Abstract
Existing models of the role of posttraumatic stress disorder (PTSD) symptoms and smoking have almost exclusively examined mean symptom levels, rather than the acute elevations that might trigger smoking lapse immediately or increase risk of a smoking lapse in the next few hours. We examined ecological momentary assessments (EMA) of PTSD symptom clusters and smoking in the first week of a quit attempt in 52 people with PTSD. In multilevel models including PTSD symptom means, acute elevations, and lagged acute elevations together as simultaneous predictors of odds of smoking in the same models, pre-quit smoking occasions were significantly related to acute elevations in symptoms, including PTSD totals (OR = 1.20; 95% CI, 1.10 to 1.31), PTSD re-experiencing symptoms (OR = 1.16; 95% CI, 1.06 to 1.27), PTSD avoidance symptoms (OR = 1.20; 95% CI, 1.10 to 1.31), PTSD numbing symptoms (OR = 1.14; 95% CI, 1.04 to 1.24), and PTSD hyperarousal symptoms (OR = 1.20; 95% CI, 1.09 to 1.31). In contrast, post-quit smoking was related to lagged acute elevations in PTSD re-experiencing (OR = 1.24, 95% CI, 1.03 to 1.50) avoidance (OR = 1.27, 95% CI, 1.05 to 1.53), and numbing symptoms (OR = 1.24, 95% CI, 1.02 to 1.51). During a quit attempt, individuals with PTSD delayed smoking in response to acute elevations in PTSD re-experiencing and Avoidance. This period presents an opportunity to use mobile health interventions to prevent smoking lapse and to use coping skills acquired in trauma-focused therapy to respond to acute PTSD symptom elevation.
Keywords: Tobacco use cessation, posttraumatic stress disorder, comorbidity, ecological momentary assessment, relapse prevention
INTRODUCTION
People with post-traumatic stress disorder (PTSD) smoke at an increased rate relative to the general population. Though people with PTSD endorse a desire and intent to quit smoking (Kirby, 2008), desire and intention to quit are not enough to prevent smokers with PTSD from lapsing (i.e., smoking a cigarette) or relapsing (i.e., return to baseline smoking levels) when making a quit attempt. In addition, smokers with PTSD tend to lapse more quickly during quit attempts (Zvolensky et al., 2008), making the first week of the quit attempt a critical time period for understanding the factors influencing smoking lapse. If the lapse process can be fully understood, then more effective treatments could be developed (Piasecki et al., 2000).
Factor analyses of PTSD symptoms have identified clusters of symptoms that reflect distinct dimensions of PTSD (Asmundson et al., 2000) that could guide smoking cessation treatment. In a sample of veterans completing intake questionnaires at a mental health clinic, approximately half of whom met criteria for PTSD, overall PTSD symptom severity was related to being a current smoker and to smoking heavily (defined as ≥ 20 cigarettes/day). When examining symptom clusters to gain a more nuanced understanding of the relationship of PTSD to smoking, the numbing cluster was related to smoking heavily, and other clusters were unrelated to smoking intensity (Cook, 2009). This analysis is complemented by a sample of veterans with PTSD who enrolled in a smoking cessation trial. Examination of data from self-report questionnaires administered at baseline revealed that the only PTSD cluster related to smoking intensity was numbing, which was positive correlated with both cigarettes per day and nicotine dependence level (Joseph, 2012).
Though these retrospective reports provide information on self-reported associations between PTSD symptom clusters and smoking intensity among current smokers, they do not elucidate the smoking cessation and lapse process. Fine-grained analyses of smoking cessation attempts and lapses are needed to determine the role of numbing in the maintenance of smoking in PTSD. These process-level analyses are enabled by an experience sampling method called ecological momentary assessment (EMA), which uses prompts from electronic devices to collect ecologically valid self-report data on quickly changing phenomena such as thoughts, feelings, immediate environment, and substance use behavior (Shiffman, 2009). By collecting data in an individual’s naturalistic environment on phenomena as they occur, EMA reduces retrospective report bias and provides rich data on smoking triggers during critical short-term periods in smoking lapse and relapse.
Existing models of the role of PTSD symptoms and smoking have almost exclusively examined mean symptom levels, rather than the emotional lability and symptom instability. To provide clarity to models of smoking in PTSD, it is important to explore whether lapses occur as a function of 1) individual differences in mean PTSD symptoms that leave the individual vulnerable to lapse, 2) acute elevation in PTSD symptoms that trigger impulsive lapse at a specific and critical time, or 3) lagged acute elevation in PTSD symptoms that does not trigger lapse immediately, but increases risk of lapse in the following hours of the day.
