Abstract
Background
Traumatic axonal injury (TAI) may be reversible, yet there are currently no clinical imaging tools to detect axonal recovery in patients with traumatic brain injury (TBI). We used diffusion tensor imaging (DTI) to characterize serial changes in fractional anisotropy (FA) within TAI lesions of the corpus callosum (CC). We hypothesized that recovery of FA within a TAI lesion correlates with better functional outcome.
Methods
Patients who underwent both an acute DTI scan (≤ day 7) and a subacute DTI scan (day 14 to inpatient rehabilitation discharge) at a single institution were retrospectively analyzed. TAI lesions were manually traced on the acute diffusion-weighted images. Fractional anisotropy (FA), apparent diffusion coefficient (ADC), axial diffusivity (AD), and radial diffusivity (RD) were measured within the TAI lesions at each time point. FA recovery was defined by a longitudinal increase in CC FA that exceeded the coefficient of variation for FA based on values from healthy controls. Acute FA, ADC, AD and RD were compared in lesions with and without FA recovery, and correlations were tested between lesional FA recovery and functional recovery, as determined by Disability Rating Scale score at discharge from inpatient rehabilitation.
Results
Eleven TAI lesions were identified in 7 patients. DTI detected FA recovery within 2 of 11 TAI lesions. Acute FA, ADC, AD, and RD did not differ between lesions with and without FA recovery. Lesional FA recovery did not correlate with Disability Rating Scale scores.
Conclusions
In this retrospective longitudinal study, we provide initial evidence that FA can recover within TAI lesions. However, FA recovery did not correlate with improved functional outcomes. Prospective histopathological and clinical studies are needed to further elucidate whether lesional FA recovery indicates axonal healing and has prognostic significance.
Keywords: Traumatic Brain Injury, Diffusion Tensor Imaging, Traumatic Axonal Injury, Fractional Anisotropy, Apparent Diffusion Coefficient
Introduction
Traumatic axonal injury (TAI) is a common cause of neurological dysfunction in patients with head trauma [1–3]. Axons affected by TAI may undergo acute axotomy, the permanent disconnection of an axon terminal from the cell body [4, 5]. Wallerian degeneration of the distal axonal segment ensues, with irreversible loss of neuronal function [6]. Histopathological studies reveal diffuse white matter atrophy after TAI, [7, 8] and magnetic resonance imaging (MRI) studies show that TAI is associated with neurocognitive dysfunction [9, 10] and physical disability [11, 12]. Furthermore, TAI is implicated in the pathogenesis of traumatic disorders of consciousness, including coma, the vegetative state, and the minimally conscious state [12–15].
Despite the potential for devastating neurological sequelae after TAI, evidence from animal models and human histopathological studies suggests that not all axons undergo acute axotomy. Rather, the biomechanical shearing forces of TAI may cause partial, reversible axonal injury. This reversible injury involves disruption of intracellular neurofilaments, alteration of microtubules, and loss of transmembrane ionic homeostasis, but preservation of the axonal membrane and its myelin sheath [16–18]. Incompletely injured axons may experience one of two fates: 1) secondary (i.e. delayed) axotomy and Wallerian degeneration [19]; or 2) axonal healing [18, 20]. Currently, there are no clinical imaging tools to detect reversibly injured axons or to predict which axons will heal. The development of such a tool could enable clinicians to provide more accurate prognoses and could be used to guide the development of therapies aimed at promoting axonal recovery in patients with traumatic brain injury (TBI).
The advanced MRI technique diffusion tensor imaging (DTI) detects TAI with greater sensitivity than CT or conventional MRI [21] and therefore has potential to identify axonal recovery after TAI. DTI measures the directional dependence of water diffusion (i.e. anisotropy) within a voxel of tissue. Scalar indices of anisotropy, such as fractional anisotropy (FA), have been used to quantify the degree of directional dependence of this diffusion [22], which tends to be highest along axon bundles. FA has therefore been hypothesized to be a potential biomarker of axonal integrity. Low FA has been found to correlate with histopathological evidence of axonal injury in experimental animals [23–25]. In addition, high white matter FA predicts better long-term neurocognitive and functional outcomes in patients with TBI [10, 26]. Furthermore, longitudinal FA increases in white matter bundles have been found to be correlated with functional improvements in patients with TBI [27], an observation that provides the basis for the present investigation of longitudinal FA changes within TAI lesions and their association with functional outcomes.
In this longitudinal retrospective study conducted during the acute-to-subacute stages of TBI, we aimed to characterize serial changes in FA within TAI lesions in the corpus callosum (CC). We hypothesized that recovery of FA within a TAI lesion, as defined by an increase in lesional FA that exceeds the coefficient of variation for FA within the CC in healthy controls, correlates with improved functional outcomes, as measured by the Disability Rating Scale (DRS) score. We also aimed to identify acute DTI biomarkers associated with recovery of FA within TAI lesions.
Methods
Patients
Three hundred and fifty patients with TBI were prospectively enrolled in an outcomes database at Spaulding Rehabilitation Hospital (SRH) from 1999–2007 as part of SRH’s contribution to the TBI Model Systems (TBIMS) National Database. TBIMS is a multicenter, longitudinal TBI study funded by the U.S. National Institute on Disability and Rehabilitation Research. TBIMS National Database enrollees were 16 years or older, received TBI care in a TBIMS-affiliated hospital within 24 hours of injury, and were transferred directly from acute care to an affiliated inpatient rehabilitation hospital. Of the 350 patients in the SRH contribution to the database, 146 were treated for acute TBI at Massachusetts General Hospital (MGH) from 2003 to 2007. Spaulding enrollees who underwent an acute (day 1–7) and a subacute (day 14 to inpatient SRH discharge) DTI scan at MGH were retrospectively identified for inclusion in this study. Our definition of the acute time period was based upon prior TBI imaging studies [10, 28, 29]. Definitions of the subacute period vary in the TBI literature and may begin as early as day 7. We excluded DTI scans that were performed between days 7 and 14 because longitudinal changes in lesional FA between the first and second week, if present, are likely to be smaller in magnitude than between the first week and subsequent weeks.
