Abstract
The ribosome is a large two-subunit ribonucleoprotein machine that translates the genetic code in all cells, synthesizing proteins according to the sequence of the mRNA template. During translation, the primary substrates, transfer RNAs (tRNAs), pass through binding sites formed between the two subunits. Multiple interactions between the ribosomal subunits, termed intersubunit bridges, keep the ribosome intact and at the same time govern dynamics that facilitate the various steps of translation such as tRNA-mRNA movement. Here, we review the molecular nature of these intersubunit bridges, how they change conformation during translation, and their functional roles in the process.
Graphical abstract
In all cells, ribosomes translate the genetic code, synthesizing proteins based on the information content of mRNA. The translation process can be divided into four stages: initiation, elongation, termination, and ribosome recycling. During initiation, the two subunits of the ribosome come together to form an initiation complex in which the initiator tRNA is paired to the start codon (typically AUG) of the mRNA. This establishes the reading frame for protein synthesis. The elongation phase of translation occurs in repetitive cycles. In each cycle, the ribosome uses elongator tRNA to read the mRNA codon (decoding), catalyzes the incorporation of the amino acid into the nascent peptide chain (peptidyl transfer), and moves along the mRNA to the next codon (translocation). When the elongation complex encounters a stop codon (UAA, UAG, or UGA) in the mRNA, translation terminates and the polypeptide is released from the ribosome-bound tRNA and folds into a functional protein. The post-termination ribosome is then split into separate subunits, which are re-used in subsequent rounds of translation.
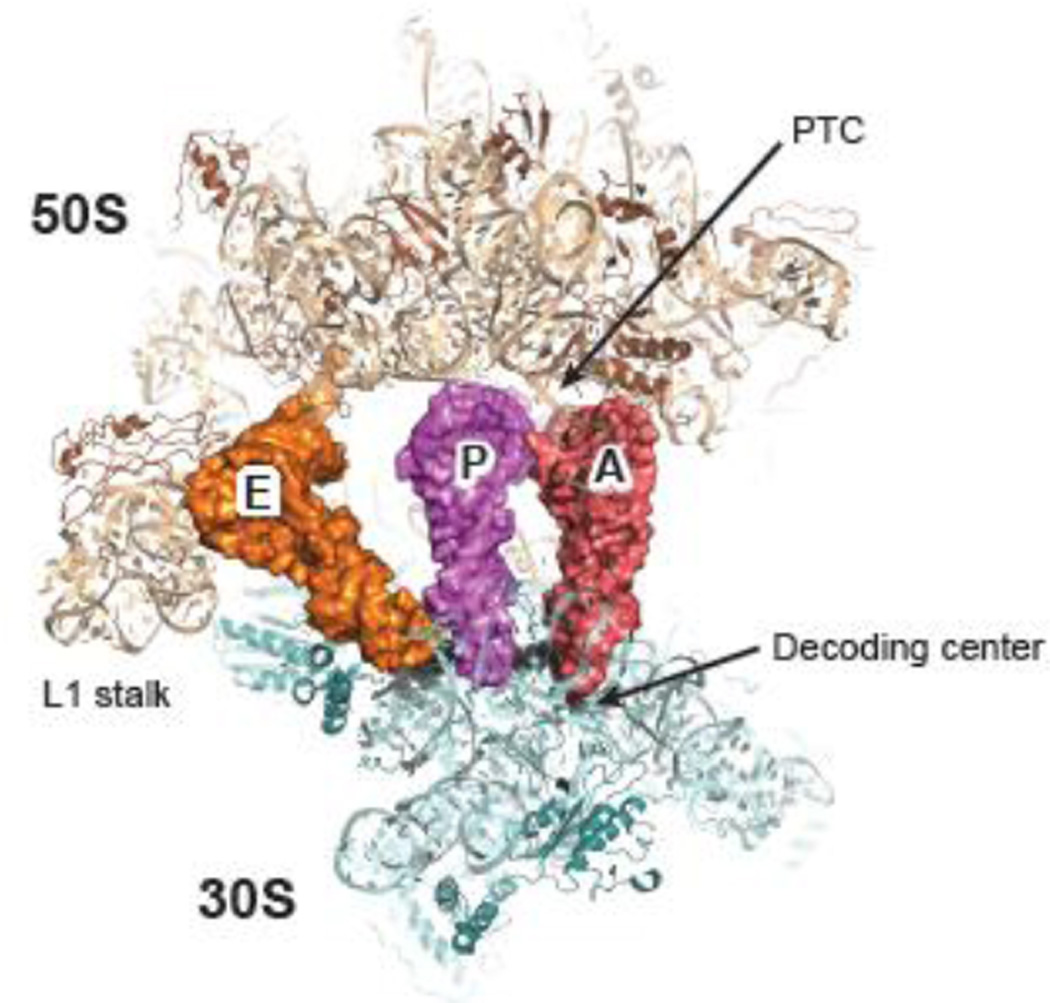
The ribosome is a megadalton ribonucleoprotein complex of two subunits. In bacteria, the small (30S) subunit is composed of one RNA molecule (16S ribosomal RNA, or 16S rRNA) and about 20 proteins, while the large (50S) subunit consists of two RNAs (23S and 5S rRNAs) and about 30 proteins. The 30S subunit binds the mRNA and facilitates decoding, whereas the 50S subunit catalyzes peptide bond formation at the peptidyl transfer center (PTC). Together, the two subunits form the 70S ribosome, which has three tRNA binding sites, the aminoacyl (A) site, the peptidyl (P) site, and the exit (E) site (Figure 1). The essential core of the ribosome, which provides its major functional sites, is highly conserved in all domains of life 1; 2; 3.
Figure 1. Structure of 70S ribosome.
The 30S (pale cyan, RNA; teal, proteins) and 50S (wheat, RNA; brown, proteins) subunits bind tRNAs in three sites: the aminoacyl (A) site (red), the peptidyl (P) site (purple), and the exit (E) site (orange). The decoding center is equivalent to 30S A site; the peptidyl transfer center (PTC) comprises the 50S A and P sites. This figure was generated in PyMol using PDB entries 2WDK and 2WDL 39.
Numerous biochemical and biophysical studies have provided information about the bacterial ribosome. Since the first atomic-resolution crystal structures were solved at the end of the last century, structural models of dozens of ribosomal complexes, with various substrates and/or protein factors, have been published (subset listed in Table 1). These complexes represent different functional stages of the translation apparatus, and help illustrate how the ribosome performs its tasks. These structures confirm earlier evidence that the major functional sites of the ribosome, including the PTC, decoding center (DC), and tRNA binding sites, are formed predominantly or solely by rRNA 4; 5; 6; 7; 8. Most of the ribosomal proteins are located on the exterior face of the 70S ribosome, with basic polypeptide extensions inserted into the rRNA tertiary structure, stabilizing rRNA folds through electrostatic interactions.
Table 1.
List of atomic-resolution structures discussed in this review
| Functional stage | tRNA(s)1 | Other ligands | Head2 | Rotation2 | Organism | Access code3 | Resolution (Å) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Pre-peptidyl transfer | A/A, P/P, E/E |
- | 0 | 0 | T. thermophilus | 2WDK, 2WDL | 3.5 | Voorhees et al. 2009 |
| Decoding | A/T, P/P, E/E |
EF-Tu, GDP, kirromycin, paromomycin |
0 | 0 | T. thermophilus | 2WRN, 2WRO | 3.6 | Schmeing et al. 2009 |
| Decoding | A/T, P/P, E/E |
EF-Tu, GDP, kirromycin | 0 | 0 | E. coli | EMD-2847 | 2.9 | Fischer et al. 2015 |
| Translocation | P/E | EF-G•GDPCP | 6.5 | 6.3 | T. thermophilus | 4JUW, 4JUX | 2.9 | Tourigny et al. 2013 |
| Post-termination | P/E | RF3•GDPCP | 6 | 8.8 | T. thermophilus | 3ZVO, 3ZVP | 3.8 | Jin et al. 2011 |
| Post-termination | - | RF3•GDPNP | 16.3 | 6.8 | E. coli | 3SFS, 3SGF | 3.2 | Zhou et al. 2012 |
| Post-termination | P/P | - | 1.5 | −1.2 | E. coli | 3R8O, 3R8T | 3.2 | Dunkle et al. 2011 |
| Recycling | P/E | RRF | 4.8 | 8.4 | E. coli | 3R8N, 3R8S | 3.2 | Dunkle et al. 2011 |
Each bound tRNA is listed in its classical or hybrid configuration.
Degrees of head swiveling (Head) and intersubunit rotation (Rotation) are obtained from Mohan et al. 2014.
Access codes are for PDB (crystal structures) or EMDB (cryo-EM reconstructions) entries.
The ribosome is dynamic, based on X-ray crystallography, cryo-electron microscopy (cryo-EM), and single-molecule Föster resonance energy transfer (smFRET) studies. For example, codon-anticodon pairing in the 30S A site induces movement of the 30S head and shoulder domain towards each other around the A site, resulting in a “closed” conformation 9, which is thought to activate elongation factor EF-Tu during the decoding process 10; 11; 12; 13. Moreover, two major large-scale rearrangements, rotation of 30S head domain relative to the rest of the subunit, as well as the rotation between 30S and 50S subunits, have been attributed to various steps of translation, including subunit joining 14, translocation 15; 16; 17; 18; 19; 20; 21, peptide release 22; 23, and ribosome recycling 24. Indeed the structural flexibility of the ribosome appears crucial for many aspects of translation, and may also be key to mechanisms of regulation.
The ribosome is intrinsically competent for protein synthesis, as shown by poly-uridine directed poly-phenylalanine (poly-Phe) synthesis in the absence of any protein factors 25; 26. But this factor-free translation is slow and relies on a noncanonical mode of initiation. Protein factors are critical for translation of natural heteropolymeric mRNA in vitro and for rapid and accurate translation in vivo. In the first stage of translation, three initiation factors—IF1, IF2, and IF3— help control the selection of initiator fMet-tRNAfMet (fMet-tRNA) and mRNA start codon during initiation complex assembly. Elongation factors EF-Tu and EF-G utilize the free energy of GTP hydrolysis to speed the processes of decoding and translocation, respectively. Although peptide bond formation is ribosome-catalyzed and thermodynamically favorable, peptidyl transfer involving certain substrates (e.g. synthesis of proline-proline) is kinetically unfavorable, and requires elongation factor EF-P to prevent translational stalling 27; 28. Other protein factors, including SmpB, ArfA, and YaeJ, are also indispensable for ribosome rescue in certain situations 29. Stop codons are recognized by class I release factors RF1 (UAA and UAG) and RF2 (UAA and UGA), which bind to the A site and facilitate the hydrolysis of peptidyl group from the P-site tRNA 30. The class II release factor RF3, related to EF-G, triggers dissociation of RF1/RF2 in a GTP-dependent manner 22; 23; 31; 32. Finally, ribosome recycling factor (RRF), EF-G, and IF3 split the two subunits after termination to release the mRNA and tRNA, in the process of ribosome recycling 33; 34. All protein factors introduced here bind sites between the two ribosomal subunits, as do the primary substrates of translation (i.e., mRNA and tRNA). Ligand binding usually masks certain regions of the subunit interface or triggers conformational rearrangement between the two subunits, which impact particular steps of translation. Indeed, understanding the molecular mechanisms of translation requires knowledge of the intersubunit interactions, the interplay of various factors and/or ligands, and their dynamics.
1. Intersubunit bridges of the ribosome
Purified 30S and 50S subunits are capable of forming 70S ribosomes in the absence of tRNA and protein factors, in a Mg2+-dependent manner 35. Low concentrations of Mg2+ favor dissociation of the two subunits, while high Mg2+ or polyamines (which effectively substitute for Mg2+) promote subunit association. Early cryo-EM studies identified several contacts between the two subunits at the interface, which were proposed to be RNA-RNA “bridges” 36; 37. These intersubunit bridges were later confirmed in higher-resolution structures 38, and most bridges are conserved in eukaryotic ribosomes 1; 2. However, the bridge interactions are not limited to RNA-RNA contacts, and in some cases involve RNA-protein or protein-protein contacts. Since ligand binding usually triggers conformational changes in intersubunit bridges, we will refer to the 70S ribosome with mRNA and three tRNAs bound, which represents the pre-peptidyl-transfer state of the elongation complex (Table 1, PDB entries 2WDK and 2WDL)39, as the classic state and use this complex as a starting point to discuss the molecular interactions of individual intersubunit bridges (Figure 2). Throughout the text below, helices of the large subunit will be denoted with an uppercase “H” and those of the small subunit with a lowercase “h.” Protein residues are numbered based on the mature (N-terminally processed) form of the protein.
