Abstract
BACKGROUND
Several lines of evidence suggest the presence of abnormalities in the endocannabinoid (eCB) system in schizophrenia (SCZ). However, there are limited in vivo measures of the eCB system in SCZ.
METHODS
Twenty five male SCZ subjects (SCZs), 18 antipsychotic treated [SCZ-MED] and 7 antipsychotic free [SCZ-UNMED]) were compared to 18 age- matched male healthy control subjects (HCs). Subjects underwent one Positron Emission Tomography (PET) scan each with the cannabinoid receptor-1 (CB1R) selective radiotracer [11C]OMAR on the High Resolution Research Tomography (HRRT) scanner. Regional volume of distribution (VT) values were determined using kinetic modeling of PET data as a measure of CB1R availability. Group differences in mean composite [11C]OMAR VT values were compared between SCZs and HCs. Exploratory comparisons of CB1R availability within 15 brain regions were also conducted. All analyses were covaried for age and body mass index.
RESULTS
SCZs showed significantly (p =0.02) lower composite [11C]OMAR VT relative to HCs (~12% difference, effect size d= 0.73). [11C]OMAR VT was significantly (all ps <0.05) lower in SCZs in the amygdala, caudate, posterior cingulate cortex, hippocampus, hypothalamus and insula. Composite [11C]OMAR VT was greater in HCs> SCZ-MED>SCZ-UNMED. Furthermore, composite [11C]OMAR VT was greater in HCs> SCZ smokers (n=11) > SCZ non-smokers (n=14).
CONCLUSIONS
CB1R availability is lower in males SCZs compared to HCs. Furthermore, antipsychotics and tobacco use may increase CB1R availability in this population. The findings of the study provide further evidence supporting the hypothesis that alterations in the eCB system might contribute to the pathophysiology of SCZ.
Keywords: Cannabinoid, Schizophrenia, CB1R, Antipsychotics, Smoking, OMAR
Introduction
Emerging evidence suggests the presence of abnormalities in the endocannabinoid (eCB) system in schizophrenia (i.e., the endogenous hypothesis). This is distinct from the better known exogenous hypothesis, according to which exogenous cannabinoids can induce transient schizophrenia-like effects in healthy individuals, exacerbate psychotic symptoms, trigger relapse and negatively impact the course of illness in schizophrenia (SCZ) patients, and finally, heavy exposure to exogenous cannabinoids in adolescence may contribute to the risk of later developing SCZ (1–6).
Several groups have reported elevated eCB levels in the blood or CSF of patients with SCZ (7–11). Furthermore, eCB levels are inversely correlated with psychotic symptoms, and normalize following treatment with antipsychotics (8–10) and with clinical remission (12). The results of postmortem studies have been mixed (Supplementary Table S1) with studies reporting increases, decreases or no changes in either CB1R protein or mRNA levels in SCZ(13–23). While 4/6 studies that used in vitro autoradiography reported significantly increased CB1R binding in SCZ subjects (SCZs) compared to healthy control subjects (HCs), 5/6 studies using immunodetection methods reported either a decrease or no change in CB1R density. These mixed results could be due to differences in methodologies, the regions studied, or the presence of comorbidities in the patient groups.
A related issue is the effect, if any, that antipsychotic treatment may have on the eCB system. Reports on the effects of antipsychotics on CB1R availability are mixed (24–26). Eggan et al. reported that CB1R mRNA and protein levels were significantly lower in the dorsolateral prefrontal cortex of SCZs as compared to matched HCs in a post mortem human study (17, 18, 23), but no differences between medicated (n=19) and unmedicated (n=4) SCZs (18). Uriguen et al reported reduced post mortem CB1R density in SCZs compared to HCs that was primarily noted in antipsychotic treated but not in drug free SCZs(19). In a second cohort that also included unmedicated SCZs (n=6), Eggan et al. suggested that antipsychotic treatment might blunt the decrease in CB1R immunoreactivity levels observed in SCZ (17). Others have shown in animals that treatment with haloperidol (27), risperidone (28) and clozapine (29) resulted in altered CB1R density in various brain regions.
Positron emission tomography (PET) imaging provides a means to study CB1R availability in SCZs in vivo. Several CB1R ligands have been developed for PET, including [18F]MK-9470, and [11C]OMAR (30). In the first PET study of CB1R in SCZ, Wong et al. (2010) reported elevated [11C]OMAR binding across the brain in SCZs (n= 10), all receiving antipsychotic medications (31). The mean difference was small and only significant in the pons. More recently Ceccarini et al (32) reported CB1R binding in a larger sample of SCZs on (n=51) and off (n=16) antipsychotics, and 12 cannabis naïve, age and gender-matched controls (n=12) using [18F]MK-9470 PET. Of note [18F]MK-9470 shows primarily irreversible binding, while [11C]OMAR showing reversible binding, a characteristic favored for quantitative receptor imaging (33). Compared to HCs, SCZs displayed increased global gray-matter [18F]MK-9470 uptake. Volume of interest analyses revealed significant increases of [18F]MK-9470 uptake in several brain regions in SCZs. Further, cannabis or tobacco use and antipsychotic medication type did not appear to influence [18F]MK-9470 uptake.
While this study has drawn attention to the possibility of CB1R alterations in SCZ, the kinetic characteristics of [18F]MK-9470 pose challenges in quantification and furthermore, the validity of the modified standardized uptake values (mSUV) technique used has been challenged by several groups (34, 35).