Thirteen of the 146 patients underwent both an acute and a subacute DTI scan. Seven patients had at least 1 TAI lesion in the CC (n=11 lesions total), as identified by an experienced neuroradiologist (W.A.C). All MRIs were performed at the discretion of the treating clinicians. The 8 acute MRI scans (1 patient had 2 acute scans) were ordered to diagnose intracranial pathology relating to the TBI. The 11 subacute MRI scans (2 patients had ≥ 2 subacute scans) were ordered for prognostication (n=4), suspected intracranial or subgaleal infection (n=5), change in mental status (n=1), or new right hemibody numbness (n=1). No new parenchymal lesions were identified on the subacute scans.
Functional Outcome Assessments
The TBIMS National Database included prospectively collected functional outcome data on all subjects at the time of discharge from inpatient rehabilitation. Functional recovery was assessed by trained examiners using the Disability Rating Scale (DRS) score, which ranges from 0 (no disability) to 29 (vegetative state) [30].
Imaging Acquisition and Processing
MRI data were obtained on 1.5 Tesla GE scanners (General Electric Medical Systems, Waukesha, WI), which were upgraded several times at our institution over the 4-year study period. The DTI sequence was either a single-shot, spin-echo echo-planar imaging (SE-EPI) sequence (n=10) or a twice refocused SE-EPI sequence (n=12) [31]. All DTI data were acquired with at least six diffusion-encoding directions at b=1000 s/mm2, and at least one non-diffusion-weighted acquisition with b=0 s/mm2 (b0). For 5 of the 7 patients, the DTI parameters that may potentially affect FA estimates – the number of diffusion-encoding directions and the b value [32] – were the same for the acute and subacute scans. However, for 2 patients (patients 6 and 7), 6 diffusion-encoding directions were used for the acute DTI scan and 25 diffusion-encoding directions for the subacute DTI scan. All DTI sequences used minimum echo time (72.9 to 99.3 ms), repetition time = 5000 to 7500 ms, in-plane resolution = 0.86 x 0.86 mm to 1.72 x 1.72 mm and acquisition matrix of 128 x 128 (with some acquisitions interpolated to 256 x 256). All DTI data were corrected for eddy-current distortions [33]. The isotropic diffusion-weighted image (DWI) was calculated as the geometric mean of the b=1000 s/mm2 acquisitions. Maps of the apparent diffusion coefficient (ADC), FA and eigenvalues were calculated as previously described [33]. The largest eigenvalue was used to define the axial diffusivity (AD), while the mean of the second and third eigenvalues was used to derive the radial diffusivity (RD).
The T2*-weighted gradient-recalled echo (GRE) sequences utilized the following parameters: echo time = 25 ms, repetition time = 750 to 817 ms, in-plane resolution = 0.86 x 0.86 mm, slice thickness = 5 to 6 mm, averages = 2, matrix = 256 x 192. The T2-weighted fluid-attenuated inversion recovery (FLAIR) sequences utilized the following parameters: echo time = 122.5 to 148.5 ms, repetition time = 10,002 ms, slice thickness = 5 mm, interslice gap = 1 mm, in-plane resolution = 0.86 x 0.86 mm, flip angle = 90°, matrix = 256 x 192.
Traumatic Axonal Injury Lesion Analyses
The CC was selected as the neuroanatomic region of interest for three reasons: 1) it is a common site of TAI [34, 35] due to its susceptibility to tissue deformation by rotational shearing forces [36, 37]; 2) CC TAI lesions are typically larger than punctate TAI lesions at the cortical gray-white junction or in the brainstem [8], which makes coregistration of acute and subacute CC lesions more reliable; and 3) CC fibers primarily travel in parallel along the medial-to-lateral diffusion axis, which enhances the reliability of measuring AD and RD. Each CC TAI lesion was manually traced on the acute DWI maps by an investigator blinded to the clinical data (B.L.E.) using Analyze 10.0 image display software (Mayo Clinic Biomedical Imaging Resource, Rochester, Minnesota, USA). Lesion borders and contours were defined by voxels that contained DWI hyperintensity (DWI+). Confirmation of lesion localization was performed by an experienced neuroradiologist (W.A.C.), who also assessed each GRE and FLAIR dataset for the presence of microhemorrhage (GRE+) and hyperintensity (FLAIR+), respectively. Coregistration of subacute to acute DTI datasets was performed using a two-step procedure: 1) the subacute b0 volumes were coregistered to the corresponding acute b0 volumes using FMRIB’s Linear Image Registration Tool (FLIRT) [38] with an affine transformation; 2) this affine transformation was then used to coregister the subacute diffusion volumes (FA, ADC, AD, and RD) to their corresponding acute diffusion volumes. Once the subacute diffusion data were transformed into the space of the acute data (i.e. “acute diffusion space”), localization of the acute CC TAI lesions on the subacute datasets was confirmed for accuracy using neuroanatomic landmarks. Finally, the mean and standard deviation of FA, ADC, AD, and RD were measured for all voxels within each CC TAI lesion on the acute DTI datasets and the subacute DTI datasets.
Control Datasets
One control DTI dataset was prospectively acquired for this study to determine whether intrasubject variability in the number of diffusion-encoding directions confounded the longitudinal measurements of lesional FA changes [32], since 2 of the 7 TBI patients underwent DTI scans that had different numbers of diffusion-encoding directions between the acute scan (6 directions) and the subacute scan (25 directions). We performed 5 DTI scans on a 36-year-old male healthy control subject using a 1.5 Tesla Siemens MRI scanner (Siemens Medical Solutions, Erlangen, Germany). The first 4 DTI scans were acquired with 6 diffusion-encoding directions and were performed during 3 separate scanning sessions (i.e. the subject was removed from the MRI scanner between sessions). The fifth DTI scan was acquired with 25 diffusion-encoding directions, thereby replicating the variability in the numbers of diffusion-encoding directions used in the acute and subacute DTI scans for patients 6 and 7. All other control DTI acquisition parameters (e.g. b value and spatial resolution) were matched to those of the TBI patients’ DTI scans, as previously reported. The control subject provided informed consent in accordance with our hospital’s institutional review board.
