Abstract
During translation, the two eukaryotic ribosomal subunits remain associated through 17 intersubunit bridges, five of which are eukaryote-specific. These are mainly localized to the peripheral regions and are believed to stabilise the structure of the ribosome. The functional importance of these bridges remains largely unknown. Here the essentiality of the eukaryote-specific bridge eB12 has been investigated. The main component of this bridge is ribosomal protein eL19 which is composed of an N-terminal globular domain, a middle region and a long C-terminal α-helix. Analysis of deletion mutants demonstrated that the globular domain and middle region of eL19 are essential for cell viability, most likely functioning in ribosome assembly. The eB12 bridge, formed by contacts between the C-terminal α-helix of eL19 and 18S rRNA in concert with additional stabilising interactions involving either eS7 or uS17, is dispensable for viability. Nevertheless, eL19 mutants impaired in eB12 bridge formation displayed slow growth phenotypes, altered sensitivity/resistance to translational inhibitors and enhanced hyperosmotic stress tolerance. Biochemical analyses determined that the eB12 bridge contributes to the stability of ribosome subunit interactions in vitro. 60S subunits containing eL19 variants defective in eB12 bridge formation failed to form 80S ribosomes regardless of Mg2+ concentration. The reassociation of 40S and mutant 60S subunits was markedly improved in the presence of deacetylated tRNA, emphasising the importance of tRNAs during the subunit association. We propose that the eB12 bridge plays an important role in subunit joining and in optimizing ribosome functionality.
Keywords: Saccharomyces cerevisiae, ribosome subunit association, eukaryote-specific bridges, ribosome assembly, stress tolerance
Graphical Abstract

INTRODUCTION
Ribosomes consist of two unequal subunits in all three domains of life: 30S and 50S (Archaea and Bacteria) or 40S and 60S (Eukarya). The rotation of subunits relative to one another enables translocation of tRNAs and mRNAs across the ribosome1; 2; 3; 4. The 3.0 Å resolution Saccharomyces cerevisiae 80S ribosome crystal structure reveals that rotation of the yeast ribosomal subunits is regulated by 17 intersubunit bridges5. Twelve of these are conserved among all three domains of life and contain three types of interactions: rRNA-rRNA, protein-rRNA and protein-protein. The conserved bridges are located close to, and coordinate the activity of the ribosomal functional centres5; 6. The five eukaryote-specific intersubunit bridges are composed of the protein-rRNA and protein-protein type interactions between eukaryote-specific rRNA expansion segments (ES) and eukaryote-specific protein domains. They are localized to peripheral regions and are thought to structurally stabilise the 80S ribosome. Two eukaryote-specific proteins, eL19 and eL24, contain long α-helices extending from the 60S subunit E- and A-site sides, respectively (Fig. 1A). These helices form the distinctive eB12 and eB13 intersubunit bridges. Bridge eB12 is formed by contacts between the C-terminal α-helix of eL19 and eukaryote-specific expansion segment 6 (ES6S) of 18S rRNA (Fig. 1B, 1C). The dynamic structure of eB12 is dependent on conformational changes that occur during ribosomal translocation. In the pre-translocational state, eB12 is formed by the contacts between eL19 and uS17. In contrast, these contacts are replaced by interactions between eL19 and eS7 when the ribosome occupies the post-translocational state5. The essential eL19 protein is encoded by two paralogous genes, RPL19A and RPL19B7. Deletion of either RPL19A or RPL19B is not lethal for yeast cells7; 8. However, both genes are required for optimal growth. Cells lacking RPL19A or RPL19B are characterised by 1.2- and 1.5-fold increased doubling rates, respectively9. Deletion of both RPL19A and RPL19B causes a synthetic lethality phenotype7. The 189 amino acid residue long eL19 proteins encoded by these genes are identical. Yeast eL19 protein exhibits 57.5% homology with mammalian eL19, and 35.8 % homology with archaeal eL19, although there is no eL19 counterpart in bacterial ribosome7; 10. eL19 contains three structural domains: the N-terminal globular domain, the middle region and the long C-terminal α-helix (Fig. 1B).
Figure 1. Functional analysis of eL19 deletion mutants in vivo.
A. S. cerevisiae 80S ribosome. The large subunit (60S) is shown in blue and the small subunit (40S) is grey. Proteins eL19 and eL24 forming the eB12 and eB13 bridges, respectively, are shown in red and orange.
B. Domain structure of eL19. The C-terminal α-helix of eL19 (dark blue) is shown in detail. Amino acid residues forming contacts with eukaryote-specific expansion segment 6 (ES6S) of 18S rRNA and 40S subunit proteins (eS7, uS17) are coloured green and yellow, respectively. Arrows indicate the positions of the last amino acids of the respective eL19 deletion alleles.
C. Close up view of the eB12 intersubunit bridge. Detailed views of contacts formed between α-helix of eL19 (dark blue) and uS17 (red), 18S rRNA ES6S (orange) and eS7 (purple) are shown. Ribosomal structures were generated by PyMol using coordinates from5.
D. Analysis of the functionality of eL19 deletion mutants using plasmid shuffle assay. An rpl19AΔrlp19BΔ strain (TYSC237) containing RPL19A/URA3/CEN plasmid was transformed with either an empty LEU2/CEN plasmid (indicated as vector) or plasmids harbouring wild type eL19 or indicated eL19 mutant alleles. Growth of the transformants was tested on control plates (SC-Leu) or on plates containing 5-fluoroorotic acid (SC-Leu+5FOA) which selects for yeast cells that have lost the RPL19A/URA3/CEN plasmid. Plates were incubated at 30°C for 3 days.
The eB13 bridge contains contacts between the C-terminal α-helix of eL24 and eS6 with assistance from uL3 in both conformational states5. However, an additional interaction between eL24 and the 18S rRNA helix 10 occurs in the post-translocational state. The main component of this bridge – ribosomal protein eL24 - is also encoded by two paralogous genes RPL24A and RPL24B11. These genes produce two near identical proteins differing in 5 out of 155 amino acid residues. Similar to eL19, eL24 is present only in archaeal and eukaryotic ribosomes10. Interestingly, eL24 is one of the few ribosomal proteins that are not essential for viability9; 11. Deletion of the both paralogous genes slightly reduces the growth rate of the cells. Given that eL24 is nonessential, the eB13 bridge is dispensable for viability.
In the current study several eL19 deletion mutants were constructed in order to evaluate the essentiality of the eB12 bridge and of various domains of the eL19. In vivo depletion of eL19 demonstrated that eL19 has an important role during pre-rRNA processing and ribosome assembly. Analysis of eL19 mutants deficient in eB12 formation demonstrated that the eB12 bridge is not essential for cell viability. However, our results indicated that eB12 plays a substantial role in shaping cell growth in sub-optimal conditions, while biochemical analyses determined that eB12 contributes to the stability of ribosome subunit interaction in vitro. Consequently, although the eB12 intersubunit bridge is dispensable for cell growth per se, it plays an important role in subunit joining and in optimizing ribosome functionality.