If mean PTSD symptom severity is related to lapse, then intervention studies might investigate whether PTSD treatment alone could result in smoking cessation. Alternatively, if acute elevations in PTSD symptoms trigger smoking lapse, then anticipating the type and timing of lapse triggers and intervening at that critical time could be needed for individuals with PTSD to achieve sustained smoking abstinence. Finally, if acute symptom elevation produces a delayed lapse as the individual is unable to successfully cope with the elevated symptoms, then it could be necessary to explicitly link PTSD-specific coping skills to high risk of smoking lapse situations.
To investigate this difference and inform treatment, we examined ecological momentary assessments (EMA) of PTSD symptom clusters and smoking in the first week of a quit attempt in 52 people with PTSD. Collecting multiple assessments throughout the day allowed calculation of interindividual (between-person) differences in mean symptoms and intraindividual (within-person) deviation of each assessment from an individual’s mean symptom level. This distinction allows exploration of relative contributions of mean symptom level, acute elevation, and lagged acute elevation in predicting risk of smoking occasions. We hypothesized that acute elevation in PTSD symptoms would be most strongly associated with smoking occasions in both the pre-quit and post-quit time periods.
MATERIAL AND METHODS
2.1. Participants
This study included 52 smokers with PTSD who provided EMA assessments. For data and more procedural details, refer to an earlier publication comparing lapse in PTSD and non-PTSD smokers (Beckham et al., 2012). Eligibility criteria included smoking at least 10 cigarettes daily for the past year, willingness to make a smoking cessation attempt, and age 18–65. Participants were excluded for use of non-cigarette forms of nicotine, major unstable medical problems (e.g., cancer, unstable angina, poorly controlled diabetes, respiratory disorders requiring frequent hospitalization), use of bupropion or benzodiazepines, or homelessness. Though individuals who are homeless are willing to make smoking cessation attempts, more intensive intervention is likely needed (Carpenter et al., 2015). Individuals who are homeless have differences in their smoking cessation process, such as pervasiveness of others smoking in the vicinity (Tsai & Rosenheck, 2012) and difficulty gaining access and transportation to treatment resources (Connor, Scharf, Jonkman, & Herbert, 2014). All participants reported being in either the contemplation (n = 35) or preparation (n = 17) stage of quitting smoking at the screening session, which means they were planning to make a quit attempt in the next six months.
2.2. Procedures
Participants were recruited through their providers, IRB-approved flyers, and/or brochures advertising a study on stress and smoking. In addition, we recruited by obtaining a list of veterans who smoke from computerized medical records at the Durham VA Medical Center. Participants were also recruited using a series of invitational letters, as described by Dillman, Smith, and Christian (Dillman, Smyth, & Christian, 2009). Participants completed a screening session, two smoking cessation counseling sessions based on the National Cancer Institute Fresh Start program (Lando, McCovern, & Barrios, 1990), and two weeks of electronic diary monitoring. All participants had quit dates scheduled to occur the week of the second counseling session. Following the quit date, participants returned to the laboratory every two days for bioverification of smoking abstinence (expired carbon monoxide and salivary cotinine) for one week after the quit date. At the screening session, each participant provided sociodemographic information, smoking history, and completed the Fagerström Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Baseline presence of PTSD was assessed with the Clinician Administered PTSD Scale (CAPS; (Blake et al., 1995), using the 1/2 frequency/intensity rule for symptom threshold (Blake et al., 1995). Participants not meeting criteria for PTSD who were otherwise eligible were placed into a non-PTSD comparison group that is not included in this report because there is no clear way to interpret the influence of PTSD symptoms in individuals without PTSD. Psychiatric disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1994). Each rater was trained using SCID and CAPS standardized training (i.e., manual, videotapes, and co-rating training with a trained rater). Interrater reliability as determined by Fleiss’ kappa (Fleiss & Cohen, 1973) for diagnoses based on videotapes of patient interviews was excellent (κ = 0.96).