A second control dataset was based on values from 15 healthy control subjects (age [mean+/−SD] = 35+/−10 years old) reported in a previously published paper [28] to measure intersubject variability in FA measurements within the corpus callosum. This previously published control dataset was selected because the DTI data were acquired using the same field strength (1.5 Tesla) and using a similar DTI protocol (6 diffusion-encoding directions and b=1221 s/mm2) as was used for the TBI patients in this study.
In both the 15-subject control dataset and the single-subject control dataset, FA was measured using manually traced regions of interest in the splenium of the CC, since this was the region of the CC within which lesional FA recovery was observed for 2 of the 11 TAI lesions (see results below). Intersubject and intrasubject variability in FA within the splenium of the CC was calculated in the control datasets using the coefficient of variation (standard deviation/mean) [39], which was compared to longitudinal FA changes within the patients’ TAI lesions.
Statistical Analysis
For patients with multiple acute DTI scans, the first scan was analyzed. For patients with multiple subacute scans, the last scan was analyzed. We defined FA recovery by an increase in lesional FA that exceeded the coefficient of variation of FA in both control datasets. This criterion is more stringent (i.e. more biased toward the null hypothesis) than alternative approaches, such as defining FA recovery by any longitudinal increase in FA.
An unpaired t-test was used to compare acute FA, ADC, AD, and RD values in the lesions with and without FA recovery. Fisher’s exact test was used to compare the number of FLAIR+ and GRE+ lesions in both groups. To determine which diffusion parameter (e.g. ADC, AD, or RD) was the strongest determinant of longitudinal changes in FA, we tested correlations between changes in ADC, AD, and RD and changes in FA using Pearson’s correlation coefficient. For these correlation tests, diffusion data from all DTI scans were analyzed, including those acquired between day 7 and day 14 (see Table 1). Statistical analyses were performed using GraphPad Prism version 6.05 (GraphPad Software; La Jolla CA), and the threshold for statistical significance was set at p<0.05.
Table 1.
Clinical and Longitudinal Imaging Data.
| Pt # | Age/ Sex | TBI Mechanism | GCS | DTI Scan Days | DWI | FLAIR | GRE | FA | ADC (x10−3 mm2/s) | AD (x10−3 mm2/s) | RD (x10−3 mm2/s) | Days to RH D/C | DRS at RH D/C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| SPLENIUM | |||||||||||||
|
| |||||||||||||
| 1 | 57M | Bicyclist hit by car | 10 | 5 | + | − | − | 0.70 | 0.62 | 1.24 | 0.31 | 63 | 2.5 |
| 14 | − | − | − | 0.65 | 0.72 | 1.35 | 0.40 | ||||||
|
| |||||||||||||
| 2 | 21M | MVA | 6T | 2 | + | + | − | 0.61 | 0.49 | 0.86 | 0.30 | 241 | 11 |
| 13 | + | + | − | 0.42 | 0.84 | 1.27 | 0.63 | ||||||
| 26 | + | + | + | 0.44 | 0.90 | 1.34 | 0.68 | ||||||
|
| |||||||||||||
| 3 | 31F | MVA | 7T | 1 | − | − | − | 0.55 | 0.94 | 1.57 | 0.63 | 45 | 3.5 |
| 6 | + | + | + | 0.52 | 0.75 | 1.24 | 0.51 | ||||||
| 19 | − | + | N/A | 0.57 | 1.31 | 2.17 | 0.88 | ||||||
|
| |||||||||||||
| 4 | 16F | Pedestrian hit by car | 6T | 2 | + | + | − | 0.67 | 0.54 | 1.02 | 0.31 | 102 | 4.5 |
| 11 | + | + | − | 0.61 | 0.56 | 0.98 | 0.36 | ||||||
| 78 | − | − | − | 0.60 | 0.75 | 1.33 | 0.45 | ||||||
|
| |||||||||||||
| 5 | 20M | MVA | 7T | 7 | + | + | − | 0.58 | 0.55 | 0.94 | 0.36 | 31 | 1 |
| 15 | + | + | − | 0.64 | 0.67 | 1.24 | 0.38 | ||||||
|
| |||||||||||||
| 6 | 17M | MVA | 3 | 2 | + | + | + | 0.28 | 0.95 | 1.24 | 0.81 | 178 | 23 |
| 19 | − | + | N/A | 0.60 | 0.79 | 1.44 | 0.47 | ||||||
| 31 | − | + | N/A | 0.50 | 0.85 | 1.39 | 0.58 | ||||||
| 48 | − | − | N/A | 0.57 | 0.90 | 1.57 | 0.57 | ||||||
| 88 | − | − | + | 0.52 | 0.81 | 1.32 | 0.56 | ||||||
|
| |||||||||||||
| 7 | 63M | Fall 15 feet from roof | 15 | 5 | + | + | − | 0.51 | 0.71 | 1.13 | 0.50 | 108 | 8.5 |
| 32 | + | + | + | 0.36 | 1.04 | 1.42 | 0.85 | ||||||
| 38 | + | + | + | 0.39 | 1.03 | 1.49 | 0.79 | ||||||
|
| |||||||||||||
| BODY | |||||||||||||
|
| |||||||||||||
| 3 | 31F | MVA | 7T | 1 | − | + | − | 0.43 | 0.98 | 1.47 | 0.73 | 45 | 3.5 |
| 6 | + | + | + | 0.32 | 0.68 | 0.91 | 0.56 | ||||||
| 19 | − | + | N/A | 0.24 | 1.46 | 1.80 | 1.29 | ||||||
|
| |||||||||||||
| 4 | 16F | Pedestrian hit by car | 6T | 2 | + | − | − | 0.33 | 0.82 | 1.09 | 0.69 | 102 | 4.5 |
| 11 | + | + | + | 0.41 | 1.02 | 1.51 | 0.77 | ||||||
| 78 | − | − | + | 0.17 | 1.28 | 1.52 | 1.16 | ||||||
|
| |||||||||||||
| GENU | |||||||||||||
|
| |||||||||||||
| 4 | 16F | Pedestrian hit by car | 6T | 2 | − | − | + | 0.64 | 0.68 | 1.31 | 0.40 | 102 | 4.5 |
| 11 | + | + | + | 0.62 | 0.61 | 1.07 | 0.40 | ||||||
| 78 | − | − | + | 0.53 | 0.78 | 1.30 | 0.52 | ||||||
|
| |||||||||||||
| 7 | 3M | Fall 15 feet from roof | 15 | 5 | + | + | − | 0.40 | 0.83 | 1.16 | 0.67 | 108 | 8.5 |
| 32 | + | − | − | N/A | N/A | N/A | N/A | ||||||
| 38 | + | − | − | 0.35 | 0.94 | 1.27 | 0.77 | ||||||
The shaded rows indicate traumatic axonal injury lesions in which axons recovered, as defined by a longitudinal increase in fractional anisotropy (FA) that exceeded the coefficient of variation for FA within the corpus callosum in healthy controls. Abbreviations: “+” indicates that a lesion was visible; “−“ indicates that a lesion was not visible; AD = axial diffusivity; ADC = apparent diffusion coefficient; DRS = Disability Rating Scale score; DWI = diffusion-weighted imaging; FLAIR = T2-weighed fluid-attenuated inversion recovery sequence; GCS = admission Glasgow Coma Scale score; GRE = T2*-weighted gradient-recalled echo sequence; MVA = motor vehicle accident; Pt = patient; RD = radial diffusivity; RH = rehabilitation hospital.
