cholinergic, rather than GABAergic, neurons.
Figure 3.
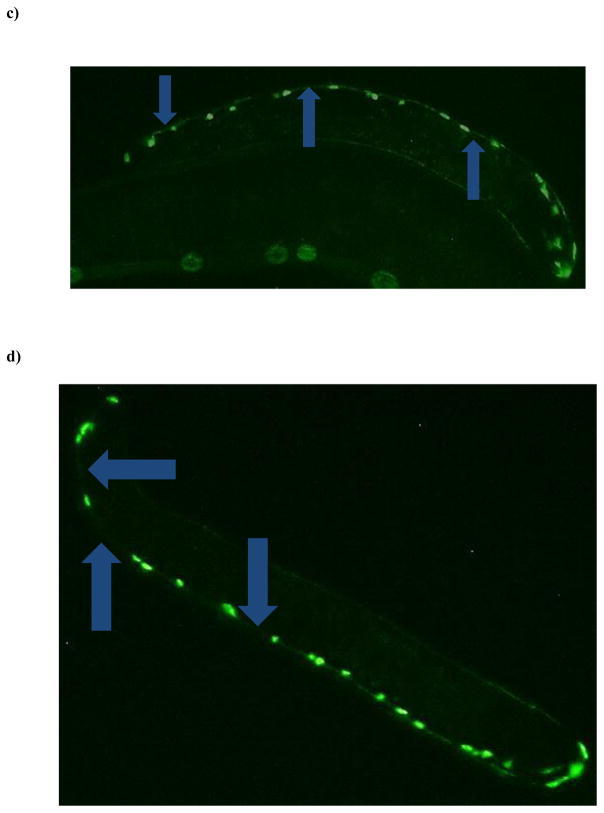
Chronic TD treatment of eggs from GABA::GFP (EG1285) worms at mid- and high-TD concentrations leads to decreased green pixel numbers at the L4 stage. (a) Eggs from EG1285 worms treated chronically with a low concentration of TD (as percent glyphosate) did not lead to decreased pixel numbers or apparent morphological changes in worms at any life stages. (b) When TD concentrations (as percent glyphosate) were increased, however, a statistically significant decrease in pixel number was observed compared to CN worms. Data are presented as mean ± SEM; *p < 0.05. Visual comparison of CN worms (c) to those treated the highest concentration of TD (d) indicated that loss of fluorescence was likely due to neurite degeneration (blue arrows).
Worms lacking the ability to synthesize or package acetylcholine are often smaller than their wild-type counterparts, and they are usually found on plates in a coiled pattern instead of a sinusoidal line [49]. This distinctive coiled pattern was observed via light microscopy for many of the treated worms. Conversely, an increased head angle, the phenotypic hallmark of loss of GABA neurons [34], was not observed in treated worms. These observations also support the hypothesis that decreased GFP in the ventral nerve cord is likely due to damage to the cholinergic neurons.
When treatment concentrations, however, were increased to 5.5% or 9.8% glyphosate (as TD), a statistically significant decrease in pixel number (*p < 0.05) was found in EG1285 larva only at the L4 stage (Figure 3b). Visual inspection of worms in the higher treatment concentration groups did have the characteristic ‘hooked’ head, indicative of the abnormally sharp head angle associated with GABAergic neuronal damage, when compared to CN worms in the same life stage (Figures 3c and 3d). Many treated worms also showed the coiled body orientation as seen before in the lower concentrations. Both of these informal observations are consistent with degeneration of acetylcholine and GABA neurons at higher TD concentrations.
