Abstract
Background
Both respiratory and non-respiratory hospitalizations are common and costly events in older subjects with obstructive lung disease. Prevention of any hospitalization in these subjects is essential. We aimed to construct a prediction model for all-cause hospitalization risk in community-dwelling older subjects with obstructive lung disease.
Methods
We studied 268 community-dwelling subjects with obstructive lung disease (defined as FEV1/FVC<LLN) who participated in the observational Health, Aging and Body Composition Study and constructed a prediction model for 9-year all-cause hospitalization risk using a weighted linear combination based upon beta coefficients.
Results
There were 225 subjects with ≥1 hospitalizations and 43 subjects free from hospitalization during the follow-up. Heart and vascular disease (H), objectively measured lower extremity dysfunction (O), systemic inflammation (S), dyspnea (P), impaired renal function (I), and tobacco exposure (T) were independent predictors for all-cause hospitalization (ALL). These factors were combined into the HOSPITALL score (0–23 points), with an area under the curve in ROC analysis of 0.70 (p<0.001). The hazard ratio for all-cause hospitalization per one-point increase in the HOSPITALL score was 1.15 (95% confidence interval, 1.11–1.19, p=0.001). Increasing HOSPITALL score was further associated with shorter time to first admission, increased admission rate, and more respiratory admissions.
Conclusion
The HOSPITALL score is a multidimensional score to predict all-cause hospitalization risk in community-dwelling older subjects with obstructive lung disease, that may aid in patient counseling and prevention to reduce burden and health care costs.
Keywords: COPD, Pulmonary disease, Older age persons, Cox Proportional Hazards Modeling
Introduction
Chronic obstructive pulmonary disease (COPD) in particular is common in aged persons, and is associated with significant functional limitations1,2 and high health care costs.3 During the course of their disease, patients with COPD are frequently hospitalized not only for exacerbations or pneumonia,4,5 but also for a wide range of non-respiratory causes.6,7 All-cause hospitalization rates in COPD range from approximately 0.4 to 0.7 admissions per person per year, depending on the population studied.6–10 Whereas hospital stays drive direct costs of COPD-related care,11 hospitalizations for non-respiratory reasons constitute the greatest expense in patients with COPD.3 Moreover, 72% of the 30-day readmissions after exacerbated COPD are primarily for non-respiratory problems.12
Irrespective of the primary reason for admission, hospitalization may trigger a progressive physical decline,13,14 which has recently also been shown in patients with COPD.10 Patients with COPD hospitalized for respiratory and non-respiratory causes experienced an equal rate of accelerated decline in six minute walking distance after discharge compared to non-hospitalized patients.10 Furthermore, all-cause hospitalization in COPD is associated with high mortality.15 Preventing any hospitalization in patients with COPD is therefore crucial. Although several studies have suggested that the physical activity level and exercise capacity may be predictors of all-cause hospitalization in patients with COPD,10,16,17 a thorough investigation of potential risk factors has not been undertaken. Moreover, these were studies conducted in secondary and tertiary care center populations, whereas strategies to prevent hospitalization should ideally commence in the pre-clinical setting.
In the current study, we analyzed baseline and 9-year follow-up data from community-dwelling older subjects with obstructive lung disease participating in the observational Health, Aging and Body Composition (ABC) Study to identify risk factors for all-cause hospitalization. We subsequently constructed a risk prediction model that may aid in patient counseling in the pre-clinical setting. The Health ABC Study cohort was selected as it provides a rich characterization of community-dwelling older subjects with long follow-up data available.
Methods
A detailed methodology can be found online in the supplemental material.
Study population
This study was performed using data from the Health ABC Study which is a longitudinal observational study of 3075 community-dwelling black and white men and women, 70 to79 years of age, residing in Pittsburgh, Pennsylvania and Memphis, Tennessee. Participants were included if they reported no difficulty walking a quarter mile, climbing 10 steps without resting, or performing mobility-related activities of daily living. The Health ABC Study protocol was approved by the Institutional Review Boards of the clinical sites. All participants gave written informed consent.