Results
Patient Demographics and Clinical Characteristics
The study sample was comprised of 4 men and 3 women who were admitted to the neurocritical care unit, surgical intensive care unit, or pediatric intensive care unit at our institution. Average age was 32.1 years (range 16 to 63 years). The mechanisms of TBI were motor vehicle accident (n=6) and fall (n=1). Median admission Glasgow Coma Scale (GCS) score was 7 (range 3 to 15). Five patients were classified as severe TBI based upon an initial GCS score between 3 and 8. One patient was classified as moderate TBI (GCS=10), and 1 as “complicated mild” TBI based upon a GCS score of 15 with a left frontal contusion, for which a left-sided hemicraniectomy was performed on post-TBI day 2. The diffuse axonal injury grade was at least 2 for all patients, consistent with the presence of a CC TAI lesion, and three patients met criteria for diffuse axonal injury grade 3 based upon the presence of brainstem TAI lesions (patients 2, 4, and 6) [8]. All 7 patients underwent intracranial pressure monitoring with either a parenchymal intracranial pressure monitor (patients 1, 2, 3, 6, and 7) or an external ventricular drain (patients 4 and 5). The median [range] day of the acute DTI scan was 2 [1 to 6]. The median [range] day of the subacute DTI scan was 38 [14 to 78]. All patients were admitted to SRH for inpatient rehabilitation at the time of MGH discharge (median day 24, range 11 to 57 days). The median time to discharge from SRH inpatient rehabilitation was 102 days post-TBI (range 31 to 241 days). All clinical and demographic data are summarized in Table 1.
Intersubject and Intrasubject FA Variability in Controls
In the previously published 15-subject control dataset, the intersubject coefficient of variation for splenium FA was 7.4%. In the single-subject prospective control dataset, the intrasubject coefficient of variation for splenium FA measurements on the 5 DTI scans was 1.5% (Table 2).
Table 2.
Comparison of Healthy Control DTI Data and Longitudinal Patient DTI Data
| ID | DTI Session # | DTI Scan # | # Diffusion-Encoding Directions | Splenium CC FA |
|---|---|---|---|---|
| Prospective Control Cohort (n=1) | 1 | 1 | 6 | 0.83 |
| 2 | 2 | 6 | 0.82 | |
| 3 | 6 | 0.80 | ||
| 3 | 4 | 6 | 0.80 | |
| 5 | 25 | 0.82 | ||
| Control Mean ± SD | 0.81 +/− 0.01 | |||
| Control CV | 1.5% | |||
| Published Control Cohort (n=15) | Control Mean ± SD | 0.81 +/− 0.06 | ||
| Control CV | 7.4% | |||
| Patient 5 | Acute-to-Subacute Change in FA within Splenium CC TAI Lesion | 0.58 to 0.64 (10.3%) | ||
| Patient 6 | Acute-to-Subacute Change in FA within Splenium CC TAI Lesion | 0.28 to 0.52 (85.7%) | ||
Abbreviations: CC, corpus callosum; CV, coefficient of variation; FA, fractional anisotropy; SD, standard deviation.