RESULTS
Loss of the eB12 bridge leads to reduced cell growth
The eukaryote-specific eB12 intersubunit bridge is formed by contacts between the C-terminal α-helix of eL19 and 18S rRNA ES6S (Fig. 1C). Additional stabilising interactions between eL19 and eS7 or uS17 are also involved depending upon the rotational state of the ribosome. Several eL19 mutants deficient in eB12 formation were constructed to analyse the importance of eB12 in terms of ribosome functionality (Fig. 1B, Table 1). In mutant eL191–183, the C-terminal 6 amino acid residues which interact with ribosomal protein eS7 were deleted. In mutant eL191–154, the C-terminal 35 amino acid residues were removed, eliminating the bridge forming region in which eL19 interacts with 18S RNA ES6S and ribosomal proteins eS7 and uS17. Mutant eL191–146, harbouring a deletion of 43 amino acid residues, mimicked the archaeal version of eL1912. In mutant eL191–133, the entire C-terminal α-helix (56 amino acid residues) was deleted. Cells expressing only mutant variants of eL19 were selected by the plasmid shuffling method (Fig. 1D). The eL19 mutant deficient in eS7 binding (eL191–183) displayed wild type growth, while growth was impaired in the mutants deficient in eB12 bridge formation (eL191–154 and eL191–146). In contrast, deletion of entire α-helix (eL191–133) was lethal. To study the growth characteristics of the viable eL19 deletion mutants in detail, temperature sensitivity analysis by serial dilutions spot-test on rich medium at different temperatures was performed (Fig. 2A). The eL19 mutant deficient in eS7 binding exhibited nearly wild type growth rates at all temperatures assayed. Loss of the eB12 bridge conferred slow growth at 30°C and 36°C. The slow growth phenotype was enhanced at lower temperatures. Ribosome-polysome profile analysis of mutant cells grown in rich medium at 30°C and 20°C was performed to assess the effects of the eL19 deletions on ribosome biogenesis (Fig. 2B). No significant changes in monosome and polysome levels compared to wild type cells were observed in cells expressing eL191–183. In contrast, mutants lacking the eB12 bridge displayed considerable increases of free 60S subunit levels at 20°C. Altogether, these results indicate that loss of interactions between eL19 and eS7 has a minor influence on cell viability. The inability to form the eB12 bridge leads to reduced cell growth and accumulation of free 60S subunits indicating a reduction in ribosome functionality.
Table 1.
Description of eL19 variants analysed in this study.
| eL19 variant | Length of eL19 (aa) | Deleted contacts/region | Viability |
|---|---|---|---|
| eL19 | 189 | − | + |
| eL191–183 | 183 | eS7 | + |
| eL191–154 | 154 | eS7, ES6S, uS17 | + |
| eL191–146 | 146 | eS7, ES6S, uS17 | + |
| eL191–133 | 133 | entire α-helix | − |
Figure 2. Phenotypic analysis of eL19 deletion mutants.
A. Growth phenotypes of eL19 mutants. Serial dilutions of wild type (WT, TYSC144) and rpl19AΔrlp19BΔ strains carrying eL19 wild type (eL19, TYSC278) or mutant alleles (eL191–183, TYSC280; eL191–154, TYSC282; eL191–146, TYSC283) were spotted onto YPD medium and incubated at the indicated temperatures for 2–4 days.
B. Analysis of ribosome-polysome profiles by sucrose density gradient centrifugation. The rpl19AΔrlp19BΔ strains carrying eL19 wild type or mutant alleles were grown in YPD medium at indicated temperatures. The whole cell extracts were prepared from cycloheximide treated cells and analysed in 7%–47% sucrose gradients. The absorbance at 260 nm (A260nm) was recorded. Sedimentation is from left to right. The peaks of free 60S ribosomal subunits, monosomes (80S) and polysomes are indicated.
The C-terminal domain of eL19 is dispensable for pre-rRNA processing
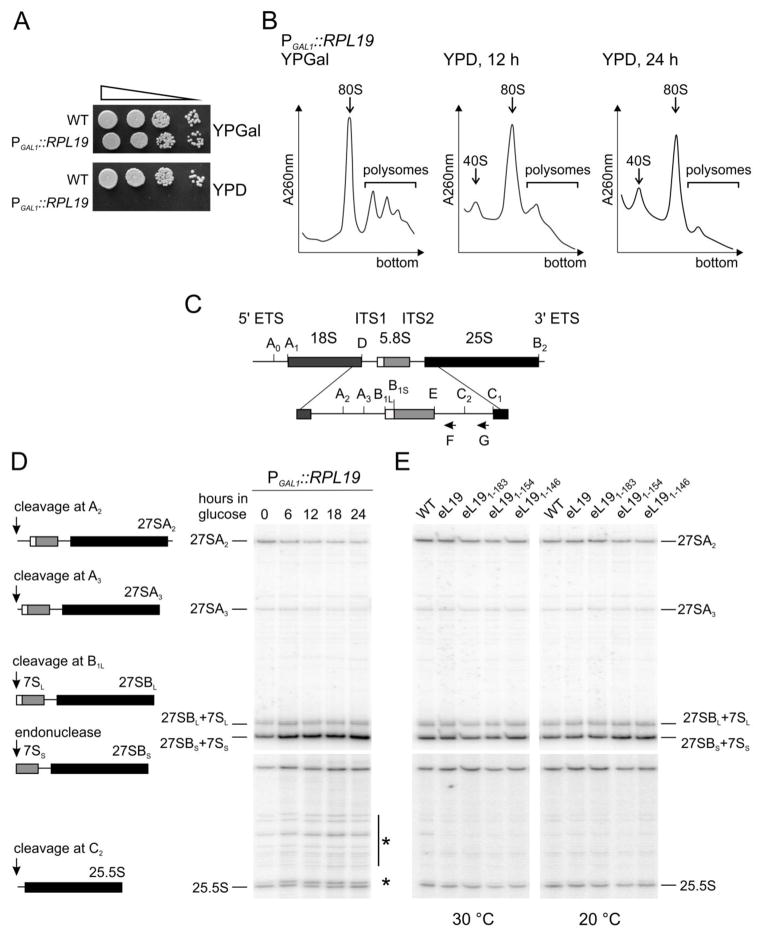
A conditional null mutant system (rpl19AΔrpl19BΔ+ [pRSpGAL-RPL19A]), where RPL19A was ectopically expressed under the control of the galactose inducible GAL1 promoter was constructed to enable analysis of the impact of eL19 C-terminal deletions on ribosome assembly and pre-rRNA processing. While growth of this strain was indistinguishable from wild type cells on permissive galactose containing medium, shut-down of eL19 expression on glucose containing medium resulted cell death (Fig. 3A), consistent with previous reports that eL19 is essential for yeast growth13. Polysome profile analysis of this strain subsequent to 12 h or 24 h shift to glucose containing medium revealed the dramatic consequences of eL19 depletion: reduction of polysome and monosome levels and accumulation of free 40S subunits (Fig. 3B). This phenotype suggests a defect in 60S subunit assembly. Next, the relative changes in steady-state levels of 60S precursor rRNAs (Fig. 3C) were analysed by primer extension analysis in cells shifted to glucose containing medium up to 24 hours. Examination of pre-rRNA species revealed very slight reductions in levels of 27SA2 and 27SA3 pre-rRNAs (Fig. 3D). In contrast, the levels of 27SBL and 27SBS pre-rRNAs were significantly increased under eL19 depletion conditions. Unfortunately, this specific primer (Probe F, Fig. 3C) does not enable one to distinguish whether pre-rRNA processing is affected at the level of 27SB, 7S pre-rRNAs, or both during depletion of eL19. Interestingly, enhancement of numerous nonspecific stops in the internal transcribed spacer region 2 (ITS2) close to the C2 cleavage site was detected (Fig. 3D, shown by asterisks). In agreement with previous analysis, these results indicate that eL19 is important for efficient conversion of 27SB pre-rRNAs13. Furthermore, an RNA pulse labelling experiment demonstrated that depletion of eL19 caused accumulation of 25SB pre-rRNAs13. Primer extension analysis of pre-rRNA species in eL19 mutant cells grown in rich medium at 30°C and 20°C was performed to investigate whether the truncated variants of the C-terminal α-helix affected pre-rRNA processing (Fig. 3E). The deletion mutants showed no changes in maturation patterns of the 27SA2, 27SA3, 27SBL, 27SBS and 25.5S pre-rRNAs compared to the wild type. These results indicate that deletion of the eB12 forming region in the C-terminal α-helix of eL19 has no influence on pre-rRNA processing. In contrast, the globular and middle domains of eL19 are essential for cell growth, suggesting that this protein is essential during large subunit assembly.
Figure 3. Processing of pre-rRNAs is not perturbed in eL19 mutants.
A. Serial dilutions of wild type (WT, TYSC144) and rpl19AΔrlp19BΔ strain carrying pGAL-RPL19 plasmid (PGAL1::RPL19, TYSC291) were spotted onto YPGal (galactose) or YPD (glucose) medium and incubated at 30°C for 3 days.