For the current analyses we used baseline data, obtained in 1997/1998 through in-person interview and clinic based examination, and 9-year follow-up hospitalization data. We analyzed the subjects (n=268) who met the criterion for obstructive lung disease (reduced [i.e. < lower limit of normal, LLN] forced expiratory volume in 1s [FEV1]/forced vital capacity [FVC] as determined by age, sex, and race-normalized values)18 at baseline. Pre-bronchodilator lung function was assessed according to international standards.19
Hospitalizations and survival
Subjects were asked to report any hospitalizations and every 6 months they were asked directed questions about interim events. When an event was reported, medical records were collected (admission, discharge dates, and primary reason for hospitalization (e-Table 1)).
Table 1.
Baseline characteristics of the 268 subjects with obstructive lung disease
| Total (n=268) | Subjects with ≥1 hospitalizations (n=225) | Subjects free from hospitalization (n=43) | p-value | |
|---|---|---|---|---|
| Age, y | 73.2 ± 2.9 | 73.2 ± 2.8 | 73.0 ± 3.1 | 0.580 |
| Gender, %male | 57.5 | 58.2 | 53.5 | 0.565 |
| Race, %white | 55.6 | 55.1 | 58.1 | 0.714 |
| Site, %Memphis | 51.1 | 49.8 | 58.1 | 0.315 |
| Previous all-cause hospitalization, % | 13.1 | 13.8 | 9.3 | 0.419 |
| FEV1, %pred* | 63 ± 18 | 62 ± 18 | 67 ± 20 | 0.121 |
| Dyspnea | ||||
| None, % | 50.6 | 48.6 | 60.5 | 0.245 |
| Mild, % | 39.2 | 40.0 | 34.9 | |
| Moderate, % | 10.3 | 11.4 | 4.7 | |
| Physical activity, kcal/kg/wk† | 65 (36–100) | 62 (35–95) | 77 (44–106) | 0.068 |
| Inactive | 27.6 | 30.2 | 14.0 | 0.085 |
| Lifestyle active | 56.3 | 54.7 | 65.1 | |
| Exercisers | 16.0 | 15.1 | 20.9 | |
| Tobacco exposure | ||||
| Ever, % | 82.8 | 85.8 | 67.4 | 0.003 |
| Never, % | 17.2 | 14.2 | 32.6 | |
| Quadriceps strength, Nm | 103 ± 40 | 102 ± 40 | 107 ± 39 | 0.466 |
| Grip strength, kg | 64 ± 22 | 63 ± 21 | 66 ± 23 | 0.421 |
| SPPB, %<10 | 31.3 | 33.8 | 21.4 | 0.115 |
| BMI, kg/m2 | 25.4 ± 4.7 | 25.3 ± 4.7 | 26.0 ± 4.8 | 0.379 |
| FFMI, kg/m2 | 17.0 ± 2.5 | 17.0 ± 2.5 | 17.1 ± 2.4 | 0.666 |
| CRP, μg/ml† | 2.06 (1.16–3.64) | 2.16 (1.24–3.78) | 1.17 (0.79–2.39) | <0.001 |
| Heart and vascular disease, % | 27.2 | 30.5 | 14.3 | 0.032 |
| Chronic kidney disease, % | 20.1 | 22.6 | 9.5 | 0.054 |
| Diabetes, % | 11.6 | 12.5 | 7.0 | 0.300 |
| Cancer, % | 17.2 | 15.1 | 27.9 | 0.041 |
| Cognitive impairment, % | 13.1 | 12.9 | 14.3 | 0.806 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; FEV1, forced expiratory volume in 1 second; FFMI, fat free mass index; FMI, fat mass index; IL-6, interleukin-6; SPPB, short physical performance battery; TNF-α, tumor necrosis factor-α.
Data are mean (SD) unless indicated otherwise.
From reference equations40
Median (IQR).
