Abstract
Background
Most non-oncologic clinical practice guidelines recommend restrictive allogeneic blood transfusion practices; however, there is a lack of consensus regarding the best transfusion practice in oncology. We conducted a systematic review of the literature to compare the efficacy and safety of restrictive versus liberal transfusion strategies in patients with cancer.
Methods
A literature search using MEDLINE, PUBMED and EMBASE identified all controlled studies comparing the use of restrictive with liberal transfusion in adult oncology participants up to August 10, 2015. Two review authors independently assessed studies for inclusion, extracted data and appraised the quality of the included studies. The primary outcomes of interest were blood utilization and all-cause mortality.
Results
Out of 4241 citations, six studies (3 randomized and 3 non-randomized) involving a total of 983 patients were included in the final review. The clinical context of the studies varied with 3 chemotherapy and 3 surgical studies. The overall risk of bias in all studies was moderate to high. Restrictive transfusion strategies were associated with a 36% reduced risk of receiving a perioperative transfusion (risk ratio (RR) 0.64, 95% confidence interval (CI) 0.49 to 0.83). There was no difference in mortality between the strategies (RR 1.00, 95% CI 0.32 to 3.18). There were no differences in adverse events reported between the restrictive and liberal transfusion strategies.
Conclusion
Restrictive strategy appears to decrease blood utilization without increasing morbidity or mortality in oncology. This review is limited by a paucity of high quality studies on this topic. Better designed studies are warranted.
Introduction
Anemia in cancer patients is pervasive with studies reporting rates up to 90%.[1–3] The etiology of anemia in cancer patients is multifactorial and involves multiple different mechanisms including nutritional deficiencies, surgical blood loss and myelosuppressive effects of chemotherapy and radiation.[3, 4] Numerous studies have demonstrated that anemia is a prognostic indicator of poor clinical and oncologic outcomes.[5–10]
A combination of clinical studies revealing the adverse impact of anemia and animal models demonstrating optimal oxygen transport at hemoglobin levels greater than 10 g/dL has resulted in the historical trend towards liberal use of red cell transfusions to correct anemia in oncology patients.[9, 11, 12] Despite the liberal use of transfusion in many oncology studies, there are little data to support the efficacy of correcting anemia with transfusion.[13] In fact, there is evidence that suggests that blood transfusions are independently associated with worse perioperative and oncologic outcomes.[14–17] Furthermore, there is evidence from other subspecialty fields that a liberal blood transfusion strategy does not improve clinical outcomes over a restrictive strategy.[18–20] As such, many subspecialty societies have developed specific clinical practice guidelines that recommend restrictive red cell transfusion.[21–23] Evidence from institutional quality improvement initiatives has demonstrated that restrictive strategies have similar clinical outcomes while utilizing less blood.[21–27]
Despite the widespread adoption of restrictive transfusion strategies seen in other fields, the oncology community has been resistant to change. This is in part because oncology patients are perceived to be different than non-oncology patients. The use of anticancer treatments such as radiotherapy and chemotherapy can lead to anemia and subsequent treatment delays if the anemia is not corrected quickly. Furthermore, the high incidence of fatigue in this patient population requires different transfusion strategies than other acutely ill populations to improve quality of life.
There is a lack of consensus regarding best transfusion practices resulting in in wide variability in blood utilization.[28–30]. Therefore, we conducted a systematic literature review to compare the efficacy and safety of restrictive versus liberal transfusion strategies in patients with cancer. The purpose of this review was to find, evaluate and summarize the existing literature to fill a gap in knowledge regarding restrictive transfusion strategies in oncology.
Methods
Study design, protocol and registration
We adhered to the Cochrane Collaboration methodology for conducting this review.[31] Study methodology was defined a priori and our protocol was registered online in advance (PROSPERO CRD42015019732). We report our results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations for reporting (PRISMA) statement.[32]
Eligibility criteria
Controlled studies comparing a liberal allogeneic packed red blood cell transfusion strategy to a restrictive allogeneic packed red blood cell transfusion strategy in adult oncology patients were considered. This included randomized and non-randomized studies. It was anticipated that the exact trigger or strategy may vary between studies. Patients could be receiving treatment with curative or palliative intent. Curative intent may involve surgical or medical treatment including chemotherapy or radiotherapy. Studies involving infants or neonates were excluded.
Data sources and search strategy
A literature search was performed with guidance from an experienced public health research librarian (HV). We searched the following databases: MEDLINE (Ovid), PUBMED (National Library of Medicine), EMBASE (Ovid) from inception until August 10, 2015. Additionally, all highly relevant studies were searched in Scopus (Elsevier) to determine if any unique studies were missed by the database searches. Bibliographies of the included studies were examined for highly relevant citations. Our search was restricted to adult patients and controlled studies published in English. No other restrictions were applied. The Medline search strategy is provided in Appendix A.
