Abstract
Introduction
Gender differences in cannabis use and cannabis use disorder have been established. Regarding treatment, some evidence suggests women are less responsive, though the mechanisms are not well understood. Motivation to change and self-efficacy are associated with better outcomes overall, and may help explain gender differences in cannabis use outcomes.
Methods
A secondary data analysis of a double-blind placebo controlled trial of buspirone treatment for cannabis dependence (N = 175) was conducted. Self-report assessments of motivation for change, self-efficacy, and other clinical correlates were completed at baseline, and cannabis use was measured throughout the study.
Results
There was a significant interaction between gender and taking steps on abstinence. Counter to hypothesis, higher taking steps reduced likelihood of achieving abstinence among women; there was no association among men. Subsequently, taking steps was associated with self-efficacy and quantity of use among men, and cannabis related problems among women. There was a significant interaction between gender and readiness to change on creatinine adjusted cannabinoid levels. Change readiness was positively associated with cannabinoid levels among women, but not men.
Conclusions
Motivation to change and initiation of change behavior predicts worse cannabis outcomes in women. Men and women differ in what motivates change behavior. Social desirability, neurobiology, and treatment type may impact these effects. Gender differences in cannabis use and treatment responsiveness must be considered in future studies.
Keywords: cannabis, motivation, taking steps, gender, sex differences, treatment
1. Introduction
Gender differences in cannabis use and cannabis use disorder (CUD) have been found in both preclinical and clinical studies. Men are more likely to initiate cannabis use and develop lifetime CUD compared to women (Khan et al., 2013; Wagner & Anthony, 2007). However, women demonstrate a “telescoping effect” progressing from first use to disorder faster than men (Hernandez-Avila, Rounsaville, & Kranzler, 2004; Khan et al., 2013). Likewise, research suggests greater abuse-related effects among women (Cooper & Haney, 2014) and greater physiological withdrawal symptoms (Copersino et al., 2010) compared to men. In preclinical studies, female rodents show greater sensitivity to anxiogenic, reinforcing, and sedative effects of cannabinoids than males (Fattore, Fadda, & Fratta, 2009; Fattore et al., 2007; Harte-Hargrove & Dow-Edwards, 2012). Neuroimaging studies show activation of amygdalar/hippocampal regions in women, but not men, in response to subliminal marijuana cues, as well as an association between craving and activation in executive control related brain regions (Wetherill, Jagannathan, Hager, Childress, & Franklin, 2015). Despite evidence of gender differences in behavioral and neural correlates of cannabis use, few studies have examined gender differences in CUD treatment.
Motivation has been defined as “personal considerations, commitments, reasons, and intentions that move individuals to perform certain behaviors” (DiClemente, Schlundt, & Gemmell, 2004). Interventions that enhance motivation are considered essential to effective substance abuse treatment outcomes (Miller & Rollnick, 2013). Motivation to change has been associated with treatment-seeking behaviors, treatment attendance, and positive clinical outcomes across settings (Amaro et al., 2010; Capone & Wood, 2009; DiClemente et al., 2004; Simpson, Joe, Rowan-Szal, & Greener, 1995). However, few studies examine gender differences in motivation to change and clinical outcomes. One study interviewed 511 adults in treatment for drug and alcohol dependence and found that among women, but not men, treatment readiness was associated with abstinence at 1-year follow-up (Hser, Huang, Teruya, & Anglin, 2003). Motivation to change is a potentially critical factor to understanding gender differences in CUD treatment.
Self-efficacy refers to the strength of an individual's belief that they will successfully engage in a specific planned behavior (i.e. meet their stated goals). In the context of addiction, self-efficacy is one of the best predictors of using behavior. Higher self-efficacy is associated with significantly better treatment outcomes (Greenfield et al., 2000; Litt, Kadden, & Petry, 2013), and has recently been identified as an essential mechanism of therapeutic change (Forcehimes & Tonigan, 2008; Kadden & Litt, 2011; Litt et al., 2013). Yet, there is a dearth of evidence examining how gender impacts self-efficacy and substance abuse, and existing evidence is inconsistent. Among 100 inpatient alcohol-dependent individuals, self-efficacy was associated with fewer days abstinent during a 12-month follow-up period for men, but not women (Greenfield, et al., 2000). However, a recent study combining data from three clinical trials, examined mediators of self-efficacy and found that coping skills partially mediated the association between self-efficacy and cannabis outcomes, but gender did not moderate this mediation relationship as expected (Litt & Kadden, 2015). While self-efficacy seems to play a key role in substance use treatment outcomes, it remains unclear how gender impacts this effect.
In a randomized placebo-controlled trial, McRae-Clark, et al. (2015), investigated buspirone treatment for CUD in treatment seeking adults in conjunction with brief motivational enhancement therapy (MET). While there was no main effect of buspirone, there was a gender by treatment interaction; women randomized to buspirone had significantly worse outcomes than men randomized to buspirone. Motivational models of behavior change may provide a framework for understanding this finding. Specifically, the transtheoretical model (TTM; Prochaska & DiClemente, 1984; 1992), which consists of five stages of behavior change: precontemplation, contemplation, preparation, action, and maintenance. Progression through the five stages reflects increasing motivation to change, which has been shown to predict better therapy outcomes (Norcross, Krebs, & Prochaska, 2011). A critical factor in actualizing a person's motivation to change is the belief that they can perform a specific behavior in a specific situation, or self-efficacy. Self-efficacy is consistently associated with positive substance use outcomes (Kadden & Litt, 2011) and likewise is important to consider alongside motivation to change.
