Abstract
Sleep disturbance is a reported side effect of antidepressant drugs in children. Using a nonhuman primate model of childhood selective serotonin reuptake inhibitor (SSRI) therapy, sleep was studied quantitatively with actigraphy. Two 48-h sessions were recorded in the home cage environment of juvenile male rhesus monkeys at two and three years of age, after one and two years of treatment with a therapeutic dose of the SSRI fluoxetine, and compared to vehicle treated controls. A third session was conducted one year after discontinuation of treatment at four years of age. During treatment, the fluoxetine group demonstrated sleep fragmentation as indexed by a greater number of rest-activity transitions compared to controls. In addition fluoxetine led to more inactivity during the day as indexed by longer duration of rest periods and the reduced activity during these periods. The fluoxetine effect on sleep fragmentation, but not on daytime rest, was modified by the monkey’s genotype for polymorphisms of monoamine oxidase A (MAOA), an enzyme that metabolizes serotonin. After treatment, the fluoxetine effect on nighttime rest-activity transitions persisted, but daytime activity was not affected. The demonstration in this nonhuman primate model of sleep disturbance in connection with fluoxetine treatment and specific genetic polymorphisms, and in the absence of diagnosed psychopathology, can help inform use of this drug in children.
Keywords: sleep, nonhuman primate, juvenile fluoxetine exposure, MAOA genotype
1. Introduction
Fluoxetine was introduced as an antipsychotic in 1987 (Perez-Caballero et al., 2014) under the trade name Prozac® and became the premier member of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants. It is estimated that 10% of US adults (> 12 years) are treated with antidepressants (Pratt et al., 2011). Fluoxetine was the third most prescribed antidepressant in a 2008 Medicare survey (Chen et al., 2008) and is widely used in children (O'Sullivan et al., 2015). The favorable efficacy and safety profile of fluoxetine in clinical practice has sustained high levels and expanded range of use of this drug. In addition to its competitive binding to the serotonin reuptake transporter, fluoxetine has been found to influence a number of biological processes important to brain development including neurogenesis, BDNF regulation, spine synapse formation and neurosteroid production (De Foubert et al., 2004; Norrholm and Ouimet, 2000; Pinna et al., 2006; Wu et al., 2014; Oberlander et al., 2009).
Drowsiness, insomnia and sleepiness are all symptoms commonly reported with SSRI therapy in adults. Early studies in depressed patients showed that fluoxetine increased nighttime awakenings, decreased rapid eye movement (REM) sleep, and increased leg movements and oculomotor activity (EOG) (Slater et al., 1978; Armitage et al., 1997). In normal adult subjects, similar electroencephalography (EEG) and EOG findings were seen with short term fluoxetine administration (Vasar et al., 1994; Saletu et al., 1991; Feige et al., 2002). These studies indicate that fluoxetine affects biological pathways regulating sleep independent of underlying psychopathology.
Less is known about fluoxetine effects on sleep in children. Fluoxetine is approved by the FDA for treating depression (MDD) and obsessive compulsive disorder (OCD) in children (FDA, 2003) and is also widely used to treat a number of more common childhood behavior disorders including autism, Down’s syndrome, conduct disorder, separation anxiety, anorexia, and social anxiety (Dorks et al., 2013; Williams et al., 2013; Costa and Scott-McKean, 2013; El-Chammas et al., 2013; Masi, 2004; Connor and Steingard, 1996; Markowitz, 1992; Riddle et al., 1990). It is valuable to know whether this widely used drug (O'Sullivan et al., 2015) influences sleep regulation in children. Sleep disturbances in children are associated with poorer performance on standardized tests, specifically cognitive function and hyperactivity-impulsivity (Touchette et al., 2007). More importantly, developmental sleep disturbance may predict long-term impairment of sleep regulation and executive function (Turnbull et al., 2013). Long-term changes in the brain’s serotonin system, a well known regulator of sleep (Ursin, 2002), were recently reported in rhesus monkeys 1.5 years after the end of a one year juvenile treatment with fluoxetine (Shrestha et al., 2014).
A limited number of studies of children diagnosed with MDD or OCD have addressed the effects of fluoxetine on sleep. A small study (N= 31) reported 13% incidence of insomnia in depressed children treated with fluoxetine (Jain et al., 1992). Later research showed that fluoxetine in six children with depression increased nighttime arousals and oculomotor activity and also dramatically increased leg movement (Armitage et al., 1997). In a chart review of 82 children and adolescents treated with SSRIs, 35% reported sleep disturbance (Wilens et al., 2003). No studies of sleep in children not treated for depression were located in the published literature. When children are treated “off-label”, side effects may vary depending on the underlying biology of the disorder. A detailed study of fluoxetine and sleep disturbance in an appropriate animal model of childhood not specific to one of these disorders would allow identification of the undesirable side effects associated with this drug in the absence of childhood psychopathology. To address the question of whether fluoxetine treatment affects sleep regulation during childhood stages of brain maturation, we employed a juvenile nonhuman primate model.
Rhesus monkeys provide a valuable nonhuman primate model of both juvenile brain development and sleep architecture. Nonhuman primates, like children, have a prolonged period of brain development after infancy and prior to puberty that is not seen in rodent models (Pagel and Harvey, 2002). The consolidated pattern of nighttime sleep seen in humans is also seen in nonhuman primates but not in rodents (Lesku et al., 2006; Balzamo et al., 1977). Rhesus monkeys are particularly well studied as a model for human sleep (Balzamo et al., 1998; Benca et al., 2000; Daley et al., 2006; Barrett et al., 2009; Hsieh et al., 2008; Andersen et al., 2013; Andersen et al., 2010; Balzamo, 1995; Balzamo, 1997; Weitzman et al., 1968). Although most studies use adults, juvenile rhesus have also been studied (Barrett et al., 2009; Benca et al., 2000; Pryce et al., 2011).