2.2.1. Ecological Momentary Assessment Procedures
Participants completed EMA readings for seven days of ad lib smoking and the first seven days after their quit date. Procedures are described in more detail in a previous publication (Beckham et al., 2012). Diary entries were time-stamped to ensure temporal accuracy (i.e., participants could not clump entries) and assess protocol adherence. Data for this report were derived from random alarm readings. To maximize the number of smoking occasions that were captured, these alarm-initiated readings asked whether the participant had smoked in the interval between readings. In addition to alarm-initiated readings, participants were asked to self-initiate readings whenever they resisted a smoking urge or had just experienced a smoking lapse. Participants were asked to complete these readings before smoking the cigarette so that EMA assessments reflected the symptoms experienced immediately prior to smoking a cigarette.
Alarm-initiated readings were designed to occur randomly between 2–3 hours after a completed assessment during the pre-quit period, and between 1–2 hours during the post-quit period to provide more assessments during this critical period. Participants had a two-minute window after the alarm to begin the assessment. They were instructed to ignore or suspend (interrupt the ringing sound) during an activity in which responding would be dangerous (e.g., driving) or too burdensome (e.g., religious services). Additionally, participants were able to delay an assessment with a 5-minute delay function. Finally, participants were able to inactivate alarms for 15–120 minutes when they expected to be unavailable and for 4–11 hours overnight for sleeping. Following any missed or skipped alarm, the next alarm was designed to go off within 30–45 minutes.
Participants were paid $75 for screening, $25/day for providing EMA readings, and up to an additional $45 in incentive pay during the pre-quit week ($25 for missing < 3 alarms per day in all days between sessions, and $20 for completing ≥ 3 entries that were self-initiated before each smoking occasions during the pre-quit phase) and another $45 in incentive pay during the post-quit week ($25 for missing ≤ 3 alarms in all days between sessions, and $20 for completing all evening post-quit week EMA readings). Participants were paid $25 for each post-quit visit with no relapse (> 5 cigarettes/day for at least three consecutive days), verified by self-report and CO reading. This could total a maximum of $750 if all incentive payments were earned.
2.2.2. Electronic Diary Assessments
All EMA readings included a question about whether the participant had smoked and PTSD symptoms.
PTSD Symptoms
Aside from CAPS at the screening visit, presence and severity of 15 of the 17 DSM-IV(American Psychiatric Association, 1994) PTSD symptoms were assessed using the Davidson Trauma Scale (DTS; (Davidson et al., 1997) throughout the study. The nightmare and sleep disturbance items were omitted because EMA was designed to measure symptoms during waking time. Severity was assessed on a 5-point scale with anchors ranging from “not at all” to “extremely,” and frequency of symptoms from DTS was omitted from analysis because multiple EMA readings captured frequency of symptoms. The severity items were summed to derive a PTSD overall score and symptom cluster scores at each reading.
2.3. Analysis Plan
For analysis purposes, EMA data were divided into two phases: pre-quit and post-quit. The pre-quit phase included all EMA readings before the quit attempt, which was defined as overnight abstinence bioverified by CO reading. The post-quit phase included readings between the quit attempt and the end of data collection one week after the quit attempt. To analyze EMA data, we used multilevel modeling (MLM). This technique is often used to model interindividual differences in intraindividual change [e.g., (Neupert, Mroczek, & Spiro, 2008)]. MLM is also uniquely suited for unbalanced data (i.e., missing data and data with differing numbers of cases per individual) because of the computation of adjusted means based on the data clustered at the individual level rather than on simple arithmetic means.
PTSD symptoms were z-scored to facilitate interpretation of effects and comparative analyses across measures. We calculated three summary variables. Grand-mean standardized symptom levels were calculated by taking the mean of all ecological momentary assessment symptom ratings by the participant across a given smoking phase (pre-quit or post-quit) and dividing by the sample standard deviation. Acute elevation was calculated for each reading by calculating the number of standard deviations a given reading was of above that participant’s individual mean symptom level, thus generating individual-mean standardized scores. This means that acute elevation measurements coincided with assessment of whether the participant had a smoking occasion. Lagged acute elevation used the acute elevation from the assessment before the one being analyzed. Due to the strong theoretical association of nicotine dependence with cigarette smoking behavior, nicotine dependence was included as a covariate in all models of symptom levels and variability. To prevent overfitting, other potential covariates with conceptual relevance to PTSD symptoms and smoking were not included, but analyses of antidepressant medication use, mean post-lapse cigarettes per day, and veteran status found that they did not change the pattern of results. We conducted sensitivity analyses of the influence of number of readings by including that covariate in models of the three summary predictor variables. Analyses were conducted using PROC GLIMMIX, available via SAS (Version 9.2).