Given the potential for motivation to change and self-efficacy to impact substance use outcomes, the purpose of this study is to examine the role these factors may play differentially across genders in that trial. We hypothesized that gender would moderate the effect of treatment motivation and self-efficacy on cannabis outcomes, such that women who report higher treatment motivation and self-efficacy at baseline would be more likely to achieve abstinence and have lower cannabinoid levels during treatment than women low in motivation and self-efficacy.
2. Materials and Methods
2.1 Participants
The current study is a secondary analysis of double-blind, placebo-controlled trial of buspirone in cannabis-dependent individuals conducted between November 2009 to March 2014. Eligible participants met DSM-IV criteria for current cannabis dependence and were between 18-65 years of age. Exclusion criteria included current dependence on another substance (except caffeine or nicotine), current major depression, current suicidal risk, current treatment with psychotropic medication (except stimulants and non-benzodiazepine sedative/hypnotics), history of psychotic, bipolar, or eating disorder, major medical illness or disease, significant cognitive impairment, hypersensitivity to buspirone or other product component, current consumption of any substance that inhibits or induces CYP3A4, and pregnancy, lactation, or inadequate birth control.
2.2 Procedures
Stratified randomization by gender and amount of daily cannabis use (one joint and above or less than one joint) determined treatment assignment. Both groups received three adjunctive Motivational Enhancement Therapy sessions during the first four weeks of the trial. Personalized Feedback Reports (PFRs) gleaned from the initial worksheets (Steinberg, et al., 2005) were used to guide MET sessions and focused on participants’ frequency of use, reasons for quitting, marijuana-related problems, behavioral contingencies of use, and short- and long-term goals. Escalating scale contingency management (CM) was used for session attendance, starting at $5 and increasing by $5 each week beginning at week 1; compensation was reset to $5 in the event of a missed session. For full original study procedures please see McRae-Clark et al., (2015).
2.3 Assessments
The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES; Miller & Tonigan, 1996) was used to assess motivation to change. The SOCRATES assesses an individual's level of motivation or readiness to change, and is comprised of three subscales: Recognition, Ambivalence, and Taking Steps. Recognition reflects the level of awareness that a problematic use pattern exists, ambivalence reflects the simultaneous desire to stop versus continue using, and taking steps reflects initiation of behavior change. Higher scores reflect greater recognition, less ambivalence, and taking more steps towards change. The SOCRATES is widely used in substance abuse research, including with cannabis using populations (Serafini, Shipley, & Stewart, 2016; Simons, Clarke, Simons, & Spelman, 2016), and has demonstrated reliability and validity across settings (Campbell, 1997; Long & Hollin, 2009). Self-efficacy to resist marijuana was assessed using the Self-Efficacy Questionnaire (SEQ; Stephens, Wertz, & Roffman, 1993), which consists of a total self-efficacy score and has also been validated with marijuana abusing populations (Stephens, Wertz, & Roffman, 1995). Additional assessments used to examine clinical characteristics included the Marijuana Ladder (Slavet et al., 2006), a single item analog measure of readiness to change marijuana use; the Inventory of Drug Taking Situations (IDTS; Turner, Annis, & Sklar, 1997), a self-report measure that provides a profile of situations in which a person has used a drug in the past year; the Marijuana Effect Expectancy Questionnaire (MEEQ; Schafer & Brown, 1991), a measure assessing the expected effect of marijuana use; and the Marijuana Problems Scale (MJPS; Stephens, Roffman, & Simpson, 1994), which assesses the negative consequences of marijuana use. Pre-treatment cannabis use was assessed with the Time-Line Follow-Back (TLFB; Sobell & Sobell, 1992), a widely used measure capturing frequency and quantity of substance use for the preceding 30 days.
Outcome measures included point prevalence abstinence and creatinine adjusted cannabinoid levels assessed by weekly urine cannabinoid tests (UCTs). Cannabinoid levels were adjusted for concurrently measured creatinine, which can be used to standardize cannabinoid concentration, thus providing a more accurate assessment (Huestis & Cohen, 1998). Cannabinoid levels were then naturally log transformed to correct for skewness.
2.4 Statistical Analysis
Primary outcomes included likelihood of point prevalence abstinence and creatinine adjusted cannabinoid levels. An intent-to-treat approach was used for primary analyses. Descriptive statistics summarize baseline demographic and clinical characteristics. Pearson chi-square test was used to assess categorical and ordinal variables between genders, while continuous measures were assessed using Wilcoxon rank sum test.
