Abstract
Significant public health disparities exist surrounding teen and unplanned pregnancy in the U.S. Women of color and those with lower education and socioeconomic status are at much greater risk of unplanned pregnancy and the resulting adverse outcomes. Unplanned pregnancies reduce educational and career opportunities and may contribute to socioeconomic deprivation and widening income disparities. Long-acting reversible contraception (LARC), including intrauterine devices (IUDs) and implants, offer the opportunity to change the default from drifting into parenthood to planned conception. LARC methods are forgettable; once placed they offer highly effective, long-term pregnancy prevention. Increasing evidence in the medical literature demonstrates the population benefits of use of these methods. However, barriers to more widespread use of LARC methods persist, and include educational, access, and cost barriers. With increasing insurance coverage under the Affordable Care Act, and more widespread, no-cost coverage of methods, more and more women are choosing IUDs and the contraceptive implant. Increasing the use of highly effective contraceptive methods may provide one solution to the persistent problem of the health disparities of unplanned and teen pregnancies in the U.S., and improve women and children's health.
INTRODUCTION
Unintended pregnancy and teen pregnancy continue to be significant public health challenges in the United States, and are listed among the priorities of Healthy People 2020.1 Approximately half of all pregnancies in the U.S. are unintended,2 and approximately half of those end in abortion, resulting in 1.2 million abortions per year.3 The risk of experiencing a pregnancy before age 20 has fallen from 4 in 10 in the 1990s to the current rate of 3 in 10, but continues to be high relative to other developed countries.4
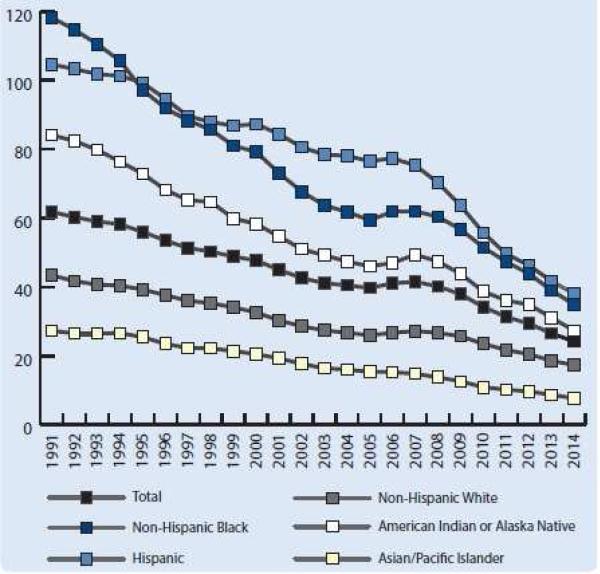
There is significant disparity in the rates of teen and unintended pregnancy by race/ethnicity, education, and income level. Figure 1 displays changes in teen birth rates over the past two decades, stratified by race/ethnicity. Black and Latina teenagers are more than twice as likely as white teenagers to experience a pregnancy, with half of black and Latina teens becoming pregnant before age 20. Half of teen mothers don't receive a high school diploma by age 22, perpetuating a cycle of lower educational attainment and poverty.5 The unintended pregnancy rate is 5 times higher in poor women compared to their wealthier counterparts, and almost 5 times higher in women with less than a high school degree compared to college graduates.2
Figure 1.
Teen Birth Rate (Per 1,000 Girls Age 15-19) 1991-2014, by Race/Ethnicity Source: The National Campaign to Prevent Teen and Unplanned Pregnancy
Unintended, teen, and rapid repeat pregnancies have substantial negative health and socioeconomic impacts on women and their families. These outcomes include higher rates of maternal depression, intimate partner violence, and low birth weight infants and lower rates of breastfeeding. Long-term developmental outcomes include poorer behavioral, mental, and physical health for the children. In addition, lower educational attainment for the mothers, fathers, and their children lead to higher rates of poverty and need for federal aid.6-9 There is evidence that unintended pregnancies and births are increasing in poorer and less educated women, a very concerning trend given the societal costs associated with these births.10
The public health cost of births resulting from unintended pregnancies in 2006 was estimated to be $11 billion in maternity and infant care alone, not accounting for the costs of abortion care, additional care required due to poorer perinatal outcomes, lost productivity, and government benefits.11 Contraception has been shown to be a highly cost-effective public health measure, with every $1 in public funding for family planning saving taxpayers $3.74 in pregnancy-related costs alone. Additionally, the most effective methods of contraception, intrauterine devices (IUDs) and subdermal contraceptive implants (long-acting reversible contraceptive [LARC] methods: See Table 1), are the most cost-effective methods; in one analysis, LARC methods were more cost-effective than the use of short-acting methods or no method, with savings of over $7 for each $1 spent.12,13 This benefit is seen because LARC methods are able to overcome the estimated 53% of annual costs of unintended pregnancy that are due to imperfect contraceptive adherence.14
Table 1.