Study selection
The PRIMARY Excel Workbook for Systematic Reviews was used to screen titles and abstracts of items found through database searching.[33] Two reviewers (LP and JT) independently screened titles and abstracts in which they were blinded to authors and journal titles. Full texts were retrieved for relevant citations. In cases of disagreement, the reviewers reached a consensus through discussion or through third party adjudication (MLO).
Data collection
Two review authors (LP and JT) independently abstracted study characteristics and outcomes using a data extraction form. All characteristics and outcomes were reviewed together and discrepancies were resolved through discussion. In case of persistent disagreement, MLO served as an adjudicator. LP entered all data into RevMan version 5.3.19[34] and data were verified by JT and MM. Dichotomous outcomes were collected according to number of patients affected. Study authors were not contacted for missing data.
Outcomes
The primary outcomes of interest were blood utilization and all-cause mortality. Secondary outcomes included cancer-related mortality, perioperative morbidity (infection, venous thromboembolism, pneumonia, unintended intubation, renal failure, stroke, cardiac arrest, myocardial infarction, flap failure, and prolonged ventilator use), transfusion-related adverse events, and other adverse events. We collected all outcome data reported in each study.
Quality assessment
Two reviewers (LP and JT) independently appraised the quality of the included studies. The Cochrane risk of bias tool was used to assess the randomized studies.[31] The tool judges the risk of 5 types of bias (i.e., selection, detection, performance, attrition, reporting) and other potentials to validity threats (e.g., funding, imbalanced use of co-intervention, etc.). Each potential source of bias was graded as low, unclear or high. Non-randomized studies were also independently appraised with A Cochrane Risk of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI).[35] Studies were judged for the potential for bias due to confounding, selection of participants, measurement of interventions, departures from intended interventions, missing data, measurement of outcomes, and selection of the reported result. Each potential source of bias was graded low, moderate, serious, critical risk of bias or no information at the outcome level. An overall risk of bias judgment for each non-randomized study across all domains was determined based on the level of bias of each of the aforementioned components. Studies were only determined to be low risk if they met criteria for low risk on all domains. Otherwise they were judged to be at moderate risk of bias or higher. Disagreements were resolved through discussion.
Summary measures, synthesis of results and analysis
We performed our meta-analysis using RevMan.[34] We calculated the risk ratio for dichotomous variables and the mean difference for continuous variables. Data were synthesized using fixed effects models except when significant heterogeneity was found. We used the I2 statistic to examine heterogeneity among the studies.[36] In the presence of significant heterogeneity (P<0.05), we fit a random effects model based on the method of Der Simonian and Laird.[37] We analyzed only the available data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions chapter on missing data.[36] The patient was the unit of analysis. If insufficient data existed (< 2 studies reporting on the same outcome), descriptive statistics were utilized to report outcomes.
Results
Study selection
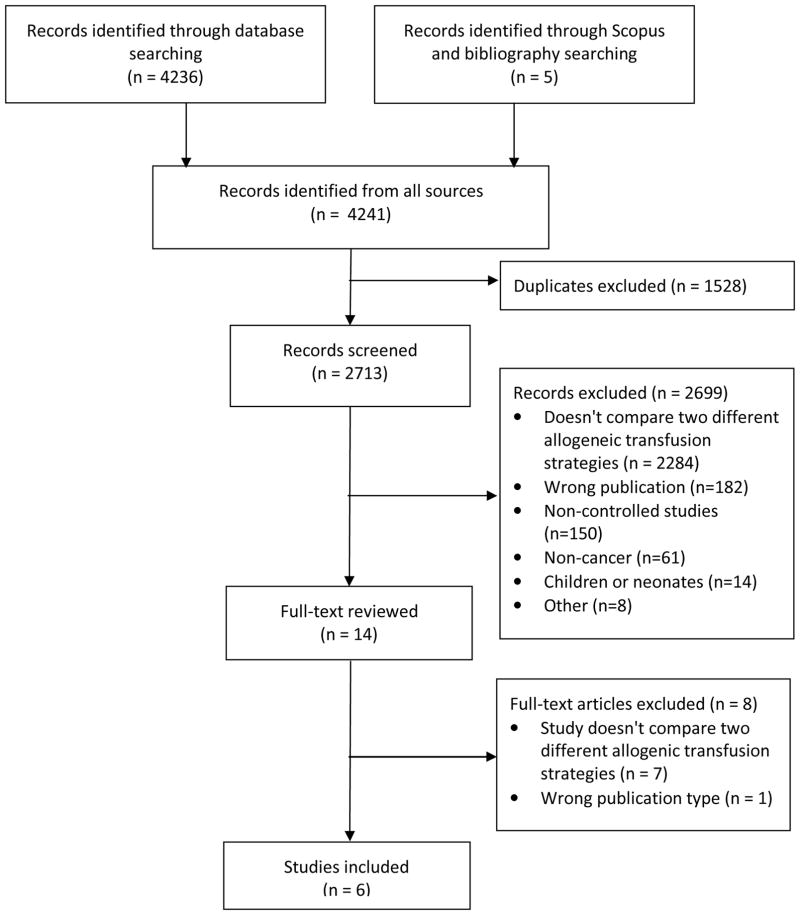