A repeated measures logistic regression model using methods of generalized estimating equations (Zeger & Liang, 1986) was used to examine the effects of treatment motivation, self-efficacy and other clinical characteristics on UCT results during the study. To determine potential clinical characteristics that predict abstinence across gender, model building procedures included only those factors that differed by gender at baseline (p < .05), or were univariately associated with abstinence (p < .20). Since participants were not randomized by gender it was important to first examine baseline gender differences. Working correlation structures were compared independently and the final model correlation structure (autoregressive) was chosen using the quasi-likelihood under the independence criterion statistic (Pan, 2001). Next, we used a linear mixed effects model to examine creatinine-adjusted cannabinoid levels. A similar process of model building was used in this analysis. Stepwise selection was used to build adjusted models. All factors included were tested for multicollinearity with VIF < 2. Logistic regression results are reported as OR(95% CI) and linear models are reported as beta(s.e.). Study retention was reported across gender using proportion of study completers and the mean number of days retained in the study. Time to study drop-out across gender was assessed using a Cox Proportional Hazards regression analysis where randomization was considered study day 1 and the final day of study enrollment was the last available study visit day. All statistical analyses were conducted using IBM SPSS version 23.
3. Results
3.1 Baseline demographics and clinical characteristics
One hundred and seventy-five participants were enrolled in the study and were on average 24 years old (SD = 6.4), predominantly Caucasian (64%, n = 112), and had earned at least a high school degree (90%, n = 158). Men and women did not differ significantly on these demographics. Demographics and clinical characteristics are presented in Table 1. The following characteristics differed by gender and were included in model building procedures for primary outcomes. Compared to men, women reported lower levels of SOCRATES Taking Steps [M = 22.13(6.4) vs. M = 24.89(6.9), p = .02]. Compared to men, women reported higher IDTS subscales; unpleasant emotions [M = 60.68(22.2) vs. M = 45.51(24.2), p = .001], physical discomfort [M = 45.47(23.4) vs. M = 36.35(20.2), p = .02], and urges/temptations [M = 58.46(22.8) vs. M = 48.05(24.4), p = .02], and conflict with others [M = 37.09(25.2) vs. M = 25.43(22.8), p = .007). Women reported significantly higher levels than men on MEEQ relaxation subscale [M = 3.96(0.48) vs. M = 3.60(0.73), p = .01].
Table 1. Baseline demographics and clinical characteristics.
| Factor | Whole sample | Male (n = 134) | Female (n = 41) | p-value |
|---|---|---|---|---|
| Age M(SD) | 24.0 (6.4) | 24.3 (6.0) | 23.0 (7.3) | .250 |
| Caucasian N(%) | 112 (64) | 86 (64) | 26 (63) | .929 |
| Marital status N(%) | ||||
| HS graduate N(%) | 158 (90) | 119 (89) | 39 (95) | .232 |
|
| ||||
| Clinical characteristics M(SD) | ||||
|
| ||||
| SOCRATES | ||||
| Recognition | 19.75 (6.3) | 20.02 (6.4) | 18.85 (6.1) | 0.29 |
| Ambivalence | 11.92 (3.8) | 11.88 (3.8) | 12.08 (3.7) | 0.80 |
| Taking steps | 24.25 (6.9) | 24.89 (6.9) | 22.13 (6.4) | 0.04 |
|
| ||||
| Self-efficacy | 3.51 (1.0) | 3.58 (1.0) | 3.28 (0.7) | 0.12 |
|
| ||||
| Past month use (ave oz./week) | 5.25 (2.6) | 5.41 (2.7) | 4.70 (2.4) | 0.17 |
|
| ||||
| Marijuana related problems | 7.37 (3.7) | 7.35 (3.9) | 7.46 (3.2) | 0.79 |
|
| ||||
| Inventory of Drug Taking Situations | ||||
| Unpleasant emotions | 49.01 (24.5) | 45.51 (24.2) | 60.68 (22.2) | 0.001 |
| Physical discomfort | 38.46 (21.3) | 36.35 (20.2) | 45.47 (23.4) | 0.02 |
| Pleasant emotions | 57.12 (20.6) | 56.36 (20.9) | 59.66 (19.5) | 0.45 |
| Testing personal control | 24.22 (22.0) | 23.89 (21.6) | 25.30 (23.6) | 0.87 |
| Urges and temptations | 50.45 (24.3) | 48.05 (24.4) | 58.46 (22.8) | 0.01 |
| Conflict with others | 28.12 (23.8) | 25.43 (22.8) | 37.09 (25.2) | 0.008 |
| Social pressure | 42.56 (25.7) | 41.38 (25.8) | 46.49 (25.4) | 0.22 |
| Pleasant time with others | 62.20 (20.9) | 61.95 (21.5) | 63.07 (18.9) | 0.79 |
|
| ||||
| Marijuana Effect Expectancy Questionnaire | ||||
| Cognitive behavioral impairment | 2.97 (.75) | 2.93 (.75) | 3.07 (.76) | 0.41 |
| Relaxation | 3.69 (.69) | 3.60 (.73) | 3.96 (.48) | 0.04 |
| Social/sexual | 3.06 (.63) | 3.01 (.65) | 3.20 (.54) | 0.22 |
| Perceptual and cognitive enhancement | 3.29 (.68) | 3.25 (.66) | 3.45 (.73) | 0.11 |
| Global negative effects | 1.77 (.57) | 1.75 (.57) | 1.85 (.59) | 0.45 |
| Craving/physical | 3.92 (.74) | 3.86 (.75) | 4.07 (.73) | 0.22 |
|
| ||||
| Marijuana Ladder | 7.11 (1.5) | 7.15 (1.6) | 7.00 (1.1) | 0.48 |
Baseline clinical characteristics were then examined as univariate predictors of abstinence using repeated measures logistic regression model. Self-efficacy predicted greater likelihood of achieving abstinence [OR = 1.73(1.1-2.6), p = .008]. MEEQ subscale physical craving predicted increased likelihood of abstinence [3.77(1.4-9.8), p = .007], while relaxation marginally predicted abstinence [0.38(0.14-1.03), p = .06]. IDTS subscales conflict with others and unpleasant emotions significantly predicted abstinence [0.96(0.93-0.98), p = .005 and 0.96(0.93-1.00), p = .05; respectively), while the pleasant emotions subscale was marginally significant [0.97(.94-1.00), p = .12]. Likewise, the marijuana ladder was marginally associated with abstinence [0.65(0.35-1.20), p = .17].
