Abstract
Impairments in memory and cognitive function are characteristic symptoms of stress-related disorders, but the relationship between stress and memory is complex. Recent findings suggest that hippocampal inflammatory processes contribute to spatial memory deficits in the rodent social defeat model that can be reversed by anti-inflammatory treatment with minocycline.
Keywords: Inflammation, stress, memory, neurogenesis
At least 20% of patients with stress-related disorders meet criteria for cognitive impairment [1]. Cognitive symptoms include diminished concentration, impaired working memory, and an enhanced negative bias in thinking [2]. These impairments are often maintained in remission, indicating that current antidepressant treatments insufficiently address cognitive symptoms. Reduced hippocampal activation and neurogenesis along with hormonal and inflammatory processes have been implicated in stress-related cognitive impairments; however, the precise mechanisms remain incompletely defined. A recent paper by McKim and colleagues [3] provides important new insight, demonstrating a prominent role for neuroinflammation, but not hippocampal neurogenesis, in memory impairments induced by social defeat stress. Social defeat stress, which involves the repeated social subordination of an experimental mouse by a larger, physically aggressive mouse, recapitulates many of the symptoms of depression, including anhedonia, social avoidance, weight change, and circadian disruption [4, 5]. Importantly, social defeat also activates the immune system, producing lasting changes in peripheral and central cytokine levels, microglial activation, number and sensitivity of circulating leukocytes, and monocyte recruitment to the brain [6–9].
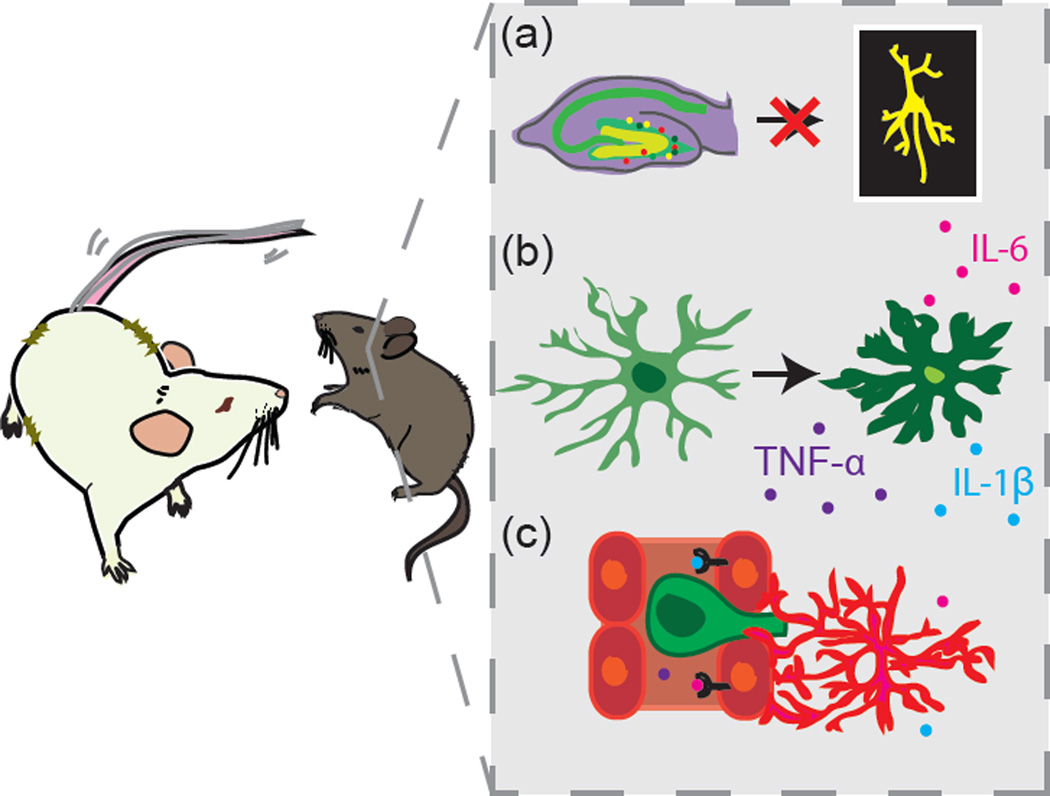
McKim et al. assessed the effects of social defeat-induced neuroinflammatory processes on learning and memory, hippocampal neurogenesis, and social avoidance behavior. Mice were exposed to Repeated Social Defeat (RSD), a version of social defeat stress in which a novel, aggressive CD-1 intruder mouse is placed in the home cage of three C57BL/6 mice for a two hour period over six consecutive nights. The intruder mouse disrupts the established social hierarchy of the trio and elicits submissive behaviors. The authors found that RSD induced a pro-inflammatory profile along with neurogenesis deficits in the hippocampus of mice exposed to