LARC Methods Currently Available in the United States
| Currently Available LARC Methods | Clinical points |
|---|---|
| 52mg levonorgestrel-releasing intrauterine system | • 99.8% effective1 • FDA-approved for 5 years of contraceptive coverage2 • FDA-approved for the treatment of heavy menstrual bleeding2 • Effective treatment for dysmenorrhea3 |
| 13.5mg levonorgestrel-releasing intrauterine system | • 99.8% effective1 • FDA-approved for 3 years of contraceptive coverage4 |
| Copper T380A intrauterine device | • 99.2% effective1 • FDA-approved for 10 years of contraceptive coverage5 • Only non-hormonal method of LARC • Most effective form of emergency contraception up to 5 days after unprotected sex6 |
| Single-rod 68mg etonogestrel implant | • 99.9% effective1 • FDA-approved for 3 years of contraceptive coverage7 • Because no pelvic exam is required, it may be more desirable for teenagers8 |
Trussell, 2011, Contraceptive efficacy
Mirena Package Insert
Jensen JT. Noncontraceptive applications of the levonorgestrel intrauterine system. Curr Womens Health Rep 2002;2:417–22.
Skyla Package Insert
Paragard Package Insert
Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: A prospective, multicenter, cohort clinical trial. BJOG 2010;117:1205-1220
Nexplanon package insert
Rosenstock JR, Peipert JF, Madden T, et al. Continuation of reversible contraception in teenagers and young women. Obstet Gynecol 2012;120:1298-1305.
Unintended pregnancy most often occurs due to non-use, including gaps in use, or inconsistent or incorrect use of contraception. The most commonly used reversible contraceptive methods are the oral contraceptive pill and the male condom,15 which have typical-use annual failure rates of 9% and 18%, respectively. Failures with pills are two-fold greater in women less than 21 years of age compared to older women, significantly contributing to the risk of teen pregnancy.16,17 Significant disparities by race, income, and education also exist for the consistent use of reversible methods. Black, low-income women, women with less than a college education, and Medicaid-insured women are more likely to experience both gaps in their contraceptive use and method failures.18-20 The reasons behind these disparities are multi-factorial, but point to the need for increased access to contraceptive methods that decrease or eliminate gaps and method failures. By removing user-dependency, IUDs and implants are associated with annual failure rates of 0.2-0.8% (IUDs) and 0.05% (implants).16,17 They also have high continuation rates that are unaffected by race or socioeconomic factors.21,22 The use of LARC methods is increasing across all contraceptive users, from 8.5% in 2009 to 11.6% in 2012, indicating improving acceptability.23 This trend is important, given that more widespread use of the most effective methods of contraception is one potential solution to reduce the rates of unintended and teen pregnancy in the U.S.
Long-Acting Reversible Contraception (LARC)
IUDs and implants utilize one-time placement with long periods of efficacy; these methods are highly effective because they are not user-dependent (see Table 1). They are “forgettable” and their continuous use eliminates gaps in contraceptive coverage, which are common with methods requiring frequent dosing. LARC methods are ideal for women at high risk of unintended pregnancy, such as adolescents, and all women who desire highly effective methods. In addition, they do not contain estrogen and therefore have few contraindications, making them ideal for use in women with medical conditions.
Intrauterine devices (IUDs)
There are currently several hormonal IUDs and one non-hormonal IUD available in the U.S, and multiple other types are available internationally. The most commonly used hormonal IUD is the 52mg levonorgestrel-releasing intrauterine system (LNG-IUS), which is FDA-approved for up to 5 years of use.24 A newer 52mg LNG-IUS was recently FDA-approved for up to 3 years of use and is a lower cost alternative for organizations that qualify for 340B pricing and for uninsured women. A lower dose (13.5mg) levonorgestrel-releasing IUD with a smaller frame is FDA-approved and effective for up to 3 years. This smaller hormonal IUD was originally marketed for nulliparous women; however, the 52mg LNG-IUS has also been shown to be safe and acceptable in this population, with high continuation rates.25 The Copper T380A IUD, the non-hormonal IUD, contains copper and has been shown to be effective for up to 10 years, possibly longer.26 The primary mechanism of action of all IUDs is the prevention of fertilization. Levonorgestrel-containing IUDs achieve this by thickening of the cervical mucus and inhibition of sperm motility and function. The copper ions released from the copper IUD, along with products released in the inflammatory reaction it induces, are toxic to both sperm and oocytes, preventing the formation of viable embryos.27,28 All IUDs are greater than 99% effective, with 0.8% of women using the copper IUD and 0.2% of women using the LNG-IUS experiencing a pregnancy in the first year of use.16 Previous concern about the risk of pelvic infection and ectopic pregnancy with IUDs has prevented more widespread use of these methods. However, multiple studies have shown that these risks are minimal. 