Diffusion Tensor Imaging Detection of Lesional FA Recovery
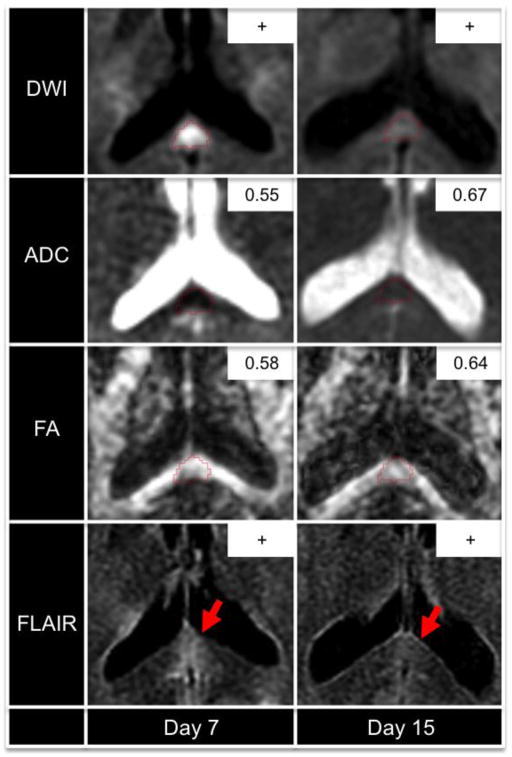
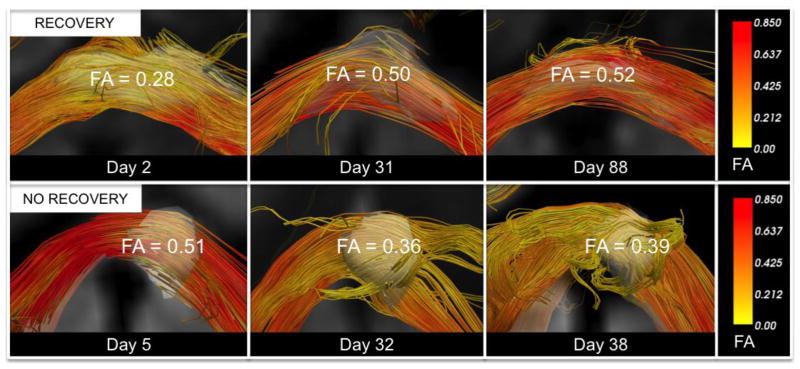
Eleven CC TAI lesions were identified in the 7 patients: 7 within the splenium, 2 within the body, and 2 within the genu. The DTI, DWI, GRE, and FLAIR signal characteristics of the CC TAI lesions at each time point are summarized in Table 1. FA increased longitudinally in 3 splenium lesions (patients 3, 5, and 6). For two of these three lesions, the increase in FA exceeded the coefficient of variation for FA in the control cohorts: the splenium lesion in patient 5 (10.3% increase) and the splenium lesion in patient 6 (85.7% increase) (Table 2). In contrast, for the other 9 lesions, the mean FA changed from 0.54 +/− 0.14 acutely to 0.44 +/− 0.16 subacutely. Acute imaging biomarkers and longitudinal biomarker changes for the lesions with and without FA recovery are summarized in Table 3. Longitudinal change in lesional RD (ΔRD) was significantly different in TAI lesions with and without recovery: ΔRD mean [range] = −0.11 [−0.25 to +0.02] x 10−3 mm2/s for lesions with FA recovery versus +0.27 [+0.09 to +0.56] x 10−3 mm2/s for lesions without recovery (p=0.02). Representative DWI, ADC, FA, and FLAIR data from a patient with lesional FA recovery (patient 5) are displayed in Figure 1. Post-hoc DTI tractography analyses of longitudinal FA changes are provided in Figure 2 to demonstrate the impact of TAI lesions on FA within extralesional segments of affected axons.
Table 3.
Imaging Biomarkers of Axonal Recovery
| Imaging Biomarker | Lesions with FA Recovery (n=2) | Lesions without FA Recovery (n=9) | P Value |
|---|---|---|---|
| Acute FLAIR+ | 2/2 (100%) | 5/9 (56%) | 0.49 |
| Acute GRE+ | 1/2 (50%) | 2/9 (22%) | 0.49 |
| Acute FA | 0.43 +/− 0.21 | 0.54 +/− 0.14 | 0.34 |
| ΔFA | +0.15 [0.07 to 0.24] | −0.05 [−0.02 to −0.19] | -- |
| Acute ADC | 0.75 +/− 0.29 | 0.73 +/− 0.17 | 0.92 |
| ΔADC | −0.02 [−0.16 to +0.12] | +0.28 [+0.10 to +0.48] | 0.70 |
| Acute AD | 1.09 +/− 0.21 | 1.21 +/− 0.22 | 0.53 |
| ΔAD | +0.18 [+0.07 to +0.29] | +0.30 [−0.02 to +0.60] | 0.45 |
| Acute RD | 0.58 +/− 0.32 | 0.50 +/− 0.18 | 0.62 |
| ΔRD | −0.11 [−0.25 to +0.02] | +0.27 [+0.09 to +0.56] | 0.02 |
None of the acute imaging biomarkers were associated with FA recovery within traumatic axonal injury (TAI) lesions. Changes (Δ) in diffusion biomarkers are presented as mean change from the subacute to acute DTI scan [range]. Continuous variables (FA, ADC, AD, and RD) were compared in the TAI lesions with and without recovery using a T-test, and categorical variables (FLAIR+, GRE+) were compared using Fisher’s exact test. Abbreviations: “+” indicates that a lesion was visible; AD = axial diffusivity (x10−3 mm2/s); ADC = apparent diffusion coefficient (x10−3 mm2/s); FA = fractional anisotropy; FLAIR = T2-weighed fluid-attenuated inversion recovery sequence; GRE = T2*-weighted gradient-recalled echo sequence; RD = radial diffusivity (x10−3 mm2/s).
Figure 1. Longitudinal Imaging Changes within a Splenium Lesion with FA Recovery.
A traumatic axonal injury lesion in the splenium of the corpus callosum is outlined in red for patient 5. Acutely (day 7), the lesion is hyperintense on diffusion-weighted imaging (DWI) and T2-weighted fluid-attenuated inversion recovery (FLAIR). Subacutely (day 15), the DWI hyperintensity has diminished, the FLAIR hyperintensity has diminished (red arrows), the apparent diffusion coefficient (ADC) has normalized (inset, units = 10−3 mm/s2), and the fractional anisotropy (FA) has increased (inset) by a magnitude that exceeded intersubject and intrasubject FA variability in healthy controls. Abbreviations: “+” indicates that a lesion was visible; “−“ indicates that a lesion was not visible.
Figure 2. Longitudinal Fractional Anisotropy Changes within White Matter Tracts Affected by Traumatic Axonal Injury.
A traumatic axonal injury (TAI) lesion in the splenium of the corpus callosum of patient 6 (top row) demonstrates FA recovery, as defined by a longitudinal increase in FA that exceeds the coefficient of variation for FA within the splenium of the corpus callosum in the two control datasets. A splenium TAI lesion for patient 7 undergoes a longitudinal decline in FA (bottom row). Each TAI lesion is rendered in 3-dimensions as a semi-transparent white region of interest so that tracts can be visualized passing through the lesions. Fiber tracts were generated using the lesions as seeds. Tracts are color-coded using mean tract FA as a scalar (right panels). All tracts were reconstructed using Diffusion Toolkit version 0.6.2 and TrackVis version 5.2 (Wang & Wedeen, Athinoula A. Martinos Center for Biomedical Imaging, www.trackvis.org).