3.4 Worms from Treated dat-1::GFP (BZ555) Eggs Show Neurodegeneration
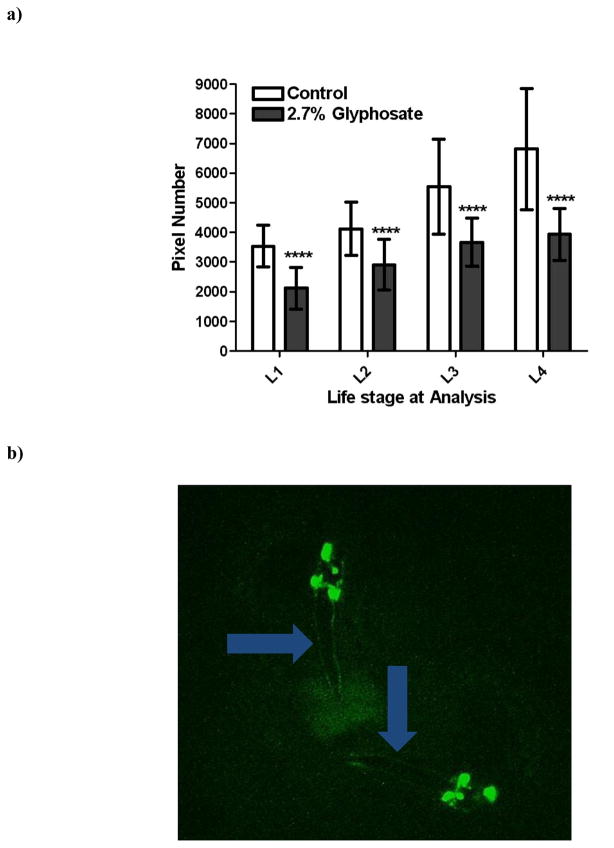
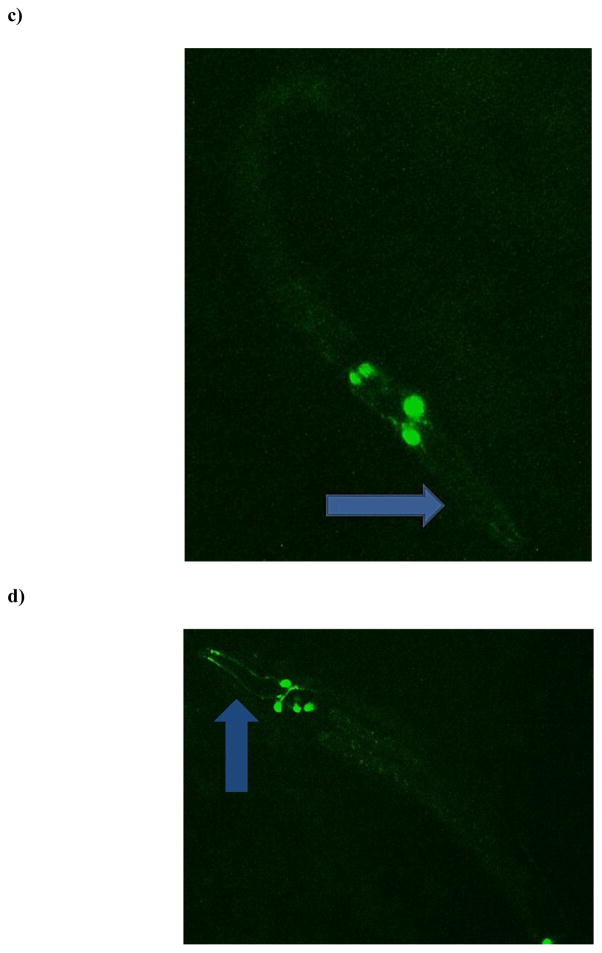
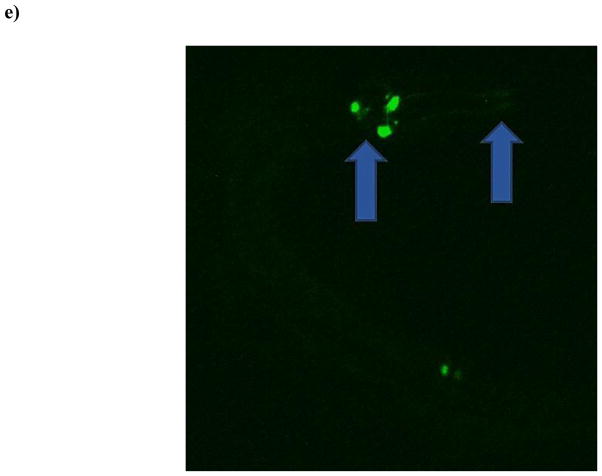
Although no statistically significant decreases in pixel number associated with neurons in the nerve ring were observed in the pan-neuronal-GFP strain, we were still interested in a greater characterization of DAergic neurons in this region. This is also particularly important since many neurological disorders that appear later in life are known to result from perturbations in dopamine levels or changes in DAergic neuronal viability [1,21,23]. Thus, we next treated eggs from the BZ555 strain (all DAergic neurons tagged with GFP) with TD. Interestingly, beginning with L1-staged worms there was a statistically significant (***p < 0.001) decrease in the number of pixels associated with the DAergic neurons in treated worms compared to CN (Figure 4a). This decrease remained consistent through all four larval stages, with the greatest difference observed at L4. Visual inspection of photomicrographs (Figures 4b–4e) suggested that the decrease was at least partially due to loss of GFP associated with the neuronal processes or neurites (blue arrows) rather than morphologic changes of the somas.
Figure 4.