29-32 The IUD does not increase the risk of pelvic inflammatory disease (PID) beyond a small increase in risk in the first 20 days after insertion. Women with current infection with Neisseria gonorrhoeae or Chlamydia trachomatis are at slightly higher risk for pelvic infection, but routine antibiotic prophylaxis at insertion is unnecessary. 29,30 Evidence shows that risk-based screening for N. gonorrhoeae and C. trachomatis at the time of insertion and treatment of women found to have infection, while leaving the IUD in place, is safe and effective. 30,33 Additionally, IUD use significantly lowers the risk of the ectopic pregnancy, because it lowers the risk of any pregnancy. However, if a woman with an IUD does become pregnant, there is a higher chance of an ectopic location than if she were not using an IUD.32 Most importantly, there is evidence that use of IUDs does not increase the risk of subsequent infertility.31,34
The main complication associated with IUD insertion is the risk of perforation. The European Active Surveillance Study on Intrauterine Devices (EURAS IUD) evaluated over 60,000 women at 1230 centers and found perforation rates of 1.4 and 1.1 per 1000 insertions for the LNG-IUS and copper IUD, respectively. Most of these were complete perforations and were removed by laparoscopy with no serious complications. The strongest risk factors for perforation were breastfeeding at the time of insertion and being less than 9 months postpartum, but even in women with both risk factors, perforation was rare.35
Subdermal contraceptive implants
Various types of progestin-based subdermal contraceptive implants have been available for almost 50 years. There are several types available globally, but currently only the single-rod etonogestrel-releasing implant is available in the U.S. It is inserted in a subdermal location in the inside of the upper arm and is effective for up to 3 years.36 The current version of this implant includes a simpler insertion device to prevent deep insertion and barium to allow for radiographic visualization.37 The prevention of pregnancy occurs by thickening of the cervical mucus and suppression of ovulation. It is more than 99% effective, with 0.05% of women experiencing a pregnancy within the first year of use.16 The insertion and removal process requires a company-sponsored training program. The average insertion and removal times are less than one and 4 minutes, respectively, and complications associated with insertion and removal have been shown to be 1% and 1.7%, respectively, and are expected to be lower with the redesigned inserter.37
The main side effect related to all LARC methods is the complaint of bleeding abnormalities. In one analysis of 12-month contraceptive continuation and satisfaction, 14% of copper IUD users and 5% of LNG-IUS users discontinued use due to bleeding and/or cramping. Similarly, 10% of implant users discontinued the method due to unpredictable bleeding. However, overall continuation rates for LARC methods were still much higher than for non-LARC methods.22 There is also some evidence that women who are counseled about possible side effects, including irregular bleeding and amenorrhea, are more satisfied with and more likely to continue their method.38,39
Barriers to LARC Use
The American Congress of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) have specifically recommended that the use of LARC methods be expanded.40-42 The reason for the low uptake of the more effective LARC methods is complex and includes patient, provider, and systems-level barriers. There is data to suggest that women have low knowledge of IUDs,43 and there is persistent stigma related to the history of IUDs, including the increased risk of infection associated with the Dalkon Shield. Many providers do not offer LARC methods to all women, or offer them only under highly restrictive conditions to a small subset of eligible women, contrary to the evidence-based recommendations by the CDC and many other national and international organizations.44-46
There is a common misconception that IUDs are unsafe or contraindicated in adolescents, nulliparous women, women who are not married or who have multiple sexual partners, and women who have had a sexually transmitted infection (STI) or ectopic pregnancy. There is also some evidence that providers are less likely to recommend IUDs to women of lower socioeconomic status (SES).47 The reasons behind this are unclear, but may include a perceived increased risk of STI in women of low SES. These myths have limited the use of IUDs in those women at high risk for unintended pregnancy. In fact, there are very few contraindications to the use of IUDs and subdermal implants, and they are the safest options for women in whom estrogen-based contraception is contraindicated due to medical conditions that increase the risk of venous thromboembolism or stroke.46 In addition, there is evidence that the discontinuation of these methods is not higher in young or nulliparous women.25 See table 2 for a review of the evidence related to many of the patient and provider misconceptions about IUDs.
Table 2.