Covariates at baseline
As previously reported,18,20 clinic site, gender, race (black/white), age, tobacco exposure, dyspnea, body mass index (BMI), fat free mass index (FFMI), daily physical activity, knee extensor strength, hand grip strength, Short Physical Performance Battery (SPPB), and plasma C-reactive protein (CRP) were determined following standardized methodology. Comorbid heart and vascular disease (coronary heart disease, cerebrovascular disease, and congestive heart failure), chronic kidney disease (CKD),21 diabetes, cancer, and cognitive impairment22 were recorded. Furthermore, all-cause hospitalizations in the year prior to inclusion were reported by Centers for Medicare and Medicaid Sevices.
Statistical analysis
Baseline differences between subjects with ≥1 hospitalizations and subjects free from hospitalizations during the follow-up were tested using Student t-test for continuous variables, χ2 test for categorical variables and Kruskal-Wallis test for continuous variables with skewed distributions. Univariate Cox proportional hazards models using bootstrap estimation (1000 replications; resampling with replacement) were performed to identify the association of candidate variables with all-cause hospitalization. All covariates with a p-value ≤0.10 were considered for inclusion in a multivariable model with a backward elimination approach using bootstrap estimation again allowing variables with a p-value ≤0.10 to be retained in the model. FEV1, physical activity, BMI and CRP were modeled in categories.23–25 The bias-corrected beta coefficients from the final multivariate model were subsequently standardized and rounded to the closest integer in order to assign weighted scores to each of the remaining variables.26 Summation of these scores led to the final risk score. Receiver-Operating Curve (ROC) analysis was performed for the newly developed risk score to estimate its sensitivity and specificity in classifying persons by means of the Cox model as either hospitalized or not. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 22 for Windows, SPSS Inc.).
Results
Of the 268 subjects, 225 (84%) had ≥1 hospitalizations and 43 (16%) were free from hospitalization during the 9-year follow-up, with a total of 1944 person years. In total, those with ≥1 hospitalizations were hospitalized 811 times for any cause. The majority of these hospitalizations were for non-respiratory reasons (72.3%). Subjects had a median number of 3 (interquartile range [IQR] 1–5) hospitalizations, corresponding with 0.44 (IQR 0.22–0.78) hospitalizations per year. The median length of stay per hospitalization was 5 (IQR 4–9) days, and the median time to the first all-cause hospitalization was 2.2 (IQR 1.0–4.4) years.
Compared to subjects free from hospitalization, those with ≥1 hospitalizations had a higher prevalence of heart and vascular disease, a lower prevalence of cancer (particularly driven by a lower prevalence of prostate cancer [data not shown]) and a tendency towards a higher prevalence of CKD (Table 1). The latter group also had higher CRP levels and were more often ever-smokers, while no differences were found in body composition or muscle strength.
Univariate Cox proportional hazards analysis indicated that all-cause hospitalization was significantly predicted by clinic site, previous all-cause hospitalization, heart and vascular disease, FEV1, dyspnea, CRP, daily physical activity level, SPPB, CKD, and tobacco exposure (Table 2).
Table 2.