Early Imaging Biomarkers of Lesional FA Recovery
Lesions that were acutely FLAIR+ or GRE+ did not have a significant difference in their rates of FA recovery. Similarly, acute FA, ADC, AD and RD did not correlate with lesional recovery. See Table 3 for a summary of the acute FLAIR, GRE, FA, ADC, AD and RD measurements for lesions with and without FA recovery.
Longitudinal Correlations of FA with ADC, AD, and RD
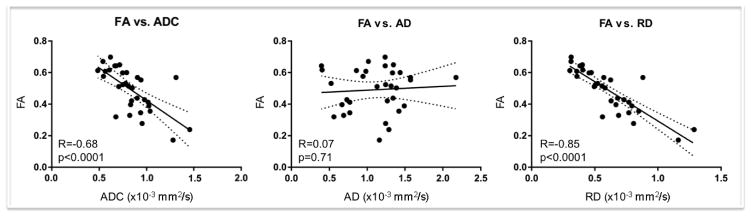
Longitudinal FA changes correlated strongly with RD (R=−0.85, p<0.0001), moderately with ADC (R=−0.68, p<0.0001), and not with AD (R=0.07, p=0.71). See Figure 3 for scatter plots and linear regression results.
Figure 3. Fractional Anisotropy Correlations with Apparent Diffusion Coefficient, Axial Diffusivity, and Radial Diffusivity.
Scatter plots and linear regression lines are displayed for all diffusion tensor imaging lesional measurements during both the acute and subacute stages of traumatic axonal injury (TAI). Individual data points are shown for each lesion at each time point, and regression lines are shown with 95% confidence intervals (dotted lines). Fractional anisotropy (FA) correlates strongly with radial diffusivity (RD), moderately with apparently diffusion coefficient (ADC), and not with axial diffusivity (AD). The strong correlation between FA and RD suggests that longitudinal changes in RD play a primary role in driving changes in FA within TAI lesions.
Lesional FA Recovery and Clinical Outcomes
There was no association between lesional FA recovery and DRS score at rehabilitation hospital discharge. Rather, DRS scores in the two patients with lesional recovery were at opposite ends of the DRS scale (1 and 23, respectively).
Discussion
The main finding of this study is that DTI detected FA recovery within 2 of 11 CC TAI lesions (18%), as defined by a longitudinal increase in FA that exceeded intersubject and intrasubject FA variability in healthy controls. While prior DTI studies have demonstrated longitudinal increases in white matter FA within white matter bundles after TBI [27, 40], we provide initial evidence that FA can recover within TAI lesions. This finding suggests that an increase in lesional FA is a potential imaging biomarker of axonal healing after TAI, since FA is associated with histopathological measures of axonal integrity [23–25]. Nevertheless, lesional FA recovery was not associated with improved clinical outcomes in this small retrospective cohort study. Given that our study may have lacked the statistical power to detect an association between lesional FA recovery and improved functional outcome, larger prospective studies are needed to determine whether lesional FA changes have prognostic significance.
The clinical rationale for developing an imaging biomarker of axonal recovery is highlighted by recent studies showing that TAI is a heterogeneous process, with lesions containing variable combinations of reversibly and irreversibly injured axons [17]. Similarly, lesions containing reversibly and irreversibly injured axons may coexist within one patient’s brain [13, 16], as evidenced by patient 3, who had a TAI lesion in the splenium of the CC in which FA remained stable (0.55 acutely to 0.57 subacutely) and a TAI lesion in the body of the CC in which FA declined (0.43 acutely to 0.24 subacutely). Further underscoring the importance of developing an imaging biomarker to distinguish reversible from irreversible TAI is that both TAI subtypes may appear similar on CT and conventional MRI due to their shared association with perilesional edema and/or microhemorrhages. Indeed, in our cohort, TAI lesions with and without FA recovery were both FLAIR+ and GRE+.
To determine the clinical potential of DTI biomarkers of axonal recovery, larger prospective studies are needed to elucidate whether a single acute DTI scan is sufficient to predict TAI recovery, or whether longitudinal imaging during the acute-to-subacute period is necessary. It would be preferable for a biomarker to be obtained early enough to be useful for prognostication and to select patients for therapies aimed at preventing secondary axotomy of incompletely injured axons. However, unlike the stereotypical temporal progression of intracellular edema in ischemic stroke lesions [41], the evolution of intracellular edema within TAI lesions is dynamic, lasting in some lesions longer than 2 weeks [42, 43]. Since dynamic changes in the ratio of intracellular to extracellular edema may affect FA in unpredictable ways, it remains uncertain whether lesional recovery can be best predicted by acute FA alone, or by longitudinal acute-to-subacute FA changes. An alternative approach that is currently being tested is whether acute diffusion tractography measurements of structural connectivity provide enhanced detection of reversibly injured axons beyond that provided by DTI measurements of FA [10, 13, 14, 44, 45]. We focused on DTI measurements of lesional FA, as opposed to ADC, based upon animal studies showing that FA provides the most histologically valid measure of the structural integrity of white matter [23, 25]. Similarly, DTI measurements of white matter FA demonstrate stronger correlations with cortical grey matter volume [46] and with clinical outcome than do measurements of white matter ADC [28, 47].
Although acute AD and RD did not correlate with lesional FA recovery, several insights into the mechanisms associated with axonal recovery can be gleaned from our longitudinal AD and RD data. We observed that longitudinal recovery of FA was driven by a decline or stability in RD rather than by an increase in AD. This finding confirms and expands upon prior DTI studies showing that a decline in FA is more closely associated with changes in RD than with changes in AD after TBI [48, 49]. Since correlative histo-radiological studies indicate that RD reflects myelin integrity [23, 50, 51], our findings add to a growing body of evidence that myelin plays a critical role in supporting axonal recovery [17]. Although TAI’s effects on myelin are incompletely understood, myelin remodeling and oligodendrocyte-mediated plasticity may contribute to recovery of reversibly injured axons [52]. Post-traumatic remyelination is believed to protect injured axons against biochemical stress, provide metabolic support, and enable optimal rates of signal transduction so that injured axons can reintegrate into neural circuits [17, 50, 53].