Provider and patient misconceptions about intrauterine devices (IUDs)
| Patient and Provider Misconceptions | Evidence |
|---|---|
| IUDs cause STIs/PID | The increased risk of PID is within the first 20 days after insertion. Infection with Neisseria gonorrhoeae or Chlamydia trachomatis infection at the time of IUD placement increases the risk. After the peri-insertional period, there is no increased risk of infection compared to women without an IUD.29,30,33 |
| IUDs cause infertility | IUD users do not appear to have an increased risk of tubal infertility. The risk of tubal-factor infertility is instead due to upper genital tract infection.31 |
| IUDs are abortifacients | The primary mechanism of action of all IUDs is the prevention of fertilization. This is achieved with the LNG-IUS by thickening of the cervical mucus & inhibition of sperm motility & function. The copper IUD causes damage to sperm & oocytes, thereby preventing the formation of viable embryos.27,28 |
| IUDs increase the risk for ectopic pregnancy | IUD use significantly lowers the risk of the ectopic pregnancy because it lowers the risk of pregnancy. However, if a woman with an IUD does become pregnant, there is a higher chance of an ectopic location than if she were not using an IUD, but the absolute risk is still very low.32 |
| IUDs are not recommended for nulliparous women | No studies have shown increased risks associated with IUD insertion in nulliparous women. Some studies have found decreased rates of expulsion.66 |
| IUDs are not recommended for young women | Women less than 20 years old have similar IUD satisfaction & continuation rates (>80% at 12-months) as older women.67 |
| IUD insertion is difficult | Available evidence from a primary care setting shows that successful insertion occurs in 95% of attempted procedures. Additionally, 90% of all insertions and 80% of insertions in nulliparous women are rated by providers as “easy.”68 |
| IUD expulsion is common | Expulsion rates are between 2 and 10%. Risk factors for expulsion are age 14-19, parity, obesity, heavy periods, & immediate postpartum or postabortion insertion.66 |
| Many women request early removal of IUDs due to side effects | IUD users have the highest satisfaction and continuation rates compared to users of other methods. At 12-months, over 80% of IUD users are still using the method, compared with 57% of DMPA* users and 49-55% of pill, patch, or ring users.22 |
| IUD insertion is painful | The largest study available used a scale of 0 (no pain) to 10 (severe pain) & showed that 48% of women rated IUD insertion as less than 1, 15% rated it 1-2, 11% rated it ≥5, and 4% rated it ≥7. Older age, nulliparity, non-breastfeeding status, and >3 months since last delivery were related to greater pain rating.69 Some lidocaine formulations, naproxen, and tramadol have been found to be moderately effective in preventing pain.70 |
| You need to have testing done before getting an IUD | The only requirements prior to placing an IUD is to have a normal gynecologic exam and that the provider be reasonably sure a woman is not currently pregnant. The copper IUD can also be used for emergency contraception.46 |
| Your partner will feel the IUD | When IUD strings are cut long enough, they become soft & curl up. If they are cut too short, they may stick out of the cervix & be felt as sharp by a woman's partner. |
DMPA=depot-medroxyprogesterone acetate
Despite the fact that women using injectables, pills, the patch, or the ring have been shown to have a 20-30 times higher risk of a rapid repeat pregnancy compared to women using LARC, only 6% of women use a LARC method at 3 months postpartum.48 Immediate postpartum and post-abortal IUD insertion has been shown to be safe and effective, with acceptable expulsion rates and good continuation rates, and the postpartum time period is often ideal for initiation of LARC methods.49-51 Similarly, immediate postpartum implants have been shown to be safe and acceptable and to decrease the risk of rapid repeat pregnancy in adolescents, a population at particularly high risk for rapid repeat pregnancy.52
Another barrier at the provider and clinic level can be the requirement of a second visit for insertion. Same-day insertion protocols have been shown to lessen patient burden and cost, improve the uptake of LARC, and prevent unintended pregnancies.53 Clinics and providers should optimize their practices to make same-day insertion possible for most women, including stocking the devices in-office and using checklists to be reasonably sure a patient is not pregnant (see Table 3). The criteria in this checklist has been found to have a 99-100% negative predictive value for ruling out a pregnancy.46 By using this checklist, providers can initiate same-day contraception without routine pregnancy testing or requiring a second visit. Insurance company pre-authorization requirements also create a burden for patients and providers. Abandonment of this unnecessary obstacle would make same-day insertion economically feasible for clinics and improve patient uptake. One of the most significant barriers to LARC use is the up-front cost for the device and insertion. Even in women with insurance coverage, the out-of-pocket expenses continue to be prohibitive for many women.54,55
Table 3.
Same-Day Insertion Checklist
| If the patient answers “yes” to any one of the following questions, you may be reasonably sure she is not pregnant and insert an IUD or implant that day. On the basis of clinical judgment, you might consider the addition of a urine pregnancy test. |
|---|
| 1. Have you been using a reliable contraceptive method consistently and correctly? |
| 2. Did your last menstrual period start within the past 7 days? |
| 3. Have you abstained from sexual intercourse since your last menstrual period? |
| 4. Did you have a baby less than 6 months ago, are you fully or nearly-fully breastfeeding and had no menstrual period since then? |
| 5. Have you had a baby in the last 4 weeks? |
| 6. Have you had a miscarriage or abortion in the last 7 days? |
Source: Labbok M, Perez A, Valdez V, et al. The Lactational Amenorrhea Method (LAM): a postpartum introductory family planning method with policy and program implications. Adv Contracept 1994;10:93–109.