In the present study, actimeters were used to measure sleep disturbance. In clinical studies questionnaire and symptom report data are important indices of sleep disturbance. Both EEG and actimeter studies are used to quantify sleep disturbance in nonhuman primates and humans. The EEG provides a record of brain electrical activity but requires head restraint or implantation of sensors. The actimeter, or accelerometer, is noninvasive, records movement, and is widely used in children (Veatch et al., 2015; De Crescenzo et al., 2015; Markovich et al., 2014; Meltzer et al., 2012). The primary indices of sleep disturbance derived from actimeter data are duration of nighttime rest (insomnia), time to onset of rest at night (sleep onset insomnia), the number of nighttime awakenings (sleep maintenance insomnia) and the number of nighttime awakenings and daytime sleep episodes (sleep fragmentation) (American Academy of Sleep Medicine, 2005; Morgenthaler et al., 2007). Sleep fragmentation, a measure of the disruption of the consolidated sleep pattern, is a major index of sleep disturbance in humans (Haba-Rubio et al., 2004; American Academy of Sleep Medicine, 2005; Balzamo et al., 1977).
The design of the current study included genotyping for high and low transcription monoamine oxidase A (MAOA) polymorphisms. Monoamine oxidase (MAO) metabolizes monoamine neurotransmitters and MAOA has high selectivity for serotonin. MAOA genotype interacts with SSRI therapeutic effects (Peters et al., 2004; Yu et al., 2005) but has not been studied for interaction with side effects. MAOA genotype is emerging as an important factor interacting with environmental influences on brain development in both human and nonhuman primates. We recently found that fluoxetine and MAOA genotype interact to influence metabolomic profiles in plasma and CSF of juvenile rhesus (He et al., 2014). Also, in studies of nutritional effects on brain development, we found that MAOA genotype interacted with prenatal iron deficiency in affecting sleep fragmentation in juvenile rhesus monkeys (Golub and Hogrefe, 2014).
2. Materials and Methods
2.1. Assurance of compliance with animal codes
All animal procedures followed the Guide for the Care and Use of Laboratory Animals of the US National Research Council and were approved by the UC Davis Institutional Animal Care and Use Committee.
2.2. Subjects and dosing
Thirty-two male rhesus monkeys (Macaca mulatta) born and raised in outdoor social groups at the California National Primate Research Center (CNPRC) were enrolled at one year of age in a four year study of fluoxetine effects on growth, social interaction, emotional responsiveness, impulsivity, attention, and cognition (Supplementary Table 1). A two year dosing period was used to cover chronic treatment and also several brain developmental stages that might be differentially sensitive to disruption. The sleep assessments were first conducted after one year of dosing, repeated at the end of the two year dosing period, and repeated again one year after the end of dosing, at four years of age. Male rhesus typically reach puberty in the breeding season of the fourth year of life.
The subjects were housed together in the same indoor caging room as described previously (He et al, 2014). Each monkey lived with a compatible peer in the same dosing group in a double cage with a connecting door. The caging partners were separated for activity assessment by closing the door of the double cage. This was necessary to prevent damage to the Actitrac monitor by the cagemate. Although the monitored animal could not reach the monitor, it could readily be manipulated and damaged by another animal in the same cage. Caging partners also were separated by closing the connecting door several times per week for behavioral assessments during the day, and also for longer periods for husbandry, veterinary, and experimental procedures. It is important to note that monitoring was conducted in the usual social environment of the cage room where peers were available for auditory and visual interactions and the vocal and locomotor activity of the cagemate was also accessible. A monitoring session was minimally stressful to the social environment because different groups of four cagemate pairs were monitored regularly on successive weekends.
Monkeys were trained with successive approximations and positive reinforcement to come forward to the front of the cage and place their mouths around the end of a 3 or 6 cc syringe to receive flavored syrup (Torani®) or liquefied baby food (Gerber®). Daily oral dosing with fluoxetine (Webster Veterinary Supply, Devens, MA) dissolved in a favored vehicle was then conducted at doses which resulted in plasma levels in the range of therapeutic doses in children as determined in preliminary pharmacokinetic studies (Golub and Hogrefe, 2014). Control animals received daily dosing with the vehicle (flavored syrup or baby food) only. Syrup and food flavors were changed throughout the study to maintain interest. Plasma fluoxetine plus norfluoxetine concentrations in samples taken 23 h after a daily dosing with 2.4 mg/kg at 3 years of age were 273±31 ng/mL (mean ± s.e.m.) as compared to 363 ng/mL measured 8–12 h after dosing in a pharmacokinetic study of pediatric patients treated with fluoxetine at therapeutic doses (Wilens et al., 2002). All but two of the animals consumed 99+% of the scheduled dose over the 110 weeks of treatment; the other two consumed 85% and 97%.
Subjects were genotyped for behaviorally relevant polymorphisms of the serotonin transporter (SERT, 5HTTLPR VNTR polymorphisms) and serotonin metabolizing enzyme monoamine oxidase A (MAOA, uVNTR polymorphisms) (Kinnally et al., 2008; Capitanio et al., 2012). Treatment groups were balanced for polymorphisms resulting in different transcription rates for these genes (5HTTLPR: SS, SL, LL groups; MAOA: hi-MAOA, low-MAOA groups) (Lesch et al., 1997; Newman et al., 2005).