Investigated covariates in 268 subjects with obstructive lung disease as predictors for 9-year all-cause hospitalization using univariate Cox proportional hazards regression analysis
| Variable | No. of hospitalizations | No. at risk | HR (95% CI) | p-value |
|---|---|---|---|---|
| Age, y | 225 | 268 | 1.03 (0.98–1.07) | 0.258 |
| Gender | ||||
| Female | 94 | 114 | 1.00 | |
| Male | 131 | 154 | 1.05 (0.81–1.37) | 0.708 |
| Race | ||||
| White | 124 | 149 | 1.00 | |
| Black | 101 | 119 | 1.19 (0.91–1.55) | 0.214 |
| Site | ||||
| Memphis | 112 | 137 | 1.00 | |
| Pittsburgh | 113 | 131 | 1.38 (1.06–1.79) | 0.012 |
| Previous all-cause hospitalization | ||||
| No | 193 | 232 | 1.00 | |
| Yes | 31 | 35 | 1.58 (1.08–2.31) | 0.048 |
| FEV1 %pred | ||||
| Mild | 38 | 47 | 1.00 | |
| Moderate | 125 | 149 | 1.29 (0.89–1.85) | 0.142 |
| Severe | 62 | 72 | 1.56 (1.04–2.34) | 0.037 |
| Dyspnea | ||||
| None | 107 | 133 | 1.00 | |
| Mild | 99 | 103 | 1.20 (0.90–1.59) | 0.238 |
| Moderate | 25 | 27 | 2.49 (1.60–3.86) | 0.004 |
| Physical activity | ||||
| Exercisers | 34 | 43 | 1.00 | |
| Lifestyle active | 123 | 151 | 1.02 (0.70–1.49) | 0.914 |
| Inactive | 68 | 74 | 1.63 (1.08–2.47) | 0.020 |
| Tobacco exposure | ||||
| Never | 32 | 46 | 1.00 | |
| Ever | 193 | 222 | 1.69 (1.16–2.47) | 0.019 |
| Quadriceps strength | 198 | 236 | 1.00 (0.99–1.00) | 0.154 |
| Grip strength | 223 | 265 | 1.00 (0.99–1.00) | 0.216 |
| SPPB | ||||
| ≥10 | 147 | 180 | 1.00 | |
| <10 | 75 | 84 | 1.78 (1.34–2.35) | 0.001 |
| BMI | ||||
| ≤20.0 kg/m2 | 21 | 24 | 1.21 (0.75–1.94) | 0.377 |
| 20.0–24.9 kg/m2 | 99 | 115 | 1.00 | |
| 25.0–29.9 kg/m2 | 67 | 85 | 0.90 (0.66–1.22) | 0.479 |
| ≥30.0 kg/m2 | 38 | 44 | 1.07 (0.74–1.55) | 0.704 |
| FFMI | 222 | 265 | 1.00 (0.95–1.06) | 0.904 |
| CRP | ||||
| <5.0 μg/ml | 186 | 224 | 1.00 | |
| ≥5.0 μg/ml | 35 | 38 | 1.70 (1.18–2.44) | 0.010 |
| Heart and vascular disease | ||||
| No | 153 | 189 | 1.00 | |
| Yes | 67 | 73 | 1.86 (1.39–2.48) | 0.002 |
| Chronic kidney disease | ||||
| No | 171 | 209 | 1.00 | |
| Yes | 50 | 54 | 1.49 (1.08–2.04) | 0.013 |
| Diabetes | ||||
| No | 196 | 236 | 1.00 | |
| Yes | 28 | 31 | 1.27 (0.85–1.88) | 0.263 |
| Cancer | ||||
| No | 191 | 222 | 1.00 | |
| Yes | 34 | 46 | 0.86 (0.59–1.23) | 0.441 |
| Cognitive impairment | ||||
| No | 196 | 232 | 1.00 | |
| Yes | 29 | 35 | 1.13 (0.76–1.67) | 0.546 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FFMI, fat free mass index; HR, hazard ratio; SPPB, short physical performance battery.
In the multivariate Cox regression model heart and vascular disease, SPPB, CRP, dyspnea, CKD, and tobacco exposure were retained as significant predictors (Table 3). These variables were subsequently combined into the HOSPITALL score (Heart and vascular disease [H], Objectively measured lower extremity dysfunction [O], Systemic inflammation [S], dysPnea [P], Impaired renal function [I], and Tobacco exposure [T] predict ALL-cause hospitalization [ALL]). Each subject received points based on the presence of heart and vascular disease, SPPB (<10 or ≥10), CRP (<5.0 or ≥5.0 μg/ml), dyspnea (none/mild or moderate), the presence of CKD, and tobacco exposure (ever- or never smokers) (Table 3). None or mild dyspnea as well as current and former smoking were taken together as their beta-coefficients were comparable (data not shown). The final HOSPITALL score for each subject was obtained by summing the points corresponding to each variable. HOSPITALL scores ranged from 0 to 23. As expected, subjects with ≥1 hospitalizations had higher HOSPITALL scores than those free from hospitalization (6.2±2.7 vs 4.4±2.1, p<0.001). Per one point increase of the HOSPITALL score the all-cause hospitalization risk increased by 15% (HR 1.15 [95% CI 1.11–1.19], p=0.001). The final model did not substantially change when additionally adjusting for clinic site (data not shown).