B. Analysis of ribosome-polysome profiles of cells depleted for the eL19 by sucrose density gradient centrifugation. Cells were cultured in YPGal and shifted to YPD for 12 or 24 hours. Whole cell extracts were prepared from cycloheximide treated cells and analysed in 7%–47% sucrose gradients. The absorbance at 260 nm (A260nm) was recorded. Sedimentation is from left to right. The peaks of free 40S ribosomal subunits, monosomes (80S) and polysomes are indicated.
C. Structure and processing sites of the 35S pre-rRNA. The mature 18S, 5.8S and 25S rRNAs are shown as boxes. Two internal transcribed spacer sequences (ITS1 and ITS2) and two external transcribed spacer sequences (5′ ETS and 3′ ETS) are shown as lines. The cleavage sites and positions of the oligonucleotides (F and G) used for the primer extension analysis are indicated.
D. Analysis of pre-rRNA processing in cells depleted for eL19 by primer extension. Total RNA was extracted from cells grown at 30°C in YPGal or shifted to YPD for up to 24 hours. Samples were subjected to primer extension analysis of 5′ ends of 27S and 25.5S pre-rRNAs. Asterisks indicate the nonspecific cleavage products of 27S pre-rRNA. The origin of the pre-rRNAs analysed by primer extension are shown.
E. Analysis of pre-rRNA processing in eL19 mutants by primer extension. Total RNA was extracted from wild type (WT, TYSC144) and rpl19AΔrlp19BΔ strains carrying eL19 wild type (eL19, TYSC278) or mutant alleles (eL191–183, TYSC280; eL191–154, TYSC282; eL191–146, TYSC283) grown in YPD medium at 30°C or 20 °C.
Loss of the eB12 bridge has no effect on the rotational state of the ribosomes
The transition from the non-rotated to rotated state after peptidyltransfer leads to the changes in the interactions between the ribosomal subunits. Recently the chemical modification profiles for non-rotated and rotated yeast ribosome were determined14. SHAPE (selective 2′-hydroxyl acylation analysed by primer extension) using 1-methyl-7-nitroisatoic anhydride (1M7) was utilized to map the chemical protection patterns of base pair interactions located in several universally conserved regions. SHAPE analysis of specific 25S rRNA nucleotides that form bridges B7a (A2207) and B2a (U2258, A2262), the sarcin/ricin loop (U3023, A3027) and the aa-tRNA accommodation corridor (U2860, U2924 and A2926) was performed in order to examine the rotational status of eB12 mutant ribosomes (Fig. S1A). Salt washed, intact ribosomes (80S) were pre-treated with puromycin, chemically probed with 1M7 (or only DMSO as controls) and the base reactivities were assessed by primer extension analysis and quantified. SHAPE reactivities of seven analysed nucleotides (U2258, A2262, U2860, U2924, A2926, U3023, A3027) were similar in ribosomes purified from cells expressing mutant alleles or wild type eL19 (Fig. S1B, S1C, data not shown). In contrast, nucleotide A2207 was less reactive in mutants lacking the eB12 bridge as compared to wild type ribosomes (Fig. S1B, S1C). This nucleotide interacts with 18S rRNA nucleotide G913 in the non-rotated state, but becomes accessible to modification by 1M7 in the rotated state (Fig. S1A)14. The ribosomes in the non-rotated state are also characterised by reactive nucleotides in the aa-tRNA accommodation corridor (U2924, A2926)14. SHAPE analysis did not show the strong chemical reactivity of these nucleotides (data not shown). Therefore we conclude that ribosomes with defective eB12 bridges are free to transit between the non-rotated and rotated states in the absence of ligands as has been shown for the vacant wild type ribosomes14; 15.
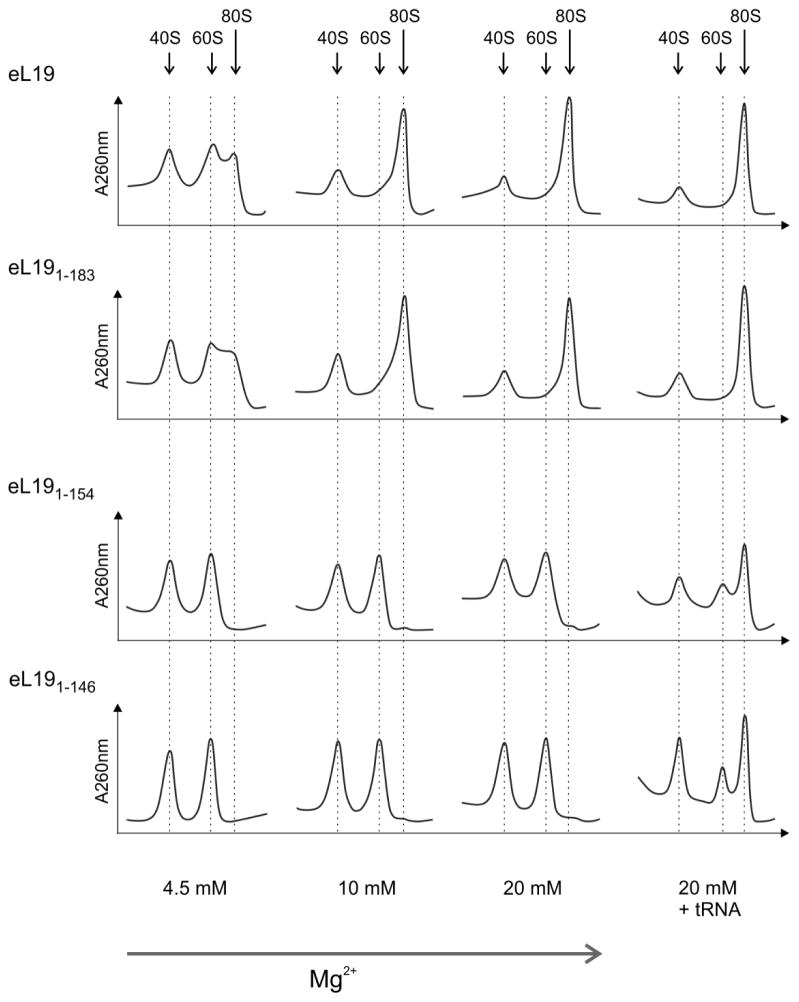
The eB12 bridge is essential for stable 40S and 60S subunit reassociation in vitro
Translation initiation in eukaryotes is a multi-step process that involves several initiation factors, mRNA and methionyl initiator tRNA in addition to the 40S and 60S subunits. Therefore, deficiencies in the intrinsic contacts between the subunits can be compensated by trans-acting factors. Wild type 40S subunits and 60S subunits containing the wild type or mutant versions of eL19 were isolated using the high salt treatment of purified monosomes to enable more direct assessment of the role of eB12 in ribosome functionality. In vitro formation of 80S ribosomes at 4.5 – 25 mM Mg2+ concentrations was examined by sucrose gradient sedimentation (Fig. 4, data not shown). No differences in the ability of 60S subunits containing either eL191–183 variant or wild type eL19 to associate with 40S subunits were observed in all conditions analysed. In contrast, 60S subunits containing either eL191–154 or eL191–146 variants failed to form 80S ribosomes, regardless of Mg2+ concentration. However, their reassociation activities were markedly improved in the presence of 20 mM Mg2+ and saturating amounts of deacylated tRNA indicating that the purified 60S subunits were functional and not damaged. This behaviour of the eL191–154 and eL191–146 mutants can be explained by the loss of contacts between the eL19 C-terminal α-helix and eS7, uS17 and 18S rRNA ES6S. We conclude that bridge eB12 is essential for the formation of 80S ribosome in vitro.
Figure 4. The eB12 intersubunit bridge is required for 80S formation in vitro.