Evidence from the Contraceptive CHOICE Project
The Contraceptive CHOICE Project (CHOICE) was a prospective cohort study that enrolled 9,256 women in the St. Louis region with a focus on eliminating barriers to LARC use. CHOICE provided structured contraceptive counseling, removal of cost barriers, same-day insertion, and post-visit contraceptive support for participants. The investigators found high rates of LARC uptake (75%) and continuation (77% at 2 years), and that non-LARC users were over 20 times more likely to have an unintended pregnancy than women using a LARC method.17,56 Additionally, 72% of adolescents aged 14-19 chose LARC methods, and 67% of them were still using their LARC method 24 months later.57 This high uptake of LARC methods translated to a 79% reduction in teen pregnancy rates.58 Other studies have confirmed that the use of LARC methods reduces the risk of pregnancy and repeat pregnancy among adolescents.52 The CHOICE Project and other studies have shown that when the barrier of cost is eliminated, women often choose LARC methods and are highly satisfied with their method. Unintended pregnancy, and the costs associated, can be averted with more widespread use of LARC.12
A recent cluster randomized trial of 40 clinics across the US provided a clinic-level intervention to improve providers’ knowledge of evidence-based eligibility and counseling, along with insertion skills. The investigators demonstrated that twice as many women chose a LARC method in the clinics randomized to the intervention. In addition, the unintended pregnancy rate was cut by 50% in women presenting for family planning visits.59 This study provides widely generalizable evidence that LARC is highly acceptable to women and that a feasible, clinic-based education program can improve outcomes. This trial also showed that women attending abortion care visits, as opposed to family planning visits, were often unable to obtain their desired LARC method due to lack of insurance coverage. This emphasizes the importance of altering insurance coverage requirements in order optimize access for all women. Together, these studies have provided significant evidence for the safety and acceptability of LARC, and the ability to prevent unintended and teen pregnancy when barriers to their use are removed.
Cutting Edge and Controversies
While LARC methods provide effective contraceptive coverage and reduce unintended pregnancies, one limitation is that they do not protect against STIs. Many providers continue to recommend against the use of IUDs for nulliparous women due to the concern about the potential for infertility related to tubal damage from PID or ectopic pregnancy. However, there is good evidence that infertility is related to a history of STIs, not IUD use.31,34 There is no evidence that women who use LARC methods are more likely to be exposed to STIs or engage in high-risk sexual behavior.58 A report from the CHOICE project found that LARC users were no more likely to experience an incident STI than users of other methods. Increased efforts to reduce the rate of STIs are needed, and use of dual methods of contraception (a barrier method plus a method that his highly effective at pregnancy prevention) is one such strategy.60
Effective contraceptive counseling by providers is key to improving the use of effective contraception. Recent research has focused on how best to provide information to patients, including the use of tiered counseling, providing information about methods from most effective to least effective, and preference-based counseling.61 It is important to avoid coercion and ensure a patient-centered model focused on shared decision-making. Additional research should evaluate how to best provide patient education and facilitate contraceptive decision0making in an individualized manner, while maintaining patient autonomy.
There is a growing body of evidence to suggest that LARC methods are actually effective for longer than their current FDA-approved period of use. McNicholas and colleagues found that both the implant and the 52mg LNG-IUS were highly effective in the year beyond FDA-approved duration.62 Prolonged effectiveness of these devices would improve their cost-effectiveness, reduce provider visits for device replacement, and save health care dollars.
No Simple Solution
While increased access to, and use of, LARC is one possible solution to the reproductive health and socioeconomic inequalities in our society, it is not the only solution. It is important to note that many factors other than use of effective contraception play a role in unintended and teen pregnancy. Therefore, LARC alone cannot solve the problem and must be one part of a larger effort to reduce social inequality. It is also important to acknowledge the history of reproductive abuse in the US and how that affects perceptions of the promotion of LARC. Our efforts to improve access to LARC must be well-integrated into a larger framework of reproductive justice for women of color and in poverty.63 Helping a woman to choose a contraceptive method that works best for her includes facilitating a discussion about her reproductive life planning. Engaging in shared decision-making allows a woman to have the information she needs to make decisions about her contraception, and how it fits into her overall health and social well-being. A recent Committee Opinion by ACOG emphasizes the important role reproductive life planning has in reducing unintended pregnancy and in improving a woman's health and her pregnancy outcomes. Additionally, the Committee Opinion recommends that discussions about a woman's reproductive life plans occur not only in the setting of a family planning visit, but in every clinical encounter. This concept is especially important for women with limited access to health care.64 Ensuring access to LARC in these various clinical settings enables women to maximize their self-efficacy regarding their reproductive life plans.
Summary
Racial/ethnic, socioeconomic, and educational disparities in rates of unplanned pregnancy is a stubborn public health problem. Despite dramatic declines over the past two decades, women of color still have a two-fold increased risk of teen pregnancy. This difference is believed to be a main driver of limited educational advancement, career progression, poverty, and socioeconomic disparities. As Isabel Sawhill states in her book, Generation Unbound, teens are drifting into parenthood, rather than planning to be parents.65 A multipronged approach including education, improved access, and affordable (or better yet, no-cost) contraception is needed to reduce these disparities. As shown by the Contraceptive CHOICE Project, removing cost, education, and access barriers can markedly reduce rates of teen and unplanned pregnancy and the need for abortion. Certainly, we can all agree on these important public health outcomes.