The PRISMA flow diagram in 1 illustrates the study identification and selection process. There were 4241 studies identified through our search, of which 14 were retrieved for full evaluation. Of the 14 articles retrieved for full-text review 6 studies involving 983 participants were included for the final review: 3 randomized controlled trials [38–40] and 3 nonrandomized studies.[26, 27, 41]
Study characteristics
The study characteristics are depicted in Table 1. Three studies took place in the context of chemotherapy [38, 40, 41] and three studies were in the context of surgery.[26, 27, 39] Transfusion strategies were evaluated in several different types of cancer including leukemia,[40, 41] gastric,[38] colorectal,[26] hepatobilliary,[27] and mixed surgical oncology patients.[39] Two studies were conducted in the United States,[26, 27] one in Brazil,[39] one in Canada,[40] one in the Netherlands[41] and one in Korea.[38] There was significant heterogeneity in the definition of restrictive and liberal transfusion strategies with overlap between studies. The restrictive strategies ranged from use of a hemoglobin trigger of 7 g/dL [27, 39] to 8 g/dL[40] to 10 g/dL.[38] One study utilized age-dependent transfusion triggers ranging from 7.2 g/dL to 8.0 g/dL[41] and one used an individualized approach with no specific trigger.[26] The liberal strategies also varied with studies utilizing a hemoglobin trigger of 9 g/dL,[39] 9.6 g/dL,[41] and 12 g/dL.[27, 38]. Two studies did not utilize specific triggers and were described as liberal blood transfusion use based on standard practice.[26, 27] In all 3 randomized trials, random allocation was at the patient level. Three trials included fewer than 100 participants.[38, 40, 41]
Table 1.
Characteristics of Studies
| Author, date | Location | Type of Cancer | Design | Sample Size | Restrictive Strategy (hgb trigger) | Liberal Strategy, (hgb trigger) | Primary Outcome | Funding |
|---|---|---|---|---|---|---|---|---|
| Froman, 2012 | USA | Colorectal | NRS | 272 | Individualized approach with no specific trigger | Liberal standard practice | Transfusion rate | None |
| Jansen, 2003 | Netherlands | Leukemia | NRS | 46 | Age-dependent transfusion triggers ranging from 7.2 g/dL to 8.0 g/dL | Hgb ≤ 9.6 g/dL | Number of units transfused and number of units given per transfusion | None |
| Park, 2008 | South Korea | Gastric | RCT | 43 | Hgb ≤ 10 g/dL | Hgb ≤ 12 g/dL | Objective response rate | Gachon University Gil Medical Center |
| Pinheiro, 2015 | Brazil | Mixed surgical oncology | RCT | 97 | Hgb ≤ 7 g/dL | Hgb ≤ 9 g/dL | All –cause mortality or severe clinical complication | Institution support |
| Webert, 2008 | Canada | Leukemia | RCT | 31 | Hgb ≤ 8 g/dL | Hgb ≤ 12 g/dL | Feasibility | Canadian Blood Services, one author supported by Novo Nordisk |
| Wehry, 2015 | USA | Hepatobiliary | NRS | 126 | Hgb ≤ 7 g/dL | Liberal standard practice | Number of transfusions | NCI |
NRS = non-randomized study; RCT = randomized controlled trial; Hgb = hemoglobin; NCI = National Cancer Institute
Risk of bias
Randomized Studies
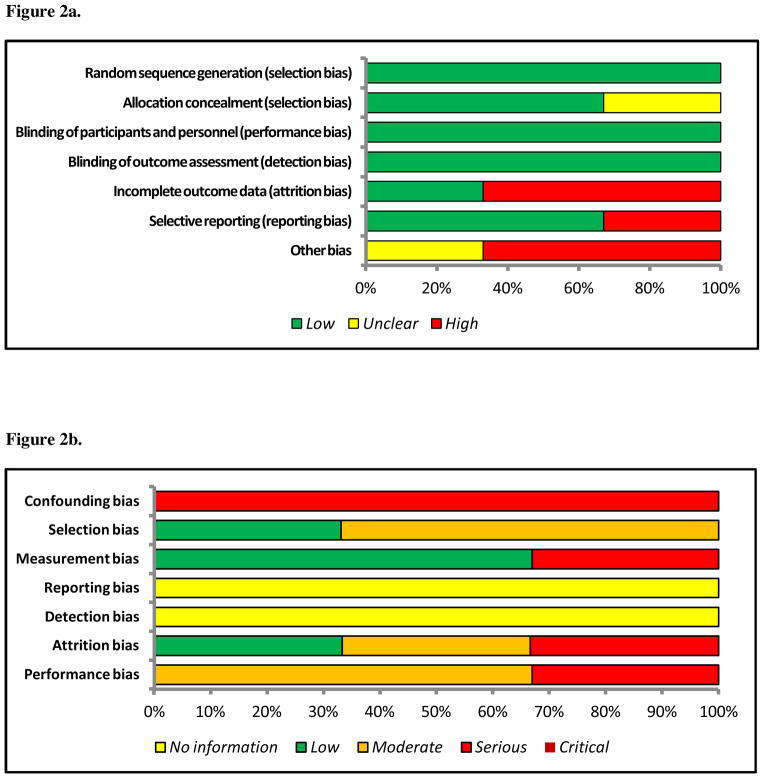
The risk of bias graph for randomized studies is depicted in Figure 2a. We judged the risk of random sequence generation (selection bias) to be low for all 3 randomized trials.[38–40] Two trials used a random table number generator [38, 39] and one trial used a computer-generated random number sequence.[40] Allocation concealment was reported for two of the three trials. In one study it was unclear if allocation occurred centrally or locally.[38] One study reported blinding of participants [39] and two studies reported blinding of outcome assessor;[39, 40] however, the primary outcomes for all three studies were judged to be objective measures that were felt by the authors to not be influenced by the presence or absence of blinding. Two of the three trials were judged as being high risk for incomplete outcome data (attrition bias) because an as-treated analysis was done with substantial departure from allocation [39] and significant cross-over between the two strategies was noted, but raw numbers were not reported.[40] Only one study performed a power calculation.[39] Two of the three studies were noted to have low reporting bias. In Park et al.,[38] one or more of the outcomes of interest were reported incompletely so they could not be entered into the meta-analysis. In the other two, all outcomes measured were reported.