Salt-washed 40S and 60S subunits were purified from rpl19AΔrlp19BΔ strains carrying eL19 wild type (eL19, TYSC278) or mutant alleles (eL191–183, TYSC280; eL191–154, TYSC282; eL191–146, TYSC283) grown in YPD medium at 30°C. Two A260 units of wild type 40S subunits were co-incubated with two A260 units of 60S subunits containing wild type or mutant eL19 in the presence of indicated magnesium acetate concentrations for 20 min at 30°C. Reactions at 20 mM magnesium acetate were also performed in the presence of saturating concentration of deacylated tRNA (20 mM + tRNA) to stimulate 80S formation. Ribosomal subunit association was analysed in 10%–30% sucrose gradients with appropriate magnesium acetate concentrations. Sedimentation is from left to right. The peaks of free 40S and 60S ribosomal subunits and monosomes (80S) are indicated.
Absence of the eB12 bridge affects stress tolerance
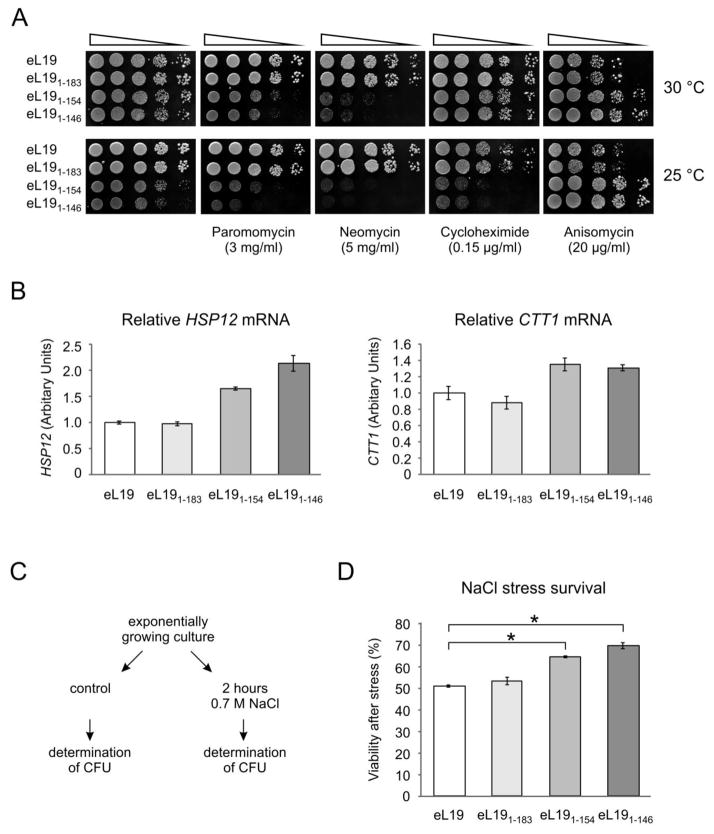
Serial dilutions spot-test analysis of sensitivity/resistance was performed in the presence of several translational inhibitors that affect different steps in protein synthesis to assess the response of eL19 mutant cells to environmental stress conditions (Fig. 5A). Two aminoglycosides, paromomycin and neomycin, bind to the A-site decoding region of the 40S subunit16; 17. Their binding inhibits ribosome translocation and compromises translational fidelity. The glutarimide inhibitor, cycloheximide, binds in the E-site on the 60S subunit18. It competes with the E-site tRNA and inhibits eEF2-mediated tRNA translocation19. Anisomycin is a macrolide that binds to the A-site in the 60S subunit and competitively inhibits the peptidyl transferase reaction thereby inhibiting translation elongation20; 21.
Figure 5. Physiological responses of eL19 deletion mutants to different stress conditions.
A. Drug resistance/sensitivity phenotypes of eL19 mutants. Serial dilutions of rpl19AΔrlp19BΔ strains carrying eL19 wild type (eL19, TYSC278) or mutant alleles (eL191–183, TYSC280; eL191–154, TYSC282; eL191–146, TYSC283) were spotted onto YPD medium without or with indicated antibiotics and incubated at the indicated temperatures for 3–4 days.
B. Analysis of HSP12 and CTT1 transcript levels with quantitative PCR in eL19 mutants. Total RNA was extracted from rpl19AΔrlp19BΔ strains carrying eL19 wild type or mutant alleles grown in YPD medium at 30°C. The results were normalised to the geometric average of two housekeeping genes (UBC6 and ARP6) and expressed relative to the strain expressing wild type eL19. Error bars represent standard deviations of two independent measurements.
C. Design of the analysis of NaCl stress survival. CFU, colony-forming unit.
D. Sensitivity to salt stress of eL19 mutants. Yeast cells were grown to early exponential phase in YPD at 30°C and exposed to 0.7 M NaCl stress for 2 hours. Cell viability was measured by counting colony-forming cells on YPD plates after the salt stress relative to untreated cells. The error bars represent the standard errors of three independent experiments. Statistical significance was evaluated with the unpaired t test (*P<0.001).
The eL19 mutant cells deficient in eS7 binding (eL191–183) exhibited similar phenotypes in response to all of the antibiotics compared to cells expressing wild type eL19 (Fig. 5A). In contrast, mutants impaired in eB12 bridge formation (eL191–154 and eL191–146) displayed hypersensitivity to both of the aminoglycosides, i.e. paromomycin and neomycin. Increased sensitivity of these mutants to cycloheximide in comparison to the cells expressing wild type eL19 was observed only at 25°C. Surprisingly, these mutants conferred increased resistance to anisomycin at both temperatures as compared to the cells expressing wild type eL19. These results suggest that the eB12 bridge may affect overall ribosomal function rather than specific steps of the protein translation cycle.
The sensitivity to translational inhibitors inducing misreading (paromomycin and neomycin) of the eL19 mutant strains lacking the eB12 bridge suggests that these mutant ribosomes are more prone to make translational errors than the wild type ribosomes. Decreased translational fidelity in the eB12 bridge mutants may activate the general stress response. Several genes containing stress-responsive elements (STRE) in their promoter regions are transcriptionally induced during the general stress response program22. STREs are found in the promoters of HSP12 and CTT123; 24; 25. HSP12 encodes a small heat shock protein and its expression is up-regulated during different stress conditions23; 26; 27; 28; 29. CTT1 encodes the cytosolic catalase T and its expression is also induced under various stresses30; 31. The levels of HSP12 and CTT1 mRNAs in cells expressing wild type and mutant alleles of eL19 grown in rich medium at 30 °C were analysed by quantitative reverse transcriptase PCR (Fig. 5B). No differences in HSP12 and CTT1 transcript levels were detected between the eL19 mutant cells deficient in eS7 binding (eL191–183) and wild type cells (Fig. 5B). However, HSP12 mRNA levels were elevated 1.6- and 2.1-fold in cells expressing the eL191–154 and eL191–146 mutants respectively compared to the cells expressing wild type eL19. Similarly, the CTT1 transcript level was 1.3-fold higher in eB12 bridge mutant cells relative to cells expressing wild type eL19. From these results we conclude that general stress response is activated in eB12 mutants in response to increased translational errors.
Several reports indicate that mistranslation enhances tolerance to different stress conditions32; 33; 34. For this reason the sensitivity to hyperosmotic stress of cells expressing wild type eL19 was compared to that of cells expressing mutant alleles of eL19. Actively growing cells were exposed to 0.7 M NaCl stress for 2 hours and the viability of cells was measured by determining the colony forming ability of stressed cells (Fig. 5C). There was no difference in sensitivity to NaCl between cells expressing wild type eL19 and eL19 variant deficient in eS7 binding (eL191–183) (Fig. 5D). In contrast, mutants lacking eB12 were less sensitive to salt stress than cells containing wild type eL19 (Fig. 5D). We propose that increased misreading by mutant ribosomes lacking eB12 induces the stress response, therefore promoting acquired stress tolerance.