Chronic TD treatment of eggs from DA::GFP (BZ555) worms leads to decreased green pixel number associated with DAergic neurons and their neurites. Following treatment with TD (as 2.7% glyphosate), number of green pixels was determined as described in Section 2.5. (a) Data analysis of pixel numbers from DAergic neurons. Data are presented as mean pixel number ± SD. ***p < 0.001 compared to CN. Photomicrographs of representative control (b) or treated (c) worms at the L1 stage, and control (d) or treated (e) worms at the L4 stage. Photomicrographs of control worms depict DAergic neurons and their projections, which are clearly visible. This is in contrast to the same projections in treated worms (blue arrows).
3.5 Worms from Treated Eggs Show Changes in Superoxide Production
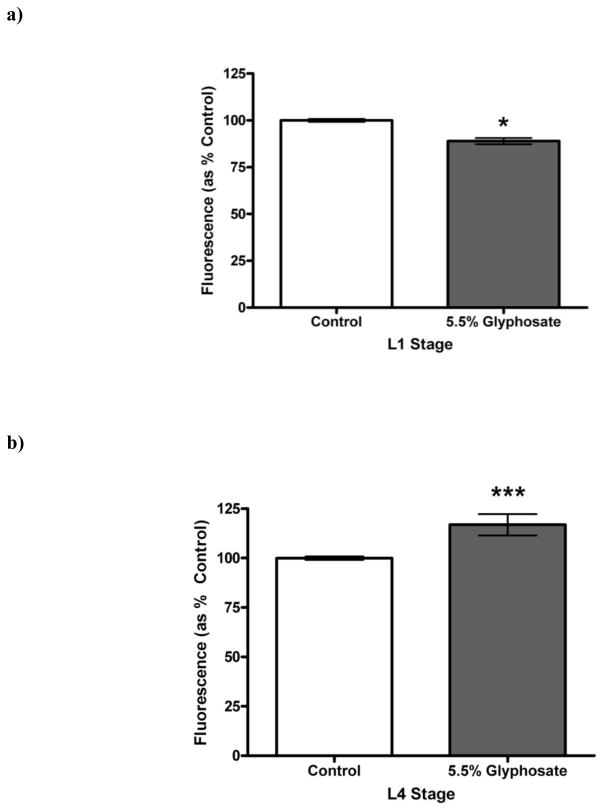
During development, multiple reactive oxygen species (ROS), particularly superoxide (•O2) [22], act as important signaling molecules. Because changes in the levels of this molecule could potentially alter offspring viability or neurodevelopment, we treated fertilized eggs from N2 (wild-type) C. elegans with TD, followed by incubation with dihydroethidium (DHE), which reacts with •O2 to produce a red fluorescent compound. Since neurodegeneration was apparent in both the BZ555 and EG1285 strains starting at a mid-range herbicide concentration, we used this amount in the DHE studies. Following treatment with 5.5% glyphosate as TD, worms were exposed to DHE to determine whether changes in •O2 levels could be detected. There was a statistically significant decrease (*p < 0.05) in DHE fluorescence in the TD-treated group compared to CN worms at the L1 stage (Figure 5a), but a statistically significant increase (***p < 0.001) in DHE fluorescence at L4 (Figure 5b). These data suggested that DHE regulation is impaired during two critical, but distinct, larval stages.
Figure 5.
Altered superoxide production following chronic treatment of eggs with TD. Eggs from N2 worms treated chronically with varying concentrations of TD (as percent glyphosate) showed a statistically significant decrease in superoxide production at the L1 stage (a), but a statistically significant increase at L4 (b) compared to control worms. Data are presented as mean ± SEM; *p < 0.05, ***p < 0.001.
4. Discussion
With the increased use of glyphosate-containing herbicides, such as Touchdown® (TD; Syngenta) and Roundup® (Monsanto), many current studies are finally beginning to examine the potential toxicity of the commercially available formulations, and not just the active ingredient or the putative surfactants and associated vehicle(s) [14,32,38]. Using C. elegans as a model organism, our lab has focused on the potential neurotoxicity of TD, as obtainable by industrial and household pesticide applicators [45,46]. In the present study, we wanted to test the hypothesis that C. elegans treated with TD during their egg stage could lead to abnormal neurodevelopment. Initially, we wanted to determine whether this exposure protocol would lead to overall and gross adverse effects on offspring viability.
Following treatment of the worm eggs with varying concentrations of TD, we observed a decrease in fecundity in a 24-h period. This decreased offspring fecundity was even observed in the absence of any additional exposure of TD to the hatched worms (Figure 1). Although there was also a reduction in the number of eggs laid, we focused our attention on potential neurodevelopmental, rather than reproductive, toxicity. As such, we were interested in using these worms to further determine whether C. elegans exposed to TD while in their egg stage would have morphologic changes associated with discrete neuronal populations. It is important to address this question because a multiple-hit hypothesis for neurodegenerative diseases predicts that environmental insults early in development, particularly during critical periods, could render offspring more vulnerable to additional insults later in life [11,57].