RESULTS
Participant characteristics at baseline are summarized in Table 1. In the pre-quit phase, inter-individual mean symptoms were 8.0 (SD = 9.7) for total PTSD symptoms, 1.5 (SD = 2.5) for Re-experiencing cluster symptoms, 1.4 (SD = 1.7) for Avoidance cluster symptoms, 2.7 (SD = 3.4) for Numbing cluster symptoms, 2.4 (SD = 3.0) for Hyperarousal cluster symptoms, and 14.0 (SD = 4.6) for Negative Affect cluster symptoms. Post quit inter-individual mean symptoms were 9.9 (SD = 12.9) for total PTSD symptoms, 1.8 (SD = 3.2) for Re-experiencing cluster symptoms, 1.7 (SD = 2.4) for Avoidance cluster symptoms, 3.6 (SD = 4.7) for Numbing cluster symptoms, 2.8 (SD = 4.1) for Hyperarousal cluster symptoms, and 14.5 (SD = 5.6) for Negative Affect cluster symptoms.
Table 1.
Baseline Sociodemographic and Psychiatric Data (N = 52).
| Frequency (%)/Means (SD) | |
|---|---|
| Female (n) | 29 (56%) |
| Racial/Ethnic Minority (n) | 30 (58%) |
| Military Veteran (n) | 13 (25%) |
| Age (years) | 42.52 (10.32) |
| Education (years) | 12.61 (1.84) |
| Socioeconomic Status (Hollingshead) | 57.23 (11.55) |
| MDD, current (n) | 17 (33%) |
| MDD, lifetime (n) | 35 (67%) |
| CAPS Total | 63.8 (20.2) |
| Re-Experiencing | 17.6 (7.9) |
| Avoidance | 9.2 (3.8) |
| Numbing | 16.7 (7.9) |
| Hyperarousal | 20.3 (6.7) |
| Negative Affect (PANAS) | 14.3 (5.8) |
| Cigarettes per day | 17.56 (8.47) |
| Total years smoked | 26.40 (11.81) |
CAPS = Clinician-Administered PTSD Scale; MDD = major depressive disorder; PANAS = positive and negative affect scale; PTSD = posttraumatic stress disorder; SES = socioeconomic status. SD = standard deviation.
Logistic MLM was used to examine the degree to which changes in PTSD symptoms were associated with smoking in the pre-quit and post-quit periods. Odds of smoking were modeled as a function of grand-mean standardized PTSD symptom levels, acute elevation above the personal mean of PTSD symptoms, or lagged acute elevation. Nicotine dependence was statistically controlled in each of the models.
3.1. Pre-Lapse: Contributions of Inter-Individual Means, Intra-Individual Variability, and Intra-Individual Variability Lags to Odds of Smoking between Pre-Quit Readings
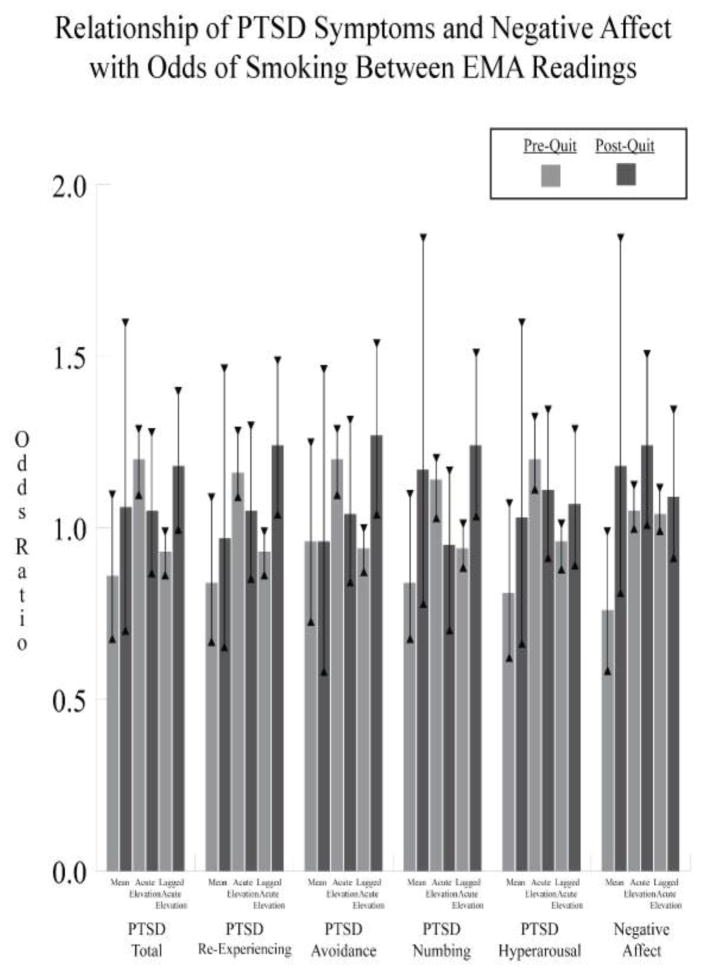
Detailed results of analyses from the pre-quit phase including nicotine dependence, PTSD mean symptoms, acute elevations, and lagged acute elevations together as simultaneous predictors of odds of smoking in the same models are presented in Table 2. In these models, acute elevations in PTSD symptom totals and each PTSD symptom cluster were the only significant predictors of smoking occasions. Smoking occasions were significantly associated with acute elevations in PTSD totals (OR = 1.20; 95% CI, 1.10 to 1.31), PTSD re-experiencing symptoms (OR = 1.16; 