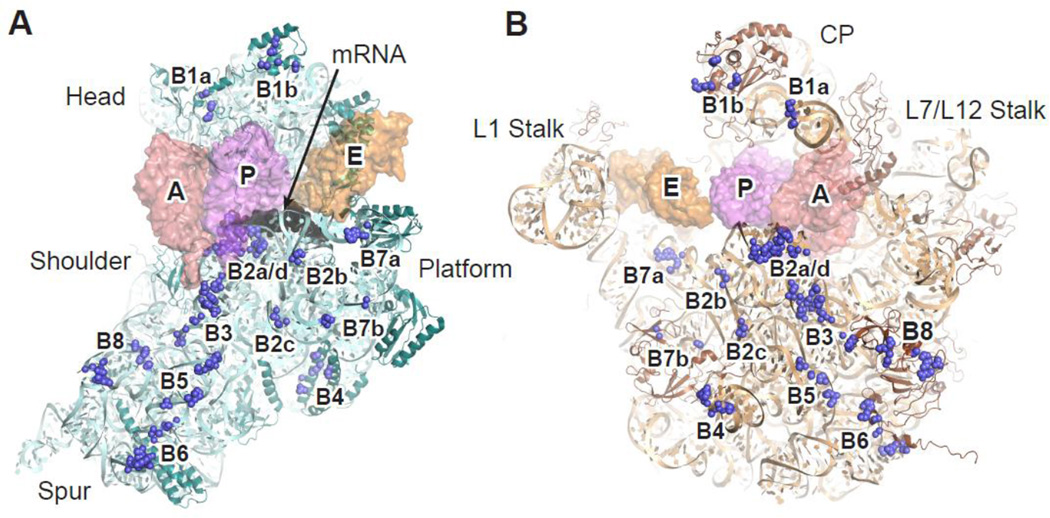
Figure 2. Intersubunit bridges of the ribosome.
Bridge components (blue spheres) of the 30S (A) or 50S (B) subunit are shown from the interface perspective. Space-filling models of the tRNAs are shown semi-transparent so the bridges remain visible. mRNA (black) is bound to the 30S neck region between the head and body domains. Color scheme is otherwise the same as in Fig. 1. CP, central protuberance. This figure was generated in PyMol using PDB entries 2WDK and 2WDL 39.
Bridge B1a and B1b
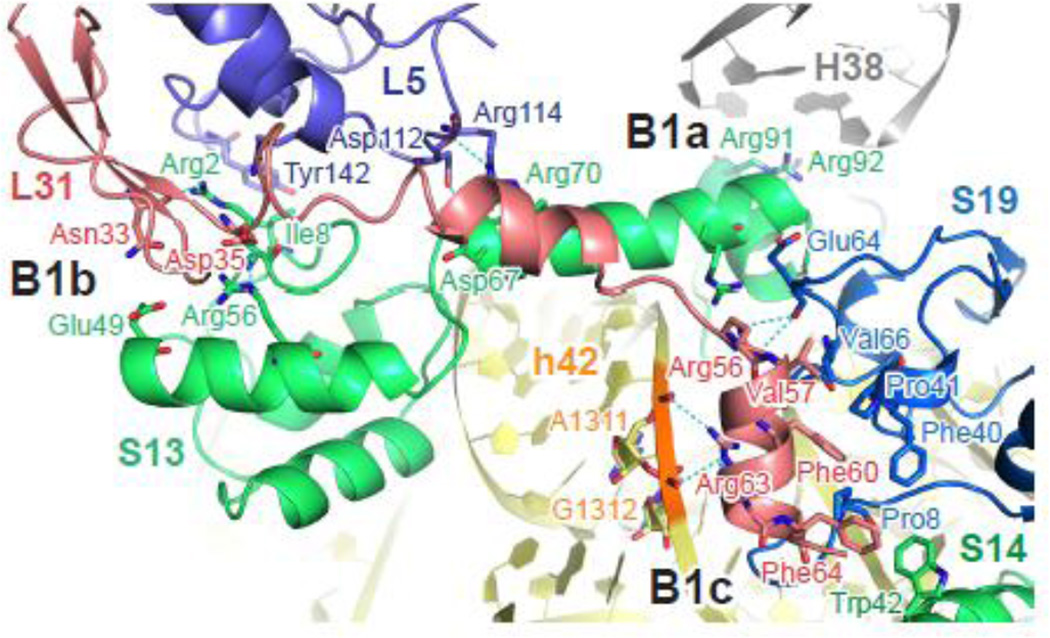
The bridge connecting 30S head domain and 50S central protuberance was one of the first intersubunit interactions identified, by ~ 40 Å resolution cryo-EM 36, and was later assigned as two adjacent interactions (Figure 3). B1a is formed between small subunit protein S13 and helix H38 of the 23S rRNA, with the side chains of Arg92 and Arg93 within hydrogen bonding distance of the phosphate backbone of A887 and C888 (Table 2). H38 is also known as the A-site finger, extending ~110 Å from the solvent side of the 50S subunit to the interface, and making direct contact with the elbow region of A-site tRNA at nucleotide (nt) 19 of the D loop and nt 55–57 of the TΨC loop. The base of H38 is intrinsically flexible 40, and contributes to movement of this helix during 30S head swiveling (see below). Electron density for the tip region of H38 is absent in available crystal structures of Escherichia coli ribosomes, consistent with H38 being mobile. Since the nature of the bridge interaction is mostly electrostatic, recognition of H38 by S13 is probably not sequence specific. In line with this idea, the loop region of H38 is less than 80% conserved among proteobacteria 41.
Figure 3. Bridges of the 30S head domain.
Residues involved in bridge interactions are shown in sticks and colored by elements (red, oxygen; blue, nitrogen). Potential hydrogen bonds are marked with dashed lines (cyan). Gray, 23S rRNA; yellow, 16S rRNA; slate, L5; salmon, L31; green, S13 or S14; blue, S19. The loop region of H38 (nt 887–889) was not included in the original atomic model due to low electron density. This figure was generated in PyMol using EMDB entry EMD-2847 43.
Table 2.
Summary of molecular interactions of the intersubunit bridges
| T. thermophilus1 | E. coli2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bridge | 30S component(s) | 50S component(s) | Interaction3 | 30S component(s) | 50S component(s) | Interaction3 | ||||
| B1a | S13 | Arg92, Arg93 | H38 | A887, C888 | Side-P | S13 | Arg91, Arg92 | H38 | Disordered | |
| B1b | S13 | Ile8 | L5 | Tyrl45 | Side-side | S13 | Ile8 | L5 | Tyrl42 | Side-side |
| Arg10 | Asp146 | Side-side | No interaction | |||||||
| Disordered | Asp67 | Arg114 | Side-side | |||||||
| Arg69, Arg70 | Asp112 | Side-side | ||||||||
| No interaction | Arg2 | L31 | Asn33 | Side-back | ||||||
| Arg56 | L31 | Glu60 | Side-side | Arg56 | Asp35 | Side-side | ||||
| B1c | L31 Disordered | S14 | Trp42 | L31 | Val57, Phe60, Phe64 |
Side-side (Hydrophobic) |
||||
| S19 | Pro8, Phe40, Pro41, Val66 |
|||||||||
| Glu64 | Arg56 | Back-side | ||||||||
| h42 | A1311, G1312 | Arg63 | Back-side | |||||||
| B2a/d | h44 | U1406, U1495 | H69 | A1919 A1912 | Base-ribose | Same as T. thermophilus | ||||
| C1407-G1494 | A1912 | A-minor | ||||||||
| A1408 | A1916 | Ribose-base | ||||||||
| A1410 | C1914 | P-base | ||||||||
| C1409 | C1914, U1915 | Ribose-base | Missing, distance change due to lack of A-site tRNA | |||||||
| Missing, conformational change due to A-site tRNA binding | h44 | A1492 | H69 | A1913 | Base-base | |||||
| h24 | U793, G1517 | H69 | C1920 | Mg | No Mg | |||||
| h45 | G1517 | A1919 | Base-ribose | Same as T. thermophilus | ||||||
| S13 | Argl24 | A1912, A1913 | Mg | No Mg, phylogenetic difference | ||||||
| B2b | h24 | C783 | H68 | C1836 | Ribose-P | Same as T. thermophilus | ||||
| Missing, distance change due to A-site tRNA binding | h45 | G1516 | H71 | U1931 | P-ribose | |||||
| B2c | h27 | C899 | H67 | C1832 | Minor-P | Same as T. thermophilus | ||||
| h24 | C770 | C1833 | Mg | No Mg | ||||||
| C770, G771, C899 |
C1832, C1833 | Mg | ||||||||
| h27 | A900, A901 | C1832 | Mg | |||||||
| B3 | h44 | A1483 | H71 | C1947-G1959 | A-minor | Same as T. thermophilus | ||||
| C1484 | A1960 | Ribose-ribose | ||||||||
| A1418 | G1948-C1958 | A-minor | ||||||||
| G1419 | G1949 | Ribose-ribose | ||||||||
| G1421 | G1950, U1951 | Mg | NoMg | |||||||
| L14 | Glu54 | h44 | G1421 | L14 | Lys54 | P-side | ||||
| G1423 | Arg49 | P-side | Same as T. thermophilusss | |||||||
| B4 | S15 | Ser39, His52 | H34 | G715 | Side-base | S15 | Gln39 | H34 | A715 | Side-base |
| Arg63 | G715 | Side-P | Arg88 | U714, A715 | Side-P | |||||
| Lys43 | A716 | Side-ribose | Gln39 | A716 | Side-ribose | |||||
| B5 | Missing, distance difference | h44 | A1428 | H62 | C1686 | Ribose-base | ||||
| G1703 | Ribose-ribose | |||||||||
| h44 | C1429, C1430 | H62 | G1703, G1704 | Mg | A1429 | G1703 | Ribose-ribose | |||
| C1704 | Ribose-P | |||||||||
| G1475 | A1689 | P-ribose | Missing, distance difference | |||||||
| A1700 | P-base | Same as T. thermophilus | ||||||||
| B6 | h44 | G1432 | L19 | Argl08 | P-back | h44 | G1432 | L19 | Lysl05 | P-back |
| No interaction | A1433 | P-side | ||||||||
| B6 | h44 | G1464, C1465 | L19 | Argl08 | P-side | h44 | U1463, U1464 | L19 | Argl08 | P-side |
| C1463 | Arg111, Arg115 | P-side | Phylogenetic difference | |||||||
| G1442, A1444 | Arg118 | Side-side | ||||||||
| G1443 | Asp122 | Ribose-side | ||||||||
| H101 | C2864 | Base-P | ||||||||
| B6R4 | H44 | No interaction | L19 | h44 | A1431 | L19 | Thrl03 | P-side | ||
| No interaction | G1432 | Glyl04, Lysl05 | P-back | |||||||
| G1443 | Ilel21, Aspl22, Argl25 |
Base-back/side | A1441 | Glu111 | Base-side | |||||
| G1464 | Arg111 | P-side | U1463, U1464 | Argl08 | P-side | |||||
| C1465 | Argl08 | P-side | ||||||||
| Phylogenetic difference | U1471 | L14 | Argl7 | P-side | ||||||
| B7a | h23 | A702 | H68 | A1848 | Stacking, P- ribose |
Same as T. thermophilics | ||||
| G1846-C1894 | A-minor | |||||||||
| B7aR4 | H23 | A702 | H68 | G1846, A1847 | Base-P | H23 | A702 | H68 | G1846 | Base-P |
| B7b | h23 | A712 | L2 | Glnl63, Glnl65 | P-side | H23 | A712 | L2 | Glnl62 | P-side |
| No interaction | C680 | Arg268 | Ribose-Side | |||||||
| B7b | h24 | G774 | L2 | Lys201 | P-side | h24 | G774 | L2 | Met200 | P-side |
| No interaction | G775 | Argl76 | P-side | |||||||
| B7bR4 | h23 | C680 | L2 | Glnl65 | Ribose-side | Missing, distance difference | ||||
| C681 | Argl67 | Ribose-back | ||||||||
| G711 | Glyl38 | Ribose-back | h23 | G711 | L2 | Glyl37 | Ribose-back | |||
| A712 | Glnl63 | Ribose-side | A712 | Glnl62 | Ribose-side | |||||
| B7bR4 | h24 | G773 | Asn202 | P-side | h24 | U772 | Lys4 | P-side | ||
| G774, G775 | Lys201 | P-side | G773, G774 | Met200, Leu201 | P-side | |||||
| B8 | h14 | A338, C339 | L14 | Asn13, Arg97 | P-side | h14 | C339 | L14 | Asn13 | P-side |
| U340 | Thr96 | P-side | U340 | Thr97 | P-side | |||||
| No interaction | C341 | Arg98 | P-side | |||||||
| C345 | Val115, Ser116, Ala118 |
Mg | No Mg | |||||||
| G346 | Argl07 | P-side | h14 | G346 | L14 | Argl05 | P-side | |||
| No interaction | L19 | C345 | L19 | Arg38 | P-side | |||||
| G346 | Lys35, Arg41 | P-side | G346 | Arg38, Gln40 | P-side | |||||
| B8R4 | h14 | C337, A338 | L14 | Arg97 | P-side | h14 | A338, C339 | L14 | Arg98 | P-side |
| C339 | Thr96 | P-side | U340 | Thr97 | P-side | |||||
| A338, C339 | Asn13 | P-side | No interaction | |||||||
| C345 | Arg107 | P-side | C345 | Argl08 | Ribose-side | |||||
| B8R4 | h14 | C345 | L19 | Ser35, Lys36 | P-back/side | |||||
| G346 | L14 | Argl05, Argl08 | P-side | |||||||
| No interaction, phylogenetic difference | L19 | Glu33 | Ribose-side | |||||||
| Gly34 | P-back | |||||||||
| h8 | G159 | Arg38 | P-side | |||||||
| NewR4 | S7 | Argl42 | H76 | G2115 | Side-P | S7 | Argl42 | H78 | A2147 | Side-P |
Classic-state T. thermophilus bridges are assigned according to the structure of ribosome in the pre-peptidyl-transfer state [PDB entries 2WDK and 2WDL, Voorhees et al. 2009].