2.3. Animal selection, care and maintenance
Monkeys were not considered for the study if they had low birth weight, history of diarrhea or BioBehavioral Assessment (BBA) scores outside colony norms. The BBA scores were obtained during a 25 h period of behavioral assessment performed on most 3–4 month old rhesus in the CNPRC colony. Data from several thousand infants are available to compare potential experimental subjects to historical norms. Excluded monkeys had scores greater or lesser than two standard deviations from the CNPRC colony mean for factors derived from multivariate analysis of behavioral observation data from the BBA database (Golub et al., 2009). The male rhesus monkeys meeting these criteria were randomly assigned to treatment groups (control and fluoxetine), balancing for weight, cage of origin, MAOA polymorphism genotype and 5HTTLPR polymorphism genotype. Group sizes (N=16/group) were based on previous studies of effect sizes for independent variables administered to rhesus monkeys during development at levels relevant to human populations. The Vehicle group size was reduced to 14 for the third session at 4 years of age due to displacement of the monitors during the session. All animals were cared for following standard CNPRC animal husbandry and health care protocols, as described previously (Golub et al., 2015).
2.4. Activity monitoring
Activity was recorded from individual animals in the home cage over a 48 h (weekend) period using actimeters (Actitrac, IM Systems, Baltimore, MD) (Golub et al., 2004; Golub et al., 2007; Golub et al., 2006; Golub et al., 2005). The actimeters were packaged in tape and attached to the upper back between the shoulders, out of reach of the animals, with a harness. Actimeters were placed on Friday afternoon and monitoring was initiated the next morning. Methods for attaching and removing the monitor were designed to minimize stress to the animals. Light anesthesia (5 mg/kg ketamine, i.m.) was administered, and the animals were quickly removed from the cage, held for harness attachment and returned to the cage. For removing the monitors, animals were isolated in the front of the cage and briefly restrained by hand while the tie holding the harness in place was cut. Ketamine anesthesia was also used for removal in the oldest (4 year old) animals.
Three monitoring sessions were conducted one and two years after initiation of fluoxetine treatment, and one year after ending fluoxetine treatment, when the animals were two, three and four years old. All animals in the study were housed in the same indoor cageroom with a 12:12 (6 a.m. – 6 p.m.) cycle of artificial light and no access to sunlight. Lux levels at the front of the cage averaged 347 during the day and 0 at night. Actitrac software provides measures of onset, duration and level of each active and inactive period (see Supplemental Figure 1 for an illustration of an actigram). An inactive period is defined as a two min period (epoch) for which that epoch and the epoch prior to and following it average to be below a software-defined activity threshold of 18 counts/2 min. This index of sleep was developed for humans (Gorney et al., 1996). The relationships between EEG and actimeter indices of sleep have not been elucidated in young rhesus. Thus we are designating activity below the threshold as “rest”.
2.5. Statistical approach
Data sets were screened for normal distribution prior to analysis. To minimize Type 1 error, a tiered approach to analysis was used, looking first at total amount of rest at night, onset of the first rest period at night, and number of rest-activity transitions over the 48 h period (fragmentation index). Other endpoints were then examined to aid in interpretation.
Potential covariates (body weight, cage location, etc.) were screened for relevance to the sleep parameters but few were significant and none influenced fluoxetine findings when included in ANOVAs with the exception of MAOA genotype. Thus selected parameters were analyzed with two-way ANOVA (fluoxetine, MAOA genotype) including the interaction (JMP, SAS, Cary, NC). Post hoc planned comparisons looked at the effect of fluoxetine within the two MAOA genotype subgroups. 5HTTLPR alleles were also examined for main effects and interaction with fluoxetine treatment by comparing SL and LL subgroups but no effects were identified. SS genotypes could not be evaluated due to limited number of subjects with this genotype (n=4).
3. Results
During dosing, maturational changes were seen in the diurnal activity pattern of the monkeys from two to three years of age. The amount of sleep was similar in two-year olds (10% rest during the day, 58% rest at night) and three-year olds (6% rest day, 56% rest night). However, three-year olds had a 35% longer delay to their first rest period at night (F1,30=9.74, p=0.004), and 29% more rest-activity transitions during the 48 h monitoring period (F1,30=36.32, p<0.0001). They also were 36% less active overall than two-year olds, had 45% less activity during active periods, and differed in the average durations of active and rest periods. Because of the different structure of activity in two-and three-year olds, the two monitoring sessions were analyzed separately for the effects of fluoxetine during dosing.
3.1 Fluoxetine did not affect total sleep or sleep onset during dosing
The total duration of rest at night, though lower in the fluoxetine group, was not significantly different from controls at either age (Table 1). Time to sleep onset was also somewhat shorter on the average but not significantly different from controls at either age.
Table 1.
Comparison of fluoxetine effects during and after the two year dosing period. Veh=vehicle, Flx=fluoxetine. N=16/group, except N=14 for the vehicle group for the 1 y post-dosing session.
| Session | ||||
|---|---|---|---|---|
| 1 y dosing | 2 y dosing | 1 y post-dosing | ||
| Nighttime active periods (#) | Veh | 52±2 | 54±2 | 54±3 |
| Flx | 55±1§ | 58±2 | 63±2* | |
| Nighttime rest duration (min) | Veh | 779±34 | 839±35 | 783±35 |
| Flx | 763±20 | 793±27 | 674±33* | |
| Time to first rest period at night (min) |
29±3 23±3 |
39±4 32±4 |
49±4 40±3 |
|
| Daytime rest duration (min) | Veh | 32±8 | 99±12 | 175±20 |
| Flx | 70±16* | 123±14 | 161±19 | |
| Daytime activity during rest periods (activity counts) |
Veh | 4.5±0.5 | 5.6±0.4 | 3.1 ±0.2 |
| Flx | 3.8±0.5 | 4.6±0.2* | 2.4±0.1** | |
fluoxetine*MAOA interaction p=.03, hi-MAOA Flx vs Veh p=.02
p<.05,
p<.02 main effect of fluoxetine
3.2. Fluoxetine increased sleep fragmentation during dosing
Sleep consolidation and continuity in primate species are reflected in a small number of transitions between rest and activity, that is few awakenings at night and few rest periods during the day. The sleep fragmentation index sums all transitions in both day and night segments of the monitoring session. It has long been used as a clinical index of sleep disturbance (Stepanski et al., 1984), or sleep-maintenance insomnia (as opposed to sleep-onset insomnia).