Table 3.
Final multivariable model as 9-year risk predictor for all-cause hospitalization: HOSPITALL score
| Covariate | Coefficient | Hazard Ratio (95% CI) | P-value | HOSPITALL points |
|---|---|---|---|---|
| Heart and vascular disease | ||||
| No | 1.00 | 0 | ||
| Yes | 0.44 | 1.55 (1.14–2.10) | 0.011 | 3 |
| Short Physical Performance Battery | ||||
| ≥10 | 1.00 | 0 | ||
| <10 | 0.55 | 1.73 (1.28–2.35) | 0.001 | 4 |
| CRP | ||||
| <5.0 μg/ml | 1.00 | 0 | ||
| ≥5.0 μg/ml | 0.37 | 1.45 (0.99–2.13) | 0.058 | 3 |
| Dyspnea | ||||
| None or mild | 1.00 | 0 | ||
| Moderate | 0.98 | 1.71 (1.71–4.17) | 0.001 | 7 |
| Chronic kidney disease | ||||
| No | 1.00 | 0 | ||
| Yes | 0.30 | 1.34 (0.96–1.88) | 0.070 | 2 |
| Tobacco exposure | ||||
| Never | 1.00 | 0 | ||
| Ever | 0.55 | 1.73 (1.16–2.58) | 0.018 | 4 |
Abbreviation: CRP, C-reactive protein.
The AUC of the ROC curve for the HOSPITALL score was 0.70 (95% CI 0.62–0.78, p<0.001). For comparison, we also analyzed the AUC’s for two previously validated mortality predictor scores in COPD and for FEV1 alone. In the same Health ABC Study cohort, Mehrotra et al. described the PILE index (a combination score of FEV1, IL-6, and knee extensor strength) and a modified version of the BODE index (mBODE, a combination score of BMI, FEV1, dyspnea, and time to complete 400 meter walking).20 The AUC’s for the PILE index, mBODE index and FEV1 to predict time to first all-cause hospitalization were 0.60 (95% CI 0.50–0.70, p=0.052), 0.58 (95% CI 0.49–0.67, p=0.105), and 0.44 (95% CI 0.35–0.53, p=0.216), respectively, indicating superiority of the HOSPITALL score in this population.
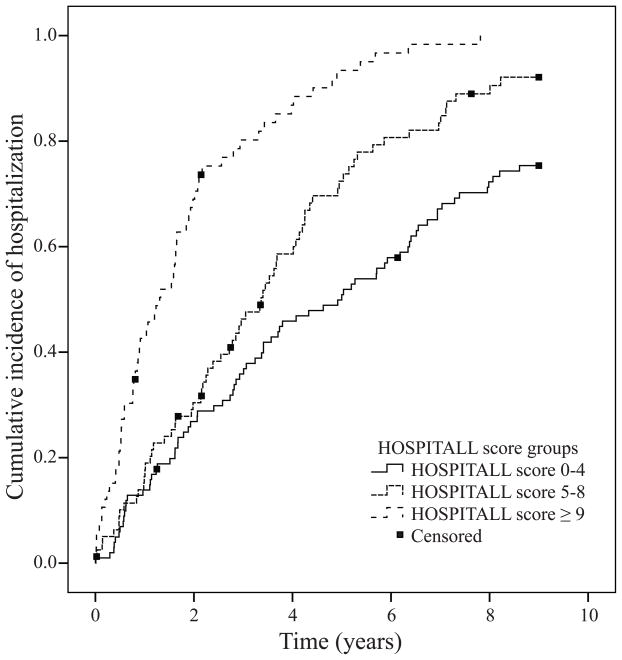
When we divided the subjects into three HOSPITALL score risk strata (low, average and high risk with HOSPITALL scores of 0–4, 5–8, and ≥9, respectively), we identified significant differences between these groups not only in their risk for all-cause hospitalization (Figure 1), but also in time to the first admission, hospitalization rate, and primary cause for hospitalization (Table 4).