RSD compared to unstressed controls. They observed increased hippocampal cytokine and growth factor gene expression (IL-1β, TNF-α, IL-6, VEGF), enhanced microglial Iba-1 immunoreactivity in the dentate gyrus (DG), and an increase in DG CD45-positive cells in RSD mice, suggesting recruitment of peripheral monocytes to the brain (Figure 1b–c). While the authors found no differences between RSD and control mice in cell proliferation in the DG, they reported reduced colocalization of BrdU with DCX (doublecortin, an immature neuronal marker) and NeuN (a mature neuronal marker) at 10 and 28 days post-stress, respectively, in RSD mice, indicating impaired differentiation of neural progenitor cells (NPCs) into mature neurons (Figure 1a). These cellular changes were most pronounced in the caudal hippocampus and accompanied behavioral changes in memory and mood. RSD mice showed a mild, short-lived impairment in Morris water maze working memory stemming from an anxiety-related thigmotactic search strategy. However, unrelated to anxiety as validated in the Barnes Maze, RSD mice also demonstrated impaired short-term spatial memory recall, but not acquisition or long-term recall, 28 days after stress. RSD mice also displayed increased social avoidance in a social interaction test. To determine the necessity of neuroinflammation for RSD-induced behavioral changes, the authors treated mice with minocycline, an antibiotic with anti-inflammatory properties, prior to RSD exposure. Minocycline abrogated the RSD-induced increase in Iba-1 immunoreactivity and monocyte recruitment, and prevented spatial memory recall deficits in the Barnes Maze. However, minocycline did not rescue social avoidance or NPC differentiation into mature neurons in RSD mice, leading the authors to conclude that neuroinflammation mediates the cognitive, but not long-term emotional, impairments produced by RSD.
Figure 1. Overview of hippocampal changes following exposure to Repeated Social Defeat (RSD).
A recent publication by McKim et al. [3] finds that RSD produces numerous cellular and molecular consequences in the caudal hippocampus relevant to cognitive function. Changes include reduced differentiation of neural progenitor cells into mature neurons in the dentate gyrus (A), enhanced microglial activation and increased expression of pro-inflammatory cytokines (B), and trafficking of monocytes into the brain from the peripheral circulation (C).