3.2 Point prevalence abstinence
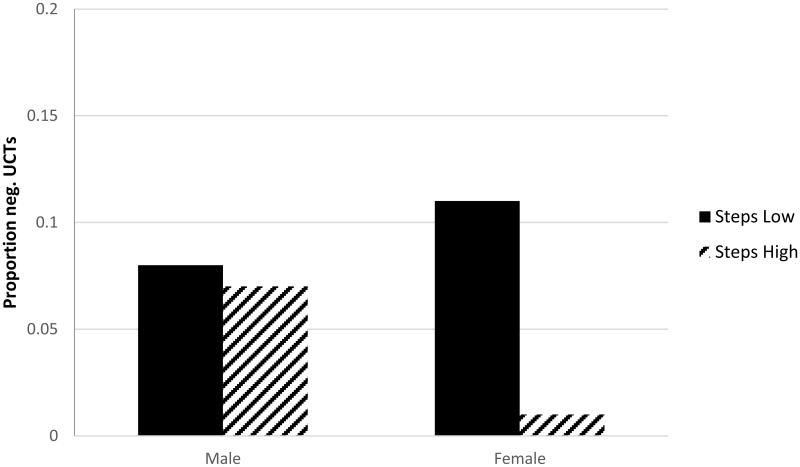
Total number of negative UCTs during treatment was 7.7% (n = 151/1953) across all participants. In our initial unadjusted repeated measures logistic regression model this proportion differed by gender (8.0% among men vs. 7.1% among women; X21 = 4.57, p = .03), however there was a significant interaction between gender and taking steps (X21 = 5.06, p = .024). In the final adjusted model (final model: gender, treatment condition, average weekly use (oz.) in past month, self-efficacy, SOCRATES-taking steps, gender*taking steps) the gender by taking steps interaction remained significant (X21 = 5.56, p = .018). Level of taking steps was associated with abstinence among females, but not males. Counter to our hypothesis, post-hoc analysis revealed that women high in taking steps were less likely to achieve abstinence than women low in taking steps [OR = .08(.02-.37), p = .001]; among men taking steps was not associated with abstinence [.91(.37-2.24), p = .84] (Figure 1). Moderating effects of gender on other factors of interest (IDTS, MEEQ, past month use) were tested and none were found to reach the level of statistical significance.
Figure 1.
Proportions of negative UCTs by gender and SOCRATES – Taking Steps.
Note: Estimated marginal means represented in figure reflect interaction of gender by Taking Steps on proportion of negative UCTs. SOCRATES – Taking Steps median split was used to illustrate interaction. Women low in Taking Steps had greater proportion of negative UCTs (M = .11, SE = .15) than women high in Taking Steps (M = .01, SE = .01); OR = .08(.02-.37), p = .001, while among men there was no significant difference by level of Taking Steps [low M = .08, SE = .09 vs. high M = .07, SE = .08; OR = .91(.37-2.24), p = .84]. Proportion neg. UCTs reflects the number of negative urine cannabinoid tests over the total number of UCTs during the study period, adjusted for non-independence.
Consequently, we explored factors that were associated with taking steps. Using multiple regression we regressed the same clinical characteristics examined above onto SOCRATES - Taking Steps. Overall, greater self-efficacy was positively associated with greater step taking (b = 1.54, SE = .54, p = .005). Likewise, average weekly marijuana use in the previous month (b = .75, SE = .19, p = .000) and marijuana-related problems (b = .33, SE = .14, p = .02) were both positively correlated with taking steps.
We then examined the model separately for males and females. For males, self-efficacy and quantity of use were positively associated with taking steps (b = 1.66, SE = .59, p = .006 and b = .82, SE = .22, p = .000; respectively); these factors were not significant among women (b = .62, SE = 1.54, p = .70 and b = .45, SE = .51, p = .38; respectively). Among women, taking steps was positively associated with marijuana related problems (b = .76, SE = .36, p = .04), which was not significant among men (b = .25, SE = .15, p = .10).