DISCUSSION
In the work presented here, we have evaluated the functional importance of the eukaryote-specific eB12 bridge at the biological, biochemical and structural levels. The core component of this bridge is the eukaryote-specific protein eL195. eL19 is composed of an N-terminal globular domain, a middle region and a long C-terminal α-helix (Fig. 1B). The C-terminal α-helical domain forms the eB12 intersubunit bridge through its interactions with 18S rRNA ES6S, eS7 and uS17 (Fig. 1C). The N-terminal globular domain and middle region are embedded in domain III of 25S rRNA. eL19 is an essential protein7. A previous report indicates that eL19 has an essential role in pre-rRNA processing13. Expression shut down of eL19 leads to a delay in the endonucleolytic cleavage of 27SB pre-RNA in the ITS2 region at site C2, which produces 7S pre-RNA and 25.5S pre-RNA. Our results show that depletion of eL19 in its entirety is lethal, and that this impairs pre-rRNA processing and leads to a ribosome 60S subunit assembly defect (Fig. 3A–D). Specifically, primer extension analysis revealed accumulation of 27SB pre-rRNAs and increased the frequency of nonspecific cleavage events in the ITS2 region. Our results also show that deletion of the eB12 moiety of eL19 is dispensable for viability although this affected cellular growth rates (Fig. 2A). Analysis of pre-rRNA processing in these mutants demonstrated that the region responsible for eB12 formation is not required for ribosome biogenesis (Fig. 3E). In contrast, a deletion mutant lacking the entire C-terminal α-helix was inviable (Fig. 1D), indicating that the first 13 amino acids of this helix are required for ribosome functionality. According to the X-ray structure of the yeast 80S ribosome5, the beginning of the C-terminal α-helix of eL19 resides inside the large subunit and is probably required for correct eL19 folding or for its association with 25S rRNA. Thus, eL19 has two specific roles. The essential one, carried by the N-terminal globular domain and middle region, is in ribosome biogenesis. The second, provided by the C-terminal α-helical domain, involves structural support for ribosomal subunit joining.
Examination of phenotypes conferred by the eL19 mutant alleles revealed several eB12 bridge specific effects, i.e. slow growth, altered sensitivity/resistance to translational inhibitors and enhanced hyperosmotic stress tolerance (Fig. 2A, Fig. 5A, 5D). Loss of the eB12 bridge had a stronger impact on A-site specific functions, i.e. the mutants were sensitive to aminoglycosides and resistant to anisomycin. The aminoglycosides paromomycin and neomycin bind to the A-site decoding region of the small subunit. The canonical aminoglycoside binding site is located within the internal loop of helix 44 of 18S rRNA and contains the universally conserved nucleotides A1755 and A1756. Two nonconserved nucleotides (G1645 in yeast, A1408 in bacteria and A1754 in yeast, G1491 in bacteria) have been shown to be responsible for the greater resistance of eukaryotes to aminoglycosides35. Mutational analyses have shown that yeast strain carrying G1645A and A1754G mutations exhibited increased sensitivity to neomycin and paromomycin. Consistent with these findings, replacement of the bacterial A-site helix 44 with its counterpart from eukaryotes conferred aminoglycoside resistance of these mutants comparable to eukaryotic ribosomes36. Recent structural analysis of translational inhibitor binding sites in the yeast ribosome explained this difference between prokaryotes and eukaryotes18. In the yeast ribosome, nucleotides G1645 and A1754 act as a barrier by preventing the accommodation of aminoglycosides such as neomycin and paromomycin. It is tempting to speculate that loss of the eB12 in mutant ribosomes changes the conformation of the G1645 and A1754 nucleotides, allowing binding of the aminoglycosides, and thus causing the observed increased sensitivity to neomycin and paromomycin. Additionally, the absence of the eB12 intersubunit bridge may result in conformational changes affecting the structure of the A-site, perhaps leading to increased affinity for aa-tRNA. In support of this, a correlation between increased affinity for aa-tRNA at the A-site and decrease in translational accuracy has been previously demonstrated37. Yeast ribosomes lacking eL39 displayed increased affinity for A-site tRNA and showed a 4-fold increase in the error frequency compared to the wild type ribosomes37. Paromomycin also induces translational misreading errors in yeast. In yeast cell free extracts the misreading of poly(U) was stimulated38; 39. Furthermore, the phenotypic suppression of both nonsense and missense mutations was induced38; 39. Both mechanisms lead to the translational misreading and this cumulative effect may cause the sensitivity to aminoglycosides. Similarly, the resistance to anisomycin can result from conformational changes in the ribosome. Anisomycin competes with the amino acid side chains of aa-tRNAs for binding to the A-site cleft of the large subunit21. It is possible that the absence of eB12 bridge may affect the structure of the A-site to favour aa-tRNA binding over the antibiotic.
Interestingly, cells containing mutant ribosomes that are not able to form the eB12 bridge exhibited higher tolerance to hyperosmotic stress when compared with wild type cells. This observation is suggestive of a phenomenon called cross-protection, in which adaptation to one form of stress often helps cells to survive other stress conditions. It is possible that compromise of ribosome function due to lack of the eB12 bridge leads to activation of general stress response. This activation may provide protection against various environmental stress conditions in the eB12 intersubunit bridge mutants.
Little is known about the functional importance of the eukaryote-specific intersubunit bridges. All five of these bridges are composed of protein-rRNA and protein-protein type of contacts5. The eB8 intersubunit bridge is formed by contacts between eS1 and 25S rRNA ES31L and is located proximal to the mRNA exit tunnel. The eB11 bridge is mainly formed by interactions between eS8 and 25S rRNA ES41L. The eB14 bridge is the only eukaryote-specific intersubunit bridge that is located in the centre of the ribosome. This bridge is formed by eL41 and 18S rRNA helix 44. The 25 amino acid long eL41 is highly positively charged and forms a single α-helix. Interestingly, eL41 is also a nonessential ribosomal protein, indicating that eB14 is dispensable for viability40. However, its deletion results in changes in peptidyltransferase activity and translational fidelity41; 42.
Structural studies of the human 80S ribosome describe large conformational changes in the eB12 and eB13 bridges during the transition from pre- to post-translocation states43; 44. In the case of eB12, the C-terminal helix of eL19 undergoes a structural transition from a straight (post-translocation state) to a bent (pre-translocation state) protein helix. Similarly, the C-terminal region of eL24 is relocalised and interacts with an entirely different region of the 18S rRNA. Given that eB12 is not essential for viability and that yeast cells survive loss of eL24 and consequently the eB13 bridge9; 11, it is possible that the dynamic nature of these bridges is not required for regulation of the information flow between subunits as previously hypothesised5; 45; 46, but rather that may be required for additional stabilisation during 40S subunit rotation. However, analysis of 80S ribosome formation in vitro revealed that 60S subunits devoid of the eL19 C-terminal region which is required for eB12 formation are strongly defective in subunit reassociation in the absence of additional factors. These results lead us to propose that tRNA molecules associated with 80S ribosomes may function as structural elements, helping to stabilise the dynamic subunit interactions in translating ribosomes. We speculate that stabilisation of ribosome subunit association by tRNA helps to ensure translational processivity.
MATERIALS AND METHODS
Yeast strains and media
All S. cerevisiae strains used in this study were isogenic derivatives of strain S288C47 and are listed in Supplemental Table S1.
Cells were grown in either YPD (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose), YPGal (1% Bacto yeast extract, 2% Bacto peptone, 2% galactose) or synthetic complete medium (0.67% Bacto yeast nitrogen base without amino acids, 2% glucose or 2% galactose) supplemented with the appropriate amino acids and bases48. Agar (2%) was added to solid media. When required, the following concentrations of antibiotics were used: 200 μg/ml geneticin, 3 mg/ml paromomycin, 5 mg/ml neomycin, 0.15 μg/ml cycloheximide or 20 μg/ml anisomycin.