95% CI, 1.06 to 1.27), PTSD avoidance symptoms (OR = 1.20; 95% CI, 1.10 to 1.31), PTSD numbing symptoms (OR = 1.14; 95% CI, 1.04 to 1.24), and PTSD hyperarousal symptoms (OR = 1.20; 95% CI, 1.09 to 1.31). Smoking occasions were significantly associated with negative affect mean symptoms over the pre-quit phase (OR = 0.76; 95% CI, 0.59–0.99). Sensitivity analyses of all models including the number of readings as an additional covariate did not change the pattern of results, except to eliminate the significant association of negative affect mean symptoms with odds of smoking (OR = 0.78; 95% CI, 0.60 to 1.01; F(1,2779) = 3.51).
Table 2.
Models of Pre-Quit Relationship of Mean Symptoms, Acute Elevation, and Lagged Acute Elevation Models to Odds of Smoking. (N = 52)
| PTSD Total Symptoms | ||||
|---|---|---|---|---|
| Predictor | OR | 95% CI | F-value | p-value |
| Mean Symptoms | 0.86 | 0.67–1.11 | 1.40 | .238 |
| Acute Elevation | 1.20 | 1.10–1.31 | 15.77 | <.001 |
| Lagged Acute Elevation | 0.93 | 0.86–1.01 | 3.22 | .073 |
| PTSD Re-Experiencing Cluster | ||||
| Mean Symptoms | 0.84 | 0.65–1.10 | 1.61 | .205 |
| Acute Elevation | 1.16 | 1.06–1.27 | 9.64 | .002 |
| Lagged Acute Elevation | 0.93 | 0.85–1.01 | 3.07 | .080 |
| PTSD Avoidance Cluster | ||||
| Mean Symptoms | 0.96 | 0.74–1.25 | 0.09 | .763 |
| Acute Elevation | 1.20 | 1.10–1.31 | 16.95 | <.001 |
| Lagged Acute Elevation | 0.94 | 0.87–1.02 | 2.36 | .125 |
| PTSD Numbing Cluster | ||||
| Mean Symptoms | 0.84 | 0.65–1.08 | 1.81 | .179 |
| Acute Elevation | 1.14 | 1.04–1.24 | 7.85 | .005 |
| Lagged Acute Elevation | 0.94 | 0.86–1.02 | 2.38 | .123 |
| PTSD Hyperarousal Cluster | ||||
| Mean Symptoms | 0.81 | 0.62–1.07 | 2.25 | .136 |
| Acute Elevation | 1.20 | 1.09–1.31 | 14.34 | <.001 |
| Lagged Acute Elevation | 0.96 | 0.88–1.05 | 0.74 | .389 |
| Negative Affect | ||||
| Mean Symptoms | 0.76 | 0.59–0.99 | 4.28 | .039 |
| Acute Elevation | 1.05 | 0.97–1.15 | 1.43 | .232 |
| Lagged Acute Elevation | 1.04 | 0.96–1.13 | 0.93 | .335 |
Each variable in the Predictor column represents an odds ratio generated in a model that included baseline nicotine dependence as a covariate and each of the listed predictors (Mean Symptoms, Acute Elevation, and Lagged Acute Elevation). There was not a statistically significant relationship of nicotine dependence to smoking occasions in any of the models presented. PTSD = posttraumatic stress disorder. OR = Odds Ratio. CI = Confidence Interval. Mean Symptoms = mean of all ecological momentary assessment symptom ratings by the participant across the pre-quit phase. Acute Elevation = magnitude of elevation of a given assessment above that participant’s individual mean symptom level. Lagged Acute Elevation = Acute elevation from the assessment before the one being analyzed.