Our observation that AD increased in lesions with and without FA recovery calls into question the relevance of AD for measuring longitudinal changes in axonal integrity. An increase in AD can reflect a variety of adaptive or maladaptive processes after TAI, such as an increase in directional water diffusion due to axonal membrane restoration or an increase in extracellular water content due to axonal membrane degradation. In the former case, AD increases without a substantial change in RD, whereas in the latter case, RD increases along with AD [12, 27]. Since axonal healing and axonal degeneration can both be associated with increased AD, longitudinal changes in RD may provide a more biologically relevant marker of TAI recovery than changes in AD.
Several methodological limitations should be considered when interpreting the results of this study. The small sample size likely limited our statistical power to detect an association between lesional FA recovery and functional outcomes. However, obtaining multiple acute-to-subacute DTI scans on patients with severe TBI is challenging, and there are not currently larger published cohorts of severe TBI patients with early serial DTI data acquisition to our knowledge. The retrospective study design and acquisition of DTI data on clinical MRI scanners precluded us from standardizing the DTI sequence, and thus the acquisition parameters differed between the acute and subacute DTI scans for 2 patients. While this variability in the DTI sequence may increase the generalizability of the results to clinical settings in which the DTI sequence is likely to vary, it may also confound the longitudinal FA measurements [32]. To account for this potential confounder, we prospectively analyzed intrasubject FA variability in the splenium of the CC in a healthy control and found minimal variability in FA attributable to changing the DTI sequence. Moreover, we used a rigorous definition for lesional FA recovery that required the magnitude of lesional FA change to exceed that of the coefficients of variation in two control datasets. Notably, FA undergoes variable changes during the acute stage of TAI, with FA either decreasing due to extracellular edema [28, 54], or increasing due to intracellular edema [29, 47]. In the subacute and chronic periods, however, FA within TAI lesions declines if there is loss of axonal integrity [48]. Thus, a longitudinal increase in FA from the acute-to-subacute period that exceeds the normal range of FA variability provides strong inferential evidence for axonal recovery.
Another limitation of this study is that there have been significant advances in the acquisition, processing, and analysis of diffusion data since these DTI data were acquired. Diffusion data acquired at higher field strength (i.e. 3 Tesla) and with higher angular resolution (i.e. ≥30 diffusion-encoding directions) may provide more reliable measurements of FA than the acquisition methods used here (1.5 Tesla, 6–25 diffusion-encoding directions) [32]. Furthermore, reconstruction of diffusion data into fiber tracts (i.e. diffusion tensor tractography and high angular resolution diffusion tractography) generates biomarkers of traumatic axonal injury that may predict functional and cognitive outcomes more accurately than do regional FA measurements [10, 14, 55]. We show diffusion tensor tractography results for visualization purposes (Figure 2), but we analyzed lesional FA rather than tract-based biomarkers because the latter require higher angular resolution data for reliable quantitative analysis [56]. It is also important to consider that the timing of the acute and subacute DTI scans in this study varied. Since extracellular and intracellular edema may progress at variable rates, it is possible that this temporal variability confounded the relationship between acute and subacute FA measurements. Nevertheless, it is more likely that variable timing of DTI data acquisition biased our results toward the null hypothesis rather than biasing the results toward a longitudinal increase in FA.
Conclusion
This longitudinal, retrospective DTI study provides proof of principle that FA may recover within TAI lesions. We did not identify correlations between lesional FA recovery and functional outcomes in patients with TBI, nor did we find acute imaging biomarkers that predict lesional FA recovery. Prospective studies utilizing higher resolution DTI data acquired at standardized time points are needed to further elucidate the mechanisms of axonal recovery and identify early biomarkers of reversibly injured axons. These biomarkers have the potential to improve the accuracy of prognostication for patients with TBI and facilitate the development of neuroprotective therapies aimed at promoting axonal recovery [17, 57].
Acknowledgments
Study Funding:
This study was supported by the National Institutes of Health (R25NS065743, K23NS094538, R01NS059775, and P41EB015896), the American Academy of Neurology/American Brain Foundation, and the National Institute on Disability, Independent Living, and Rehabilitation Research, Administration for Community Living, U.S. Department Health and Human Services to Spaulding Rehabilitation Hospital (H133A120085). However, the contents of this manuscript do not necessarily represent the policy of the Department of Health and Human Services and endorsement by the Federal Government should not be assumed.