Figure 2.
Figure 2a. Risk of Bias Graph for Randomized Studies
Figure 2b. Risk of Bias Graph for Randomized Studies
Non-randomized studies
The risk of bias graph for non-randomized studies is depicted in Figure 2b. Significant confounding was present in all three studies with differences between strategies not accounted for in analysis. Judgments on the other domains ranged from low to serious. No studies were regarded as critical. Selection bias was low in one study as all patients who underwent liver resection were included in a prospectively collected database. Intervention status was well defined for two studies.[27, 41] None of the three studies reported effect size and no information was provided regarding departure from intended interventions. All three studies were noted to have overall high risk of bias. This judgment was dominated by the high risk of confounding in all three studies.
Results of individual studies and synthesis of results
The studies varied in the outcomes reported and therefore we were unable to pool data for all outcomes of interest. We were able to provide aggregate data for the following outcomes: all-cause mortality, surgical site infections (SSI), urinary tract infections (UTI), venous thromboembolism (VTE), objective response, bleeding, myocardial infarction (MI) and proportion of patients transfused during the perioperative period. Blood utilization data were reported using different measures for each study and thus we report descriptive results for transfusion rates and blood usage. The summary of effect estimates and findings by outcomes are listed in Table 2. Forrest plots for all analyses not provided in the manuscript are available in Appendix B.
Table 2.
Summary of Effect Estimates and Findings from Meta-analysis
| Outcome | Studies | Participants | RR 95% CI) |
|---|---|---|---|
| Perioperative blood transfusions* | 3 | 812 | 0.64 [0.49, 0.83] |
|
| |||
| Mortality (30-day) | 5 | 896 | 1.00 [0.32, 3.18] |
| Perioperative morbidityǂ | |||
| SSI | 3 | 752 | 1.37 [0.94, 1.99] |
| UTI | 2 | 566 | 0.43 [0.13, 1.42] |
| VTE | 2 | 566 | 2.62 [0.64, 10.73] |
| Objective response | 2 | 170 | 0.91 [0.71, 1.15] |
| Bleeding (CTC grade 2+) | 2 | 144 | 0.85 [0.66, 1.09] |
| Myocardial infarction (30-day) | 2 | 566 | 1.21 [0.16, 9.19] |
RR = risk ratio; CI = confidence interval; SSI = surgical site infection; UTI = urinary tract infection; VTE = venous thromboembolism; CTC = common toxicity criteria
Proportion of Patients transfused. Includes only surgical studies.
Froman and Pinheiro report 30-day perioperative morbidity; Wehry no follow-up time specified.
Blood utilization
The three surgical studies reported blood utilization data and the proportion of patients transfused. Of the three surgical studies, Froman et al.,[26] reported the transfusion rate during three different time periods (preoperative, intraoperative, postoperative). Pinheiro et al.,[39] reported transfusion events post-operatively and Wehry et al.,[27] evaluated all transfused patients, but did not specify the time period of transfusion. When we pooled the outcomes for proportion of patients transfused during the perioperative period, the restrictive strategy was associated with a 36% decreased risk of transfusion (RR 0.64, 95% CI 0.49 to 0.83).