3.3 Creatinine adjusted cannabinoid levels
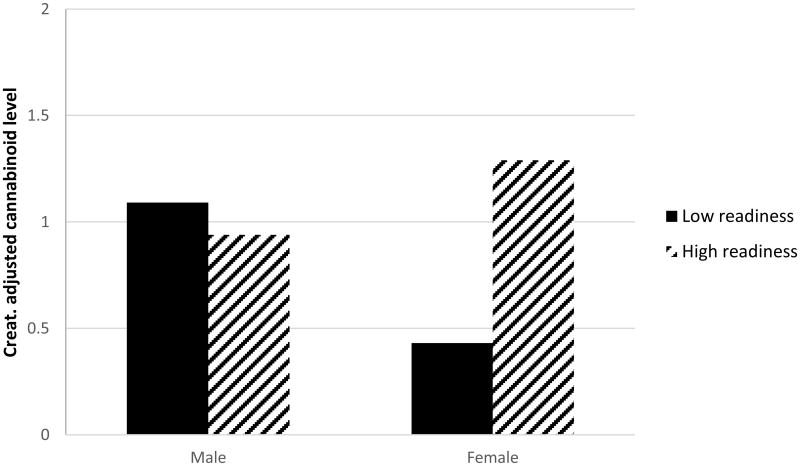
Linear mixed models for repeated measures were used to examine creatinine adjusted cannabinoid levels and gender. In the unadjusted model cannabinoid levels did not differ by gender (b = -.14, SE = .16, p = .38). Following similar stepwise model building procedures used in previous analyses, the final adjusted model (final model: gender, treatment condition, self-efficacy, MJ ladder, SOCRATES-ambivalence, MEEQ-social/sexual facilitation, IDTS-physical discomfort, MCQ-compulsivity, Gender*MJ ladder) revealed significant results. The MEEQ subscale social and sexual facilitation and SOCRATES – ambivalence were both positively associated with cannabinoid levels (b = .32, se = .14, p = .03 and b = .06, se = .02, p = .008; respectively). There were also main effects of gender and readiness to change (MJ ladder), which were superseded by a significant interaction of gender by MJ ladder (b = -.50, se = .17, p = .004). As shown in Figure 2, post-hoc analyses revealed a significant association between readiness to change and cannabinoid levels among women (b = .35, se = .08, p = .000), but not men (b = -.08, se = .07, p = .20). Moderating effects of gender on other factors of interest (SOCRATES, MEEQ, IDTS) were tested and none were found to reach the level of statistical significance.
Figure 2.
Creatinine adjusted cannabinoid levels by gender and readiness to change.
Note: Figure 2 illustrates estimated marginal means for creatinine adjusted cannabinoid levels for men and women across high and low levels of readiness to change. Median split was applied to demonstrate the interaction. Women high on readiness to change had higher creatinine adjusted cannabinoid levels during treatment (M = 1.3, SE = .21) than women low on readiness (M = .43, SE = .31; b = .35, se = .08, p = .000), while this association was not significant among men (high M = .94, SE = .12 vs. low M = 1.1, SE = .22; b = -.08, se = .07, p = .20).
3.3 Retention
Of the 175 participants randomized into the study, 92 (53%) completed the study. This percentage did not differ by gender (X21 = .61, p = .43), nor did mean number of days in the study (M = 48, SD = 38 for men vs. M = 46, SD = 39 for women, p = .85). Additionally, time to study drop out was examined across gender and found than men and women did not differ (HR=1.10 95% CI=0.68-1.78; p=0.71).
4. Discussion
In this study we examined clinical correlates of cannabis use and creatinine adjusted cannabinoid levels across gender in a clinical trial for cannabis use disorder. Men were more likely to achieve point prevalence abstinence than women; however, level of taking steps moderated this relationship. Counter to our hypothesis, women who reported higher levels of taking steps were less likely to achieve abstinence during treatment than women low in taking steps. Exploratory analysis revealed gender differences in factors associated with taking steps – that is, what motivated initiation of behavior change appeared to vary by gender. For women, cannabis-related problems were associated with taking steps, while for men, greater self-efficacy and recent use were associated with taking steps. Regarding creatinine adjusted cannabinoid levels, readiness to change was associated with cannabinoid levels for women, but not men. Creatinine adjusted cannabinoid levels were higher among women who reported greater readiness to change compared to those who reported low readiness to change.