Classic-state E.coli bridges are assigned according to the structure of ribosome bound with a P-site tRNA [PDB entries 3R8O and 3R8T, Dunkle et al. 2011], except that B1b, B1c, and B7b are inferred from the structure of a decoding complex bound with the ternary complex and P- and E-site tRNAs [EMDB entry EMD-2847, Fischer et al. 2015].
The detailed molecular interaction(s) between 30S-50S components. For protein components: side, side chains; back, backbone. For RNA components: P, phosphate backbone; A-minor, interaction of an adenine with the minor groove. Mg, Mg2+-coordinated interaction.
Bridge interactions in the rotated conformation are assigned according to the structure of RF3-bound [T. thermophilus, PDB entries 3ZVO and 3ZVP, Jin et al. 2011] or RRF-bound [E.coli, PDB entries 3R8N and 3R8S, Dunkle et al. 2011] ribosome with a P/E-site tRNA.
Bridge B1b connects S13 and large subunit proteins L5 and L31 in the classic-state T. thermophilus ribosome, through electrostatic and possibly hydrogen-bond interactions (Table 2). The position of L31 remained ambiguous until recent structural studies uncovered its interactions within both the 50S and 30S subunit 42; 43. In the model of full-length L3143, its N-terminal domain (NTD), together with L5, forms bridge interactions with S13 essentially as seen in T. thermophilus ribosomes (Table 2, Figure 3). Additionally, the C-terminal domain (CTD) of L31 docks onto a hydrophobic surface formed by S14 and S19, which is further stabilized by hydrogen bonding between Arg56 and backbone of S19, as well as electrostatic interaction between Arg63 side chain and the phosphate backbone of helix h42 (A1311 and G1312). Since the interaction between S14/S19/h42 and L31 has only recently been identified, we refer to it here as B1c (Figure 3, Table 2). The extensive protein-protein interactions of bridge B1c may contribute to the stability of 30S head domain during intersubunit movement (see below).
Truncation of H38 44 or mutating the C-terminal region of S13 that contributes to B1a (R92E/R93E or S13 1–84 45) has a relatively modest effect on cell growth, with the former mutation slightly stimulating translation in vitro. In contrast, S13 mutations partially disrupting B1b (R3A/I4A or S13 1–69) cause substantial growth defects and increase −1 frameshifting in vivo 45. Although S13 is not essential, depletion of S13 significantly impairs cell growth and leads to a subunit association defect in vitro. Similar to the H38 truncation, removal of S13 from the small subunit stimulates factor-independent poly-Phe synthesis 46. Hence, these head bridges were proposed to act as “translocation attenuators”, controlling this step of elongation. The more severe phenotype caused by B1b mutations can be rationalized in the context of the structure. The B1b interaction is sequence-specific and may depend on cooperative binding between S13, L5, and L31. Disrupting interactions between S13 N-terminus and either L5 or L31 could interfere with the association of L31 NTD with the 50S, as well as its CTD with the 30S. Future studies targeting specific regions of L31 may clarify the contributions of individual protein-protein contacts.
Bridge B2a/d, B2b, and B2c
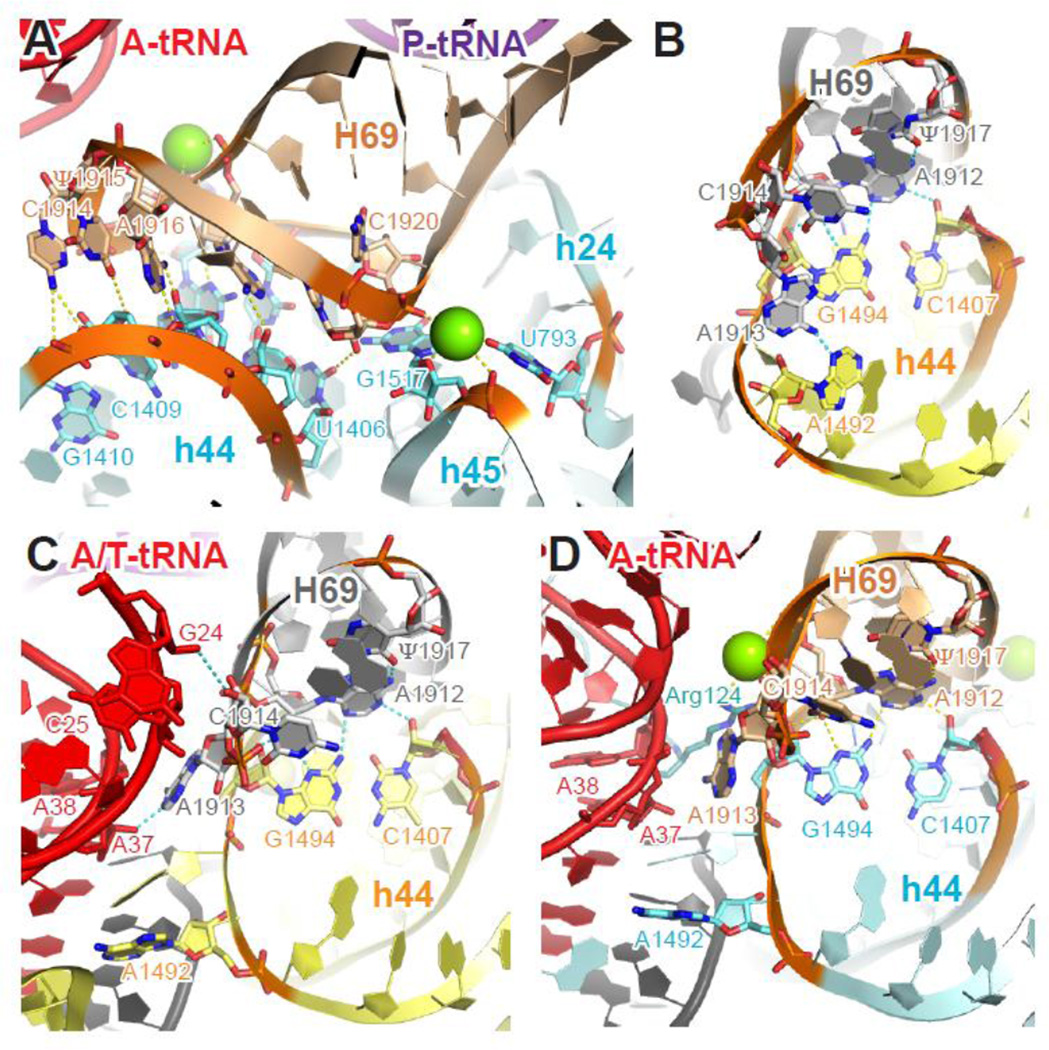
Another of the earliest intersubunit interactions identified 36; 47 is B2, which spans a large interface area of 30S platform. B2 has since been subdivided into several adjacent contacts. B2a/d is close to the decoding center and formed between the tip of helix H69 of 23S rRNA (nt 1912–1920) and several elements of 16S rRNA (base of helix h44, nt 1406–1410 / 1494–1495; loop of h24, nt 793; loop of h45, nt 1517). These contacts are made mostly through hydrogen bonding and Mg2+-coordinated interactions (Table 2, Figure 4A). In the T. thermophilus ribosome, H69 makes additional contacts with the C-terminus of S13 through the side chain of Arg124, although this interaction is not conserved in E. coli 24.
Figure 4. Molecular interactions and conformational changes at bridge B2a/d.
(A) Tertiary structure of H69 of the 23S rRNA and h24, h44, h45 of the 16S rRNA in the context of classic-state 70S ribosome. (B–D) Conformational changes of h44 (A1492) and H69 (A1913 and C1914) during decoding. In the ribosome with a vacant A site (B), A1492 of h44 interacts with A1913 of H69 at bridge B2a/d. In the ternary complex-bound ribosome trapped with kirromycin (C), the aa-tRNA is in a distorted A/T conformation, A1492 flips out of h44 and loses its interaction with H69, while A1913 and C1914 contact nt 37–38 and 24–25 of the A/T-site tRNA, respectively. When the aa-tRNA has been accommodated into the A/A site (D), A1492 of h44 and A1913 of H69 remains interact with the A-site tRNA, while C1914 of stacks back into H69. Cyan (T. thermophilus), yellow (E. coli), 16S rRNA; wheat (T. thermophilus), gray (E. coli), 23S rRNA; red, A- or A/T- site tRNA; purple, P-site tRNA; black, mRNA; green sphere, Mg2+. Residues involved in tertiary interactions are shown in sticks and colored by elements (red, oxygen; blue, nitrogen). Potential hydrogen bonds are marked with yellow (T. thermophilus) or cyan (E. coli) dashed lines. Panels A and D were generated using PDB entries 2WDK and 2WDL 39, panel B was generated using PDB entries 3R8O and 3R8T 24, and panel C was generated using EMDB entry EMD-2847 43.
Bridge B2a/d undergoes a large conformational change during tRNA selection. When aa-tRNA binds the ribosome as part of the ternary complex (EF-Tu•GTP•aa-tRNA), A1492 and A1493 of h44 flip out of the helix and dock into the minor groove of the codon-anticodon helix. This rearrangement disrupts the interaction between A1492 of h44 and A1913 of H69, and places A1913 into a pocket formed between the backbone of h44 (nt 1493–1494) and A-site tRNA (nt 37–38), while the adjacent C1914 of H69 makes direct contact with the D stem (nt 25–26) of aa-tRNA (Figure 4B–C) 24; 42; 43; 48. The aa-tRNA in this decoding state adopts a highly distorted (A/T) conformation, and the interactions made by h44 and H69 appear to help stabilize A/T-tRNA. This A-site rearrangement is highly unfavorable with a mismatch between codon and anticodon and hence is key to the fidelity of decoding 12; 49. Consistent with the importance of these interactions in decoding, various mutations affecting translation fidelity localize to the loop region of H69 50; 51; 52; 53.
Both h44 and H69 retain their contacts with the accommodated A/A-site tRNA, except that C1914 stacks back into H69 (Figure 4D, Table 2). Compared to ribosomes with a vacant A site, H69 adopts a less-bent conformation and shifts slightly closer to the minor grove of h44 in the pre-peptidyl-transfer complex 54. As a result, extra interactions are established between C1409 of h44 and C1914/U1915 of H69, presumably compensating the loss hydrogen bonding between A1492 and A1913. Other bridge interactions, including the highly conserved A-minor interaction between A1912 of H69 and C1407-G1494 of h44, are largely unchanged upon A-tRNA binding. The specific tertiary structure of H69 seems to be critical for its flexibility in adopting different conformations during intersubunit movement, while maintaining the bridge interaction with h44 (see below). This is corroborated by the deleterious effects of mutations in the H69 loop region 53; 55, and the fact that nucleotides Ψ1911-A1919 and A1912 are > 98% conserved in all domains of life 41. Post-transcriptional modification of H69 has also been shown be critical for its conformation 56, and may be important for bridge interactions and/or structural flexibility.