Contributor Information
Brian L. Edlow, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA. Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA
William A. Copen, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Saef Izzy, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Andre van der Kouwe, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA. Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Mel B. Glenn, Department of Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Steven M. Greenberg, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
David M. Greer, Department of Neurology, Yale-New Haven Hospital, Yale School of Medicine, New Haven, CT, USA
Ona Wu, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA. Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
References
- 1.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988;150:663–72. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 2.Adams JH, Jennett B, Murray LS, et al. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma. 2011;28:701–9. doi: 10.1089/neu.2010.1733. [DOI] [PubMed] [Google Scholar]
- 3.Blumbergs PC, Scott G, Manavis J, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–6. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 4.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18:307–16. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Christman CW, Grady MS, Walker SA, et al. Ultrastructural studies of diffuse axonal injury in humans. J Neurotrauma. 1994;11:173–86. doi: 10.1089/neu.1994.11.173. [DOI] [PubMed] [Google Scholar]
- 6.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–98. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 7.Strich SJ. Shearing of Nerve Fibers as a Cause of Brain Damage due to Head Injury: A Pathological Study of Twenty Cases. Lancet. 1961;2:443–448. [Google Scholar]
- 8.Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Gupta RK, Husain M, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 2009;23:675–85. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- 10.Wang JY, Bakhadirov K, Abdi H, et al. Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology. 2011;77:818–26. doi: 10.1212/WNL.0b013e31822c61d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer PW, Huisman TA, Sorensen AG, et al. Diffusion-weighted MR imaging in closed head injury: high correlation with initial glasgow coma scale score and score on modified Rankin scale at discharge. Radiology. 2004;233:58–66. doi: 10.1148/radiol.2323031173. [DOI] [PubMed] [Google Scholar]
- 12.Newcombe V, Chatfield D, Outtrim J, et al. Mapping traumatic axonal injury using diffusion tensor imaging: correlations with functional outcome. PLoS One. 2011;6:e19214. doi: 10.1371/journal.pone.0019214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edlow BL, Haynes RL, Takahashi E, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72:505–23. doi: 10.1097/NEN.0b013e3182945bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newcombe VF, Williams GB, Scoffings D, et al. Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. J Neurol Neurosurg Psychiatry. 2010;81:552–61. doi: 10.1136/jnnp.2009.196246. [DOI] [PubMed] [Google Scholar]
- 15.Jennett B, Adams JH, Murray LS, Graham DI. Neuropathology in vegetative and severely disabled patients after head injury. Neurology. 2001;56:486–90. doi: 10.1212/wnl.56.4.486. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–40. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp Neurol. 2015 doi: 10.1016/j.expneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148:181–93. doi: 10.1007/s00701-005-0674-4. discussion 193–4. [DOI] [PubMed] [Google Scholar]
- 19.Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- 20.Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12:555–64. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- 21.Edlow BL, Wu O. Advanced neuroimaging in traumatic brain injury. Seminars in Neurology. 2012;32:372–398. doi: 10.1055/s-0032-1331810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Li XY, Feng DF, Gu L. Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur J Neurosci. 2011;33:933–45. doi: 10.1111/j.1460-9568.2010.07573.x. [DOI] [PubMed] [Google Scholar]
- 24.Mac Donald CL, Dikranian K, Song SK, et al. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205:116–31. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Looij Y, Mauconduit F, Beaumont M, et al. Diffusion tensor imaging of diffuse axonal injury in a rat brain trauma model. NMR Biomed. 2012 doi: 10.1002/nbm.1721. [DOI] [PubMed] [Google Scholar]
- 26.Galanaud D, Perlbarg V, Gupta R, et al. Assessment of white matter injury and outcome in severe brain trauma: a prospective multicenter cohort. Anesthesiology. 2012;117:1300–10. doi: 10.1097/ALN.0b013e3182755558. [DOI] [PubMed] [Google Scholar]
- 27.Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–72. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 28.Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25:370–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–55. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 30.Rappaport M, Hall KM, Hopkins K, et al. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–23. [PubMed] [Google Scholar]
- 31.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 32.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51:807–15. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen AG, Wu O, Copen WA, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–92. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- 34.Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum: MR features. AJNR Am J Neuroradiol. 1988;9:1129–38. [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Rasmussen IA, Lagopoulos J, Haberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J Neurotrauma. 2007;24:753–65. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- 36.Gennarelli TA, Thibault LE, Adams JH, et al. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–74. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 37.McAllister TW, Ford JC, Ji S, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng. 2012;40:127–40. doi: 10.1007/s10439-011-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 39.Kessler LG, Barnhart HX, Buckler AJ, et al. The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat Methods Med Res. 2015;24:9–26. doi: 10.1177/0962280214537333. [DOI] [PubMed] [Google Scholar]
- 40.Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 41.Schwamm LH, Koroshetz WJ, Sorensen AG, et al. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29:2268–76. doi: 10.1161/01.str.29.11.2268. [DOI] [PubMed] [Google Scholar]
- 42.Liu AY, Maldjian JA, Bagley LJ, et al. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol. 1999;20:1636–41. [PMC free article] [PubMed] [Google Scholar]
- 43.Pasco A, Ter Minassian A, Chapon C, et al. Dynamics of cerebral edema and the apparent diffusion coefficient of water changes in patients with severe traumatic brain injury. A prospective MRI study. Eur Radiol. 2006;16:1501–8. doi: 10.1007/s00330-005-0086-0. [DOI] [PubMed] [Google Scholar]
- 44.Jang SH, Kim SH, Lim HW, Yeo SS. Recovery of injured lower portion of the ascending reticular activating system in a patient with traumatic brain injury. Am J Phys Med Rehabil. 2015;94:250–3. doi: 10.1097/PHM.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Espejo D, Soddu A, Cruse D, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. 2012;72:335–43. doi: 10.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- 46.Warner MA, Marquez de la Plata C, Spence J, et al. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J Neurotrauma. 2010;27:2121–30. doi: 10.1089/neu.2010.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24:1447–59. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 48.Kumar R, Husain M, Gupta RK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26:481–95. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- 49.Newcombe VF, Williams GB, Nortje J, et al. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br J Neurosurg. 2007;21:340–8. doi: 10.1080/02688690701400882. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan GM, Mierzwa AJ, Kijpaisalratana N, et al. Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum. J Neuropathol Exp Neurol. 2013;72:1106–25. doi: 10.1097/NEN.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong RC, Mierzwa AJ, Sullivan GM, Sanchez MA. Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Mierzwa AJ, Marion CM, Sullivan GM, et al. Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol. 2015;74:218–32. doi: 10.1097/NEN.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang JY, Bakhadirov K, Devous MD, Sr, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65:619–26. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- 55.Shin SS, Verstynen T, Pathak S, et al. High-definition fiber tracking for assessment of neurological deficit in a case of traumatic brain injury: finding, visualizing, and interpreting small sites of damage. J Neurosurg. 2012;116:1062–9. doi: 10.3171/2012.1.JNS111282. [DOI] [PubMed] [Google Scholar]
- 56.Wang JY, Abdi H, Bakhadirov K, et al. A comprehensive reliability assessment of quantitative diffusion tensor tractography. Neuroimage. 2012;60:1127–38. doi: 10.1016/j.neuroimage.2011.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith DH, Hicks R, Povlishock JT. Therapy development for diffuse axonal injury. J Neurotrauma. 2013;30:307–23. doi: 10.1089/neu.2012.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]