Figure 1.
Kaplan Meier plot using HOSPITALL score groups as stratum (p < 0.001 by log-rank test).
Table 4.
Stratification of HOSPITALL scores
| HOSPITALL score groups | p-value | |||
|---|---|---|---|---|
| Low risk | Average risk | High risk | ||
| HOSPITALL score | 0–4 | 5–8 | ≥9 | |
| n | 101 | 80 | 66 | |
| Risk of all-cause hospitalization, HR (95%CI) | 0.60 (0.43–0.84)† | 1 | 2.36 (1.66–3.34)‡ | - |
| Time to first all-cause hospitalization, y (IQR)* | 3.06 (1.26–5.87) | 2.92 (1.16–4.38) | 1.27 (0.52–2.15) | <0.001 |
| All-cause hospitalizations per person per year, n (IQR)* | 0.11 (0.00–0.39) | 0.40 (0.13–0.73) | 0.66 (0.35–1.23) | <0.001 |
| Primary cause of first hospitalization | ||||
| Respiratory, n (%) | 8 (10.7) | 17 (24.6) | 21 (32.8) | 0.006 |
| Non-respiratory, n (%) | 67 (89.3) | 52 (75.4) | 43 (67.2) | |
Median (IQR)
p=0.002
p<0.001
Discussion
Preventing hospitalizations is key in the management of patients with obstructive lung disease. The alarming admission and readmission rates urge physicians to break and preferably prevent this vicious cycle of hospitalizations. In community-dwelling older subjects with obstructive lung disease participating in the Health ABC Study, we have constructed the HOSPITALL score to predict all-cause hospitalization. The score comprises heart and vascular disease, lower extremity dysfunction, systemic inflammation, dyspnea, CKD, and tobacco exposure. The score may aid in the guidance for risk reduction in a population of older obstructive lung disease patients in the pre-clinical setting. The HOSPITALL score was more discriminative in predicting all-cause hospitalization than existing multidimensional risk scores. While the HOSPITALL risk factors may not necessarily be disease-specific and may also predict hospitalization and associated poor outcomes in general populations, the score underlines the broad multidimensional scope needed in obstructive lung disease care.
Moderate dyspnea was the strongest predictor amongst the HOSPITALL risk factors. Mild dyspnea did not increase the risk above no dyspnea. The definition of moderate dyspnea as applied in the Health ABC Study is comparable to a modified Medical Research Council grade of 2.27 In our population the percentage of subjects with moderate dyspnea was relatively low with 10.3% as was to be expected given the community-dwelling nature of the population and that subjects needed to be well-functioning upon inclusion. Our results stress the need for documenting dyspnea severity in primary care obstructive lung disease subjects as it is a simple and sensitive measure that provides insight into the risk of a variety of poor outcomes including all-cause hospitalization.
Heart and vascular disease is the most prevalent comorbidity in COPD in the majority of studies, ranging from 28–70%.28,29 Furthermore, a recent meta-analysis showed a two to five times higher risk of major cardiovascular diseases in patients with COPD compared with a non-COPD population.30 Further stressing the clinical importance of comorbid heart and vascular disease in COPD, we found that it was a strong predictor in the HOSPITALL score. This suggests that preventing the development of heart and vascular disease in COPD may decrease the risk of hospitalization. Early identification of cardiovascular risk factors in COPD is therefore essential. Not only should we focus on common risk factors such as smoking and age, but metabolic syndrome status, systemic inflammation, adipose tissue distribution, and skeletal muscle oxidative capacity have been proposed as key mediators in COPD,31 that need further investigation in future studies. Also, interventions to modify cardiovascular risk in COPD are urgently warranted.