In order to determine whether damage occurred to the nervous system, we exposed eggs from pan-neuronal GFP-tagged worms (NW1229) to TD. Our initial screen, using visual inspection of neurons and pixel number analysis, indicated that treated worms were similar to CN worms until the L4 stage. At this point in development a statistically significant decrease (*p < 0.05) in the number of green pixels associated with neurons were observed (Figure 2). These results paralleled the loss of neurites and abnormal soma morphology observed using light and fluorescence microscopy. While these data suggested that the developing nervous system of C. elegans might be vulnerable to TD exposure, it did not provide information as to whether specific neuronal populations were differentially vulnerable. In our previous studies assessing worms treated with TD at the L1/L2 stage, we observed that GABAergic and DAergic neurons were among the first to show morphological changes associated with loss of pixel numbers [45,46]. Since it is difficult to clearly identify these two populations in the pan-neuronal::GFP strain, we continued our studies in GABA neurons::GFP worms (EG1285) and DA neurons::GFP worms (BZ555).
Although GABAergic neurons are not typically associated with signs or symptoms of major neurodegenerative or neurodevelopmental diseases, recent research has demonstrated their importance in the early symptomology of Parkinson’s disease [8,17,35] and Alzheimer’s disease [39,47,54]. In our herbicide model, however, these GABAergic ventral nerve cord neurons do not appear to contribute significantly (Figure 3) to the loss of green pixels observed in the pan-neuronal::GFP studies (Figure 2). Rather, behavioral data, as well as absence of statistically significant decreased pixel number in the EG1285 worms, suggest that TD exposure in C. elegans results in the loss of cholinergic neurons and/or their processes at the lower TD concentrations. Because various ventral cholinergic neurons are responsible for innervating muscles associated with egg laying [2], loss or dysfunction of these neurons could potentially explain the decreased number of eggs observed in our initial viability studies. In the absence of this innervation, fewer eggs are laid [61]. Thus, loss or dysfunction of choline neurons could also potentially explain the decreased number of eggs observed in our initial viability studies (Figure 1). In humans, however, the cholinergic neurons of the pedunculopontine nucleus complex and thalamus, and the interneurons of the striatum are thought to contribute to some of the postural instability observed in PD patients [8,44]. Thus, changes in cholinergic neurons would be expected to exacerbate signs and symptoms associated with degeneration of the DAergic system.
In addition to PD, however, many neurodegenerative and behavioral diseases are associated with changes in DA levels. Thus, we also took a closer look at the extent of DAergic neurodegeneration in C. elegans following exposure to TD using the DAergic neurons::GFP (BZ555) worm strain. Visual confirmation of decreased neuron size throughout all larval stages (L1–L4) was corroborated by green pixel analysis (Figure 4). We also noted that these worms did not move in the normal sinusoidal pattern consistent in control worms. Rather, they tended to move into a ‘comma’ pattern, and lacked high-angle turns in feeding, both of which are consistent with behavior associated with worms lacking DA or DA receptors [13]. The damage to the DAergic neurons was much more extensive than that observed in other populations we assessed. Furthermore, the gaps between total pixel number and associated changes in neuronal morphology were greater at the L4 stage than at the earlier larval stages, which suggest that the treated worms were unable to compensate for the damage associated with the early TD exposure. While the results should be repeated in higher model organisms, our data from C. elegans do suggest that early exposure to TD could render a developing organism more vulnerable to DAergic insults later in life.
Finally, in order to identify a potential mechanism to explain our results, we treated N2 eggs with the mid-range concentration of TD that decreased GABAergic neuronal pixel numbers early in development. In particular, we wanted to determine whether we could detect changes in superoxide production, which can lead to hydrogen peroxide formation. We assayed for changes in this particular ROS because it and hydrogen peroxide regulate various aspects of signaling and cellular differentiation [4,20,60]. At the L1 stage we detected a statistically significant decrease (*p < 0.05) in superoxide levels in treated worms compared to CN (Figure 5). This could result from an inhibition in superoxide production, an increased turnover to hydrogen peroxide, or activation/upregulation of antioxidant proteins such as superoxide dismutase. This was beyond the scope of our study, but such mechanisms are consistent with similar endpoints reported following exposure to other pesticides [15,16] By the L4 stage, the decrease completely reversed, and a statistically significant increase (***p < 0.001) in superoxide was detected. In this case, it could be that any protection afforded by upregulation in antioxidant proteins had been overwhelmed by this later larval stage. Although the TD concentration in these studies was higher than the 2.7% glyphosate used on the transgenic worm strains, it is still well within the manufacturer recommended levels [6,43] for spot spraying weeds, and has been used in other studies from this lab [45,46].