CONDENSATION.
Long-acting reversible contraception (LARC) changes the default from drifting into pregnancy to planning for pregnancy, and may help reduce disparities in unintended pregnancy rates.
ACKNOWLEDGEMENT
This publication was supported by the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1 TR000448 and TL1 TR000449. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Presented in part at the 2013 Annual Meeting of the American Gynecological and Obsterical Society (AGOS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: Dr. Peipert receives research funding/support from Bayer, Teva, and Merck, and serves on advisory boards for Teva and Perrigo Pharmaceuticals. Dr. Parks has no disclosures.
References
- 1.Healthy People 2020 [September 21, 2015];Family Planning. 2015 (at http://www.healthypeople.gov/2020/topics-objectives/topic/family-planning.)
- 2.Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478–85. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventura SJ, Abma JC, Mosher WD, Henshaw SK. Estimated pregnancy rates for the United States, 1990-2005: an update. Natl Vital Stat Rep. 2009;58:1–14. [PubMed] [Google Scholar]
- 4.Kost K, Henshaw S. US teenage pregnancies, births and abortions, 2010: National and state trends by age, race and ethnicity. Guttmacher Institute; New York: 2014. [Google Scholar]
- 5.Perper K, Peterson K, Manlove J. Diploma attainment among teen mothers. Washington, DC: 2010. [Google Scholar]
- 6.Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing. Child Trends and National Campaign to Prevent Teen Pregnancy; Washington, DC: 2007. pp. 142–51. [Google Scholar]
- 7.Cheng D, Schwarz EB, Douglas E, Horon I. Unintended pregnancy and associated maternal preconception, prenatal and postpartum behaviors. Contraception. 2009;79:194–8. doi: 10.1016/j.contraception.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Gipson JD, Koenig MA, Hindin MJ. The effects of unintended pregnancy on infant, child, and parental health: a review of the literature. Stud Fam Plann. 2008;39:18–38. doi: 10.1111/j.1728-4465.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 9.D'Angelo D, Williams L, Morrow B, et al. Preconception and Interconception Health Status of Women who Recently Gave Birth to a Live-born Infant: A Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 Reporting Areas. US Department of Health & Human Services, Centers for Disease Control and Prevention; 2004. 2007. [PubMed] [Google Scholar]
- 10.Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(Suppl 1):S43–8. doi: 10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonfield A, Kost K, Gold RB, Finer LB. The public costs of births resulting from unintended pregnancies: national and state-level estimates. Perspect Sex Reprod Health. 2011;43:94–102. doi: 10.1363/4309411. [DOI] [PubMed] [Google Scholar]
- 12.Cleland K, Peipert JF, Westhoff C, Spear S, Trussell J. Family planning as a cost-saving preventive health service. N Engl J Med. 2011;364:e37. doi: 10.1056/NEJMp1104373. [DOI] [PubMed] [Google Scholar]
- 13.Foster DG, Rostovtseva DP, Brindis CD, Biggs MA, Hulett D, Darney PD. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Public Health. 2009;99:446–51. doi: 10.2105/AJPH.2007.129353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87:154–61. doi: 10.1016/j.contraception.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniels K. Current contraceptive status among women aged 15–44: United States, 2011–2013. NCHS Data Brief. 2014;173:1–8. [PubMed] [Google Scholar]
- 16.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 18.Kost K, Singh S, Vaughan B, Trussell J, Bankole A. Estimates of contraceptive failure from the 2002 National Survey of Family Growth. Contraception. 2008;77:10–21. doi: 10.1016/j.contraception.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trussell J, Vaughan B. Contraceptive Failure, Method-Related Discontinuation and Resumption of Use: Results from the 1995 National Survey of Family Growth. Family planning perspectives. 1999;31:64–72. [PubMed] [Google Scholar]
- 20.Frost J, Singh S, Finer LB. Factors Associated with Contraceptive Use and Nonuse, United States, 2004. Perspectives on sexual and reproductive health. 2007;39:90–9. doi: 10.1363/3909007. [DOI] [PubMed] [Google Scholar]
- 21.Diedrich J, Madden T, Zhao Q, Peipert J. Long-term utilization and continuation of intrauterine devices: the LUCID study. American journal of obstetrics and gynecology. 2015 doi: 10.1016/j.ajog.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peipert J, Zhao Q, Allsworth JE, et al. Continuation and Satisfaction of Reversible Contraception. Obstetrics and gynecology. 2011;117:1105–13. doi: 10.1097/AOG.0b013e31821188ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kavanaugh ML, Jerman J, Finer LB. Changes in Use of Long-Acting Reversible Contraceptive Methods Among U.S. Women, 2009-2012. Obstet Gynecol. 2015;126:917–27. doi: 10.1097/AOG.0000000000001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirena Package Insert. Bayer; Whippany, NJ: 2015. [Google Scholar]