We found that objectively measured lower extremity dysfunction measured by the SPPB was a strong predictor for all-cause hospitalization. The SPPB is commonly used in older age populations but has recently also been shown to be a valid and simple assessment tool to measure functional impairment in COPD, independent of FEV1.32 SPPB scores <10 have been associated with disability in aged persons,33 with hyperinflation and with an increased proportion of type 2 quadriceps muscle fibers in COPD patients indicative of decreased oxidative capacity in skeletal muscle.32 Also, SPPB scores at discharge after hospitalization have been inversely correlated with the rate of decline in activity of daily living performance in older persons.34 Clinical use of the SPPB test in COPD patients warrants further investigation.
It is well established that persistent low-grade systemic inflammation is present in some patients with COPD but its origin is still unclear.35 Recent studies indicate that plasma levels of the clinical inflammatory marker CRP are at least partly influenced by adipose tissue mass.36,37 which is modifiable by lifestyle adaptations. In addition to previously reported associations between high CRP and low exercise capacity,38 respiratory hospitalizations and increased mortality,39 the current study also shows that high levels of CRP are predictive of all-cause hospitalization risk.
Although CKD is less common in COPD patients than heart and vascular disease, it has recently been shown that COPD patients with CKD had the highest incidence of all-cause ER visits which led to hospitalizations, and had the highest incidence of all-cause hospitalizations.28 In addition, that study also showed that all-cause total healthcare costs were highest in COPD patients with CKD compared with other comorbidities.28 Therefore, future studies need to increase the understanding of the relation between COPD and CKD. In our study, CKD was defined based on the CKD-Epidemiology Collaboration creatinine-cystatin C equation, which, in comparison to equations based on creatinine or cystatin C alone, has been shown to be more precisely and accurately in estimating the glomerular filtration rate (GFR) across the range of GFR.21
It is well-known that smoking is a major risk factor for developing COPD and many other chronic diseases, but smokers in this population still had an additional risk for all-cause hospitalization. This may be related to known effects of smoking on other organ systems and could reflect the influence of an overall unhealthy lifestyle.
Strengths and weaknesses
The unique design of the Health ABC Study enabled us to thoroughly investigate risk factors for all-cause hospitalization during a long follow-up of 9 years in community-dwelling older subjects with obstructive lung disease. An advantage of the long follow-up duration was that we could identify subjects free from any hospitalization during the entire follow-up. By comparing their characteristics to those with at least one all-cause hospitalization we were able to construct a solid risk score. Also, it was possible to compare the HOSPITALL score with other existing multidimensional risk scores showing superiority of the HOSPITALL score.
A limitation of this study is that no COPD diagnosis based on Global Initiative of Obstructive Lung Disease criteria could be given because post-bronchodilator pulmonary function was not available. To define obstructive lung disease, we used stringent criteria based on age-, sex-, and race-adjusted LLN cutoffs, as recommended by previous studies.40 Nevertheless, it would be of interest to validate the HOSPITALL score in comparable cohorts with post-bronchodilator spirometry. Furthermore, it should be noted that the Health ABC Study included subjects without disability or mobility impairment which has implications for the generalizability of our results.
Conclusion
The HOSPITALL score is a multidimensional score to predict the risk of all-cause hospitalization in community-dwelling older subjects with obstructive lung disease. The HOSPITALL score may aid in patient counseling and prevention to reduce burden and health care costs.
Supplementary Material
Acknowledgments
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2103; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. This research was financially supported by the Lung Foundation Netherlands (grant number: 3.4.12.023).