Bridge B2b is formed by hydrogen bonding between 2’-OH of C783 of helix h24 and the phosphate backbone of C1836 at the base of helix H68. In structures of the E. coli ribosome lacking A-site tRNA, additional interaction is seen between the phosphate backbone of G1516 of helix h45 and the 2’-OH of U1931 of helix H71. Loss of this interaction in the pre-peptidyl-transfer complex is likely due to the domain closure movement of 30S subunit upon aa-tRNA accommodation, which strengthens the interaction of h45 with H69 and shifts it away from H71. In line with this idea, similar widening between h45 and H71 was also seen in structures of the E. coli ribosome trapped at the decoding stage 43.
At bridge B2c, the phosphate backbone of C1832 of helix H67 is within hydrogen-bonding distance to the minor groove of C899 at the loop region of helix h27, which forms tertiary interaction with the base of helix h24. Three Mg2+ ions coordinate additional interactions between the backbone of H67 and h24/h27 in the classic-state T. thermophilus ribosome. In the E. coli ribosome with a vacant A site, although Mg2+ ions are not reported at B2c, the distance between H67 and h24/h27 backbones is very similar to that seen in the T. thermophilus structure. Therefore, Mg2+ or other divalent cations may similarly coordinate interactions of B2c in the E. coli ribosome as well. Involvement of phosphate backbone of h24 in bridge interaction is supported by the observation that phosphorothioate substitution at C770 of E. coli 16S rRNA abolishes subunit association 57. In any case, stable tertiary interaction between the GCAA tetraloop of h27 and the stem region of h24 (nt 770–772, 809–811) seems to be important for the formation of bridge B2c, as indicated by the subunit association defect when such interactions are disrupted 58; 59. Interestingly, disruption of h24-h27 interaction from either helix also compromises translation fidelity. The stem region of h27 (nt 887–888, 892–893, 909–912) interacts directly with h44 (nt 1413–1415, 1488–1489) and S12 protein (Arg97 and Arg89), both of which are important for the accuracy of decoding 9; 60. Mutations targeting the base of h27 also confer fidelity phenotypes 61; 62; 63, further implicating this region in the decoding process.
Bridge B3
Helix h44, the longest helix of 16S rRNA, spans the 30S interface from the neck region to the spur, and contributes to multiple bridge interactions, including B2a, B3, B5, and B6. One common feature of these bridges is that helices of 23S rRNA or side chains of large subunit protein dock into the minor groove of h44, as discussed above for B2a/d. In bridge B3, the tip region of helix H71 (nt 1947–1950, 1958–1960) of 23S rRNA interacts with h44 (nt 1418–1421, 1483–1484) through extensive hydrogen bonding, including two highly-conserved A-minor interactions (Table 2). Several nucleotides at B3 (A1483 and C1484 of h44, C1947 and A1960 of H71) have been shown to be critical for subunit association 64; 65; 66, and essentially no differences in RNA-RNA interactions are observed between T. thermophilus and E. coli ribosomes. Additional bridge contacts are mediated through Mg2+ and large subunit protein L14 through electrostatic interactions. In the T. thermophilus ribosome, a Mg2+ ion coordinates the interaction between the phosphate backbone of G1421 of h44, G1950 and U1951 of H71, and the side chain of Glu54. In the E. coli ribosome, however, the corresponding residue of L14 is replaced with a lysine. As result, Lys54 interacts directly with the phosphate backbone of G1421 of h44, which probably excludes Mg2+ from B3. As seen with other bridge interactions (e.g. B1b, B4, and B7a), ribosomal protein components generally exhibit more variations than the rRNA components.
Bridge B4
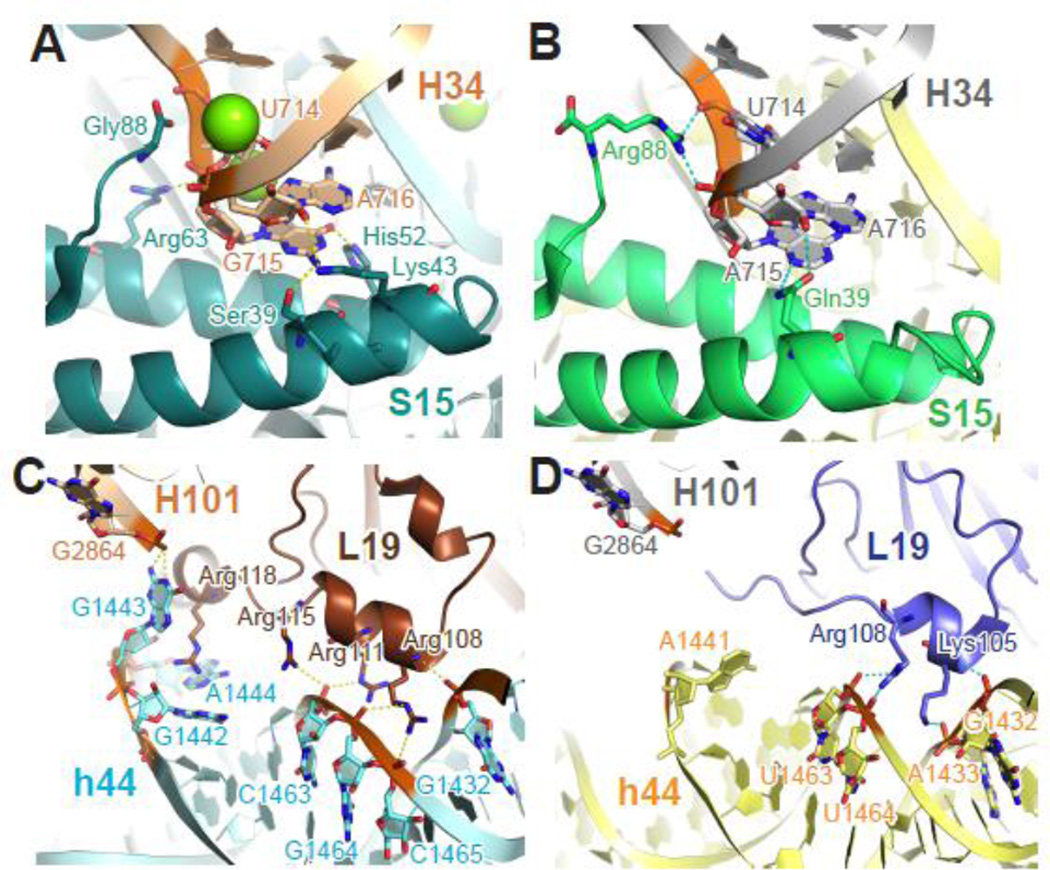
Bridge B4 was identified from low-resolution crystallography and hydroxyl radical probing as an interaction between the loop region of helix H34 and the small subunit protein S15 67. Although nucleotides contributing to B4 are highly conserved (Table 2), replacing the loop region of H34 with various tetraloops minimally affects ribosome function and cell growth 66, which probably reflects the lack of base-specific interactions at this bridge. In addition, S15 residues contributing to the bridge vary considerably across different species. For example, Arg63 of T. thermophilus S15 and Arg88 of E. coli S15, although unrelated phylogenetically, make similar electrostatic contacts to the phosphate backbone of the 715 loop (Figure 5A–B). Similarly, Gln39 of E. coli S15 and Ser39 and Lys43 of T. thermophilus S15 interact with H34 in analogous ways. It is striking that the overall structure of B4 remains largely unchanged despite these differences in the detailed molecular interactions. Indeed, although evolutionarily quite plastic, the presence of bridge B4 is needed for optimal subunit association and translation activity44.
Figure 5. Phylogenetic differences at bridges B4 and B6.
Bridge B4 (A–B) and B6 (C–D) from T. thermophilus (A, C) and E. coli (B, D) ribosomes share the same overall structure despite the phylogenetic differences in protein S15 and L19. In the T. thermophilus B6 (C), additional interactions form due to the unique C-terminal extension of L19 and a three-nucleotide bulge (nt 1442–1444) of h44. Teal (T. thermophilus), green (E. coli), small subunit proteins; brown (T. thermophilus), slate (E. coli), large subunit proteins; otherwise color scheme is the same as Figure 4. Panel A and C were generated using PDB entries 2WDK and 2WDL 39, and panels B and D were generated using PDB entries 3R8O and 3R8T 24.
Bridge B5
Further from the decoding center, a third bridge forms between h44 (nt 1428 –1431, 1472 –1476) and helix H62 of 23S rRNA. In contrast to B2a/d and B3, the tertiary interaction at B5 lacks base-specific minor-groove interactions, and the nucleotide sequence is much less conserved, with the only exception being the hydrogen bonding between N6 of the universally conserved A1700 of H62 and the phosphate backbone of G1475 of h44 (Table 2). In agreement with the sequence variability, introducing base substitution at nucleotides 1429–1430 or 1474–1476 of h44 does not affect cell growth and shows little-to-no defect in subunit association 65. The same mutations do increase miscoding, which is likely due to the disruption of h44 base pairing rather than bridge interaction.
Bridge B6
The last bridge contributed by h44 is formed mainly through electrostatic interactions between its phosphate backbone (nt 1432–1433, 1463–1465) and basic residues from large subunit protein L19, with very little sequence conservation. In the T. thermophilus ribosome, a unique 30-residue C-terminal extension of L19 makes extra interactions with h44 (nt 1463, 1442–1444), and a three-nucleotide bulge of h44 (nt 1442–1444, equivalent to the nt 1441 bulge in E. coli) brings its phosphate backbone closer to helix H101 of the 23S rRNA, compared to their E. coli counterparts (Figure 5C–D). As seen with B4, although residues contributing to the bridge interaction vary between different organisms, the overall architecture of the bridge remains largely unchanged. The extra intersubunit contacts seen in the T. thermophilus ribosome likely contribute to the thermostability of this organism.
Bridge B7a and B7b
Bridge B7 links the 30S platform domain and the base of the 50S L1 stalk, close to the E site. At bridge B7a, A702 flips out of helix h23, stacks with an A-A platform (nt 1847–1848) at the stem region of helix H68, and interacts with the minor groove of G1846–C1894 and the 2’-OH of A1848 (Figure 6A, Table 2). Base substitution of the highly conserved A1848 has minimal effects on cell growth and subunit association, while disruption of H68 secondary structure is more deleterious 66. In the 70S ribosome, H68 stacks coaxially with helix H71 and forms a long continuous arm spanning the 50S interface below the tRNA binding cleft, with the stem region of H68 (nt 1850–1852) directly contacting the acceptor stem of E-site tRNA (nt 70–72) (Figure 6C). Interactions with H68 may facilitate movement of P-site tRNA into the P/E hybrid site during translocation, as methylation of tRNA at the 2’-OH of position 72 strongly inhibits this process 68. A truncation of H68, predicted to eliminate B7a and compromise the 50S E site, reduces both subunit association and translation activity in vitro 44.
Figure 6. Interactions at bridge B7a and B7b in response to E-site occupancy.
Compared to ribosomes with a vacant E site (A–B), the presence of E-site tRNA (C–D) strengthens bridge interactions at B7b and has minimal effect on B7a. Nucleotide A1848 of H68, which stacks with A702 of h23 at bridge B7a, is highlighted in purple. Regions involved in the interaction between H68 and E-site tRNA are highlighted in pink. Residues of interest are shown in sticks and colored by elements (red, oxygen; blue, nitrogen). Dashed cyan lines indicate potential hydrogen bonds, with Å distances indicated in panel D. In panel B, larger gaps between the identical atoms are pointed out with double-headed black arrows. Gray, 23S rRNA; yellow, 16S rRNA; slate, L2; orange, E-site tRNA. Panels A-B were generated using PDB entries 3R8O and 3R8T 24, and panels C-D were generated using EMDB entry EMD-2847 43.