Combined, results suggest an interesting, yet unexpected phenomenon whereby women who report greater readiness to change and initiation of change show worse cannabis use outcomes. One possible explanation is person-centered. Women and men may differ in what motivates them to change behavior or enter treatment. In our sample, women appeared to be driven by external factors (e.g. cannabis-related problems) while men were driven by internal factors (e.g. self-efficacy). A large survey study (N = 385) of cannabis using adults in part supports this conclusion finding that women are more likely to seek treatment due to social acceptability, self-image/self-control, and health concerns compared to men (Chauchard, Levin, Copersino, Heishman, & Gorelick, 2013). According to self-determination theory (Deci & Ryan 1985; Ryan & Deci, 2000) intrinsic motivation is associated with greater attendance and involvement in substance abuse treatment, as well as overall behavioral effectiveness, and enhanced subjective well-being; thus poorer outcomes would be expected for extrinsically motivated persons. Relatedly, step taking may be associated with poor outcomes because of social desirability bias. In a study of 200 individuals with substance use disorder, (Zemore, 2012) found that when accounting for social desirability (“faking good”), change readiness was positively associated with treatment outcome, whereas the association was null before the adjustment. Zemore (2012) did not examine gender which may have further clarified this effect. Some women report avoiding treatment due to stigma involving social and familial ostracism, questions of maternal fitness, and negative images of sexuality (Greenfield & Grella, 2009), which could enhance social desirability bias when they do enter treatment.
A second possible explanation is treatment-centered. Evidence suggests differential substance use treatment outcomes by gender, with women often faring worse than men (DeVito, Babuscio, Nich, Ball, & Carroll, 2014; Litt, Kadden, & Tennen, 2015; McRae-Clark AL, 2015). Although men are more likely to be diagnosed with CUD, women may be more treatment resistant given their greater sensitivity, more rapid progression to disorder, and greater abuse potential. To date, the most effective treatment for CUD involves some combination of MET/CBT/CM, as such, the current study included a brief MET/CM intervention for all participants. However, a limitation of these interventions is that efficacy trials included predominantly male participants (Greenfield, Back, Lawson, & Brady 2010). Attempts at gender specific substance use treatment development were promising (see Women's Recovery Group; WRG; Greenfield et al., 2007), but unsuccessful in stage II trials (Greenfield et al. 2014). However, while the content of WRG carefully addressed substance-related issues unique to women, it was still based on cognitive-behavioral principles, which may be more effective for men.
Neuroimaging studies provide some evidence for this claim. Using fMRI, Wetherill and colleagues (2015) found activation in limbic regions (left hippocampus/amygdala) in women, but not men, in response to subliminal cannabis cues. In addition, structural MRI has revealed associations between emotion regulation ability and the dorsolateral PFC in men, and the hippocampus, amygdala, and insular cortex in women (Kong et al., 2014). These results demonstrate anatomical and functional differences in emotion regulation ability and activation in response to cannabis cues. In turn, it may be that women would benefit more from treatment that includes an emotion regulation component, while men would benefit more from cognitive-behavioral interventions (Wetherill et al., 2015).
Strengths of our study include internal validity as a double-blind, placebo-controlled clinical trial, strict inclusion/exclusion criteria, and a large sample size. However, certain limitations must be noted. The study was a secondary data analysis and therefore not powered to examine these specific associations. Second, point prevalence abstinence rates were markedly low in either gender, which limited our ability to examine predictors of abstinence. While other pharmacotherapy trials for CUD have achieved somewhat higher abstinence rates ranging from 15.6% to 19.0% (Levin, Mariani, Brooks, Pavlicova, Cheng, & Nunes, 2011; Weinstein, Miller, Bluvstein, et al., 2014), these trials included weekly psychotherapy whereas the current study only included 3 sessions of MET. Third, our retention rate was low (53%) limiting our ability to detect treatment effects; however, similar clinical trials for CUD have shown equally low (50%, Weinstein, et al., 2014) or lower rates (43%, Carpenter McDowell, Brooks, Cheng, & Levin, 2009). Despite these limitations we found evidence that men and women differ in what may motivate behavior change, and how motivation to change impacts treatment outcome.
5. Conclusion
Gender differences in cannabis use and cannabis use disorder are well-established. Men are more likely to initiate use, use with greater severity, and be diagnosed with lifetime CUD, while women escalate from first use to dependence quicker and report greater withdrawal symptoms. Despite these differences, few clinical trials have systematically examined gender as a correlate of clinical outcomes, though some evidence suggests that women fare worse than men. The current study found that greater readiness and initiation of change was associated with worse cannabis outcomes for women. This may be explained, in part, by the finding that women and men may differ on what motivates change behavior in the first place. These findings highlight the challenges of developing effective treatments for CUD that account for gender differences across a range of clinical correlates. Moreover, treatments that seek to capitalize on neurobiologically-based differences may prove particularly fruitful.
Highlights.
We examined gender differences in cannabis outcomes from a clinical trial
Women high in taking steps were less likely to achieve abstinence during treatment
Greater readiness to change predicted higher cannabinoid levels in women
What motivates change behavior may vary by gender
Attention to gender differences in cannabis use treatment is needed
Acknowledgments
Role of Funding Sources: Funding for this study was provided by NIDA Grant T32DA007288 (BJS) and K24DA038240 (AMC). NIDA had no role in the study design, collection, analysis, or interpretation of data, writing the manuscript, and the decision to submit the manuscript for publication.
Footnotes
Contributors: Author AMC designed the study and wrote the protocol. Author BJS conducted the statistical anaslysis and wrote the first draft of the manuscript. Author NLB provided statistical consultation. All authors contributed to and have approved the final manuscript.