Abbreviation list
- AUC
Area under the curve
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CI
Confidence Interval
- CKD
Chronic kidney disease
- CRP
C-reactive protein
- FEV1
Forced expiratory volume in 1 second
- FFMI
Fat free mass index
- FVC
Forced vital capacity
- Health ABC Study
Health, Aging, and Body Composition Study
- HR
Hazard ratio
- IQR
Interquartile range
- LLN
Lower limit of normal
- ROC
Receiver operating curve
- SPPB
Short physical performance battery
Footnotes
Conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 2.Watz H, Waschki B, Boehme C, et al. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177:743–751. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- 3.Jansson SA, Backman H, Ronmark E, et al. Hospitalization due to co-morbid conditions is the main cost driver among subjects with COPD-A report from the population-based OLIN COPD Study. COPD. 2015;12:381–389. doi: 10.3109/15412555.2014.974089. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001–2012 and Nationwide Emergency Department Sample 2006–2011. Chest. 2015;147:989–998. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized Exacerbations of COPD: Risk Factors and Outcomes in the ECLIPSE Cohort. Chest. 2015;147:999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 6.Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 7.Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156:673–683. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 9.Lainscak M, Kadivec S, Kosnik M, et al. Discharge coordinator intervention prevents hospitalizations in patients with COPD: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:450e.1–6. doi: 10.1016/j.jamda.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Ramon MA, Gimeno-Santos E, Ferrer J, et al. Hospital admissions and exercise capacity decline in patients with COPD. Eur Respir J. 2014;43:1018–1027. doi: 10.1183/09031936.00088313. [DOI] [PubMed] [Google Scholar]
- 11.Dalal AA, Christensen L, Liu F, et al. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341–349. doi: 10.2147/COPD.S13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah T, Churpek MM, Coca Perraillon M, et al. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83–91. doi: 10.1016/s0167-4943(02)00061-4. [DOI] [PubMed] [Google Scholar]
- 14.Wong RY, Miller WC. Adverse outcomes following hospitalization in acutely ill older patients. BMC Geriatr. 2008;8:10. doi: 10.1186/1471-2318-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holguin F, Folch E, Redd SC, et al. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128:2005–2011. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 16.Moberg M, Vestbo J, Martinez G, et al. Validation of the i-BODE index as a predictor of hospitalization and mortality in patients with COPD Participating in pulmonary rehabilitation. COPD. 2014;11:381–387. doi: 10.3109/15412555.2013.836171. [DOI] [PubMed] [Google Scholar]
- 17.Zanoria SJ, ZuWallack R. Directly measured physical activity as a predictor of hospitalizations in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10:207–213. doi: 10.1177/1479972313505880. [DOI] [PubMed] [Google Scholar]
- 18.van den Borst B, Koster A, Yu B, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax. 2011;66:961–969. doi: 10.1136/thoraxjnl-2011-200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61:10–16. doi: 10.1136/thx.2004.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrotra N, Freire AX, Bauer DC, et al. Predictors of mortality in elderly subjects with obstructive airway disease: the PILE score. Ann Epidemiol. 2010;20:223–232. doi: 10.1016/j.annepidem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 23.World Health Organization. BMI classification. 2006 [cited 2015 September 24]. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 24.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 25.Lange-Maia BS, Strotmeyer ES, Harris TB, et al. Physical Activity and Change in Long Distance Corridor Walk Performance in the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2015;63:1348–1354. doi: 10.1111/jgs.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 27.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 28.Mannino DM, Higuchi K, Yu TC, et al. Economic Burden of COPD in the Presence of Comorbidities. Chest. 2015;148:138–150. doi: 10.1378/chest.14-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullerova H, Agusti A, Erqou S, et al. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144:1163–1178. doi: 10.1378/chest.12-2847. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Thomas J, Sadatsafavi M, et al. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 31.van den Borst B, Gosker HR, Schols AM. Central fat and peripheral muscle: partners in crime in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:8–13. doi: 10.1164/rccm.201208-1441OE. [DOI] [PubMed] [Google Scholar]
- 32.Patel MS, Mohan D, Andersson YM, et al. Phenotypic characteristics associated with reduced short physical performance battery score in COPD. Chest. 2014;145:1016–1024. doi: 10.1378/chest.13-1398. [DOI] [PubMed] [Google Scholar]
- 33.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 36.Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutten EP, Breyer MK, Spruit MA, et al. Abdominal fat mass contributes to the systemic inflammation in chronic obstructive pulmonary disease. Clin Nutr. 2010;29:756–760. doi: 10.1016/j.clnu.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Broekhuizen R, Wouters EF, Creutzberg EC, et al. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahl M, Vestbo J, Lange P, et al. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–255. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 40.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.