Bridge B7b is formed between the stem regions of helix h23 and h24 of 16S rRNA and large subunit protein L2. In the T. thermophilus classic-state ribosome, several polar and positively charged residues are within hydrogen-bonding distance of the phosphate backbone of nucleotides 712–713 (h23) and 772–774 (h24) (Table 2). In the E. coli ribosome bound with P-site tRNA, however, the distance between L2 and h23/h24 is significantly larger, with the closest polar residue of L2 more than 4.5 Å away from the 16S rRNA (Figure 6B). The loss of interaction at B7b in the E. coli complex could be due to the absence of tRNA in the E site. E-site tRNA interacts with S7 and S11 of the 30S, as well as the L1 stalk and helix H88 of the 50S, bringing the two subunits closer in this region. In line with this idea, in the cryo-EM reconstruction of the E. coli ribosome with E-site tRNA, there is clear evidence for bridge interaction between L2 (Gln162, Arg174, Arg176, Met200, Arg268) and h23/h24 backbone at similar region as seen in T. thermophilus ribosome (Figure 6D, Table 2). The dynamics at B7b in response to E site occupancy might contribute to the reported effects of E-site tRNA on decoding 69, translocation 70, and frame maintenance 71; 72.
Bridge B8
At bridge B8, large subunit protein L14 and L19 make contact with the loop (nt 345–346) and stem (nt 339–341) regions of helix h14 of 16S rRNA, mainly through electrostatic interactions with the phosphate backbone (Table 2). A Mg2+ ion coordinates additional interactions between the peptide backbone at the C-terminal region of L14 and the non-bridging phosphate oxygen of the h14 loop. Three universally conserved nucleotides (A344, G346, G347) from h14 also interact with the loop region (A160 and A161) of helix h8 of the 16S rRNA. Truncation of h8 or h14, as well as point mutations targeting either tetraloop, strongly decreases translation fidelity 63; 73. Crystal structure of ribosomes with G347U mutation in the 16S rRNA showed a distortion of both h8 and h14. As a result, the phosphate backbone of h14 moves 2–6 away from the large subunit, with the bridge interaction presumably disrupted 74. Therefore, the integrity of h8-h14 structure seems to be critical for the formation of B8. In support of this notion, replacing the entire tetraloop of h8 or h14 while maintaining the tertiary structure has only mild effect on translation 75.
Disruption of B8 is also seen in the ribosome complex trapped at the decoding stage 48, suggesting that the bridge normally separates during GTPase activation of EF-Tu. Indeed, 16S rRNA mutations disrupting bridge B8 have been shown to stimulate GTP hydrolysis by EF-Tu, an effect more prominent in the presence of a codon-anticodon mismatch 63; 74. These observations indicate that bridge B8 negatively regulates GTPase activation of EF-Tu to increase the stringency of decoding. Several sites within L14 and L19, including Gln40 of L19, which directly interacts with the phosphate backbone of h14, have also been implicated in decoding 76. The role of B8 in decoding might explain the unusually high degree of conservation for both RNA (h8/h14) and protein (L14/L19) components of this bridge.
2. The formation and disruption of bridge interactions
Formation of bridges during subunit joining
Comparison of the structures of 70S ribosome and the isolated subunits suggest that substantial conformational changes occur upon subunit joining. In the 30S subunit, the head domain tilts towards the subunit interface 38; 77; 78, while at the body domain, h8, h14, and h44 of the 16S rRNA move laterally away from the platform domain by 4–7Å 77. In the 50S subunit, structural changes are observed mainly at H34 and H69 of the 23S rRNA, as well as protein L2, L14, and L19, while other flexible regions, such as H38, L1 and L11 stalks, become restrained in the 70S ribosome 77. Most of these mobile elements are located at the subunit interface and contribute to the bridge interactions. For example, compared to that seen in the free 50S, the tip region of H69 moves about 14 Å towards the 30S in the 70S ribosome, forming bridge B2a/d with h44 79.
During translation initiation, subunit joining occurs between the 50S subunit and the 30S initiation complex (30SIC), in which mRNA, fMet-tRNA, and three initiation factors are bound. Formation of intersubunit bridges in the context of 70S initiation complex (70SIC) formation is controlled in part by the initiation factors. IF1 occupies 30S A site and interacts directly with the base of h44, causing conformational changes down the helix that presumably impact bridge interactions 80; 81. IF3 binds the 30S platform and is predicted to occlude the formation of central bridges B2b and B2a/d 80; 82; 83. Functional studies show that both IF1 and IF3 negatively regulate subunit joining and enhance the fidelity of start codon selection 84; 85; 86; 87; 88; 89; 90; 91; 92. IF2 is a GTPase that interacts with the 30S shoulder domain and the acceptor end of fMet-tRNA, extending more than 100 angstroms across the interface side of the 30S subunit 14; 83. IF2•GTP promotes fMet-tRNA binding and stimulates 50S docking, speeding the initiation process while ensuring accurate tRNA selection 84; 88; 93. Binding of fMet-tRNA and the initiation factors induces a clockwise rotation of 30S head domain relative to the body domain 83. Analysis of 70SIC trapped by GDPNP with all initiation factors bound, which represents the intermediate after initial 50S docking, showed that the 30S subunit is rotated 4° counterclockwise relative to the 50S subunit, compared to the classic-state 70S ribosome 14. Similar head swiveling and intersubunit rotation have been observed during other steps of translation (see below) and most bridges adopt different conformations in response to intersubunit movement. It is plausible that during 70SIC formation, the 50S subunit approaches 30SIC in a rotated position, so that bridge interactions could begin to form without steric clash from bound fMet-tRNA and initiation factors. For example, domain C1 of IF2 interacts with h14 of 16S rRNA and protein L14 in the 70SIC 14; 83, and intersubunit rotation may facilitate formation of bridge B8 in the presence of IF2. In addition, as seen with cryo-EM reconstruction of the 30SIC, the C-terminal domain (CTD) of IF3 binds close to the loop region of h24 at the 30S platform domain, which overlaps with the binding site of the stem region of H69 in the classic-state 70S ribosome 83. In the rotated conformation of 70SIC, however, since the acceptor stem of fMet-tRNA is titled towards the 50S E site, a gap between H69 and fMet-tRNA would appear to accommodate IF3 CTD without interfering with the formation of bridge B2a/d and B2c. It is evident that the stability and conformation of initiation factors in the 30SIC is affected by the identity of codon-anticodon pairing in the 30S P site, as well as the interaction between 30S and translation initiation region (TIR) of the mRNA 85; 92; 94; 95. For example, competition between IF3 and H69 for 30S binding controls 70SIC formation and is strongly influenced by codon-anticodon pairing, explaining the fidelity function of IF3 92. Indeed, key functions of the initiation factors in regulating bridge formation allow the initiation process to be both fast and accurate.
The kinetics of bridge formation during subunit association (in the absence of other ligands) has drawn the attention of several groups. Time-resolved chemical probing experiments provided evidence that h27 and h44 (A1408) are among the first regions of 16S rRNA to be protected by 50S, suggesting that B2c and B2a/d establish early in the process, followed by B3 (A1418 of h44), B6 (A1441 of h14) and B7a (A702 of h23) 96. In a time-resolved cryo-EM study, it was concluded that bridge B2c, B4, B5, and B6 take longer to form than the other bridges 97. Although there are some discrepancies between the two studies, both suggest that bridges at the center of subunit interface (e.g. B2a/d and B3) form earlier than those at the periphery (e.g. B4, B5, B6). This conclusion has been challenged, though, by a recent cryo-EM study of higher-resolution, which finds no evidence for step-wise bridge formation at pre-equilibrium (e.g., 60 ms) time points 98. Clearly, further work will be needed to resolve these incongruities. Even so, development of these methods to obtain time-resolved structural information is progressing rapidly, and future high-resolution studies in the presence of initiation ligands may soon reveal the real-time process of subunit joining.
Disruption of bridges during ribosome recycling
After translation termination, the 70S ribosome is left with a single deacylated tRNA oscillating between the classic P/P and hybrid P/E sites (P/E-tRNA occupies the 30S P site and the 50S E site) 99; 100; 101. RRF binds the post-termination complex (PoTC) with P/E-tRNA, in which the 30S head is swiveled by 6° towards the E site, and the subunit is rotated by 8°, compared to the classic-state ribosome 24. When bound to PoTC, the elongated domain I of RRF interacts directly with H69 of the 23S rRNA and shifts the tip of the helix ~ 4 Å away from h44 of the 16S rRNA, disrupting bridge B2a/d 102; 103. RRF also contacts H71 of the 23S rRNA in the PoTC, although without inducing any structural changes. In the complex with the 50S subunit and EF-G, RRF packs H69 and H71 closer to each other 33. This rearrangement at the center of 50S interface seems to be the reverse of that seen in subunit joining, and suggests that the function of RRF in subunit splitting may lie in disrupting bridge B2a/d and B3. In line with this idea, deletion of H69, which abolishes interactions at B2/d, allows ribosome recycling in the absence of RRF 104. The role of EF-G in ribosome recycling is less well understood, but it has been shown that both GTP hydrolysis and inorganic phosphate release are required before subunit splitting occurs 105. Presumably, both events promote conformational changes in EF-G, which affect its interaction with RRF and the ribosome. For example, based on a cryo-EM reconstruction of PoTC•EF-G•RRF complex stabilized with fusidic acid, EF-G triggers rearrangement between the two domains of RRF similar to that seen in the 50S-bound state 33, and reverses head swiveling and intersubunit rotation of the ribosome. This structure may resemble the transition state intermediate that precedes splitting of the two subunits 102. Binding of RRF and EF-G also induces a conformational change at the central protuberance of the 50S subunit, which may weaken the bridge interaction with the 30S head domain to promote subunit separation 102; 106.
3. Rearrangement of bridge interactions during translation
A “ratchet-like” intersubunit movement was first observed in cryo-EM reconstructions of EF-G-bound ribosomes 107. As shown later in higher-resolution structures, this movement actually entails a counterclockwise rotation of the 30S relative to the 50S, as well as a swiveling motion of 30S head (Figure 7). Similar movement has been implicated not only in translocation but also in subunit joining, peptide release, and ribosome recycling (see above). This “racheting” is strongly influenced by the acylation state of the bound tRNAs. In particular, the P-site tRNA needs be deacylated (lack a peptidyl group) for racheting to be thermodynamically favorable 20; 99; 108; 109. This is because racheting is coupled to the movement of tRNA into the P/E hybrid site, which is strongly inhibited by a peptidyl group. Intersubunit rotation and head swiveling cause most bridges seen in the classic-state 70S ribosome to undergo conformational change or to break and form new interactions between the two subunits. These bridge rearrangements help explain how the integrity of 70S ribosome is maintained during classic-to-hybrid transitions.
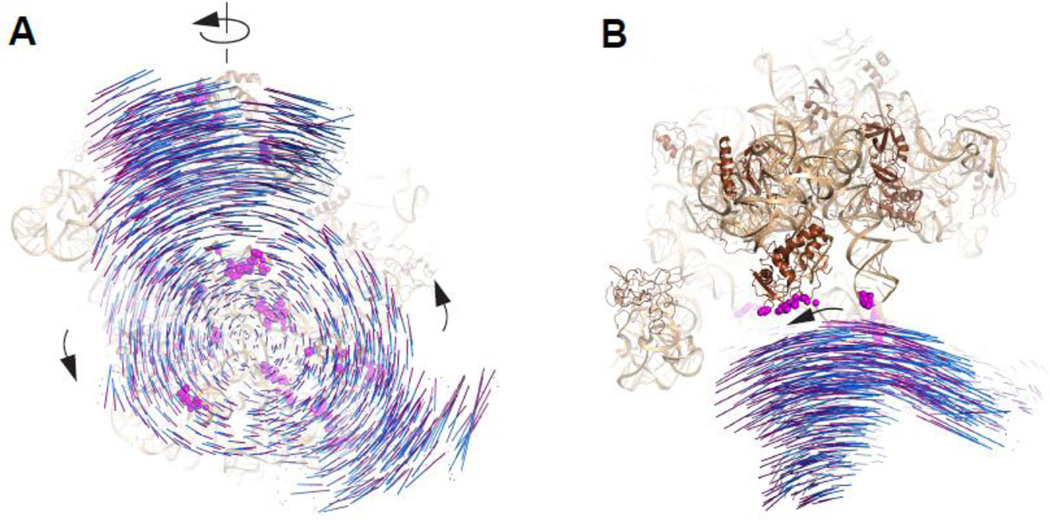
Figure 7. Intersubunit movement of the ribosome.