Conflict of Interest: All authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaro H, Reed E, Rowe E, Picci J, Mantella P, Prado G. Brief screening and intervention for alcohol and drug use in a college student health clinic: Feasibility, implementation, and outcomes. Journal of American College Health. 2010;58(4):357–364. doi: 10.1080/07448480903501764. [DOI] [PubMed] [Google Scholar]
- Campbell WG. Evaluation of a residential program using the Addiction Severity Index and stages of change. Journal of Addictive Diseases. 1997;16(2):27–39. doi: 10.1300/J069v16n02_03. [DOI] [PubMed] [Google Scholar]
- Capone C, Wood MD. Thinking about drinking: Need for cognition and readiness to change moderate the effects of brief alcohol interventions. Psychology of Addictive Behaviors. 2009;23(4):684–688. doi: 10.1037/a0016235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng W, Levin FR. A preliminary trial: Double-blind comparison of nefazodone, buproprion-SR and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18(1):53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauchard E, Levin KH, Copersino ML, Heishman SJ, Gorelick DA. Motivations to quit cannabis use in an adult non-treatment sample: are they related to relapse? Addict Behav. 2013;38(9):2422–2427. doi: 10.1016/j.addbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and Alcohol Dependence. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, et al. Gorelick DA. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. The American Journal of Drug and Alcohol Abuse. 2010;36(6):311–319. doi: 10.3109/00952990.2010.503825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci E, Ryan RM. Intrinsic motivation and self-determination in human behavior. New York: Springer; 1985. [Google Scholar]
- DeVito EE, Babuscio TA, Nich C, Ball SA, Carroll KM. Gender differences in clinical outcomes for cocaine dependence: Randomized clinical trials of behavioral therapy and disulfiram. Drug and Alcohol Dependence. 2014;145:156–167. doi: 10.1016/j.drugalcdep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiClemente CC, Schlundt D, Gemmell L. Readiness and Stages of Change in Addiction Treatment. The American Journal on Addictions. 2004;13(2):103–119. doi: 10.1080/10550490490435777. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34(Suppl 1):S227–S236. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. British Journal of Pharmacology. 2007;152(5):795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcehimes AA, Tonigan JS. Self-efficacy as a factor in abstinence from alcohol/other drug abuse: A meta-analysis. Alcoholism Treatment Quarterly. 2008;26(4):480–489. doi: 10.1080/07347320802347145. [DOI] [Google Scholar]
- Greenfield S, Hufford M, Vagge L, Muenz L, Costello M, Weiss R. The relationship of self-efficacy expectancies to relapse among alcohol dependent men and women: A prospective study. Journal of Studies on Alcohol. 2000;61:345–351. doi: 10.15288/jsa.2000.61.345. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatric Clinics of North America. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, et al. Miele GM. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug and Alcohol Dependence. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Grella CE. What is ‘women-focused’ treatment for substance use disorders? Psychiatric Services. 2009;60(7):880–882. doi: 10.1176/appi.ps.60.7.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Sugarman DE, Freid CM, Bailey GL, Crisafulli MA, Kaufman JS, et al. Fitzmaurice GM. Group therapy for women with substance use disorders: results from the Women's Recovery Group Study. Drug Alcohol Depend. 2014;142:245–253. doi: 10.1016/j.drugalcdep.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Trucco EM, McHugh RK, Lincoln M, Gallop RJ. The women's recovery group study: A stage I trial of women-focused group therapy for substance use disorders versus mixed-gender group drug counseling. Drug and Alcohol Dependence. 2007;90(1):39–47. doi: 10.1016/j.drugalcdep.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL. Withdrawal from THC during adolescence: Sex differences in locomotor activity and anxiety. Behavioural Brain Research. 2012;231(1):48–59. doi: 10.1016/j.bbr.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and Alcohol Dependence. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hser YI, Huang D, Teruya C, Anglin MD. Gender comparisons of drug abuse treatment outcomes and predictors. Drug and Alcohol Dependence. 2003;72(3):255–264. doi: 10.1016/j.drugalcdep.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cohen EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of Analytical Toxicology. 1998;22(6):445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD. The role of self-efficacy in the treatment of substance use disorders. Addictive Behaviors. 2011;36(12):1120–1126. doi: 10.1016/j.addbeh.2011.07.032. doi: http://dx.doi.org/10.1016/j.addbeh.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: Results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug and Alcohol Dependence. 2013;130(1-3):101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Zhen Z, Li J, Huang L, Wang X, Song Y, Liu J. Sex-related neuroanatomical basis of emotion regulation ability. PLoS ONE. 2014;9(5):e97071. doi: 10.1371/journal.pone.0097071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1-3):142–50. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM. (2015). Willpower versus “skillpower”: Examining how self-efficacy works in treatment for marijuana dependence. Psychol Addict Behav. 