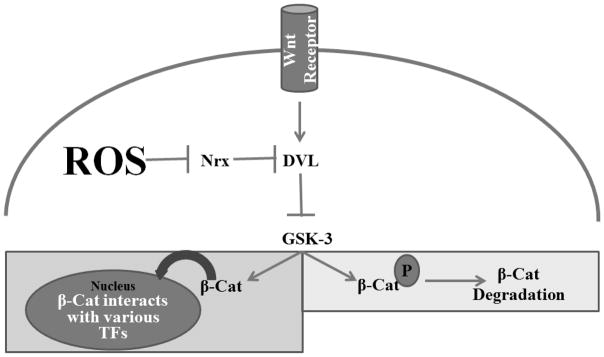
Hermaphroditic C. elegans only have eight DAergic neurons (CEPDL, CEPDR, CEPVL, CEPVR, ADEL, ADER, PDEL, PDER) in their nervous system [13]. Four of these (ADEL/R, and PDEL/R) develop post-embryonically during the second larval (L2) stage [3]. Furthermore, the polarity of the PDE cells are under the control of the Wnt pathway, as is the general position of the nerve ring [53], where many of the DAergic neurons are located [13]. It is also known that changes in the levels of ROS, particularly superoxide and hydrogen peroxide, can regulate the ability of Wnt-related transcription factors to translocate to the nucleus [18]. The other DAergic neurons (CEPDL/R and CEPVL/R), as well as the ADEL/R neurons, all originate from embryonic precursor cells identified as AB, which are formed during the first cell division [26]. Thus, we suggest that the changes in ROS status documented in our studies could provide a mechanistic explanation as to why the DAergic neurons were more vulnerable to changes when compared to other neuronal populations (Figure 6).
Figure 6.
Simplified Wnt/ β-catenin pathway in C. elegans showing how altered ROS production could disrupt normal transcription of genes associated with neurodevelopment of DAergic neurons in C. elegans. In the absence of Wnt ligands, glycogen synththase kinase-3 (GSK-3) will phosphorylate β-catenin (β-Cat), leading to its degradation (light gray box). When Wnt ligands bind to Wnt receptors, GSK-3 is prevented from phosphorylating β-Cat, allowing it to translocate to the nucleus where it can activate various transcription factors (TFs) in the nucleus (dark gray box). The abnormal presence of reactive oxygen species (ROS) can inhibit nucleoredoxins (of which R05H5.3 and C32D5.8 are orthologs in C. elegans), which leads to the phosphorylation of β-Cat by GSK-3.
Although C. elegans are removed from humans on the evolutionary ladder, their nervous system shares a remarkable similarity to that of humans [10,12]. These include important aspects such as homologous and orthologous genes for neurotransmitter receptors, synthesis enzymes, and vesicular and membrane transporters [30]. Furthermore, while the eggshell protecting the early embryonic development of C. elegans does not have the same chemical composition as the human placenta [33], its function is roughly that of its mammalian counterpart: to protect the growing larva/fetus from environmental toxicants. As such, we suggest that our egg-treatment studies serve as a good first-approximation to model in utero exposure to agrochemicals. Although these results should be replicated in higher model organisms, our data suggests initial areas of interest for further studies. In conclusion, we have demonstrated that eggs from C. elegans exposed to TD results in larva with abnormal neuronal cell bodies and/or neurites, and that these developmental abnormalities could be regulated by early larval-stage alterations in superoxide concentrations. These data may also shed light on why prenatal exposure to various pesticides could lead to greater vulnerability to neurodevelopmental or neurodegenerative diseases later in life.
Highlights.
C. elegans hatched from eggs exposed to Touchdown have decreased fecundity compared to non-treated worms;
General changes in neurodevelopment are not observed until the fourth larval (L4) stage;
GABAergic neurons are less vulnerable than DAergic neurons to exposure to Touchdown;
DAergic neurons show altered neurodevelopment as early as the first larval (L1) stage;
Abnormal reactive oxygen species levels may be responsible for the observed toxicity.
Acknowledgments
Worm strains and bacteria were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). The authors would also like to thank Holly Hatfield and Sarah Orfield for their expert editorial help in the manuscript preparation.
Funding
This work was supported by the National Institute of Environmental Health Sciences (R15 ES015628-01A1 and ES015628-02A1 to VF).
Footnotes
The authors have taken the liberty to modify the generally accepted abbreviations for gene::gfp or protein::GFP in such a way as to provide information for the neuronal populations tagged with GFP. This is meant to provide a short-hand for readers who may be unfamiliar with the abbreviations while concurrently providing information about the actual worm strain. For detailed gene::gfp information, please see the Section 2.1 in Materials and Methods.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kenneth A McVey, Email: kamcvey@liberty.edu.