The three studies evaluating transfusion strategies in chemotherapy patients used different variables to report blood utilization and thus we were unable to combine these results. Webert et al.,[40] reported the liberal group received more transfusions/patient-day than the restrictive group (0.233 v. 0.151; RR 1.56; 95% CI 1.16 to 2.10). Jansen et al.,[41] reported the total number of RBC transfusions per patient (9.6 v. 10.8; RR −1.20; 95% CI −2.70 to 0.30) and the number of RBC units given per transfusion (1.3 v. 1.8; RR −0.50; 95% CI −0.70 to −0.30). Park et al.,[38] reported the total number of transfusions given before each cycle of chemotherapy which resulted in a total of 110 units compared to 222 units of blood.
Mortality
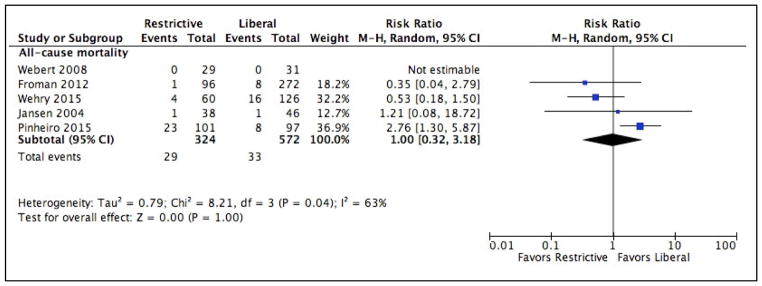
All-cause 30-day mortality data were reported in 5 studies.[26, 27, 39–41] One study, Pinheiro et al.,[39] noted an increased risk of mortality in the restrictive arm. (RR 2.76, 95% CI 1.30 to 5.87). There were no deaths in Webert et al.,[40], thus the effect was not estimable and it was not included in the meta-analysis. There was no difference in mortality in the other three studies. Overall, there was no difference in mortality between the two transfusion strategies. (RR 1.00, 95% CI 0.32 to 3.18). Heterogeneity between these studies was statistically significant (P = 0.04). Only one study[27] reported cancer-specific mortality and in this study there was no significant difference in cancer-specific mortality between the two intervention strategies (RR 0.21, 95% CI 0.03 to 1.60). The Forrest plot for mortality is demonstrated in Figure 3.
Figure 3.
Forrest plot depicting individual studies, risk ratios and mean differences for the primary outcome, all-cause mortality.
Perioperative Morbidity
The outcomes reported by the studies varied and thus we were only able to pool data on a few of the outcomes of interest. All three of the perioperative studies reported data on surgical site infections (SSIs). Overall there was no difference in SSIs (RR 1.37, 95% CI 0.94 to 1.99). Heterogeneity between these studies was not statistically significant (P = 0.92).
Two studies reported data on UTIs and VTEs.[26, 39] There was no difference in incidence of UTIs or VTEs. (RR 0.43, 95% CI 0.13 to 1.42 and RR 2.62, 95% CI 0.64 to 10.73). Heterogeneity between these studies was not statistically significant (P = 0.21; P = 0.37). One study in colorectal cancer patients reported data on anastomotic leaks.[26] Reduction in transfusion was not associated with increased leak rate (RR 1.70, 95% CI 0.41 to 6.98). One study that randomized post-operative patients in the ICU reported data on pneumonia, acute kidney injury, septic shock, stroke, and need for mechanical ventilation during ICU stay.[39] There was no significant difference between study strategies in any of these adverse events.
Transfusion-specific adverse events
Only one study reported transfusion-specific adverse events.[38] There was no difference in patients who experienced: fever (RR 0.78, 95% CI 0.34 to 1.79), allergy with urticaria (RR 0.87, 95% CI 0.37 to 2.04), pulmonary edema (RR 0.20, 95% CI 0.01 to 3.96), or new alloantibodies (RR 1.95, 95% CI 0.18 to 20.77).
Chemotherapy-specific adverse events
Only one study reported chemotherapy-specific adverse events.[38] There was no difference between the two strategies with respect to chemotherapy-specific adverse events which included: neutropenia (RR 1.19, 95% CI 0.83 to 1.70), neutropenic infection (RR 0.86, 95% CI 0.34 to 2.15), thrombocytopenia (RR 0.89, 95% CI 0.42 to 1.87), nausea and vomiting (RR 1.06, 95% CI 0.73 to 1.55), oral mucositis (RR 0.73, 95% CI 0.39 to 1.36), diarrhea (RR 0.91, 95% CI 0.48 to 1.70), constipation (RR 1.19, 95% CI 0.55 to 2.59) and fatigue (RR 1.19, 95% CI 0.55 to 2.59).