Strains TYSC207 and TYSC227 were generated by one-step PCR-based gene disruption49 of the RPL19A and RPL19B genes, respectively. Deletion cassettes containing kanMX4 were PCR amplified from the genomic DNA isolated from respective EUROSCARF deletion mutants8 and transformed into diploid TYSC184 followed by sporulation and tetrad dissection. Strain TYSC237 was constructed by crossing TYSC207 and TYSC227. The resulting diploid was transformed with pRS316-RPL19 plasmid, sporulated and tetrads were dissected. Strains TYSC278, TYSC280, TYSC282, and TYSC283 were constructed using plasmid shuffling on glucose containing SC-Leu plates supplemented with 1 g/l 5-floroorotic acid (5FOA) at 30°C after TYSC237 transformation with plasmids pRS315-RPL19, pRS315-rpl191–183, pRS315-rpl191–154, or pRS315-rpl191–146, respectively. Strain TYSC291 was constructed using plasmid shuffling on galactose containing SC-Leu plates with 5FOA after TYSC237 transformation with plasmid pGAL-RPL19.
Plasmids
Plasmids used in this study are listed in Supplemental Table S2. The RPL19A coding region with its 5′ upstream (799 bp) and 3′ downstream (682 bp) regions was amplified with PCR from genomic DNA and cloned between BamHI and Cfr42I restriction sites into the pRS316 or pRS315 vector47. The resulting plasmids were named pRS316-RPL19 and pRS315-RPL19, respectively. To generate pRS315-rpl191–183, pRS315-rpl191–154, pRS315-rpl191–146 and pRS315-rpl191–133 the site-directed mutagenesis was performed by overlap-extension PCR to delete C-terminal coding region for 6, 35, 43 and 56 amino acid residues, respectively. For construction pGAL-RPL19, the RPL19A coding region with its 3′ downstream (235 bp) region was amplified with PCR and cloned between BamHI and SalI restriction sites into the pRS415-GAL1 vector50. All cloned DNA fragments generated by PCR amplification were verified by sequencing.
Temperature and antibiotic sensitivity/resistance assays
Yeast strains were grown in YPD or YPGal media at 30°C to mid-exponential phase. Cultures were serially diluted, spotted on YPD or YPGal plates and incubated at 16°C, 20°C, 25°C, 30°C and 36°C for 2–6 days. To analyse the sensitivity/resistance to antibiotics the serially diluted cultures were spotted on YPD plates supplemented with appropriate antibiotics and incubated at 25°C and 30°C for 3–5 days.
Salt stress assay
Triplicate samples of yeast cultures grown overnight in YPD at 30°C were diluted into fresh medium and were grown to early exponential phase (OD600 of 0.4). Cells were exposed to 0.7 M NaCl stress for 2 hours at 30°C. To determine colony-forming units the cells were plated on YPD agar plates and incubated at 30°C for 3 days. Viability was expressed as percentage of cells forming colonies after salt stress relative to the number of colonies in the untreated samples. The average and standard errors were calculated from three independent experiments. Statistical significance was evaluated with the unpaired t test.
In vivo depletion of eL19
The TYSC291 strain was grown in YPGal medium at 30°C until mid-exponential phase. Cells were washed and transferred to YPD medium. At different time points, samples were collected to perform ribosome-polysome profile analysis and RNA extraction.
Ribosome-polysome profile analysis
Ribosome-polysome profile analysis was performed as described previously51; 52 with modifications. Briefly, 100 ml of yeast cells was grown in YPD at 30°C or 20°C to mid-exponential phase. Cycloheximide was added at a final concentration of 100 μg/ml 15 min before the cells were harvested and washed with ice-cold breaking buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 30 mM MgCl2) supplemented with cycloheximide. Cells were disrupted by the glass beads in Precellys 24 homogenizer (Bertin Technologies) (program: 6000 rpm, 4°C, 3×1 min, pause 1 min). Obtained extract was centrifuged at 16060xg for 15 min at 4°C and further clarified using 20 μm pore size filter (Masherey Nagel). Thirty A260 units of extract was layered onto 7%–47% sucrose gradient containing 50 mM Tris-HCl (pH 7.5), 50 mM NH4Cl, 12 mM MgCl2, 1mM dithiothreitol and centrifuged at 4°C in SW28 rotor (Beckmann) at ω2t=1.38×1011. Gradients were monitored at 260 nm.
RNA extraction and primer extension analysis
RNA was extracted from samples corresponding to 10 OD600 units of exponentially grown cells by SDS-acidic phenol-chloroform treatment53. Primer extension reactions were performed as described previously54 using primers (Probe F: 5′-GGCCAGCAATTTCAAGTTA-3′ and probe G: 5′-GAACATTGTTCGCCTAGA-3′ from55) complementary to a region immediately upstream and downstream of the C2 cleavage site in ITS2 (Fig. 3C).
Quantitative PCR analysis
Total RNA was isolated by SDS-acidic phenol-chloroform treatment53 from triplicate cultures (15 ml) grown overnight in YPD at 30°C, diluted into fresh medium and grown to exponential phase (OD600 of 0.8). DNase I treatment, cDNA synthesis and quantitative PCR were performed as described earlier56. Oligonucleotide primers used are listed in Supplemental Table S3. For normalisation of mRNA levels, the geometric mean of two housekeeping genes (ARP6 and UBC6) was used. The average and standard deviations were calculated from two independent experiments.
Preparation of salt-washed ribosomal subunits
400 ml of yeast cells was grown in YPD at 30°C until mid-exponential phase. Cells were harvested, washed with ice-cold buffer A (30 mM Hepes-KOH [pH 7.5], 100 mM KCl, 10 mM Mg(OAc)2, 2 mM DTT, 0.5 mM EDTA), and disrupted by the glass beads in Precellys 24 homogenizer. The extract was centrifuged at 16060xg for 15 min at 4°C and filtered using 20 μm pore size filter (Masherey Nagel). One hundred and fifty A260 units of extract was layered onto 10%–30% sucrose gradient in buffer A and centrifuged at 4°C in SW28 rotor at ω2t=2.4×1011. Gradients were monitored at 260 nm. 80S ribosomes were fractionated and centrifuged at 4°C in Ti50 rotor (Beckmann) at ω2t=1.0×1012. The pellet was resuspended in buffer B (30 mM Hepes-KOH [pH 7.5], 500 mM KCl, 5 mM Mg(OAc)2, 2 mM DTT, 0.5 mM EDTA) and dialysed for 1 hour against buffer B. Forty A260 units of sample was layered onto 10%–25% sucrose gradient in buffer B and centrifuged at 4°C in SW28 rotor at ω2t=2.8×1011. Dissociated 60S and 40S subunits were fractionated and concentrated with 100 kDa Centricon Plus-70 filter (Millipore) in buffer C (30 mM Hepes-KOH [pH 7.5], 100 mM KCl, 5 mM Mg(OAc)2, 2 mM DTT, 0.5 mM EDTA). Obtained salt-washed ribosomal subunits were stored at −80°C.
In vitro reassociation of 80S
Two A260 units of wild type 40S was mixed with two A260 units of 60S subunits containing wild type or mutant eL19 in 300 μl of buffer R (30 mM Hepes-KOH [pH 7.5], 100 mM KCl, 1 mM DTT) and incubated for 20 min at 30°C. The assays with five different Mg-acetate concentrations (R buffer with 4.5 mM, 10 mM, 15 mM, 20 mM (with and without 0.08 mg of total E. coli deacylated tRNA) and 25 mM Mg-acetate) were performed. Samples were diluted to the final volume of 800 μl with ice-cold buffer R, layered onto 10%–30% sucrose gradient with corresponded Mg-acetate concentration and centrifuged at 4°C in SW28 rotor at ω2t=2.4×1011. Gradients were monitored at 260 nm.
SHAPE structural analysis
1-methyl-7-nitroisatoic anhydride (1M7) was synthesized according to the method described by Turner and co-authors57. Empty puromycin treated salt-washed ribosomes were prepared as described previously58. The ribosomes were treated with 1M7 at 30°C for 20 min59; 60. Ribosomes were precipitated, pelleted and RNA was isolated by SDS-acidic phenol-chloroform treatment53. Primer extension reactions were performed as described previously61. Oligonucleotide primers used for the primer extension analysis are listed in Supplemental Table S4. All assays were performed with two independent biological replicates. SHAPE reactivities were quantified using Image Quant TL v2005 software (GE Healthcare). Reactivities for the analysed nucleotides were normalized by dividing by the average reactivity of all nucleotides. Absolute reactivities were calculated by subtracting the non-reagent intensities from the 1M7-modified intensities. The reactivities that were less than zero were reset to zero. Representative results of SHAPE analysis are shown in Figure S2.