Isaac B Snapp, Email: snapp@musc.edu.
Megan B Johnson, Email: megan.johnson02@lmunet.edu.
Rekek Negga, Email: rnegga@utk.edu.
Aireal S Pressley, Email: adpressley@outlook.com.
References
- 1.Abi-Dargham A. Probing cortical dopamine function in schizophrenia: What can D1 receptors tell us? World Psychiatry. 2003;2(32):166–71. [PMC free article] [PubMed] [Google Scholar]
- 2.Altun ZF, Hall DH. In: Muscle system, nonstriated muscle. Herndon LA, editor. WormAtlas; 2009. [Google Scholar]
- 3.Altun ZF, Hall DH. In: Nervous system, neuronal support cells. Herndon L, editor. WormAtlas; 2010. [Google Scholar]
- 4.Bartosz G. Reactive oxygen species: Destroyers or messengers? Biochem Pharmacol. 2009;77:1303–15. doi: 10.1016/j.bcp.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Benachour N, Seralini GE. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem Res Toxicol. 2009;22:97–105. doi: 10.1021/tx800218n. [DOI] [PubMed] [Google Scholar]
- 6.Bonide; I. Bonide Products, editor. Mancozeb Flowable with Zinc Concentrate. Bonide Products, Inc; Oriskany, NY: 2010. [Google Scholar]
- 7.Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270–7. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brichta L, Greengard P, Flajolet M. Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci. 2013;36:543–54. doi: 10.1016/j.tins.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Brookes E, Shi Y. Diverse epigenetic mechanisms of human disease. Annual Rev Genetics. 2014;48:237–68. doi: 10.1146/annurev-genet-120213-092518. [DOI] [PubMed] [Google Scholar]
- 10.Calahorro F, Ruiz-Rubio M. Caenorhabditis elegans as an experimental tool for the study of complex neurological diseases: Parkinson’s disease, Alzheimer’s disease and autism spectrum disorder. Invert Neurosci. 2011;11:73–83. doi: 10.1007/s10158-011-0126-1. [DOI] [PubMed] [Google Scholar]
- 11.Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson’s disease: The multiple hit hypothesis. Cell Transplant. 2006;15:239–50. doi: 10.3727/000000006783981990. [DOI] [PubMed] [Google Scholar]
- 12.Chalfie M, White J. The Nervous System. In: Wood WB, editor. The Nematode Caeorhabditis Elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1988. pp. 337–92. [Google Scholar]
- 13.Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. The C. elegans Research Community, editor. Worm Book. 2007 doi: 10.1895/wormbook.1.132.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 14.Chaufan G, Coalova I, del Rios de Molina MC. Glyphosate commercial formulation causes cytotoxicity, oxidative effects, and apoptosis on human cells: differences with its active ingredient. Int J Toxicol. 2014;33:29–38. doi: 10.1177/1091581813517906. [DOI] [PubMed] [Google Scholar]
- 15.Coleman MD, O’Neil JD, Woehrling EK, Ndunge OBA, Hill EJ, Menache A, Reiss CJ. A preliminary investigation into the impact of a pesticide combination on human neuronal and glial cell lines in vitro. PLoS ONE. 2012;7:e42768. doi: 10.1371/journal.pone.0042768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa-Silva DG, Nunes MEM, Wallau GL, Martins IK, Zemolin APP, Cruz LC, Rodrigues NR, Lopes AR, Posser T, Franco JL. Oxidative stress markers in fish (Astyanax sp. and Danio rerio) exposed to urban and agricultural effluents in the Brazilian Pampa biome. Environmental Science and Pollution Research. 2015;22:15526–15535. doi: 10.1007/s11356-015-4737-7. [DOI] [PubMed] [Google Scholar]
- 17.Coune PG, Craveiro M, Gaugler MN, Mlynarik V, Schneider BL, Aebischer P, Gruetter R. An in vivo ultrahigh field 14.1 T (1)H-MRS study on 6-OHDA and alpha-synuclein-based rat models of Parkinson’s disease: GABA as an early disease marker. NMR Biomed. 2013;26:43–50. doi: 10.1002/nbm.2817. [DOI] [PubMed] [Google Scholar]
- 18.Covarrubias L, Hernandez-Garcia D, Schnabel D, Salas-Vidal E, Castro-Obregon S. Function of reactive oxygen species during animal development: Passive or active? Dev Biol. 2008;320:1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Desplats PA. Perinatal programming of neurodevelopment: epigenetic mechanisms and the prenatal shaping of the brain. Adv Neurobiol. 2015;10:335–61. doi: 10.1007/978-1-4939-1372-5_16. [DOI] [PubMed] [Google Scholar]
- 20.Dodson M, Darley-Usmar V, Zhang J. Cellular metabolic and autophagic pathways: Traffic control by redox signaling. Free Radic Biol Med. 2013;63:207–21. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Federoff HJ, Burke RE, Fahn S, Fiskum G, editors. NY Acad Sci. New York, NY: 2003. Parkinson’s Disease: The life cycle of the dopamine neuron. [Google Scholar]