Objective response
Two studies of patients receiving chemotherapy reported objective responses.[38, 41] Restrictive transfusion was not associated with a decreased objective response (RR 0.91, 95% CI 0.71 to 1.15). Heterogeneity between these studies was not statistically significant (P = 0.82).
Bleeding
Two studies reported CTC grade 2 bleeding events and higher.[40, 41] There was no difference in bleeding between restrictive and liberal transfusion strategies (RR 0.85, 95% CI 0.66 to 1.09). Heterogeneity between these studies was not statistically significant (P = 0.11).
Cardiac Events
Three studies reported cardiac-specific adverse events.[14,28,30] Two studies reported 30-day myocardial infarction incidence[26, 39] and one study reported cardiac rhythm dysfunction and cardiac function abnormalities.[41] There was no difference between the restrictive and liberal transfusion strategies with regards to myocardial infarction (RR 1.21, 95% CI 0.16 to 9.19). Heterogeneity between these two studies was not statistically significant (P = 0.47).
Sensitivity analyses
Park et al.,[38] explored whether use of a higher hemoglobin level would improve clinical outcomes for patients undergoing treatment with chemotherapy for advanced gastric cancer comparing hemoglobin triggers of 10 g/dL (restrictive) v. 12 g/dL (liberal). Thus, the hemoglobin trigger utilized in the restrictive arm was similar to the liberal hemoglobin trigger used in other studies. We performed a sensitivity analysis by removing the Park study. Excluding Park impacted only the analysis of objective response. Since there were only two studies that reported objective response, removal of the Park study resulted in the removal of this outcome from the meta-analysis. The resulting RR for objective response changed minimally from RR 0.91, 95% CI 0.71, 1.15 to RR 0.92, 95% CI 0.74, 1.15. Due to differences in reported outcomes we were unable to report any combined outcomes for the 3 RCTs. The outcomes of the individual RCTs were previously described. With regards to the NRS, we were able to pool outcomes for mortality, perioperative SSI and proportion of patients transfused for two of the three studies. Froman and Wehry reported mortality, SSI and proportion of patients transfused. The pooled results of these two NRS alone did not differ significantly in magnitude of effect or in statistical significance from the original reported outcomes. Specifically, the RR of mortality was 0.52, 95% CI 0.21, 1.25. The RR for SSI was 1.32, 95% CI 0.72, 2.37. The RR of the proportion of patients transfused was 0.61, 95% CI 0.42, 0.89.
Discussion
Our systematic review of the safety and efficacy of restrictive versus liberal transfusion strategies in patients with cancer demonstrates that restrictive strategies appear to decrease blood utilization without increasing morbidity or mortality in oncologic patients. Our findings are in concordance with outcomes from non-oncologic studies that have investigated the role of liberal and restrictive transfusion strategies and found restrictive transfusion strategies (defined by use of a hemoglobin trigger of 7–8 g/dL) safe and effective.[42, 43] The Transfusion Requirement in Critical Care (TRICC) trial is a landmark study that was one of the first trials to challenge the view that a hemoglobin threshold of 10 g/dL should be standard of care.[18] The TRICC trial revealed a non-significant trend towards decreased mortality in the restrictive group among critically ill patients admitted to the ICU (18.7% v. 23.3%, P = 0.1). This trial challenged the historical perspective that more blood was better and allowed for a series of trials in other clinical settings such as postoperative orthopedic surgery, active gastrointestional bleeding, cardiac surgery, neonatal intensive care unit and septic shock. In fact, aside from the Pinheiro study discussed in this review, no published RCTs to date have demonstrated an advantage to a liberal transfusion strategy.
Our findings differ from pooled data from non-oncologic randomized controlled trials in that we did not find a mortality advantage to the restrictive strategy. Salpeter et al.,[13] reported pooled data from three RCTs that utilized a hemoglobin trigger of 7 g/dL and demonstrated a reduction in adverse clinical outcomes including hospital and overall mortality, pulmonary edema and bacterial infections. Similarly, in a meta-analysis of 19 trials comparing restrictive to liberal transfusion strategies, the restrictive strategy was found to significantly reduce in-hospital mortality (RR 0.77, 95% CI 0.62 to 0.95).[42] Only the Pinheiro study was powered to detect a mortality difference; therefore, the meta-analysis could have been underpowered to detect a difference in 30-day mortality outcomes.