(A) View of the 70S ribosome from the 30S perspective, depicting the distinct intersubunit and 30S head rotations. (B) “Top” view of 70S ribosome showing the swiveling motion of the 30S head domain. Each line of movement represents distance between phosphorus atoms of the corresponding nucleotides of 16S rRNA from non-rotated [blue, PDB entries 2WDK,39] and rotated [purple, PDB entry 3SFS,23] ribosomes, with the two structure aligned to the same coordinate according to the 50S subunit120. Wheat, 23S rRNA; brown, large subunit proteins; magenta spheres, 50S components of intersubunit bridges in the classic-state (non-rotated) 70S ribosome.
Head swiveling
The 30S head domain is dynamic, presumably due to flexibility of the neck region. Multiple states of head swiveling, with rotation up to 20°, have been visualized in different functional states of the ribosome. Recent analysis of these complexes revealed two hinge points for head movement, a single-nucleotide bulge (G926) of the neck helix h28, and a three-way junction formed by helices h34, h35, and h38 of the 16S rRNA 110. In the head-swiveled ribosome, the kinked h28 is straightened, while h34 is rotated counterclockwise around h35, compared to the classic-state 70S. As a result, bridge interactions between S13 and H38 of the 23S rRNA (B1a) or L5/L31 (B1b) are disrupted, with small subunit protein S19 moving close to H38, and the C-terminal region of S13 approaching L5/L31. Comparing ribosome complexes with different degree of head swiveling suggests that, although the 30S head domain is held in approximation to the 50S central protuberance in all conformations, interactions at these interface regions are not always maintained. For instance, in an EF-G-bound complex with ~6° head swiveling, no specific interaction is established between the closely located H38 and S19, or L5/L31 and S13 17. Since 30S head undergoes structural change over a wide and continuous range of swiveling motion 110, and each conformation forms unique interface between H38/L5/L31 and S13/S19, it is unlikely that these > 40 different states can be stabilized by the few contact points at B1a and B1b, as see in the classic-state ribosome. As mentioned above, L31 protein, which binds L5 at the lateral solvent side and makes extensive contacts with S14 and S19 proteins through bridge B1c (Figure 3, Table 2), may in fact be more important for bridging the interaction at the 30S head 111. In a recent cryo-EM reconstruction of hybrid-state ribosome with 30S head swiveled, as S19 moves closer to L5, the flexible linker region of L31 becomes more compact, while the NTD and CTD maintain their interactions with L5 and S14/S19, respectively 43. Therefore, L31 might be critical in connecting 30S head and 50S during intersubunit movement. Further support of this idea comes from the crystal structure of RF3-bound hybrid-state ribosome, in which 30S head adopts a similar conformation to that seen in the EF-G-bound complex mentioned above, while the partially resolved L31 CTD (aa. 44–47) docks into a pocket formed by hydrophilic residues and peptide backbone (aa. 22–27, 43–48) from S1922
Intersubunit rotation
Similar to head swiveling, various extents of intersubunit rotation of up to 10° have been observed. Comparing different states of rotated ribosome suggests that the pivot point lies near bridge B3, which maintains largely the same conformation as seen in the classic-state 70S 22; 24. Most of the other bridges in the 30S body domain undergo rearrangement during intersubunit rotation. At bridge B2a/d, although most interactions between h44/h45 and H69 are preserved in the rotated ribosome, the base of h44 moves 6–7 Å towards the E site, and H69 is compressed by5–7Å 22; 24. As a result, A1913 at the loop region of H69 inserted further into the minor groove of A1408 and A1492 of h44, compared to a classic-state ribosome with vacant A site, while C1914 of H69 partially or completely loses its interaction with the h44 backbone. The twisted conformation of H69 in the rotated ribosome is probably stabilized by the flipping out of C1925 at the base of the helix, and the alternative base pairing formed by the universally conserved U1926 and G1929. Although a Mg2+ ion at B2a/d is not visualized in all rotated ribosomes, the distance between U793 (h24), G1517 (h45), and C1920 (H69) seems to be compatible with Mg2+-coordinated interaction, as seen in the classic-state 70S. In contrast, the gap between the backbone of h24 and H68, as well as h24/h27 and H67, is significantly widened in the rotated ribosome, resulting in complete loss of B2b and B2c.
Another bridge interaction preserved in the rotated ribosome is B4, where H34 bends by about 12° and its loop region maintains similar interaction with protein S15 as seen in classic-state ribosome 22; 24. The flexibility of H34, which protrudes from the interface of 50S subunit by ~50 Å, contributes to the stability of 30S platform region in different states of rotation. This is particularly important because another platform bridge, B7a, loses all of the base stacking and minor groove interaction, as A702 of h23 moves away from H68 by ~13 Å in the rotated ribosome 24. The weakened interaction at B7a is partially compensated by the more compact interface between h23/h24 and protein L2 at bridge B7b (Table 2). In addition, the closure of L1 stalk in the hybrid-state ribosome establishes new interaction between the backbone of 23S rRNA and small subunit protein S7 (Table 2).
Bridge B5 is located close to the pivot point and bears minimum lateral movement during intersubunit rotation. Therefore, most interactions between the backbone of h44 and H62 are retained in the rotated ribosome 17; 23; 24. In the 9°-rotated T. thermophilus ribosome with RF3 bound, however, h44 and H62 are completely separated, with the closest polar residues 4.4 Å away from each other 22. This is not likely due to phylogenetic differences or structural changes induced by specific ligands, as the RRF-bound T. thermophilus ribosome and the RF3-bound E. coli ribosome both showed retention of interactions between h44 and H62. Since B5 lacks minor groove interactions that usually restrain the tertiary structure, it is possible that this bridge could be disrupted with a larger degree of intersubunit rotation in E. coli ribosomes, which has only been visualized under low resolution 16.
Near the spur region of the 30S, intersubunit rotation packs h44 and h8/h14 of the 16S rRNA closer to large subunit protein L19 and L14, and therefore results a strengthened interaction at bridge B6 and B8 in E.coli ribosomes (Table 2) 24. Interestingly, the T. thermophilus-specific interaction between h44 and H101 of the 23S rRNA or the extended C-terminus of L19, is largely lost in the rotated ribosome, presumably resulting in a weaker B6 22. In addition, a more flexible loop formed by residues 30–40 of T. thermophilus L19 docks on h8/h14 in a much looser conformation, compared to its E.coli counterpart. This phylogenetic difference in L19 may help to explain the higher tendency for E.coli ribosomes to adopt the rotated conformation in the absence of ligand binding 24; 77; 112.
In contrast to the strict need for preserving bridge interaction at the 30S head domain, where only a single surface of contact exists, loss (e.g. B2b, B2c, and B5) or rearrangement (B7 and B8) of bridge interaction at the body domain during intersubunit movement is more tolerable, as the unchanged bridges (B2a/d, B3, B4) help to maintain subunit association. In order for B2a/d, B3, and B4 to remain intact upon intersubunit rotation, components of these bridges (H69, H71, and H34 of the 23S rRNA) need to undergo conformational change and presumably enter a higher-energy state in the rotated ribosome. This distortion is probably compensated for by a combination of alternative bridge interactions, contacts established by hybrid-site tRNAs, as well as interactions between translation factors and the ribosome. In addition, bridges at the 30S head domain also influence the equilibrium of intersubunit rotation, as shown by smFRET 113.
Relevance of head swiveling and intersubunit rotation to translocation
In each round of elongation, decoding culminates with transfer of the nascent peptide chain from the P-site tRNA to the A-site tRNA, forming the pre-translocation (PRE) complex. In the PRE complex, the tRNAs can rapidly fluctuate between the classical (P/P, A/A) and hybrid (P/E, A/P) sites, movements accompanied by the ribosomal motions of racheting. Recent biophysical studies of the PRE complex have shown that while these motions (classic-to-hybrid tRNA movement, intersubunit rotation, head swiveling) are coupled, they are loosely (or partially) coupled 20; 114. Indeed the PRE complex is a highly dynamic complex with two predominant low-energy conformers—corresponding basically to the classic state (unrotated, unswiveled, P/P-tRNA) and hybrid state (rotated, swiveled, P/E-tRNA).
Translocation entails not only movement of the tRNAs within the 50S subunit (i.e. hybrid state formation) but also movement of the codon-anticodon helices within the 30S subunit. EF-G catalyzes forward translocation, forming the post-translocation complex (with deacyl tRNA in the E/E site and peptidyl-tRNA in the P/P site). Kinetic studies have shown that codon-anticodon movement is rate-limited by a conformational change in the ribosome termed “unlocking” 115. Recent structural studies provide compelling clues to the molecular basis of this kinetically-defined unlocking step 19; 21. Under certain conditions in the presence of EF-G, the 30S head swivels dramatically (18–21°) with little-to-no accompanying intersubunit rotation. This translocates the tRNAs with respect to the 30S body into “intra-subunit hybrid sites,” denoted as pe/E and ap/P. From this putative intermediate, the only event needed to complete translocation is release of the tRNA-head contacts and resetting of the head position (i.e., unswiveling). Based on the free energy landscape of the PRE complex, formation of this intermediate is unfavorable (in the absence of EF-G), as is the unlocking step 20; 116. Head swiveling is concurrent with tRNA-mRNA movement 117, trapping the 30S head in a less-swiveled conformation with spectinomycin inhibits translocation 118; 119, and disruption of bridges specifically around the periphery of the subunit interface reduce the energy barrier of unlocking 116. All these observations are consistent with a model in which EF-G promotes formation of this unrotated swiveled conformation to unlock the ribosome and catalyze translocation.
Concluding remarks
The ribosome is a two-subunit enzyme, with binding sites for tRNAs, mRNA, and translation factors at the subunit interface. A growing body of evidence indicates that ribosome-ribosome contacts across the interface play important roles in all stages of translation. In this article, we describe these intersubunit bridges and highlight some of the ways in which they change conformation and/or contribute to various steps of translation. We focus solely on the bacterial ribosome, as the amount of data available in this case is largest. But many of these bridges are conserved in all domains of life, so lessons from the bacterial system hold broad general relevance. The wealth of structural information on the ribosome obtained in recent years has advanced our understanding of these bridges tremendously, yet questions regarding function remain open. Experiments to address these questions will further clarify the roles of the intersubunit bridges in translation.
Highlights.