Supplementary Material
HIGHLIGHTS.
Seventeen bridges are formed during association of eukaryotic ribosome subunits
The main component of the eukaryote-specific eB12 bridge is C-terminal α-helix of eL19
The globular domain and middle region of eL19 are essential during 60S assembly
Ribosomes lacking eB12 can support growth and are active in translation
eB12 has a role in subunit joining and in optimizing ribosome functionality
Acknowledgments
We thank Dr. Arturas Meskauskas and Anastassija Andrijako for their involvement in the initial stages of this study. We also thank all members of the Chair of Molecular Biology, especially Dr. Aivar Liiv and Dr. Margus Leppik, for their support and for helpful discussions and Silva Lilleorg for critical reading of the manuscript. This work was supported by grants from the Estonian Ministry of Education and Research to T.T. (Estonian Research Foundation Grant Nr. 9210), to J.R. (Estonian Research Foundation Grant Nr. 9289, Institutional Research Funding Project No. IUT20-21) and to U.M. (Institutional Research Funding Project No. IUT20-17), and from the National Institutes of Health to J.D.D. (R01 GM117177 and R01 HL119439).
ABBREVIATIONS
- 1M7
1-methyl-7-nitroisatoic anhydride
- eB12
eukaryote-specific ribosome intersubunit bridge 12
- eL19
eukaryotic ribosomal large subunit protein 19
- ES6S
eukaryote-specific rRNA expansion segment 6
- eS7
eukaryotic ribosomal small subunit protein 7
- ITS2
internal transcribed spacer region 2
- uS17
universal ribosomal small subunit protein 17
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horan LH, Noller HF. Intersubunit movement is required for ribosomal translocation. Proc Natl Acad Sci USA. 2007;104:4881–5. doi: 10.1073/pnas.0700762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulk A, Cate JH. Control of ribosomal subunit rotation by elongation factor G. Science. 2013;340:1235970. doi: 10.1126/science.1235970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Lancaster L, Donohue JP, Noller HF. Crystal structures of EF-G-ribosome complexes trapped in intermediate states of translocation. Science. 2013;340:1236086. doi: 10.1126/science.1236086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tourigny DS, Fernandez IS, Kelley AC, Ramakrishnan V. Elongation factor G bound to the ribosome in an intermediate state of translocation. Science. 2013;340:1235490. doi: 10.1126/science.1235490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. The structure of the eukaryotic ribosome at 3.0 A resolution. Science. 2011;334:1524–9. doi: 10.1126/science.1212642. [DOI] [PubMed] [Google Scholar]
- 6.Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G, Frank J. Structure of the 80S ribosome from Saccharomyces cerevisiae--tRNA-ribosome and subunit-subunit interactions. Cell. 2001;107:373–86. doi: 10.1016/s0092-8674(01)00539-6. [DOI] [PubMed] [Google Scholar]
- 7.Song JM, Cheung E, Rabinowitz JC. Organization and characterization of the two yeast ribosomal protein YL19 genes. Curr Genet. 1996;30:273–8. doi: 10.1007/s002940050132. [DOI] [PubMed] [Google Scholar]
- 8.Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian KD, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Guldener U, Hegemann JH, Hempel S, Herman Z, Jaramillo DF, Kelly DE, Kelly SL, Kotter P, LaBonte D, Lamb DC, Lan N, Liang H, Liao H, Liu L, Luo C, Lussier M, Mao R, Menard P, Ooi SL, Revuelta JL, Roberts CJ, Rose M, Ross-Macdonald P, Scherens B, Schimmack G, Shafer B, Shoemaker DD, Sookhai-Mahadeo S, Storms RK, Strathern JN, Valle G, Voet M, Volckaert G, Wang CY, Ward TR, Wilhelmy J, Winzeler EA, Yang Y, Yen G, Youngman E, Yu K, Bussey H, Boeke JD, Snyder M, Philippsen P, Davis RW, Johnston M. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–91. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 9.Steffen KK, McCormick MA, Pham KM, MacKay VL, Delaney JR, Murakami CJ, Kaeberlein M, Kennedy BK. Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics. 2012;191:107–18. doi: 10.1534/genetics.111.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecompte O, Ripp R, Thierry JC, Moras D, Poch O. Comparative analysis of ribosomal proteins in complete genomes: an example of reductive evolution at the domain scale. Nucleic Acids Res. 2002;30:5382–90. doi: 10.1093/nar/gkf693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baronas-Lowell DM, Warner JR. Ribosomal protein L30 is dispensable in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:5235–43. doi: 10.1128/mcb.10.10.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armache JP, Anger AM, Marquez V, Franckenberg S, Frohlich T, Villa E, Berninghausen O, Thomm M, Arnold GJ, Beckmann R, Wilson DN. Promiscuous behaviour of archaeal ribosomal proteins: implications for eukaryotic ribosome evolution. Nucleic Acids Res. 2013;41:1284–93. doi: 10.1093/nar/gks1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poll G, Braun T, Jakovljevic J, Neueder A, Jakob S, Woolford JL, Jr, Tschochner H, Milkereit P. rRNA maturation in yeast cells depleted of large ribosomal subunit proteins. PLoS One. 2009;4:e8249. doi: 10.1371/journal.pone.0008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sulima SO, Gulay SP, Anjos M, Patchett S, Meskauskas A, Johnson AW, Dinman JD. Eukaryotic rpL10 drives ribosomal rotation. Nucleic Acids Res. 2014;42:2049–63. doi: 10.1093/nar/gkt1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–33. doi: 10.1038/nature09206. [DOI] [PubMed] [Google Scholar]
- 16.Carter AP, Clemons WM, Brodersen DE, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature. 2000;407:340–8. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 17.Tuite MF, McLaughlin CS. The effects of paromomycin on the fidelity of translation in a yeast cell-free system. Biochim Biophys Acta. 1984;783:166–70. doi: 10.1016/0167-4781(84)90009-5. [DOI] [PubMed] [Google Scholar]
- 18.Garreau de Loubresse N, Prokhorova I, Holtkamp W, Rodnina MV, Yusupova G, Yusupov M. Structural basis for the inhibition of the eukaryotic ribosome. Nature. 2014;513:517–22. doi: 10.1038/nature13737. [DOI] [PubMed] [Google Scholar]
- 19.Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6:209–217. doi: 10.1038/nchembio.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Fonseca C, Amils R, Garrett RA. Fine structure of the peptidyl transferase centre on 23 S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J Mol Biol. 1995;247:224–35. doi: 10.1006/jmbi.1994.0135. [DOI] [PubMed] [Google Scholar]
- 21.Rakauskaite R, Dinman JD. rRNA mutants in the yeast peptidyltransferase center reveal allosteric information networks and mechanisms of drug resistance. Nucleic Acids Res. 2008;36:1497–507. doi: 10.1093/nar/gkm1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–57. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varela JC, Praekelt UM, Meacock PA, Planta RJ, Mager WH. The Saccharomyces cerevisiae HSP12 gene is activated by the high-osmolarity glycerol pathway and negatively regulated by protein kinase A. Mol Cell Biol. 1995;15:6232–45. doi: 10.1128/mcb.15.11.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchler G, Schuller C, Adam G, Ruis H. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 1993;12:1997–2003. doi: 10.1002/j.1460-2075.1993.tb05849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuller C, Brewster JL, Alexander MR, Gustin MC, Ruis H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994;13:4382–9. doi: 10.1002/j.1460-2075.1994.tb06758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone RL, Matarese V, Magee BB, Magee PT, Bernlohr DA. Cloning, sequencing and chromosomal assignment of a gene from Saccharomyces cerevisiae which is negatively regulated by glucose and positively by lipids. Gene. 1990;96:171–6. doi: 10.1016/0378-1119(90)90249-q. [DOI] [PubMed] [Google Scholar]