Only one study included in our review addressed the effectiveness of transfusion and reported no difference in fatigue between the restrictive and liberal strategies; however, this study utilized much higher transfusion triggers than what would be considered standard of liberal and restrictive strategies. Furthermore, most studies failed to report functional clinical outcomes thus making interpretations about effectiveness of transfusion not possible. We identified two studies in our search that did not meet inclusion criteria for our review, but are important to discuss regarding the effectiveness of blood transfusion. In the DAHANCA 5 and 7 trials, patients with head and neck squamous cell carcinoma and low pre-treatment hemoglobin levels were randomized to transfusion or non-transfusion while undergoing radiation.[44, 45] Patients with high baseline hemoglobin had improved clinical outcomes compared to those with low hemoglobin. However, within the low hemoglobin group, elevating hemoglobin levels with transfusion did not improve locoregional control, disease-specific survival or overall survival. In fact, patients who received transfusions during radiotherapy had worse disease-free and overall survival compared to those who did not receive transfusions.[46, 47]
There are several limitations to this meta-analysis and systematic review. First, we limited our review to published studies and we identified very few studies that met our inclusion criteria. Most of these studies were small with less than 1000 total patients. Second, we were unable to identify any high quality RCTs. All six studies included in this review had moderate to high risk of bias. Third, the included studies represent a heterogeneous group of patients, and clinical scenarios with varying hemoglobin triggers. To account for this, we fit a random-effects model for all outcomes and found no differences in effect size, direction or level of significance. No studies included in our analysis reported concomitant use of iron or epoetin alpha thus introducing increased confounding. Only one study reported the age of blood, which has been an increasing area of interest with regard to the potential adverse effects of transfusions. Despite the aforementioned limitations, this is the first meta-analysis of restrictive compared to liberal transfusion strategy in oncology. This review carefully adheres to the PRISMA guidelines with a rigorous appraisal process in adherence with the Cochrane review risk of bias tool.
Conclusion
In summary, a restrictive blood transfusion strategy appears to decrease blood utilization in oncology patients without increasing morbidity or mortality. The information from this review combined with the evidence supporting more restrictive strategies in non-oncologic patients and increased morbidity associated with transfusions portends a need for clinical practice change in oncology. However, given the variability in transfusion strategies reviewed, better studies investigating the optimal transfusion trigger or patient blood management strategy are warranted.
Supplementary Material
Figure 1.
PRISMA Flow Diagram Illustrating the Study Identification and Selection Process
Highlights.
Restrictive and liberal transfusion strategies in oncology patients were compared
Restrictive transfusion strategy is associated with decreased blood utilization
No differences in mortality between the two strategies were identified
No differences in morbidity between the two strategies were identified
Acknowledgments
This research was supported in part by the National Institutes of Health through MD Anderson Cancer Center’s Support Grant CA016672. Dr. Prescott’s work on this project was supported by a NIH T32 grant, Training of Academic Gynecologic Oncologists, from the National Cancer Institute (5T32-CA101642).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foubert J. New EORTC guidelines for the treatment of anaemia in patients with cancer: Implications for nursing practice. Eur J Oncol Nurs. 2006;10:177–86. doi: 10.1016/j.ejon.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Spivak JL. Cancer-related anemia: its causes and characteristics. Semin Oncol. 1994;21:3–8. [PubMed] [Google Scholar]
- 5.Altman AD, Liu XQ, Nelson G, Chu P, Nation J, Ghatage P. The effects of anemia and blood transfusion on patients with stage III–IV ovarian cancer. Int J Gynecol Cancer. 2013;23:1569–76. doi: 10.1097/IGC.0b013e3182a57ff6. [DOI] [PubMed] [Google Scholar]
- 6.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110:574–81. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 7.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–21. [PubMed] [Google Scholar]
- 8.Dunst J, Kuhnt T, Strauss HG, Krause U, Pelz T, Koelbl H, et al. Anemia in cervical cancers: impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol Biol Phys. 2003;56:778–87. doi: 10.1016/s0360-3016(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 9.Evans JC, Bergsjo P. The influence of anemia on the results of radiotherapy in carcinoma of the cervix. Radiology. 1965;84:709–17. doi: 10.1148/84.4.709. [DOI] [PubMed] [Google Scholar]
- 10.Shander A, Javidroozi M, Naqvi S, Aregbeyen O, Caylan M, Demir S, et al. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion. Transfusion. 2014;54:2688–95. doi: 10.1111/trf.12565. [DOI] [PubMed] [Google Scholar]
- 11.Fyles AW, Milosevic M, Pintilie M, Syed A, Hill RP. Anemia, hypoxia and transfusion in patients with cervix cancer: a review. Radiother Oncol. 2000;57:13–9. doi: 10.1016/s0167-8140(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim BD, Ver Halen JP, Mlodinow AS, Kim JY. Intraoperative transfusion of packed red blood cells in microvascular free tissue transfer patients: assessment of 30-day morbidity using the NSQIP dataset. J Reconstr Microsurg. 2014;2:103–14. doi: 10.1055/s-0033-1357275. [DOI] [PubMed] [Google Scholar]