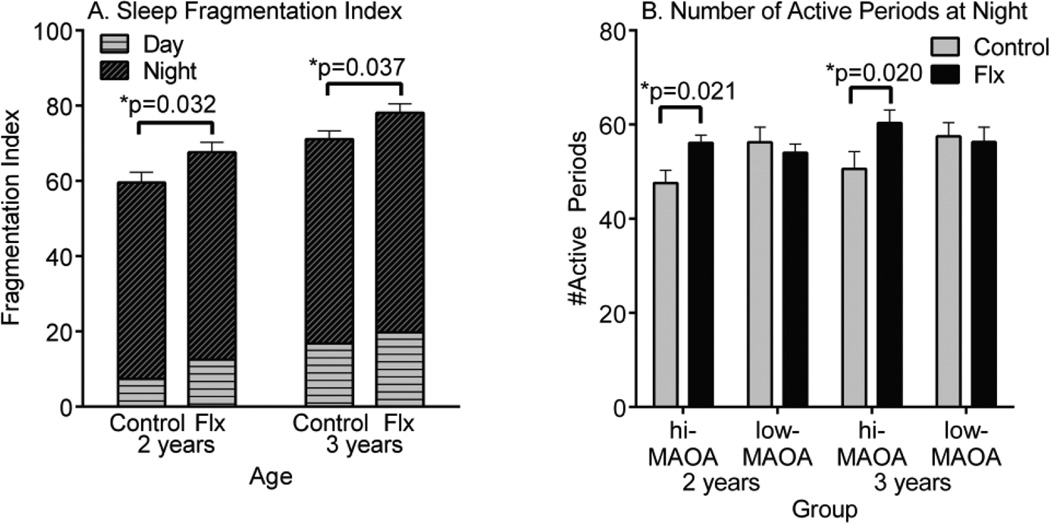
Sleep fragmentation was increased by fluoxetine at both two and three years of age (Figure 1A). In the case of the younger animals, an interaction with MAOA genotype was also suggested (p=0.057) in that the fluoxetine effect occurred primarily in the hi-MAOA genotype monkeys (p=0.006). As seen in Figure 1B, a drug*genotype interaction appeared specifically for the transitions at night at two years of age (F1,28=5.00, p=0.032), and was also suggested for three-year olds at night (F1,28=3.78, p=0.062). As was the case for the fragmentation index, the fluoxetine effect occurred in the hi-MAOA subgroup, but not the low-MAOA subgroup. The high-MAOA vehicle group initially had a lower number of active periods at night compared to the low-MAOA vehicle group, but the number increased to comparable levels when hi-MAOA genotype animals were dosed with fluoxetine.
Figure 1.
Sleep disturbance in two and three-year old male rhesus monkeys treated with fluoxetine. (A) Sleep Fragmentation Index. (B) Number of active periods at night. The sleep fragmentation is the sum of active periods initiated at night and rest periods initiated during the day. Based on 48 h continuous actimeter monitoring period. N=16 control and fluoxetine groups. N=8 hi- and low-MAOA subgroups
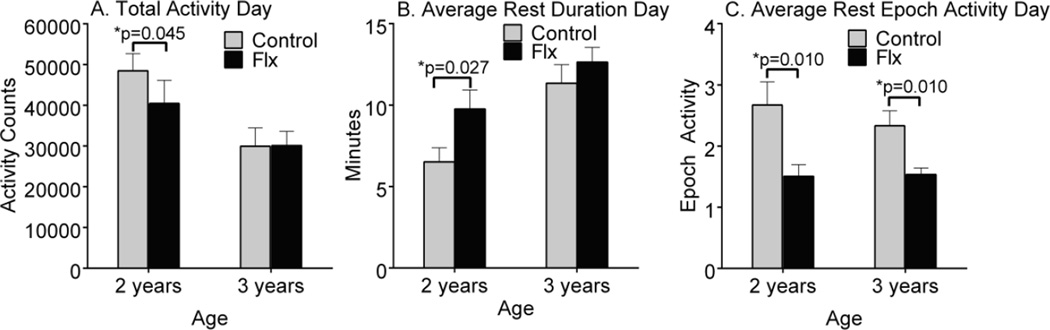
3.3. Fluoxetine-treated monkeys had less daytime activity during dosing
Daytime sleepiness is a potential concern when sleep is disrupted (Stepanski et al., 1984). Fluoxetine-treated two-year olds were less active during the day as reflected in total rest duration (F=4.40, p=0.045), average duration of rest periods (F=5.46, p=0.027), and the amount of activity during rest (F=7.75, p=0.010) (Figure 2). As three-year olds, during the first active period the fluoxetine-treated animals had a shorter amount of time to the first rest period (F=4.89, p=0.035), a longer average duration of rest periods (F=3.98, p=0.056) and less activity during rest period epochs (F=8.76, p=0.006). No statistically significant MAOA interactions were seen for these daytime indices.
Fig 2.
Fluoxetine effects on indices of daytime inactivity. Based on sum of two 12 h light periods. (A) Total activity during the day, (B) Average inactive duration during the day and (C) Average inactive epoch activity during the day.
3.4. Sleep fragmentation and daytime activity were negatively correlated during dosing
The amount of sleep fragmentation correlates with daytime sleepiness in humans (Stepanski et al., 1984). To determine whether these measures were associated in our sample, regression analyses were conducted. At two years of age, the fragmentation index was positively correlated with daytime rest (duration of rest periods r=+0.44, p=0.013), and negatively correlated with the daytime activity (total daytime activity r=−0.40, p=0.027, activity during daytime rest epochs r=−0.49, p=0.013, time to first daytime rest period r=−0.33, ns). At three years of age, these correlations were no longer significant.