The rescue of the central inflammatory and cognitive effects of RSD by minocycline highlights the role of inflammation in stress-induced behavioral changes and identifies a potential therapy for patients experiencing stress-related cognitive impairment. As such, this work has interesting implications for post-traumatic stress disorder as well as depression. Minocycline is a broad spectrum antibiotic that easily crosses the blood-brain barrier into the CNS, where is has been shown to inhibit microglial activation, cytokine signaling, and immune cell infiltration [10]. Interestingly, minocycline has shown efficacy as an adjunct to traditional antidepressants in an open label trial for psychotic depression, with patients demonstrating significant improvement in depressive and psychotic symptoms [11]. However, the effect of minocycline on cognitive symptoms has not been assessed clinically in patients with stress-related disorders. It is surprising that an anti-inflammatory compound did not alleviate social avoidance as previous work shows that manipulating the peripheral immune system can reverse social avoidance after chronic social defeat stress (CSDS). CSDS (sometimes also referred to as repeated social defeat stress or RSDS) utilizes a different stress protocol than is used in RSD. Over the course of 10 days, a C57BL/6 mouse is individually exposed to a novel, aggressive CD-1 mouse each day for a 10 minute physical encounter, followed by overnight sensory, but not physical, contact through a perforated plastic partition [4]. Social avoidance behavior is measured 24 hours after the last defeat session by a social interaction test, and mice are divided into stress resilient and stress susceptible groups based on their degree of preference for interacting with a novel CD-1 mouse versus an empty enclosure. Resilient and susceptible mice are biologically distinct populations, with resilient mice more closely resembling control mice that have never been exposed to CSDS [5]. Blocking peripheral IL-6 via treatment with a monoclonal antibody or transplantation with IL-6 knockout bone marrow promoted resilience to defeat-induced social avoidance [6]. It is possible that antibiotic treatment with minocycline produced changes to the microbiome that prevented rescue of social avoidance, as probiotic supplementation with the strains Bifidobacterium and Lactobacillus has yielded positive results in reducing anxiety- and depression-like behavior [12]. Alternatively, the general anti-inflammatory properties of minocycline may have been insufficient to reverse social avoidance deficits that potentially require more targeted immunomodulatory approaches. It would be interesting to explore the circulating cytokine profiles of minocycline-treated RSD mice in order to further understand the effect of minocycline on stressinduced peripheral inflammation.
Prior literature suggests a role for neurogenesis in mediating individual differences in social avoidance following CSDS. Lagace et al. [13] demonstrated that CSDS exposure induced phenotype specific differences in neurogenesis that mediated persistent social avoidance behavior. Immediately following stress, both susceptible and resilient mice displayed reduced DG cell proliferation that normalized within 24 hours. However, when the authors labeled proliferating cells one hour after stress cessation and then waited 4 weeks to measure cell survival, susceptible mice displayed more labeled cells 4 weeks post-CSDS than did control and resilient mice. This effect was driven by an increase in transient-amplifying progenitors. Mice in which hippocampal neurogenesis was ablated by X-irradiation 4 weeks prior to social defeat exhibited enhanced resilience for at least 4 weeks following defeat. The authors hypothesized that the greater survival of DG neurons generated after defeat stress in susceptible mice may enable stronger, persistent memory of traumatic events associated with CSDS. The differences in findings between the studies of McKim et al. and Lagace et al. may reflect differences in the time course of BrdU injections, the social defeat protocol employed, or the delineation of susceptible and resilient mice. Collectively, the two studies suggest that social defeat alters neurogenesis in a manner perhaps independent of central inflammation, and that these changes are relevant to long-term emotional memory. However, the precise contribution of neurogenesis to social avoidance and other depression- and anxiety-like behaviors in the social defeat model requires further exploration.
In conclusion, new work by McKim et al. establishes an important role for neuroinflammation in the cognitive consequences of social defeat stress, and raises important questions regarding the nature and behavioral relevance of defeat-induced changes to hippocampal neurogenesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69:1122–1130. doi: 10.4088/jcp.v69n0712. [DOI] [PubMed] [Google Scholar]
- 2.Papakostas GI, Culpepper L. Understanding and managing Cognition in the Depressed Patient. J Clin Psychiatry. 2015;76:418–425. doi: 10.4088/JCP.13086ah1c. [DOI] [PubMed] [Google Scholar]
- 3.McKim DB, et al. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J Neurosci. 2016;36:2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golden SA, et al. A standardized protocol for repeated social defeat stress in mice. Nature protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wohleb ES, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohleb ES, et al. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell ND, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido-Mesa N, et al. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyaoka T, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:222–226. doi: 10.1016/j.pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EA, et al. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagace DC, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]