- 13.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127:124–31. doi: 10.1016/j.amjmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2015;13:102–10. doi: 10.1016/j.ijsu.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 15.Prescott LS, Aloia TA, Brown AJ, Taylor JS, Munsell MF, Sun CC, et al. Perioperative blood transfusion in gynecologic oncology surgery: analysis of the National Surgical Quality Improvement Program Database. Gynecol Oncol. 2015;136:65–70. doi: 10.1016/j.ygyno.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luan H, Ye F, Wu L, Zhou Y, Jiang J. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg. 2014;14:34. doi: 10.1186/1471-2482-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acheson AG, Brookes MJ, Spahn DR. Effects of Allogeneic Red Blood Cell Transfusions on Clinical Outcomes in Patients Undergoing Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Annals of Surgery. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 18.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 19.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–91. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 20.Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 21.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 22.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 Update to The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. Am Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 23.Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P Clinical Guidelines Committee of the American College of P. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:770–9. doi: 10.7326/0003-4819-159-11-201312030-00009. [DOI] [PubMed] [Google Scholar]
- 24.Boone JD, Kim KH, Marques M, Straughn JM. Compliance rates and outcomes associated with a restrictive transfusion policy in gynecologic oncology patients. Gynecol Oncol. 2014;132:227–30. doi: 10.1016/j.ygyno.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9:745–9. doi: 10.1002/jhm.2237. [DOI] [PubMed] [Google Scholar]
- 26.Froman JP, Mathiason MA, Kallies KJ, Bottner WA, Shapiro SB. The impact of an integrated transfusion reduction initiative in patients undergoing resection for colorectal cancer. Am J Surg. 2012;204:944–50. doi: 10.1016/j.amjsurg.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Wehry J, Cannon R, Scoggins CR, Puffer L, McMasters KM, Martin RCG. Restrictive blood transfusion protocol in liver resection patients reduces blood transfusions with no increase in patient morbidity. Am J Surg. 2015;210:1197–205. doi: 10.1016/j.amjsurg.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion Medicine — Blood Conservation. N Engl J Med. 1999;340:525–33. doi: 10.1056/NEJM199902183400706. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann A, Ozawa S, Farrugia A, Farmer SL, Shander A. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013;27:59–68. doi: 10.1016/j.bpa.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Lyman GH, Berndt ER, Kallich JD, Erder MH, Crown WH, Long SR, et al. The economic burden of anemia in cancer patients receiving chemotherapy. Value Health. 2005;8:149–56. doi: 10.1111/j.1524-4733.2005.03089.x. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2013. [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Vonville H. In: Reporting Your Systematic Review Methods & Results. Prescott LS, editor. 2014. [Google Scholar]
- 34.Review Manager (RevMan) [Computer program] Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. Version 5.3. [Google Scholar]
- 35.Sterne J, Higgins J, Reeves B. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI) 2014 Sep 24, 2014. [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Park SH, Nam E, Bang SM, Cho EK, Shin DB, Lee JH. A randomized trial of anemia correction with two different hemoglobin targets in the first-line chemotherapy of advanced gastric cancer. Cancer Chemother Pharmacol. 2008;62:1–9. doi: 10.1007/s00280-007-0561-1. [DOI] [PubMed] [Google Scholar]
- 39.Pinheiro de Almeida J, Vincent JL, Barbosa Gomes Galas FR, Pinto Marinho de Almeida E, Fukushima JT, Osawa EA, et al. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology. 2015;122:29–38. doi: 10.1097/ALN.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 40.Webert KE, Cook RJ, Couban S, Carruthers J, Lee KA, Blajchman MA, et al. A multicenter pilot-randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion. 2008;48:81–91. doi: 10.1111/j.1537-2995.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 41.Jansen AJ, Caljouw MA, Hop WC, van Rhenen DJ, Schipperus MR. Feasibility of a restrictive red-cell transfusion policy for patients treated with intensive chemotherapy for acute myeloid leukaemia. Transfus Med. 2004;14:33–8. doi: 10.1111/j.0958-7578.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 42.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carson JL, Strair R. Transfusion strategies in hematologic and nonhematologic disease. Hematology Am Soc Hematol Educ Program. 2014;2014:548–52. doi: 10.1182/asheducation-2014.1.548. [DOI] [PubMed] [Google Scholar]
- 44.Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol. 1998;46:135–46. doi: 10.1016/s0167-8140(97)00220-x. [DOI] [PubMed] [Google Scholar]
- 45.Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–40. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 46.Hoff CM, Hansen HS, Overgaard M, Grau C, Johansen J, Bentzen J, et al. The importance of haemoglobin level and effect of transfusion in HNSCC patients treated with radiotherapy--results from the randomized DAHANCA 5 study. Radiother Oncol. 2011;98:28–33. doi: 10.1016/j.radonc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 47.Hoff CM, Lassen P, Eriksen JG, Hansen HS, Specht L, Overgaard M, et al. Does transfusion improve the outcome for HNSCC patients treated with radiotherapy? - results from the randomized DAHANCA 5 and 7 trials. Acta Oncol. 2011;50:1006–14. doi: 10.3109/0284186X.2011.592650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.