3.6. Effects on sleep persisted after discontinuation of dosing
A third actimeter monitoring session was conducted one year after the end of the two year dosing period. At that time the monkeys were four years old. Data analyses tested the hypothesis that the effects seen during dosing persisted (see dosing/post-dosing comparisons in Table 1). The fluoxetine effect on sleep fragmentation index was not significant (p=.072), but nighttime awakenings (nighttime active periods) were significantly more frequent in the fluoxetine group (F1,26=4.71, p=.039) (Table 1). Unlike the sessions conducted during dosing, there was no fluoxetine*MAOA interaction, but a significant main effect of MAOA genotype was seen for nighttime active periods (F1,26=6.06, p=.020). Fewer nighttime active periods were seen in the hi-MAOA vs low-MAOA group, a similar pattern as in previous sessions. Although the interaction was not significant (p=.44), the fluoxetine effect was seen in the hi-MAOA group (p=.039), but not the low-MAOA group, as during dosing. In addition, the total nighttime rest duration in the post-dosing session was significantly lower in the fluoxetine group (F1,28=4.55, p=.043). During the day, the rest duration was not affected, but the amount of activity during rest periods was lower in the fluoxetine group (F1,28=7.03, p=.013), as was the case in the two year dosing session.
4. Discussion
Juvenile rhesus macaque monkeys dosed chronically with fluoxetine demonstrated sleep fragmentation as well as increased daytime inactivity when assessed one and two years after initiation of dosing. One year after the discontinuation of dosing the same effect on sleep disruption, as reflected in nighttime active periods, was identified, but daytime inactivity was not increased.
Sleep disruption has been reported in children treated with fluoxetine, but only in patient populations and only during treatment. The present study demonstrates that fluoxetine can produce sleep disruption in an appropriate animal model (juvenile rhesus macaques) in the absence of diagnosed psychopathology, that the extent of disruption is greater at younger ages and that the effect can persist after discontinuation of treatment. This suggests that the treatment during the period of brain maturation and establishment of sleep regulation can have long-term or perhaps permanent effects on individual sleep patterns. Long-term effects on sleep from childhood exposure to fluoxetine, or indeed any other psychoactive drug, have not been studied to our knowledge.
There are a number of limitations in generalizing from the current study to a clinical setting. Only male subjects were studied to facilitate study of polymorphisms of the MAOA gene, which is X-linked. Actimeters were the only indicator of sleep; no information on EEG, EOG or leg movement was available to better translate the fluoxetine effects in juvenile primates to children. Some commercial actimeters, but not the Actritrac actimeter, have been validated with EEG measures of sleep (polysomnography) in children (Meltzer et al., 2015; Meltzer et al., 2015; Meltzer et al., 2012). Also, we are not able to assess the subjective quality of sleep disturbance and sleepiness which are the basis for symptom reports in humans. Interestingly, actigraphy data have been found to reflect clinical sleep reporting instruments better than EEG data in children, particularly for nighttime awakenings (Markovich et al., 2014). Finally, aspects of the controlled environment under which the study was conducted may have modified the treatment effect. Monkeys were housed indoors with artificial 12:12 light cycles and limited opportunity for motor activity and social interaction.
Fluoxetine effects were much less prominent at three years of age than two years of age but emerged again in the post-dosing session at four years of age. As previously described (Golub et al., 1998), prepubertal monkeys (three years old) had a longer delay to sleep onset after dark onset than younger monkeys (two years old) paralleling the later bedtimes seen in pubertal children (Carskadon et al., 1993). Older monkeys also had less daytime activity, paralleling decreases in activity with age in children as measured with actimeters (Trost et al., 2002). Nighttime sleep and daytime activity were correlated at two years of age, but not in the older animals (three and four years of age). In additional to maturational changes, time on study, length of fluoxetine dosing, and environment may have played a role in the sleep changes across sessions. Although the physical environment was the same throughout the study, all animals were housed together in the same room and the social environment and available cage space also changed as they grew and matured.
MAOA polymorphisms resulting in high gene transcription (hi-MAOA) were more susceptible to fluoxetine effects on sleep fragmentation. Fluoxetine effects on sleep fragmentation were greater in the younger hi-MAOA subjects and in the post-dosing session there was a main effect of MAOA genotype. Hi-MAOA VNTR polymorphisms were also shown to increase the risk for sleep disturbance in Alzheimer’s patients (Craig et al., 2006), and for restless leg syndrome in women (Desautels et al., 2002). Daytime sleepiness was more prominent in depressed patients with high transcribing MAOA genotypes (Ojeda et al., 2014). These studies were performed with adult patients. Understanding genetic risk for sleep disturbance as a side effect of fluoxetine use in children could be a valuable contribution to personalized medicine in children.
Notably, comparison of SL and LL genotypes did not yield any indication of effects of the S allele on the sleep measures recorded. The present study was not able to study effects of the homogeneous SS genotype as there were only two SS subjects in each treatment group. Homogeneous SS 5HTTLPR alleles showed a strong association with insomnia as a side effect of fluoxetine (Perlis et al., 2003), the was reported to interact with stress in affecting sleep quality (Brummett et al., 2007), and was more prevalent in 18 year-olds who reported low amounts of sleep and high amounts of depression (Carskadon et al., 2012).