2015 Sep;29(3):532–40. doi: 10.1037/adb0000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: randomized trial of contingency management and self-efficacy enhancement. Addict Behav. 2013;38(3):1764–1775. doi: 10.1016/j.addbeh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Tennen H. Network support treatment for alcohol dependence: Gender differences in treatment mechanisms and outcomes. Addictive Behaviors. 2015;45:87–92. doi: 10.1016/j.addbeh.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CG, Hollin CR. Assessing comorbid substance use in detained psychiatric patients: Issues and instruments for evaluating treatment outcome. Substance Use & Misuse. 2009;44(11):1602–1641. doi: 10.1080/10826080802486434. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, B N, Gray KM, Killeen TK, Wagner AM, Brady KT, DeVane CL, Norton J. Buspirone treatment of cannabis dependence: a randomized, controlled trial. Drug and Alcohol Dependence. 2015 doi: 10.1016/j.drugalcdep.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Helping people change. 3rd. New York, NY, US: Guilford Press; 2013. [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers' motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) Psychology of Addictive Behaviors. 1996;10(2):81–89. doi: 10.1037/0893-164X.10.2.81. [DOI] [Google Scholar]
- Norcross JC, Krebs PM, Prochaska JO. Stages of change. J Clin Psychol. 2011;67(2):143–54. doi: 10.1002/jclp.20758. [DOI] [PubMed] [Google Scholar]
- Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. The Transtheoretical approach: Crossing the traditional boundaries of therapy. Malabar, FL: Krieger; 1984. [Google Scholar]
- Prochaska JO, DiClemente CC. Stages of change in the modification of problem behavior. In: Hersen M, Eisler R, Miller PM, editors. Progress in behavior modification. Vol. 28. New York: Academic Press; 1992. [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55(1):68–78. doi: 10.1037/0003-066X.55.1.68. [DOI] [PubMed] [Google Scholar]
- Schafer J, Brown SA. Marijuana and cocaine effect expectancies and drug use patterns. Journal of Consulting and Clinical Psychology. 1991;59(4):558–565. doi: 10.1037/0022-006X.59.4.558. [DOI] [PubMed] [Google Scholar]
- Serafini K, Shipley L, Stewart DG. Motivation and substance use outcomes among adolescents in a school-based intervention. Addictive Behaviors. 2016;53:74–79. doi: 10.1016/j.addbeh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Clarke CJ, Simons RM, Spelman PJ. Marijuana consequences in a motivational context: Goal congruence reduces likelihood of taking steps towards change. Addictive Behaviors. 2016;52:83–90. doi: 10.1016/j.addbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal G, Greener J. Client engagement and change during drug abuse treatment. Journal of Substance Abuse. 1995;7(1):117–134. doi: 10.1016/0899-3289(95)90309-7. doi: http://dx.doi.org/10.1016/0899-3289(95)90309-7. [DOI] [PubMed] [Google Scholar]
- Slavet JD, Stein LAR, Colby SM, Barnett NP, Monti PM, Golembeske C, Jr, Lebeau-Craven R. The Marijuana Ladder: Measuring motivation to change marijuana use in incarcerated adolescents. Drug and Alcohol Dependence. 2006;83(1):42–48. doi: 10.1016/j.drugalcdep.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline Follow-Back. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Humana Press; 1992. pp. 41–72. [Google Scholar]
- Steinberg KL, Roffman RA, Carroll KM, et al. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults (Vol HHS Publication No (SMA) 12-4211) Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; Rockville: 2005. [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting and Clinical Psychology. 1994;62(1):92–99. doi: 10.1037/0022-006X.62.1.92. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Wertz JS, Roffman RA. Predictors of marijuana treatment outcomes: The role of self-efficacy. Journal of Substance Abuse. 1993;5(4):341–354. doi: 10.1016/0899-3289(93)90003-T. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Wertz JS, Roffman RA. Self-efficacy and marijuana cessation: A construct validity analysis. Journal of Consulting and Clinical Psychology. 1995;63(6):1022–1031. doi: 10.1037/0022-006X.63.6.1022. [DOI] [PubMed] [Google Scholar]
- Turner NE, Annis HM, Sklar SM. Measurement of antecedents to drug and alcohol use: Psychometric properties of the Inventory of Drug-Taking Situations (IDTS) Behaviour Research and Therapy. 1997;35(5):465–483. doi: 10.1016/S0005-7967(96)00119-2. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug and Alcohol Dependence. 2007;86(2-3):191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar-Hamburger R, Bloch M. Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: A double-blind placebo controlled study. Am J Drug Alcohol Abuse. 2014;40(1):16–22. doi: 10.3109/00952990.2013.819362. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Franklin TR. Sex differences in associations between cannabis craving and neural responses to cannabis cues: Implications for treatment. Experimental and Clinical Psychopharmacology. 2015;23(4):238–246. doi: 10.1037/pha0000036. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal Data Analysis for Discrete and Continuous Outcomes. Biometrics. 1986;42(1):121–130. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
- Zemore SE. The effect of social desirability on reported motivation, substance use severity, and treatment attendance. Journal of Substance Abuse Treatment. 2012;42(4):400–412. doi: 10.1016/j.jsat.2011.09.013. doi: http://dx.doi.org/10.1016/j.jsat.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]