- 27.Jamieson DJ, Rivers SL, Stephen DW. Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology. 1994;140( Pt 12):3277–83. doi: 10.1099/13500872-140-12-3277. [DOI] [PubMed] [Google Scholar]
- 28.Piper PW, Talreja K, Panaretou B, Moradas-Ferreira P, Byrne K, Praekelt UM, Meacock P, Recnacq M, Boucherie H. Induction of major heat-shock proteins of Saccharomyces cerevisiae, including plasma membrane Hsp30, by ethanol levels above a critical threshold. Microbiology. 1994;140( Pt 11):3031–8. doi: 10.1099/13500872-140-11-3031. [DOI] [PubMed] [Google Scholar]
- 29.Kandror O, Bretschneider N, Kreydin E, Cavalieri D, Goldberg AL. Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Mol Cell. 2004;13:771–81. doi: 10.1016/s1097-2765(04)00148-0. [DOI] [PubMed] [Google Scholar]
- 30.Hartig A, Ruis H. Nucleotide sequence of the Saccharomyces cerevisiae CTT1 gene and deduced amino-acid sequence of yeast catalase T. Eur J Biochem. 1986;160:487–90. doi: 10.1111/j.1432-1033.1986.tb10065.x. [DOI] [PubMed] [Google Scholar]
- 31.Herrero E, Ros J, Belli G, Cabiscol E. Redox control and oxidative stress in yeast cells. Biochim Biophys Acta. 2008;1780:1217–35. doi: 10.1016/j.bbagen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Pan T. Adaptive translation as a mechanism of stress response and adaptation. Annu Rev Genet. 2013;47:121–37. doi: 10.1146/annurev-genet-111212-133522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javid B, Sorrentino F, Toosky M, Zheng W, Pinkham JT, Jain N, Pan M, Deighan P, Rubin EJ. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc Natl Acad Sci USA. 2014;111:1132–7. doi: 10.1073/pnas.1317580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y, Wu J, Ung MH, De Lay N, Cheng C, Ling J. Protein mistranslation protects bacteria against oxidative stress. Nucleic Acids Res. 2015;43:1740–8. doi: 10.1093/nar/gku1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan-Minogue H, Bedwell DM. Eukaryotic ribosomal RNA determinants of aminoglycoside resistance and their role in translational fidelity. RNA. 2008;14:148–57. doi: 10.1261/rna.805208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobbie SN, Kalapala SK, Akshay S, Bruell C, Schmidt S, Dabow S, Vasella A, Sander P, Bottger EC. Engineering the rRNA decoding site of eukaryotic cytosolic ribosomes in bacteria. Nucleic Acids Res. 2007;35:6086–93. doi: 10.1093/nar/gkm658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dresios J, Derkatch IL, Liebman SW, Synetos D. Yeast ribosomal protein L24 affects the kinetics of protein synthesis and ribosomal protein L39 improves translational accuracy, while mutants lacking both remain viable. Biochemistry. 2000;39:7236–44. doi: 10.1021/bi9925266. [DOI] [PubMed] [Google Scholar]
- 38.Palmer E, Wilhelm JM, Sherman F. Phenotypic suppression of nonsense mutants in yeast by aminoglycoside antibiotics. Nature. 1979;277:148–50. doi: 10.1038/277148a0. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Ursic D, Davies J. Phenotypic suppression and misreading Saccharomyces cerevisiae. Nature. 1979;277:146–8. doi: 10.1038/277146a0. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Warner JR. Expression of a micro-protein. J Biol Chem. 2001;276:33821–5. doi: 10.1074/jbc.M103772200. [DOI] [PubMed] [Google Scholar]
- 41.Dresios J, Panopoulos P, Suzuki K, Synetos D. A dispensable yeast ribosomal protein optimizes peptidyltransferase activity and affects translocation. J Biol Chem. 2003;278:3314–22. doi: 10.1074/jbc.M207533200. [DOI] [PubMed] [Google Scholar]
- 42.Meskauskas A, Harger JW, Jacobs KL, Dinman JD. Decreased peptidyltransferase activity correlates with increased programmed -1 ribosomal frameshifting and viral maintenance defects in the yeast Saccharomyces cerevisiae. RNA. 2003;9:982–92. doi: 10.1261/rna.2165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khatter H, Myasnikov AG, Natchiar SK, Klaholz BP. Structure of the human 80S ribosome. Nature. 2015;520:640–5. doi: 10.1038/nature14427. [DOI] [PubMed] [Google Scholar]
- 44.Behrmann E, Loerke J, Budkevich TV, Yamamoto K, Schmidt A, Penczek PA, Vos MR, Burger J, Mielke T, Scheerer P, Spahn CM. Structural snapshots of actively translating human ribosomes. Cell. 2015;161:845–57. doi: 10.1016/j.cell.2015.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao H, Sengupta J, Valle M, Korostelev A, Eswar N, Stagg SM, Van Roey P, Agrawal RK, Harvey SC, Sali A, Chapman MS, Frank J. Study of the structural dynamics of the E coli 70S ribosome using real-space refinement. Cell. 2003;113:789–801. doi: 10.1016/s0092-8674(03)00427-6. [DOI] [PubMed] [Google Scholar]
- 46.Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292:883–96. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 47.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- 49.Janke C, Magiera MM, Rathfelder N, Taxis C, Reber S, Maekawa H, Moreno-Borchart A, Doenges G, Schwob E, Schiebel E, Knop M. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947–62. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 50.Mumberg D, Muller R, Funk M. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 1994;22:5767–8. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foiani M, Cigan AM, Paddon CJ, Harashima S, Hinnebusch AG. GCD2, a translational repressor of the GCN4 gene, has a general function in the initiation of protein synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3203–16. doi: 10.1128/mcb.11.6.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piir K, Tamm T, Kisly I, Tammsalu T, Remme J. Stepwise splitting of ribosomal proteins from yeast ribosomes by LiCl. PLoS One. 2014;9:e101561. doi: 10.1371/journal.pone.0101561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mai B, Breeden L. Xbp1, a stress-induced transcriptional repressor of the Saccharomyces cerevisiae Swi4/Mbp1 family. Mol Cell Biol. 1997;17:6491–501. doi: 10.1128/mcb.17.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venema J, Planta RJ, Raue HA. In vivo mutational analysis of ribosomal RNA in Saccharomyces cerevisiae. Methods Mol Biol. 1998;77:257–70. doi: 10.1385/0-89603-397-X:257. [DOI] [PubMed] [Google Scholar]
- 55.Babiano R, Gamalinda M, Woolford JL, Jr, de la Cruz J. Saccharomyces cerevisiae ribosomal protein L26 is not essential for ribosome assembly and function. Mol Cell Biol. 2012;32:3228–41. doi: 10.1128/MCB.00539-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aun A, Tamm T, Sedman J. Dysfunctional mitochondria modulate cAMP-PKA signaling and filamentous and invasive growth of Saccharomyces cerevisiae. Genetics. 2013;193:467–81. doi: 10.1534/genetics.112.147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner R, Shefer K, Ares M., Jr Safer one-pot synthesis of the ‘SHAPE’ reagent 1-methyl-7-nitroisatoic anhydride (1m7) RNA. 2013;19:1857–63. doi: 10.1261/rna.042374.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meskauskas A, Leshin JA, Dinman JD. Chromatographic purification of highly active yeast ribosomes. J Vis Exp. 2011:e3214. doi: 10.3791/3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhodin MH, Dinman JD. A flexible loop in yeast ribosomal protein L11 coordinates P-site tRNA binding. Nucleic Acids Res. 2010;38:8377–89. doi: 10.1093/nar/gkq711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhodin MH, Dinman JD. An extensive network of information flow through the B1b/c intersubunit bridge of the yeast ribosome. PLoS One. 2011;6:e20048. doi: 10.1371/journal.pone.0020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stern S, Moazed D, Noller HF. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–9. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.