Structures of intersubunit bridges
Inferred dynamics of bridges
Functional roles of bridges
Acknowledgments
We thank C. Dunham for helpful discussions. Studies on protein synthesis in our laboratory are supported by grants from the National Institutes of Health (GM 072528) and the National Science Foundation (MCB 1243997).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anger AM, Armache JP, Berninghausen O, Habeck M, Subklewe M, Wilson DN, Beckmann R. Structures of the human and Drosophila 80S ribosome. Nature. 2013;497:80–85. doi: 10.1038/nature12104. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. The structure of the eukaryotic ribosome at 3.0 A resolution. Science. 2011;334:1524–1529. doi: 10.1126/science.1212642. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DN, Doudna Cate JH. The structure and function of the eukaryotic ribosome. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moazed D, Noller HF. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989;57:585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- 5.Noller HF, Chang C, Thomas G, Aldridge J. Chemical modification of the transfer RNA and polyuridylic acid binding sites of Escherichia coli 30 s ribosomal subunits. J Mol Biol. 1971;61:669–679. doi: 10.1016/0022-2836(71)90071-4. [DOI] [PubMed] [Google Scholar]
- 6.Moazed D, Noller HF. Transfer RNA shields specific nucleotides in 16S ribosomal RNA from attack by chemical probes. Cell. 1986;47:985–994. doi: 10.1016/0092-8674(86)90813-5. [DOI] [PubMed] [Google Scholar]
- 7.Noller HF, Hoffarth V, Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science. 1992;256:1416–1419. doi: 10.1126/science.1604315. [DOI] [PubMed] [Google Scholar]
- 8.Noller HF, Chaires JB. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972;69:3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell. 2002;111:721–732. doi: 10.1016/s0092-8674(02)01086-3. [DOI] [PubMed] [Google Scholar]
- 10.Gromadski KB, Rodnina MV. Streptomycin interferes with conformational coupling between codon recognition and GTPase activation on the ribosome. Nat Struct Mol Biol. 2004;11:316–322. doi: 10.1038/nsmb742. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal D, Kamath D, Gregory ST, O'Connor M. Modulation of decoding fidelity by ribosomal proteins S4 and S5. J Bacteriol. 2015;197:1017–1025. doi: 10.1128/JB.02485-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogle JM, Ramakrishnan V. Structural insights into translational fidelity. Annu Rev Biochem. 2005;74:129–177. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- 13.Fredrick K. Another look at mutations in ribosomal protein S4 lends strong support to the domain closure model. J Bacteriol. 2015;197:1014–1016. doi: 10.1128/JB.02579-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell. 2005;121:703–712. doi: 10.1016/j.cell.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Ramrath DJ, Lancaster L, Sprink T, Mielke T, Loerke J, Noller HF, Spahn CM. Visualization of two transfer RNAs trapped in transit during elongation factor G-mediated translocation. Proc Natl Acad Sci U S A. 2013;110:20964–20969. doi: 10.1073/pnas.1320387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brilot AF, Korostelev AA, Ermolenko DN, Grigorieff N. Structure of the ribosome with elongation factor G trapped in the pretranslocation state. Proc Natl Acad Sci U S A. 2013;110:20994–20999. doi: 10.1073/pnas.1311423110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tourigny DS, Fernandez IS, Kelley AC, Ramakrishnan V. Elongation factor G bound to the ribosome in an intermediate state of translocation. Science. 2013;340:1235490. doi: 10.1126/science.1235490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulk A, Cate JH. Control of ribosomal subunit rotation by elongation factor G. Science. 2013;340:1235970. doi: 10.1126/science.1235970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Lancaster L, Donohue JP, Noller HF. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science. 2014;345:1188–1191. doi: 10.1126/science.1255030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–333. doi: 10.1038/nature09206. [DOI] [PubMed] [Google Scholar]
- 21.Ratje AH, Loerke J, Mikolajka A, Brunner M, Hildebrand PW, Starosta AL, Donhofer A, Connell SR, Fucini P, Mielke T, Whitford PC, Onuchic JN, Yu Y, Sanbonmatsu KY, Hartmann RK, Penczek PA, Wilson DN, Spahn CM. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature. 2010;468:713–716. doi: 10.1038/nature09547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin H, Kelley AC, Ramakrishnan V. Crystal structure of the hybrid state of ribosome in complex with the guanosine triphosphatase release factor 3. Proc Natl Acad Sci U S A. 2011;108:15798–15803. doi: 10.1073/pnas.1112185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Lancaster L, Trakhanov S, Noller HF. Crystal structure of release factor RF3 trapped in the GTP state on a rotated conformation of the ribosome. RNA. 2012;18:230–240. doi: 10.1261/rna.031187.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkle JA, Wang L, Feldman MB, Pulk A, Chen VB, Kapral GJ, Noeske J, Richardson JS, Blanchard SC, Cate JH. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science. 2011;332:981–984. doi: 10.1126/science.1202692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. VI. Oligopeptide synthesis and translocation on ribosomes in the presence and absence of soluble transfer factors. J Biol Chem. 1969;244:1533–1539. [PubMed] [Google Scholar]
- 26.Gavrilova LP, Kostiashkina OE, Koteliansky VE, Rutkevitch NM, Spirin AS. Factor-free ("non-enzymic") and factor-dependent systems of translation of polyuridylic acid by Escherichia coli ribosomes. J Mol Biol. 1976;101:537–552. doi: 10.1016/0022-2836(76)90243-6. [DOI] [PubMed] [Google Scholar]
- 27.Ude S, Lassak J, Starosta AL, Kraxenberger T, Wilson DN, Jung K. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339:82–85. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- 28.Doerfel LK, Wohlgemuth I, Kothe C, Peske F, Urlaub H, Rodnina MV. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339:85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- 29.Himeno H, Nameki N, Kurita D, Muto A, Abo T. Ribosome rescue systems in bacteria. Biochimie. 2015;114:102–112. doi: 10.1016/j.biochi.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Korostelev A, Asahara H, Lancaster L, Laurberg M, Hirschi A, Zhu J, Trakhanov S, Scott WG, Noller HF. Crystal structure of a translation termination complex formed with release factor RF2. Proc Natl Acad Sci U S A. 2008;105:19684–19689. doi: 10.1073/pnas.0810953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freistroffer DV, Pavlov MY, MacDougall J, Buckingham RH, Ehrenberg M. Release factor RF3 in E.coli accelerates the dissociation of release factors RF1 and RF2 from the ribosome in a GTP-dependent manner. EMBO J. 1997;16:4126–4133. doi: 10.1093/emboj/16.13.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pallesen J, Hashem Y, Korkmaz G, Koripella RK, Huang C, Ehrenberg M, Sanyal S, Frank J. Cryo-EM visualization of the ribosome in termination complex with apo-RF3 and RF1. Elife. 2013;2:e00411. doi: 10.7554/eLife.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao N, Zavialov AV, Li W, Sengupta J, Valle M, Gursky RP, Ehrenberg M, Frank J. Mechanism for the disassembly of the posttermination complex inferred from cryo-EM studies. Mol Cell. 2005;18:663–674. doi: 10.1016/j.molcel.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Peske F, Rodnina MV, Wintermeyer W. Sequence of steps in ribosome recycling as defined by kinetic analysis. Mol Cell. 2005;18:403–412. doi: 10.1016/j.molcel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Tissieres A, Watson JD. Ribonucleoprotein particles from Escherichia coli. Nature. 1958;182:778–780. doi: 10.1038/182778b0. [DOI] [PubMed] [Google Scholar]
- 36.Frank J, Penczek P, Grassucci R, Srivastava S. Three-dimensional reconstruction of the 70S Escherichia coli ribosome in ice: the distribution of ribosomal RNA. J Cell Biol. 1991;115:597–605. doi: 10.1083/jcb.115.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank J, Verschoor A, Li Y, Zhu J, Lata RK, Radermacher M, Penczek P, Grassucci R, Agrawal RK, Srivastava S. A model of the translational apparatus based on a three-dimensional reconstruction of the Escherichia coli ribosome. Biochem Cell Biol. 1995;73:757–765. doi: 10.1139/o95-084. [DOI] [PubMed] [Google Scholar]
- 38.Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 39.Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat Struct Mol Biol. 2009;16:528–533. doi: 10.1038/nsmb.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reblova K, Razga F, Li W, Gao H, Frank J, Sponer J. Dynamics of the base of ribosomal A-site finger revealed by molecular dynamics simulations and Cryo-EM. Nucleic Acids Res. 2010;38:1325–1340. doi: 10.1093/nar/gkp1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannone JJ, Subramanian S, Schnare MN, Collett JR, D'Souza LM, Du Y, Feng B, Lin N, Madabusi LV, Muller KM, Pande N, Shang Z, Yu N, Gutell RR. The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics. 2002;3:2. doi: 10.1186/1471-2105-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenner L, Demeshkina N, Yusupova G, Yusupov M. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat Struct Mol Biol. 2010;17:1072–1078. doi: 10.1038/nsmb.1880. [DOI] [PubMed] [Google Scholar]
- 43.Fischer N, Neumann P, Konevega AL, Bock LV, Ficner R, Rodnina MV, Stark H. Structure of the E. coli ribosome-EF-Tu complex at <3 A resolution by Cs-corrected cryo-EM. Nature. 2015;520:567–570. doi: 10.1038/nature14275. [DOI] [PubMed] [Google Scholar]
- 44.Komoda T, Sato NS, Phelps SS, Namba N, Joseph S, Suzuki T. The A-site finger in 23 S rRNA acts as a functional attenuator for translocation. J Biol Chem. 2006;281:32303–32309. doi: 10.1074/jbc.M607058200. [DOI] [PubMed] [Google Scholar]
- 45.Cukras AR, Green R. Multiple effects of S13 in modulating the strength of intersubunit interactions in the ribosome during translation. J Mol Biol. 2005;349:47–59. doi: 10.1016/j.jmb.2005.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cukras AR, Southworth DR, Brunelle JL, Culver GM, Green R. Ribosomal proteins S12 and S13 function as control elements for translocation of the mRNA:tRNA complex. Mol Cell. 2003;12:321–328. doi: 10.1016/s1097-2765(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell P, Osswald M, Brimacombe R. Identification of intermolecular RNA cross-links at the subunit interface of the Escherichia coli ribosome. Biochemistry. 1992;31:3004–3011. doi: 10.1021/bi00126a023. [DOI] [PubMed] [Google Scholar]
- 48.Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FVt, Weir JR, Ramakrishnan V. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326:688–694. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satpati P, Sund J, Aqvist J. Structure-based energetics of mRNA decoding on the ribosome. Biochemistry. 2014;53:1714–1722. doi: 10.1021/bi5000355. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor M. Helix 69 in 23S rRNA modulates decoding by wild type and suppressor tRNAs. Mol Genet Genomics. 2009;282:371–380. doi: 10.1007/s00438-009-0470-6. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor M, Dahlberg AE. The involvement of two distinct regions of 23 S ribosomal RNA in tRNA selection. J Mol Biol. 1995;254:838–847. doi: 10.1006/jmbi.1995.0659. [DOI] [PubMed] [Google Scholar]
- 52.Ortiz-Meoz RF, Green R. Helix 69 is key for uniformity during substrate selection on the ribosome. J Biol Chem. 2011;286:25604–25610. doi: 10.1074/jbc.M111.256255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirabayashi N, Sato NS, Suzuki T. Conserved loop sequence of helix 69 in Escherichia coli 23 S rRNA is involved in A-site tRNA binding and translational fidelity. J Biol Chem. 2006;281:17203–17211. doi: 10.1074/jbc.M511728200. [DOI] [PubMed] [Google Scholar]
- 54.Selmer M, Dunham CM, Murphy FVt, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- 55.Kipper K, Hetenyi C, Sild S, Remme J, Liiv A. Ribosomal intersubunit bridge B2a is involved in factor-dependent translation initiation and translational processivity. J Mol Biol. 2009;385:405–422. doi: 10.1016/j.jmb.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 56.Liang XH, Liu Q, Fournier MJ. rRNA modifications in an intersubunit bridge of the ribosome strongly affect both ribosome biogenesis and activity. Mol Cell. 2007;28:965–977. doi: 10.1016/j.molcel.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh S, Joseph S. Nonbridging phosphate oxygens in 16S rRNA important for 30S subunit assembly and association with the 50S ribosomal subunit. RNA. 2005;11:657–667. doi: 10.1261/rna.7224305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belanger F, Leger M, Saraiya AA, Cunningham PR, Brakier-Gingras L. Functional studies of the 900 tetraloop capping helix 27 of 16S ribosomal RNA. J Mol Biol. 2002;320:979–989. doi: 10.1016/s0022-2836(02)00550-8. [DOI] [PubMed] [Google Scholar]
- 59.Belanger F, Gagnon MG, Steinberg SV, Cunningham PR, Brakier-Gingras L. Study of the functional interaction of the 900 Tetraloop of 16S ribosomal RNA with helix 24 within the bacterial ribosome. J Mol Biol. 2004;338:683–693. doi: 10.1016/j.jmb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Demirci H, Wang L, Murphy FVt, Murphy EL, Carr JF, Blanchard SC, Jogl G, Dahlberg AE, Gregory ST. The central role of protein S12 in organizing the structure of the decoding site of the ribosome. RNA. 2013;19:1791–1801. doi: 10.1261/rna.040030.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lodmell JS, Dahlberg AE. A conformational switch in Escherichia coli 16S ribosomal RNA during decoding of messenger RNA. Science. 1997;277:1262–1267. doi: 10.1126/science.277.5330.1262. [DOI] [PubMed] [Google Scholar]