A large number of sites in the brain for fluoxetine*MAOA interactions could be proposed. Both fluoxetine and MAOA have extensive impacts throughout the monoamine systems, and influences on endocrine systems and metabolism are also well known. MAOA degrades serotonin intracellularly, and can interact with cellular uptake transport to alter extracellular serotonin levels as demonstrated in experiments in transgenic mice (Mossner et al., 2006). The relationship between serotonin and sleep is well known (Ursin, 2002). The dorsal raphe nucleus, the major origin of serotonin pathways, projects to the suprachiasmatic nucleus and is involved in sleep regulation. Serotonin is a precursor of melatonin, the hormone that regulates light-dark cycles. Interesting potential direct interactions include fluoxetine inhibition of MAOA as demonstrated in vitro (Fisar et al., 2010).
5. Conclusion
Sleep disturbance during fluoxetine treatment is recognized in adults and has been documented in studies of depressed children. This study in an appropriate animal model of juvenile sleep demonstrates fluoxetine effects during dosing independent of a diagnosed behavioral disorder and indicates that a genetic polymorphism common to human and nonhuman primates modifies this effect. Further, after discontinuation of dosing for one year, the sleep disturbance effect was still apparent. This information may help anticipate and mitigate sleep disturbance as a side effect of fluoxetine therapy in children.
Supplementary Material
Highlights.
Young monkeys were treated daily with fluoxetine
Rest-activity cycles were recorded by actimeter at two ages
Sleep fragmentation was greater in the fluoxetine than the control group
Activity during the day was lower in the fluoxetine group
Fluoxetine effects were diminished in older monkeys
Fluoxetine effects persisted after dosing ended
Acknowledgments
The authors thank the technical staff at the CNPRC for assisting with actimeter placements for monitoring and the Research Services staff at CNPRC for dosing support. This work was supported by NIH grants HD065826 (PI MSG), OD010962 (PI John Capitanio) and OD011107 (PI Harris Lewin). The funding sources had no involvement in the study design; in the collection, analysis or interpretation of data; or in the writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
Contributor Information
Mari S. Golub, Email: msgolub@ucdavis.edu.
Casey E. Hogrefe, Email: cehogrefe@ucdavis.edu.
References
- American Academy of Sleep Medicine. International classification of sleep disorders. 2nd. Westchester, IL: Diagostic and coding manual; 2005. [Google Scholar]
- Andersen ML, Diaz MP, Murnane KS, Howell LL. Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology (Berl) 2013;227:101–107. doi: 10.1007/s00213-012-2943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl) 2010;210:439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage R, Emslie G, Rintelmann J. The effect of fluoxetine on sleep EEG in childhood depression: a preliminary report. Neuropsychopharmacology. 1997;17:241–245. doi: 10.1016/S0893-133X(97)00048-1. [DOI] [PubMed] [Google Scholar]
- Armitage R, Yonkers K, Cole D, Rush AJ. A multicenter, double-blind comparison of the effects of nefazodone and fluoxetine on sleep architecture and quality of sleep in depressed outpatients. J Clin Psychopharmacol. 1997;17:161–168. doi: 10.1097/00004714-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Balzamo E. Sleep-wake cycles in rhesus monkeys during Spacelab flight simulations. J Gravit Physiol. 1995;2:P54–P55. [PubMed] [Google Scholar]
- Balzamo E. Evolution of sleep and wakefulness organization in Macaca mulatta during Spacelab flight simulation. J Gravit Physiol. 1997;4:35–41. [PubMed] [Google Scholar]
- Balzamo E, Santucci V, Seri B, Vuillon-Cacciuttolo G, Bert J. Nonhuman primates: laboratory animals of choice for neurophysiologic studies of sleep. Lab Anim Sci. 1977;27:879–886. [PubMed] [Google Scholar]
- Balzamo E, Van Beers P, Lagarde D. Scoring of sleep and wakefulness by behavioral analysis from video recordings in rhesus monkeys: comparison with conventional EEG analysis. Electroencephalogr Clin Neurophysiol. 1998;106:206–212. doi: 10.1016/s0013-4694(97)00152-1. [DOI] [PubMed] [Google Scholar]
- Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. Early adverse rearing experiences alter sleep-wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psychoneuroendocrinology. 2009;34:1029–1040. doi: 10.1016/j.psyneuen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879:130–138. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Krystal AD, Ashley-Koch A, Kuhn CM, Zuchner S, Siegler IC, Barefoot JC, Ballard EL, Gwyther LP, Williams RB. Sleep quality varies as a function of 5-HTTLPR genotype and stress. Psychosom Med. 2007;69:621–624. doi: 10.1097/PSY.0b013e31814b8de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Del Rosso LA, Calonder LA, Blozis SA, Penedo MC. Behavioral effects of prenatal ketamine exposure in rhesus macaques are dependent on MAOA genotype. Exp Clin Psychopharmacol. 2012;20:173–180. doi: 10.1037/a0026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Sharkey KM, Knopik VS, McGeary JE. Short sleep as an environmental exposure: a preliminary study associating 5-HTTLPR genotype to self-reported sleep duration and depressed mood in first-year university students. Sleep. 2012;35:791–796. doi: 10.5665/sleep.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kelton CM, Jing Y, Guo JJ, Li X, Patel NC. Utilization, price, and spending trends for antidepressants in the US Medicaid Program. Res Social Adm Pharm. 2008;4:244–257. doi: 10.1016/j.sapharm.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Connor DF, Steingard RJ. A clinical approach to the pharmacotherapy of aggression in children and adolescents. Ann N Y Acad Sci. 1996;794:290–307. doi: 10.1111/j.1749-6632.1996.tb32529.x. [DOI] [PubMed] [Google Scholar]