- 25.Abraham M, Zhao Q, Peipert JF. Young Age, Nulliparity, and Continuation of Long-Acting Reversible Contraceptive Methods. Obstet Gynecol. 2015;126:823–9. doi: 10.1097/AOG.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 26.Paragard Package Insert. Teva; Sellersville, PA: 2013. [Google Scholar]
- 27.Ortiz ME, Croxatto HB. Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception. 2007;75:S16–30. doi: 10.1016/j.contraception.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson B, Landgren BM, Eneroth P. Effects of various IUDs on the composition of cervical mucus. Contraception. 1991;43:447–58. doi: 10.1016/0010-7824(91)90135-3. [DOI] [PubMed] [Google Scholar]
- 29.Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785–8. doi: 10.1016/0140-6736(92)91904-m. [DOI] [PubMed] [Google Scholar]
- 30.Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Positive Testing for Neisseria gonorrhoeae and Chlamydia trachomatis and the Risk of Pelvic Inflammatory Disease in IUD Users. J Womens Health (Larchmt) 2015;24:354–9. doi: 10.1089/jwh.2015.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra-Infante F, Guzman-Rodriguez R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345:561–7. doi: 10.1056/NEJMoa010438. [DOI] [PubMed] [Google Scholar]
- 32.Xiong X, Buekens P, Wollast E. IUD use and the risk of ectopic pregnancy: a meta-analysis of case-control studies. Contraception. 1995;52:23–34. doi: 10.1016/0010-7824(95)00120-y. [DOI] [PubMed] [Google Scholar]
- 33.Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92:313–8. doi: 10.1016/j.contraception.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet. 2000;356:1013–9. doi: 10.1016/S0140-6736(00)02699-4. [DOI] [PubMed] [Google Scholar]
- 35.Heinemann K, Reed S, Moehner S, Minh T. Risk of uterine perforation with the levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274–9. doi: 10.1016/j.contraception.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Nexplanon Package Insert. Merck & Co, Inc; Whitehouse Station, NJ: 2011. [Google Scholar]
- 37.Grentzer J, McNicholas C, Peipert JF. Use of the etonogestrel-releasing contraceptive implant. Expert Review of Obstetrics & Gynecology. 2013;8:337–44. [Google Scholar]
- 38.Backman T, Huhtala S, Luoto R, Tuominen J, Rauramo I, Koskenvuo M. Advance information improves user satisfaction with the levonorgestrel intrauterine system. Obstetrics and Gynecology. 2002;99:608–13. doi: 10.1016/s0029-7844(01)01764-1. [DOI] [PubMed] [Google Scholar]
- 39.Canto de Cetina T, Canto P, Ordonez Luna M. Effect of counseling to improve compliance in Mexican women receiving depot-medroxyprogesterone acetate. Contraception. 2001;63:143–6. doi: 10.1016/s0010-7824(01)00181-0. [DOI] [PubMed] [Google Scholar]
- 40.Committee Opinion No. 642: Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy. Obstet Gynecol. 2015;126:e44–8. doi: 10.1097/AOG.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 41.ACOG ACOG Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118:184–96. doi: 10.1097/AOG.0b013e318227f05e. [DOI] [PubMed] [Google Scholar]
- 42.Ott MA, Sucato GS, Committee on A. Contraception for adolescents. Pediatrics. 2014;134:e1257–81. doi: 10.1542/peds.2014-2300. [DOI] [PubMed] [Google Scholar]
- 43.Madden T, Allsworth JE, Hladky KJ, Secura GM, Peipert JF. Intrauterine contraception in Saint Louis: a survey of obstetrician and gynecologists' knowledge and attitudes. Contraception. 2010;81:112–6. doi: 10.1016/j.contraception.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med. 2012;44:637–45. [PMC free article] [PubMed] [Google Scholar]
- 45.Harper CC, Stratton L, Raine TR, et al. Counseling and provision of long-acting reversible contraception in the US: national survey of nurse practitioners. Prev Med. 2013;57:883–8. doi: 10.1016/j.ypmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Division of Reproductive Health NCfCDP, Health Promotion CfDC, Prevention. U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use. MMWR Recomm Rep. (2nd edition) 2013;62:1–60. [PubMed] [Google Scholar]