- 62.Velichutina IV, Dresios J, Hong JY, Li C, Mankin A, Synetos D, Liebman SW. Mutations in helix 27 of the yeast Saccharomyces cerevisiae 18S rRNA affect the function of the decoding center of the ribosome. RNA. 2000;6:1174–1184. doi: 10.1017/s1355838200000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McClory SP, Leisring JM, Qin D, Fredrick K. Missense suppressor mutations in 16S rRNA reveal the importance of helices h8 and h14 in aminoacyl-tRNA selection. RNA. 2010;16:1925–1934. doi: 10.1261/rna.2228510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pulk A, Maivali U, Remme J. Identification of nucleotides in E. coli 16S rRNA essential for ribosome subunit association. RNA. 2006;12:790–796. doi: 10.1261/rna.2275906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Q, Vila-Sanjurjo A, O'Connor M. Mutations in the intersubunit bridge regions of 16S rRNA affect decoding and subunit-subunit interactions on the 70S ribosome. Nucleic Acids Res. 2011;39:3321–3330. doi: 10.1093/nar/gkq1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liiv A, O'Connor M. Mutations in the intersubunit bridge regions of 23 S rRNA. J Biol Chem. 2006;281:29850–29862. doi: 10.1074/jbc.M603013200. [DOI] [PubMed] [Google Scholar]
- 67.Culver GM, Cate JH, Yusupova GZ, Yusupov MM, Noller HF. Identification of an RNA-protein bridge spanning the ribosomal subunit interface. Science. 1999;285:2133–2136. doi: 10.1126/science.285.5436.2133. [DOI] [PubMed] [Google Scholar]
- 68.Feinberg JS, Joseph S. Identification of molecular interactions between P-site tRNA and the ribosome essential for translocation. Proc Natl Acad Sci U S A. 2001;98:11120–11125. doi: 10.1073/pnas.211184098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nierhaus KH. Decoding errors and the involvement of the E-site. Biochimie. 2006;88:1013–1019. doi: 10.1016/j.biochi.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Chen C, Stevens B, Kaur J, Smilansky Z, Cooperman BS, Goldman YE. Allosteric vs. spontaneous exit-site (E-site) tRNA dissociation early in protein synthesis. Proc Natl Acad Sci U S A. 2011;108:16980–16985. doi: 10.1073/pnas.1106999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leger M, Dulude D, Steinberg SV, Brakier-Gingras L. The three transfer RNAs occupying the A, P and E sites on the ribosome are involved in viral programmed −1 ribosomal frameshift. Nucleic Acids Res. 2007;35:5581–5592. doi: 10.1093/nar/gkm578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devaraj A, Shoji S, Holbrook ED, Fredrick K. A role for the 30S subunit E site in maintenance of the translational reading frame. RNA. 2009;15:255–265. doi: 10.1261/rna.1320109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClory SP, Devaraj A, Fredrick K. Distinct functional classes of ram mutations in 16S rRNA. RNA. 2014;20:496–504. doi: 10.1261/rna.043331.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fagan CE, Dunkle JA, Maehigashi T, Dang MN, Devaraj A, Miles SJ, Qin D, Fredrick K, Dunham CM. Reorganization of an intersubunit bridge induced by disparate 16S ribosomal ambiguity mutations mimics an EF-Tu-bound state. Proc Natl Acad Sci U S A. 2013;110:9716–9721. doi: 10.1073/pnas.1301585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sahu B, Khade PK, Joseph S. Functional replacement of two highly conserved tetraloops in the bacterial ribosome. Biochemistry. 2012;51:7618–7626. doi: 10.1021/bi300930r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maisnier-Patin S, Paulander W, Pennhag A, Andersson DI. Compensatory evolution reveals functional interactions between ribosomal proteins S12, L14 and L19. J Mol Biol. 2007;366:207–215. doi: 10.1016/j.jmb.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 77.Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 78.Hickerson R, Majumdar ZK, Baucom A, Clegg RM, Noller HF. Measurement of internal movements within the 30 S ribosomal subunit using Forster resonance energy transfer. J Mol Biol. 2005;354:459–472. doi: 10.1016/j.jmb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 79.Harms J, Schluenzen F, Zarivach R, Bashan A, Gat S, Agmon I, Bartels H, Franceschi F, Yonath A. High resolution structure of the large ribosomal subunit from a mesophilic eubacterium. Cell. 2001;107:679–688. doi: 10.1016/s0092-8674(01)00546-3. [DOI] [PubMed] [Google Scholar]
- 80.Moazed D, Samaha RR, Gualerzi C, Noller HF. Specific protection of 16 S rRNA by translational initiation factors. J Mol Biol. 1995;248:207–210. doi: 10.1016/s0022-2836(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 81.Carter AP, Clemons WM, Jr, Brodersen DE, Morgan-Warren RJ, Hartsch T, Wimberly BT, Ramakrishnan V. Crystal structure of an initiation factor bound to the 30S ribosomal subunit. Science. 2001;291:498–501. doi: 10.1126/science.1057766. [DOI] [PubMed] [Google Scholar]
- 82.Dallas A, Noller HF. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol Cell. 2001;8:855–864. doi: 10.1016/s1097-2765(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 83.Julian P, Milon P, Agirrezabala X, Lasso G, Gil D, Rodnina MV, Valle M. The Cryo-EM structure of a complete 30S translation initiation complex from Escherichia coli. PLoS Biol. 2011;9:e1001095. doi: 10.1371/journal.pbio.1001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors tune the rate of initiation of protein synthesis in bacteria. EMBO J. 2006;25:2539–2550. doi: 10.1038/sj.emboj.7601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milon P, Konevega AL, Gualerzi CO, Rodnina MV. Kinetic checkpoint at a late step in translation initiation. Mol Cell. 2008;30:712–720. doi: 10.1016/j.molcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 86.Qin D, Liu Q, Devaraj A, Fredrick K. Role of helix 44 of 16S rRNA in the fidelity of translation initiation. RNA. 2012;18:485–495. doi: 10.1261/rna.031203.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qin D, Fredrick K. Control of translation initiation involves a factor-induced rearrangement of helix 44 of 16S ribosomal RNA. Mol Microbiol. 2009;71:1239–1249. doi: 10.1111/j.1365-2958.2009.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors maximize the accuracy of tRNA selection in initiation of bacterial protein synthesis. Mol Cell. 2006;23:183–193. doi: 10.1016/j.molcel.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 89.Grigoriadou C, Marzi S, Pan D, Gualerzi CO, Cooperman BS. The translational fidelity function of IF3 during transition from the 30 S initiation complex to the 70 S initiation complex. J Mol Biol. 2007;373:551–561. doi: 10.1016/j.jmb.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.MacDougall DD, Gonzalez RL., Jr Translation initiation factor 3 regulates switching between different modes of ribosomal subunit joining. J Mol Biol. 2015;427:1801–1818. doi: 10.1016/j.jmb.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elvekrog MM, Gonzalez RL., Jr Conformational selection of translation initiation factor 3 signals proper substrate selection. Nat Struct Mol Biol. 2013;20:628–633. doi: 10.1038/nsmb.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Q, Fredrick K. Roles of helix H69 of 23S rRNA in translation initiation. Proc Natl Acad Sci U S A. 2015;112:11559–11564. doi: 10.1073/pnas.1507703112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pavlov MY, Zorzet A, Andersson DI, Ehrenberg M. Activation of initiation factor 2 by ligands and mutations for rapid docking of ribosomal subunits. EMBO J. 2011;30:289–301. doi: 10.1038/emboj.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Milon P, Maracci C, Filonava L, Gualerzi CO, Rodnina MV. Real-time assembly landscape of bacterial 30S translation initiation complex. Nat Struct Mol Biol. 2012;19:609–615. doi: 10.1038/nsmb.2285. [DOI] [PubMed] [Google Scholar]
- 95.Studer SM, Joseph S. Unfolding of mRNA secondary structure by the bacterial translation initiation complex. Mol Cell. 2006;22:105–115. doi: 10.1016/j.molcel.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 96.Hennelly SP, Antoun A, Ehrenberg M, Gualerzi CO, Knight W, Lodmell JS, Hill WE. A time-resolved investigation of ribosomal subunit association. J Mol Biol. 2005;346:1243–1258. doi: 10.1016/j.jmb.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 97.Shaikh TR, Yassin AS, Lu Z, Barnard D, Meng X, Lu TM, Wagenknecht T, Agrawal RK. Initial bridges between two ribosomal subunits are formed within 9.4 milliseconds, as studied by time-resolved cryo-EM. Proc Natl Acad Sci U S A. 2014;111:9822–9827. doi: 10.1073/pnas.1406744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen B, Kaledhonkar S, Sun M, Shen B, Lu Z, Barnard D, Lu TM, Gonzalez RL, Jr, Frank J. Structural dynamics of ribosome subunit association studied by mixing-spraying time-resolved cryogenic electron microscopy. Structure. 2015;23:1097–1105. doi: 10.1016/j.str.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 100.Fei J, Bronson JE, Hofman JM, Srinivas RL, Wiggins CH, Gonzalez RL., Jr Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation. Proc Natl Acad Sci U S A. 2009;106:15702–15707. doi: 10.1073/pnas.0908077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sternberg SH, Fei J, Prywes N, McGrath KA, Gonzalez RL., Jr Translation factors direct intrinsic ribosome dynamics during translation termination and ribosome recycling. Nat Struct Mol Biol. 2009;16:861–868. doi: 10.1038/nsmb.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yokoyama T, Shaikh TR, Iwakura N, Kaji H, Kaji A, Agrawal RK. Structural insights into initial and intermediate steps of the ribosome-recycling process. EMBO J. 2012;31:1836–1846. doi: 10.1038/emboj.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borovinskaya MA, Pai RD, Zhang W, Schuwirth BS, Holton JM, Hirokawa G, Kaji H, Kaji A, Cate JH. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat Struct Mol Biol. 2007;14:727–732. doi: 10.1038/nsmb1271. [DOI] [PubMed] [Google Scholar]
- 104.Ali IK, Lancaster L, Feinberg J, Joseph S, Noller HF. Deletion of a conserved, central ribosomal intersubunit RNA bridge. Mol Cell. 2006;23:865–874. doi: 10.1016/j.molcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 105.Savelsbergh A, Rodnina MV, Wintermeyer W. Distinct functions of elongation factor G in ribosome recycling and translocation. RNA. 2009;15:772–780. doi: 10.1261/rna.1592509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barat C, Datta PP, Raj VS, Sharma MR, Kaji H, Kaji A, Agrawal RK. Progression of the ribosome recycling factor through the ribosome dissociates the two ribosomal subunits. Mol Cell. 2007;27:250–261. doi: 10.1016/j.molcel.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 107.Frank J, Agrawal RK. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature. 2000;406:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 108.Cornish PV, Ermolenko DN, Noller HF, Ha T. Spontaneous intersubunit rotation in single ribosomes. Mol Cell. 2008;30:578–588. doi: 10.1016/j.molcel.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munro JB, Altman RB, O'Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell. 2007;25:505–517. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohan S, Donohue JP, Noller HF. Molecular mechanics of 30S subunit head rotation. Proc Natl Acad Sci U S A. 2014;111:13325–13330. doi: 10.1073/pnas.1413731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shasmal M, Chakraborty B, Sengupta J. Intrinsic molecular properties of the protein-protein bridge facilitate ratchet-like motion of the ribosome. Biochem Biophys Res Commun. 2010;399:192–197. doi: 10.1016/j.bbrc.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W, Dunkle JA, Cate JH. Structures of the ribosome in intermediate states of ratcheting. Science. 2009;325:1014–1017. doi: 10.1126/science.1175275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ning W, Fei J, Gonzalez RL., Jr The ribosome uses cooperative conformational changes to maximize and regulate the efficiency of translation. Proc Natl Acad Sci U S A. 2014;111:12073–12078. doi: 10.1073/pnas.1401864111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Munro JB, Altman RB, Tung CS, Cate JH, Sanbonmatsu KY, Blanchard SC. Spontaneous formation of the unlocked state of the ribosome is a multistep process. Proc Natl Acad Sci U S A. 2010;107:709–714. doi: 10.1073/pnas.0908597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Savelsbergh A, Katunin VI, Mohr D, Peske F, Rodnina MV, Wintermeyer W. An elongation factor G-induced ribosome rearrangement precedes tRNA-mRNA translocation. Mol Cell. 2003;11:1517–1523. doi: 10.1016/s1097-2765(03)00230-2. [DOI] [PubMed] [Google Scholar]
- 116.Liu Q, Fredrick K. Contribution of intersubunit bridges to the energy barrier of ribosomal translocation. Nucleic Acids Res. 2013;41:565–574. doi: 10.1093/nar/gks1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo Z, Noller HF. Rotation of the head of the 30S ribosomal subunit during mRNA translocation. Proc Natl Acad Sci U S A. 2012;109:20391–20394. doi: 10.1073/pnas.1218999109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peske F, Savelsbergh A, Katunin VI, Rodnina MV, Wintermeyer W. Conformational changes of the small ribosomal subunit during elongation factor G-dependent tRNA-mRNA translocation. J Mol Biol. 2004;343:1183–1194. doi: 10.1016/j.jmb.2004.08.097. [DOI] [PubMed] [Google Scholar]
- 119.Borovinskaya MA, Shoji S, Holton JM, Fredrick K, Cate JH. A steric block in translation caused by the antibiotic spectinomycin. ACS Chem Biol. 2007;2:545–552. doi: 10.1021/cb700100n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jarasch A, Dziuk P, Becker T, Armache JP, Hauser A, Wilson DN, Beckmann R. The DARC site: a database of aligned ribosomal complexes. Nucleic Acids Res. 2012;40:D495–D500. doi: 10.1093/nar/gkr824. [DOI] [PMC free article] [PubMed] [Google Scholar]