- Costa AC, Scott-McKean JJ. Prospects for improving brain function in individuals with Down syndrome. CNS Drugs. 2013;27:679–702. doi: 10.1007/s40263-013-0089-3. [DOI] [PubMed] [Google Scholar]
- Craig D, Hart DJ, Passmore AP. Genetically increased risk of sleep disruption in Alzheimer's disease. Sleep. 2006;29:1003–1007. doi: 10.1093/sleep/29.8.1003. [DOI] [PubMed] [Google Scholar]
- Daley JT, Turner RS, Freeman A, Bliwise DL, Rye DB. Prolonged assessment of sleep and daytime sleepiness in unrestrained Macaca mulatta. Sleep. 2006;29:221–231. [PubMed] [Google Scholar]
- De Crescenzo F, Licchelli S, Ciabattini M, Menghini D, Armando M, Alfieri P, Mazzone L, Pontrelli G, Livadiotti S, Foti F, Quested D, Vicari S. The use of actigraphy in the monitoring of sleep and activity in ADHD: A meta-analysis. Sleep Med Rev. 2015;26:9–20. doi: 10.1016/j.smrv.2015.04.002. [DOI] [PubMed] [Google Scholar]
- De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, Murray TK, Gaillard JP, Deville C, Xhenseval V, Thomas CE, O'Neill MJ, Zetterstrom TS. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Desautels A, Turecki G, Montplaisir J, Brisebois K, Sequeira A, Adam B, Rouleau GA. Evidence for a genetic association between monoamine oxidase A and restless legs syndrome. Neurology. 2002;59:215–219. doi: 10.1212/wnl.59.2.215. [DOI] [PubMed] [Google Scholar]
- Dorks M, Langner I, Dittmann U, Timmer A, Garbe E. Antidepressant drug use and off-label prescribing in children and adolescents in Germany: results from a large population-based cohort study. Eur Child Adolesc Psychiatry. 2013;22:511–518. doi: 10.1007/s00787-013-0395-9. [DOI] [PubMed] [Google Scholar]
- El-Chammas K, Keyes J, Thompson N, Vijayakumar J, Becher D, Jackson JL. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250–258. doi: 10.1001/jamapediatrics.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Approval letter: Application number 18-936/SE5-064. 2003. C. f. D. E. a. Research. [Google Scholar]
- Feige B, Voderholzer U, Riemann D, Dittmann R, Hohagen F, Berger M. Fluoxetine and sleep EEG: effects of a single dose, subchronic treatment, and discontinuation in healthy subjects. Neuropsychopharmacology. 2002;26:246–258. doi: 10.1016/S0893-133X(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Fisar Z, Hroudova J, Raboch J. Inhibition of monoamine oxidase activity by antidepressants and mood stabilizers. Neuro Endocrinol Lett. 2010;31:645–656. [PubMed] [Google Scholar]
- Golub MS, Germann SL, Hogrefe CE. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicol Teratol. 2004;26:799–809. doi: 10.1016/j.ntt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE. Fluoxetine: juvenile pharmacokinetics in a nonhuman primate model. Psychopharmacology (Berl) 2014;231:4041–4047. doi: 10.1007/s00213-014-3537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE. Sleep patterns in male juvenile monkeys are influenced by gestational iron deprivation and monoamine oxidase A genotype. Br J Nutr. 2014;112:1478–1483. doi: 10.1017/S0007114514002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Bulleri AM. Peer social interaction in rhesus monkeys treated with fluoxetine during juvenile development. Neuropharmacology submitted. 2015 doi: 10.1016/j.neuropharm.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Takeuchi PT, Hoban-Higgins TM. Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon M, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. New York: Cambridge University Press; 1998. pp. 50–68. [Google Scholar]
- Gorney S, Allen R, Krausman D, Earley C. Parametric analyses of factors affecting accuracy of detection of wake epochs after sleep onset based on wrist activity data. Sleep Res. 1996;25:490. [Google Scholar]
- Haba-Rubio J, Ibanez V, Sforza E. An alternative measure of sleep fragmentation in clinical practice: the sleep fragmentation index. Sleep Med. 2004;5:577–581. doi: 10.1016/j.sleep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- He Y, Hogrefe CE, Grapov D, Palazoglu M, Fiehn O, Turck CW, Golub MS. Identifying individual differences of fluoxetine response in juvenile rhesus monkeys by metabolite profiling. Transl Psychiatry. 2014;4:e478. doi: 10.1038/tp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh KC, Robinson EL, Fuller CA. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep. 2008;31:1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Jain U, Birmaher B, Garcia M, Al-Shabbout M, Ryan N. Fluoxetine in children and adolescents with mood disorders: a chart review of efficacy and adverse effects. J Child Adolesc Psychopharmacol. 1992;2:259–265. doi: 10.1089/cap.1992.2.259. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Lyons LA, Abel K, Mendoza S, Capitanio JP. Effects of early experience and genotype on serotonin transporter regulation in infant rhesus macaques. Genes Brain Behav. 2008;7:481–486. doi: 10.1111/j.1601-183X.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Lesku JA, Roth TC, 2nd, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am Nat. 2006;168:441–453. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- Markovich AN, Gendron MA, Corkum PV. Validating the Children's Sleep Habits Questionnaire Against Polysomnography and Actigraphy in School-Aged Children. Front Psychiatry. 2014;5:188. doi: 10.3389/fpsyt.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz PI. Effect of fluoxetine on self-injurious behavior in the developmentally disabled: a preliminary study. J Clin Psychopharmacol. 1992;12:27–31. doi: 10.1097/00001573-199202000-00005. [DOI] [PubMed] [Google Scholar]