3.2. Post-Lapse: Contributions of Inter-Individual Means, Intra-Individual Variability, and Intra-Individual Variability Lags to Odds of Smoking between Post-Quit Readings
Detailed results of analyses from the post-quit phase are presented in Table 3. In models including PTSD mean symptoms, acute elevation, and lagged acute elevation together as simultaneous predictors, neither PTSD mean symptoms nor acute elevation were related to odds of smoking. However, increased odds of smoking were related to lagged acute elevation in PTSD re-experiencing (OR = 1.24, 95% CI, 1.03 to 1.50), PTSD avoidance (OR = 1.27, 95% CI, 1.05 to 1.53), PTSD numbing (OR = 1.24, 95% CI, 1.02 to 1.51), and negative affect (OR = 1.24, 95% CI, 1.02 to 1.50). Sensitivity analyses of all models including the number of readings as an additional covariate did not change the pattern of results
Table 3.
Models of Post-Quit Relationship of Mean Symptoms, Acute Elevation, and Lagged Acute Elevation Models to Smoking Occasions (N = 52).
| PTSD Total Symptoms | ||||
|---|---|---|---|---|
| Predictor | OR | 95% CI | F-value | p-value |
| Mean Symptoms | 1.06 | 0.69–1.61 | 0.06 | .804 |
| Acute Elevation | 1.05 | 0.86–1.29 | 0.22 | .636 |
| Lagged Acute Elevation | 1.18 | 0.98–1.41 | 3.04 | .082 |
| PTSD Re-Experiencing Cluster | ||||
| Mean Symptoms | 0.97 | 0.64–1.47 | 0.02 | .879 |
| Acute Elevation | 1.05 | 0.84–1.31 | 0.20 | .659 |
| Lagged Acute Elevation | 1.24 | 1.03–1.50 | 4.97 | .026 |
| PTSD Avoidance Cluster | ||||
| Mean Symptoms | 0.96 | 0.63–1.45 | 0.04 | .845 |
| Acute Elevation | 1.04 | 0.82–1.31 | 0.11 | .742 |
| Lagged Acute Elevation | 1.27 | 1.05–1.53 | 6.29 | .012 |
| PTSD Numbing Cluster | ||||
| Mean Symptoms | 1.17 | 0.75–1.81 | 0.47 | .493 |
| Acute Elevation | 0.95 | 0.76–1.20 | 0.16 | .691 |
| Lagged Acute Elevation | 1.24 | 1.02–1.51 | 4.55 | .033 |
| PTSD Hyperarousal Cluster | ||||
| Mean Symptoms | 1.03 | 0.66–1.61 | 0.02 | .891 |
| Acute Elevation | 1.11 | 0.90–1.35 | 0.95 | .330 |
| Lagged Acute Elevation | 1.07 | 0.88–1.30 | 0.42 | .517 |
| Negative Affect | ||||
| Mean Symptoms | 1.18 | 0.77–1.81 | 0.59 | .443 |
| Acute Elevation | 1.24 | 1.02–1.50 | 4.86 | .028 |
| Lagged Acute Elevation | 1.09 | 0.90–1.31 | 0.73 | .392 |
Each variable in the Predictor column represents an odds ratio generated in a model that included only baseline nicotine dependence as a covariate and the named variable as the only predictor. There was not a statistically significant relationship of nicotine dependence to smoking occasions in any of the models presented. PTSD = posttraumatic stress disorder. OR = Odds Ratio. CI = Confidence Interval. Mean Symptoms = mean of all ecological momentary assessment symptom ratings by the participant across the pre-quit phase. Acute Elevation = magnitude of elevation of a given assessment above that participant’s individual mean symptom level. Lagged Acute
Elevation = Acute elevation from the assessment before the one being analyzed.
DISCUSSION
In this study, we used data provided by real-time EMA data collection methods to calculate variables that would reflect different processes by which PTSD symptoms might function to trigger smoking in people with PTSD before and during a quit attempt. This provided the opportunity to make comparisons between the possibility of persistent increased risk of smoking caused by chronic PTSD severity, the risk of impulsive smoking that might be triggered by acute symptom elevations, and potentiation of delayed lapse by acute symptom elevations. Results suggested that overall PTSD mean symptoms were not as strongly related to smoking occasions as the acute elevation in PTSD symptoms. The pre-quit period was characterized by smoking that coincided with acute elevation in PTSD symptom clusters. In the post-quit period, increased risk of smoking was related to lagged acute elevation in re-experiencing, avoidance, and numbing symptoms, as well as acute elevations in negative affect.