- 22.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–53. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 23.Forssberg H, Fernell E, Waters S, Waters N, Tedroff J. Altered pattern of brain dopamine synthesis in male adolescents with attention deficit hyperactivity disorder. Behav Brain Funct. 2006;2:40. doi: 10.1186/1744-9081-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu RH, Wang YC, Chen CS, Tsai RT, Liu SP, Chang WL, Lin HL, Lu CH, Wei JR, Wang ZW, et al. Acetylcorynoline attenuates dopaminergic neuron degeneration and alpha-synuclein aggregation in animal models of Parkinson’s disease. Neuropharmacology. 2014;82:108–20. doi: 10.1016/j.neuropharm.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Gapp K, Woldemichael BT, Bohacek J, Mansuy IM. Epigenetic regulation in neurodevelopment and neurodegenerative diseases. Neuroscience. 2014;264:99–111. doi: 10.1016/j.neuroscience.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert SF. Early development of the nematode Caenorhabditis elegans. In: Gilbert Scott F., editor. Developmental Biology. Sinaur Assoc; 2000. [Google Scholar]
- 27.Grube A, Donaldson D, Kiely T, Wu L. United States Environmental Protection Agency, editor. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. Biological and Economic Analysis Division, Office of Pesticide Programs; 2011. [Google Scholar]
- 28.Harrison Brody A, Chou E, Gray JM, Pokyrwka NJ, Raley-Susman KM. Mancozeb-induced behavioral deficits precede structural neural degeneration. Neurotoxicology. 2013;34:74–81. doi: 10.1016/j.neuro.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Hart AC. The C.elegans Research Community, editor. WormBook. WormBook; 2006. Behavior. [Google Scholar]
- 30.Hobert O. The C.elegans Research Community, editor. WormBook. WormBook; 2010. Neurogenesis in the nematode Caenorhabditis elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgkin J, Barnes TM. More is not better: Brood size and population growth in a self-fertilizing nematode. Proc Biol Sci. 1991;246:19–24. doi: 10.1098/rspb.1991.0119. [DOI] [PubMed] [Google Scholar]
- 32.Jasper R, Locatelli GO, Pilati C, Locatelli C. Evaluation of biochemical, hematological and oxidative parameters in mice exposed to the herbicide glyphosate-Roundup®. Interdiscip Toxicol. 2013;5:133–40. doi: 10.2478/v10102-012-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston WL, Dennis JW. The eggshell in the C. elegans oocyte-to-embryo transition. Genesis. 2012;50:333–49. doi: 10.1002/dvg.20823. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen EM. The C.elegans Research Community, editor. WormBook. WormBook; 2005. GABA. [Google Scholar]
- 35.Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson’s disease: Review of recent trials. Mov Disord. 2013;28:131–44. doi: 10.1002/mds.25273. [DOI] [PubMed] [Google Scholar]
- 36.Kitagawa H, Izumikawa T, Mizuguchi S, Dejima K, Nomura KH, Egusa N, Taniguchi F, Tamura J, Gengyo-Ando K, Mitani S, et al. Expression of rib-1, a Caenorhabditis elegans homolog of the human tumor suppressor EXT genes, is indispensable for heparan sulfate synthesis and embryonic morphogenesis. J Biol Chem. 2007;282:8533–44. doi: 10.1074/jbc.M611107200. [DOI] [PubMed] [Google Scholar]
- 37.Knobel KM, Davis WS, Jorgensen EM, Bastiani MJ. UNC-119 suppresses axon branching in C. elegans. Development. 2001;128:4079–92. doi: 10.1242/dev.128.20.4079. [DOI] [PubMed] [Google Scholar]
- 38.Koller VJ, Furhacker M, Nersesyan A, Misik M, Eisenbauer M, Knasmueller S. Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells. Arch Toxicol. 2012;86:805–13. doi: 10.1007/s00204-012-0804-8. [DOI] [PubMed] [Google Scholar]
- 39.Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc Natl Acad Sci U S A. 2012;109:10071–6. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo CL, Zhou FC. Environmental alterations of epigenetics prior to the birth. Int Rev Neurobiol. 2014;115:1–49. doi: 10.1016/B978-0-12-801311-3.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesnage R, Bernay B, Seralini GE. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. 2013;313:122–8. doi: 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Mink PJ, Mandel JS, Sceurman BK, Lundin JI. Epidemiologic studies of glyphosate and cancer: A review. Regulatory toxicology and pharmacology : RTP. 2012;63:440–52. doi: 10.1016/j.yrtph.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Monsanto. Roundup® Ready-to-Use Weed & Grass Killer III with One-Touch Wand. 2014. [Google Scholar]