- Masi G. Pharmacotherapy of pervasive developmental disorders in children and adolescents. CNS Drugs. 2004;18:1031–1052. doi: 10.2165/00023210-200418140-00006. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a Commercial Accelerometer with Polysomnography and Actigraphy in Children and Adolescents. Sleep. 2015;38:1323–1330. doi: 10.5665/sleep.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16:463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Walsh CM, Peightal AA. Comparison of actigraphy immobility rules with polysomnographic sleep onset latency in children and adolescents. Sleep Breath. 2015;19:1415–1423. doi: 10.1007/s11325-015-1138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A, Jr, Coleman J, Lee-Chiong T, Pancer J, Swick TJ. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Mossner R, Simantov R, Marx A, Lesch KP, Seif I. Aberrant accumulation of serotonin in dopaminergic neurons. Neurosci Lett. 2006;401:49–54. doi: 10.1016/j.neulet.2006.02.081. [DOI] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res. 2000;883:205–215. doi: 10.1016/s0006-8993(00)02909-7. [DOI] [PubMed] [Google Scholar]
- O'Sullivan K, Boland F, Reulbach U, Motterlini N, Kelly D, Bennett K, Fahey T. Antidepressant prescribing in Irish children: secular trends and international comparison in the context of a safety warning. BMC Pediatr. 2015;15:119. doi: 10.1186/s12887-015-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda DA, Nino CL, Lopez-Leon S, Camargo A, Adan A, Forero DA. A functional polymorphism in the promoter region of MAOA gene is associated with daytime sleepiness in healthy subjects. J Neurol Sci. 2014;337:176–179. doi: 10.1016/j.jns.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Pagel M, Harvey P. Evolution of the juvenile period in mammals. In: Pereira M, Fairbanks L, editors. Juvenile Primates:life history, development and behavior. Chicago, IL: University of Chicato; 2002. pp. 28–37. [Google Scholar]
- Perez-Caballero L, Torres-Sanchez S, Bravo L, Mico JA, Berrocoso E. Fluoxetine: a case history of its discovery and preclinical development. Expert Opin Drug Discov. 2014;9:567–578. doi: 10.1517/17460441.2014.907790. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Mischoulon D, Smoller JW, Wan YJ, Lamon-Fava S, Lin KM, Rosenbaum JF, Fava M. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54:879–883. doi: 10.1016/s0006-3223(03)00424-4. [DOI] [PubMed] [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005–2008. NCHS Data Brief, National Center for Health Statistics, CDC, US DHHS; 2011. [PubMed] [Google Scholar]
- Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology (Berl) 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MA, Hardin MT, King R, Scahill L, Woolston JL. Fluoxetine treatment of children and adolescents with Tourette's and obsessive compulsive disorders: preliminary clinical experience. J Am Acad Child Adolesc Psychiatry. 1990;29:45–48. doi: 10.1097/00004583-199001000-00008. [DOI] [PubMed] [Google Scholar]
- Saletu B, Frey R, Krupka M, Anderer P, Grunberger J, See WR. Sleep laboratory studies on the single-dose effects of serotonin reuptake inhibitors paroxetine and fluoxetine on human sleep and awakening qualities. Sleep. 1991;14:439–447. doi: 10.1093/sleep/14.5.439. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, Morse C, Henter ID, Kruger J, Zhang B, Suomi SJ, Svenningsson P, Pike VW, Winslow JT, Leibenluft E, Pine DS, Innis RB. Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry. 2014;171:323–331. doi: 10.1176/appi.ajp.2013.13020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater IH, Jones GT, Moore RA. Inhibition of REM sleep by fluoxetine, a specific inhibitor of serotonin uptake. Neuropharmacology. 1978;17:383–389. doi: 10.1016/0028-3908(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Stepanski E, Lamphere J, Badia P, Zorick F, Roth T. Sleep fragmentation and daytime sleepiness. Sleep. 1984;7:18–26. doi: 10.1093/sleep/7.1.18. [DOI] [PubMed] [Google Scholar]
- Touchette E, Petit D, Seguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost SG, Pate RR, Sallis JF, Freedson PS, Taylor WC, Dowda M, Sirard J. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc. 2002;34:350–355. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Turnbull K, Reid GJ, Morton JB. Behavioral Sleep Problems and their Potential Impact on Developing Executive Function in Children. Sleep. 2013;36:1077–1084. doi: 10.5665/sleep.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- Vasar V, Appelberg B, Rimon R, Selvaratnam J. The effect of fluoxetine on sleep: a longitudinal, double-blind polysomnographic study of healthy volunteers. Int Clin Psychopharmacol. 1994;9:203–206. doi: 10.1097/00004850-199409000-00009. [DOI] [PubMed] [Google Scholar]
- Veatch OJ, Reynolds A, Katz T, Weiss SK, Loh A, Wang L, Malow BA. Sleep in Children With Autism Spectrum Disorders: How Are Measures of Parent Report and Actigraphy Related and Affected by Sleep Education? Behav Sleep Med. 2015:1–12. doi: 10.1080/15402002.2015.1065408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Rapport MM, McGregor P, Jacoby J. Sleep patterns of the monkey and brain serotonin concentration: effect of p-chlorophenylalanine. Science. 1968;160:1361–1363. doi: 10.1126/science.160.3834.1361. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Kwon A, Chase R, Greenberg L, Mick E, Spencer TJ. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003;13:143–152. doi: 10.1089/104454603322163862. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Cohen L, Biederman J, Abrams A, Neft D, Faird N, Sinha V. Fluoxetine pharmacokinetics in pediatric patients. J Clin Psychopharmacol. 2002;22:568–575. doi: 10.1097/00004714-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2013;8:Cd004677. doi: 10.1002/14651858.CD004677.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Shamy JL, Bedi G, Choi CW, Wall MM, Arango V, Boldrini M, Foltin RW, Hen R. Impact of social status and antidepressant treatment on neurogenesis in the baboon hippocampus. Neuropsychopharmacology. 2014;39:1861–1871. doi: 10.1038/npp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW. Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology. 2005;30:1719–1723. doi: 10.1038/sj.npp.1300785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.