Results generally supported a relationship of PTSD symptoms with smoking occasions. However, rather than PTSD symptom increasing risk of smoking occasions in a consistent manner or in an entirely unpredictable, erratic manner, there was a clear relationship between the acute elevations in PTSD symptoms and ad lib smoking in the week preceding the quit attempt. In the post-quit week, smoking did not consistently coincide with acute PTSD symptom elevations. Instead, results suggested that participants were able to resist smoking immediately in response to acute elevations, but only for a limited amount of time. Analysis of lag values suggested that re-experiencing avoidance, and numbing symptoms potentiated smoking lapse, but only after a lag of several hours. It is possible that trauma-related thoughts and feelings (i.e., re-experiencing), as well as the associated avoidance of trauma reminders, are tolerated by smokers trying to quit for a short time period. Acute elevations in negative affect were associated with more immediate smoking lapse, suggesting that failure to reduce PTSD symptoms precedes negative affect in the chain of events that result in smoking. If so, this critical time period could be targeted for use of PTSD symptom coping skills by using mobile health interventions to ensure that individuals are aware of effective coping skills that will address the acute elevation in PTSD symptoms and prevent smoking lapse. Interaction with mobile health applications that assess PTSD symptoms and other smoking triggers could alert individuals to use coping skills acquired in therapy to prevent smoking lapses.
The observation that lagged acute elevations in re-experiencing and avoidance symptoms preceded the smoking lapse by up to several hours could reflect the process by which PTSD symptoms emerge over a short period of time. Individuals with PTSD tend to use avoidance to cope with distressing reminders of the trauma (Pineless, Mostoufi, Ready, Street, & Griffin, 2011). Because avoidance is ultimately an unsuccessful coping strategy for resolving distress related to re-experiencing, the result is an increase in hyperarousal and potentially in physically harmful coping methods such as substance misuse (Roberts, Roberts, Jones, & Bisson, 2015). This interpretation supports the utility of treatments that address trauma-related avoidance in facilitating smoking cessation. To date, the most successful interventions for comorbid PTSD and substance use disorder is for individual therapy that incorporates substance use into trauma-focused psychotherapy (Roberts et al., 2015). This approach has been attempted for comorbid PTSD and tobacco smoking (Dedert et al., 2015), but further research is needed to establish its effectiveness.
This study was limited by the 1 week time frame, which gives an insight into the process by which lapse occurs in the first week of a quit attempt. However, it will be important for future research to report on the influence of PTSD symptoms on long-term smoking abstinence processes. It is also likely that not all smoking occasions were initiated by participants. Though participants were asked to record symptoms before smoking cigarettes for self-initiated readings, it is possible that participants waited until after smoking to record symptoms. Due to exclusions, results do not generalize to individuals who are homeless, use non-cigarette forms of nicotine, or have unstable medical conditions.
4.1. Conclusions
In summary, this investigation of the first week of smoking occasions in PTSD found that acute elevation in symptoms were more strongly related to smoking occasions than an individual’s general symptom severity over time. In addition, we found that while acute elevation in PTSD symptoms accompanied smoking occasions before the quit date, the post-quit period was characterized by a lag time of a few hours between spikes in re-experiencing, avoidance, and numbing symptoms and lapsing to smoking. This period presents an opportunity to use mobile health interventions to prevent smoking lapse and to use coping skills acquired in trauma-focused therapy to respond to acute PTSD symptom elevation. Future research could also examine how modifiable exacerbation-triggered symptoms are, and look at whether decreases in PTSD symptoms related to trauma-focused treatment result in decreased symptom variability and decreased relationship between PTSD symptom exacerbations and smoking occasions.
Figure 1. Relationship of PTSD Symptoms and Negative Affect with Odds of Smoking Between Ecological Momentary Assessment Readings by Phase of Smoking Cessation.
Results are derived from models including nicotine dependence as a covariate and PTSD and Negative Affect mean symptoms, acute elevations, and lagged acute elevations as simultaneous predictors in all models. PTSD = posttraumatic stress disorder.
* = p < .05.
HIGHLIGHTS.