- 47.Dehlendorf C, Ruskin R, K G, et al. Recommendations for intrauterine contraception: a randomized trial of the effects of patients' race/ethnicity and socioeconomic status. American journal of obstetrics and gynecology. 2010;203:319, e1–e8. doi: 10.1016/j.ajog.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White K, Teal SB, Potter JE. Contraception after delivery and short interpregnancy intervals among women in the United States. Obstet Gynecol. 2015;125:1471–7. doi: 10.1097/AOG.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez LM, Bernholc A, Hubacher D, Stuart G, Van Vliet HA. Immediate postpartum insertion of intrauterine device for contraception. Cochrane Database Syst Rev. 2015;6:CD003036. doi: 10.1002/14651858.CD003036.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okusanya BO, Oduwole O, Effa EE. Immediate postabortal insertion of intrauterine devices. Cochrane Database Syst Rev. 2014;7:CD001777. doi: 10.1002/14651858.CD001777.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bednarek PH, Creinin MD, Reeves MF, et al. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364:2208–17. doi: 10.1056/NEJMoa1011600. [DOI] [PubMed] [Google Scholar]
- 52.Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206481:e1–7. doi: 10.1016/j.ajog.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Wang NA, Papic M, Parisi SM, Baldauf E, Rapkin R, Schwarz EB. Same-day placement of intrauterine contraception for high-risk women. Obstetrics & Gynecology. 2014;123:15S. [Google Scholar]
- 54.Gariepy AM, Simon EJ, Patel DA, Creinin MD, Schwarz EB. The impact of out-of-pocket expense on IUD utilization among women with private insurance. Contraception. 2011;84:e39–42. doi: 10.1016/j.contraception.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Homco JB, Peipert JF, Secura GM, Lewis VA, Allsworth JE. Reasons for ineffective pre-pregnancy contraception use in patients seeking abortion services. Contraception. 2009;80:569–74. doi: 10.1016/j.contraception.2009.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNicholas C, Madden T, Secura G, Peipert JF. The contraceptive CHOICE project round up: what we did and what we learned. Clin Obstet Gynecol. 2014;57:635–43. doi: 10.1097/GRF.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Neil-Callahan M, Peipert JF, Zhao Q, Madden T, Secura G. Twenty-four-month continuation of reversible contraception. Obstet Gynecol. 2013;122:1083–91. doi: 10.1097/AOG.0b013e3182a91f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Secura GM, Madden T, McNicholas C, et al. Provision of No-Cost, Long-Acting Contraception and Teenage Pregnancy. N Engl J Med. 2014;371:1316–23. doi: 10.1056/NEJMoa1400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harper CC, Rocca CH, Thompson KM, et al. Reductions in pregnancy rates in the USA with long-acting reversible contraception: a cluster randomised trial. Lancet. 2015;386:562–8. doi: 10.1016/S0140-6736(14)62460-0. [DOI] [PubMed] [Google Scholar]
- 60.Peipert J, Redding CA, Blume J, et al. Design of a stage-matched intervention trial to increase dual method contraceptive use (Project PROTECT). Contemp Clin Trials. 2007;28:626–37. doi: 10.1016/j.cct.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Madden T, Mullersman JL, Omvig KJ, Secura GM, Peipert JF. Structured contraceptive counseling provided by the Contraceptive CHOICE Project. Contraception. 2013;88:243–9. doi: 10.1016/j.contraception.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125:599–604. doi: 10.1097/AOG.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Higgins J. Celebration meets caution: Long Acting Reversible Contraception (LARC)'s boons, potential busts, and the benefits of a reproductive justice approach. Contraception. 2014;89:237–41. doi: 10.1016/j.contraception.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gynecologists CONACoOa. Reproductive life planning to reduce unintended pregnancy. Obstetrics and Gynecology. 2016;127:e66–9. doi: 10.1097/AOG.0000000000001314. [DOI] [PubMed] [Google Scholar]
- 65.Sawhill I. Generation Unbound: Drifting into Sex and Parenthood without Marriage. Brookings Institution Press; Washington, D.C.: 2014. [Google Scholar]
- 66.Madden T, McNicholas C, Zhao Q, Secura G, Eisenberg D, Peipert J. Association of age and parity with intrauterine device expulsion. Obstetrics and Gynecology. 2014;124:718–26. doi: 10.1097/AOG.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abraham M, Zhao Q, Peipert J. Young age, nulliparity, and continuation of long-acting reversible contraceptive methods. Obstet Gynecol. 2015 doi: 10.1097/AOG.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 68.Harvey C, Bateson D, Wattimena J, Black K. Ease of intrauterine contraceptive device insertion in family planning settings. Aust N Z J Obstet Gynaecol. 2012;52:534–9. doi: 10.1111/ajo.12007. [DOI] [PubMed] [Google Scholar]
- 69.Hubacher D, Reyes V, Lillo S, Zepeda A, Chen P-L, Croxatto H. Pain from copper intrauterine device insertion: Randomized trial of prophylactic ibuprofen. Am J Obstet Gynecol. 2006;195:1272–7. doi: 10.1016/j.ajog.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 70.Lopez L, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion (Review). Cochrane Database Syst Rev. 2015;7:CD007373. doi: 10.1002/14651858.CD007373.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]