- 44.Muller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, Bohnen NI. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain. 2013;136:3282–9. doi: 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negga R, Rudd DA, Davis NS, Justice AN, Hatfield HE, Valente AL, Fields AS, Fitsanakis VA. Exposure to Mn/Zn ethylene-bis-dithiocarbamate and glyphosate pesticides leads to neurodegeneration in Caenorhabditis elegans. Neurotoxicology. 2011;32:331–41. doi: 10.1016/j.neuro.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negga R, Stuart JA, Machen ML, Salva J, Lizek AJ, Richardson SJ, Osborne AS, Mirallas O, McVey KA, Fitsanakis VA. Exposure to glyphosate- and/or Mn/Zn-ethylene-bis-dithiocarbamate-containing pesticides leads to degeneration of gamma-aminobutyric acid and dopamine neurons in Caenorhabditis elegans. Neurotox Res. 2012;21:281–90. doi: 10.1007/s12640-011-9274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascual B, Prieto E, Arbizu J, Marti-Climent JM, Penuelas I, Quincoces G, Zarauza R, Pappata S, Masdeu JC. Decreased carbon-11-flumazenil binding in early Alzheimer’s disease. Brain. 2012;135:2817–25. doi: 10.1093/brain/aws210. [DOI] [PubMed] [Google Scholar]
- 48.Peixoto F. Comparative effects of the Roundup and glyphosate on mitochondrial oxidative phosphorylation. Chemosphere. 2005;61:1115. doi: 10.1016/j.chemosphere.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Rand JB. The C.elegans Research Community (Eds.), editor . WormBook. WormBook; 2007. Acetylcholine. [Google Scholar]
- 50.Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini GE. Differential effects of glyphosate and Roundup on human placental cells and aromatase. Environ Health Perspect. 2005;113:716–20. doi: 10.1289/ehp.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero DM, Rios de Molina MC, Juarez AB. Oxidative stress induced by a commercial glyphosate formulation in a tolerant strain of Chlorella kessleri. Ecotoxicol Environ Saf. 2011;74:741–7. doi: 10.1016/j.ecoenv.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 52.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawa H, Korswagen HC. The C.elegans Research Community, editor. Wnt signaling in C. elegans. WormBook; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwab C, Yu S, Wong W, McGeer EG, McGeer PL. GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease. J Alzheimers Dis. 2013;33:1073–88. doi: 10.3233/JAD-2012-121330. [DOI] [PubMed] [Google Scholar]
- 55.Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C elegans: A Practical Approach. Oxford University Press; New York: 1999. [Google Scholar]
- 56.Syngenta; Syngenta, editor. Product Label. Syngenta Crop Protection; Greensboro, NC: 2010. TouchDown Hi Tech. [Google Scholar]
- 57.Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. NeuroToxicology. 2002;23:621–33. doi: 10.1016/s0161-813x(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 58.Tomlin CDS. The Pesticide Manual: A World Compendium. 14. British Crop Protection Council; Hampshire, UK: 2006. pp. 545–8. [Google Scholar]
- 59.Tsui MTK, Wang WX, Chu LM. Influence of glyphosate and its formulation (Roundup®) on the toxicity and bioavailability of metals to Ceriodaphnia dubia. Environmental Pollution. 2005;138:59. doi: 10.1016/j.envpol.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 60.van der Vliet A, Janssen-Heininger Y. Hydrogen peroxide as a damage signal in tissue injury and inflammation: Murderer, mediator, or messenger? J Cellul Biochem. 2013 doi: 10.1002/jcb.24683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhaditis elegans, Phil Trans Royal Soc London Series B. Biol Scien. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 62.Williams GM, Kroes R, Munro IC. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol. 2000;31:117. doi: 10.1006/rtph.1999.1371. [DOI] [PubMed] [Google Scholar]
- 63.Woodburn AT. Glyphosate: Production, pricing and use worldwide. Pest Management Sci. 2000;56:309–312. [Google Scholar]
- 64.Wynder C, Stalker L, Doughty ML. Role of H3K4 demethylases in complex neurodevelopmental diseases. Epigenomics. 2010;2:407–18. doi: 10.2217/epi.10.12. [DOI] [PubMed] [Google Scholar]