Smoking interventions could be improved by process research on PTSD symptoms and lapse.
During a quit attempt, PTSD re-experiencing, avoidance, and numbing triggered lapses.
During quit attempts, participants successfully delayed PTSD-triggered lapse for short periods.
Critical periods could benefit from mobile health prompts to use coping skills.
Acknowledgments
ROLE OF FUNDING SOURCES
Funding for this study was provided by Award Number IK2CX000718 to Dr. Dedert from the CSR&D Service of the VA Office of Research and Development and NCI Grant R01 CA081595. Neither the VA nor NCI had any role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
The authors wish to thank Michelle Dennis, who provided programming of EMA readings and data management for the study. The authors also wish to thank the participants who provided data for the study.
Footnotes
CONTRIBUTORS
Drs. Beckham and Calhoun designed the study and wrote the protocol. Dr. Dedert wrote the first draft of the manuscript. Mr. Hicks conducted the literature searches and provided summaries of previous research studies. Dr. Dennis provided statistical consulting and design of analyses. All authors contributed to and have approved the final manuscript.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Asmundson GJ, Frombach I, McQuaid J, Pedrelli P, Lenox R, Stein MB. Dimensionality of posttraumatic stress symptoms: a confirmatory factor analysis of DSM-IV symptom clusters and other symptom models. Behaviour Research & Therapy. 2000;38(2):230. doi: 10.1016/S0005-7967(99)00061-3. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Johnson YC, Dedert EA. Predictors of lapse in the first week of smoking abstinence in posttraumatic stress disorder and non-posttraumatic stress disorder smokers. Nicotine & Tobacco Research. 2012 doi: 10.1093/ntr/nts252. Epub ahead of print [Nov. 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered posttraumatic stress disorder scale. Journal of Traumatic Stress. 1995;8:75–80. doi: 10.1007/BF02105408. 0894-9867/95/0100--U075507,50/1. [DOI] [PubMed] [Google Scholar]
- Carpenter VL, Hertzberg JS, Kirby AC, Calhoun PS, Moore SD, Dennis MF, … Beckham JC. Multicomponent smoking cessation treatment including mobile contingency management in homeless veterans. Journal of Clinical Psychiatry. 2015;76(7):959–964. doi: 10.4088/JCP.14m09053. doi: dx.doi.org/10.4088/JCP.14m09053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor SE, Scharf DM, Jonkman LJ, Herbert MI. Focusing on the five A's: a comparison of homeless and housed patients' access to and use of pharmacist-provided smoking cessation treatment. Research in Social & Administrative Pharmacy. 2014;10:369–377. doi: 10.1016/j.sapharm.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Davidson JRT, Book SW, Colket JT, Tupler LA, Roth S, David D, … Feldman ME. Assessment of a new self-rating scale for posttraumatic stress disorder: The Davidson Trauma Scale. Psychological Medicine. 1997;27:153–160. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Resick PA, McFall ME, Dennis PA, Olsen MK, Beckham JC. Pilot cases of combined cognitive processing therapy and smoking cessation for smokers with posttraumatic stress disorder. Behavior Therapy. 2015 doi: 10.1016/j.beth.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman DA, Smyth JD, Christian LM. Internet, mail, and mixed-mode surveys: The tailored design method. 3. New York: John Wiley; 2009. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Lando HA, McCovern PG, Barrios FX. Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. American Journal of Public Health. 1990;80:554–559. doi: 10.2105/ajph.80.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert SD, Mroczek DK, Spiro A. Neuroticism moderates the daily relation between stressors and memory failures. Psychology and Aging. 2008;23(2):287–296. doi: 10.1037/0882-7974.23.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineless SL, Mostoufi SM, Ready CB, Street AE, Griffin MG. Trauma reactivity, avoidant coping, and PTSD symptoms: A moderating relationship? Journal of Abnormal Psychology. 2011;120:240–246. doi: 10.1037/a0022123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, Bisson JI. Psychological interventions for posttraumatic stress disorder and comorbid substance use disorder: A systematic review and meta-analysis. Clinical Psychology Review. 2015;38:25–38. doi: 10.1016/j.cpr.2015.02.007. doi: http://dx.doi.org/10.1016/j.cpr.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21(4):486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Rosenheck RA. Smoking among chronically homeless adults: Prevalance and correlates. Psychiatric Services. 2012;63:569–576. doi: 10.1176/appi.ps.201100398. [DOI] [PubMed] [Google Scholar]