In the current study, we measured CB1R availability in SCZs using the reversible ligand [11C]OMAR, based on absolute quantification using arterial sampling with metabolite analysis and tracer kinetic modeling analysis that does not have the limitations associated with the mSUV method. Based on the postmortem data of Eggan et al., SCZs were hypothesized to show lower CB1R availability compared to HCs.
Methods
Approvals
This study was approved by the Yale University and VA Connecticut Healthcare System Institutional Review Boards, the Yale Magnetic Resonance Research Center, and the Yale New Haven Hospital Radiation Drug Research Committee. All subjects signed informed consent after the study was explained to them in detail.
Subjects
Male SCZs on and off antipsychotics, and age-matched (± 3 years) HCs completed a comprehensive screening process that included a psychiatric, medical, and neurological evaluation by a research physician as reported in the supplemental section.
Assessments
Positive and negative symptoms of SCZ were assessed using the Positive and Negative Syndrome Scale (PANSS)(36).
Imaging
Magnetic resonance imaging (MRI) scans were conducted before the PET scans as described in the supplemental section.
Prior to PET scanning, urine drug toxicology was repeated and IV lines and an arterial catheter were placed. PET scans were acquired as subjects rested in the HRRT scanner (207 slices, resolution better than 3 mm FWHM in 3D acquisition mode) (Siemens Medical Systems, Knoxville, TN). Procedures for PET imaging, metabolite analysis and measurement of arterial input functions were the same as those previously described (37). In addition, the fraction of plasma 11C-OMAR unbound to protein (fp) was determined by ultrafiltration.
Image Analysis
Summed PET images were registered to the subject’s T1-weighted MR images, which, in turn, were registered to an MR template. Gray matter regions of interest (ROIs) were predefined on a template (Anatomical Automatic Labeling (AAL) for SPM2). This process permitted direct, automatic determination of volume of distribution (VT) values using the MA1 method with t* = 30 min (38), the preferred kinetic modeling method for [11C]OMAR. VT is the ratio at equilibrium of total tracer to that in plasma, including free, nonspecifically bound, and specifically bound tracer. Exploratory comparisons of [11C]OMAR VT levels were examined in 15 regions, some of which are implicated in the circuitry underlying the behavioral and cognitive effects of cannabinoids. The ROIs included the amygdala, globus pallidus, caudate, putamen, hippocampus, hypothalamus, cerebellum, thalamus, and cerebral cortices (insula, anterior cingulate, posterior cingulate, temporal, frontal, parietal, and occipital).
Statistical analyses
Whole brain composite [11C]OMAR VT levels were compared among diagnostic groups (SCZs vs. HCs) using analysis of covariance (ANCOVA) with age and BMI included as covariates. Composite [11C]OMAR VT values were calculated as a mean of the regional [11C]OMAR VT values using the justification and methods in Neumeister et al (2013), because regional [11C]OMAR VT values were highly correlated (r values> 0.91 in both HCs and SCZs). Group differences in regional [11C]OMAR VT values were analyzed using linear mixed models with diagnostic group (SCZs or HCs) as a between-subjects factor and region as a within-subjects factor. The interaction between group and region was modeled and age and BMI served as covariates. The best-fitting variance-covariance structure was selected based on information criteria. Additional, exploratory analyses were conducted within levels of medication and smoking. Total PANSS score was included as a covariate in all models restricted to SCZ subjects. Correlations were explored between regional CB1R availability and PANSS total and subscale scores. Given the exploratory nature of the regional analysis and correlations, results are not corrected for multiple comparisons. All tests were significant at the alpha=0.05 threshold.
RESULTS
Twenty-five male SCZs, seven of whom were antipsychotic free (SCZ-UNMED), eighteen receiving antipsychotic treatment (SCZ-MED), and eighteen age-matched (± 3 years) male HCs were studied. Tables 1 and 2 show demographic and clinical characteristics of the groups. [11C]OMAR injection parameters did not differ among the groups.
Table 1.
All Subject Demographics
| Schizophrenia (n=25) | Controls (n=18) | Statistics | |||
|---|---|---|---|---|---|
| Variable | Mean or n | SD or % | Mean or n | SD or % | p-value |
| Age (Years) | 34.7 | 11.3 | 28.7 | 8.1 | 0.060 |
| Education (years) | 13.2 | 1.5 | 15.4 | 2.4 | 0.001* |
| BMI | 30.7 | 5.1 | 26.9 | 5.3 | 0.020* |
| NART | 109.4 | 8.5 | 113.6 | 7 | 0.096 |
| FTND | 2 | 2.6 | - | - | - |
| Injected Activity [mCi] | 14.09 | 3.65 | 15.68 | 2.87 | 0.14 |
| Injected Mass [μg] | 3.67 | 3.22 | 3.62 | 3.22 | 0.95 |
| Specific Activity [mCi/nmol] | 3.85 | 3.57 | 5.35 | 4.58 | 0.23 |
| Free Fraction (fp) | 0.001 | 0.0004 | 0.001 | 0.0004 | 0.16 |
| Antipsychotic Treatment (No/Yes) | 7/18 | 28/72 | - | - | 0.001* |
| Tobacco Smoker (No/Yes) | 14/11 | 56/44 | 18/0 | 100/0 | 0.410 |
| Race (White/AA/Other) | 15/9/1 | 60/36/4 | 14/4/0 | 78/22/0 | |
| Ethnicity (Non-Hispanic/Hispanic) | 21/4 | 84/16 | 14/4 | 78/22 | |
| Lifetime DSM-IV Cannabis Use Disorder | 0 | 0 | 0 | 0 | |
BMI: Body Mass Index; NART = National Adult Reading Test; BMI = Body Mass Index; FTND = Fagerstrom Tobacco Test for Nicotine Dependence; AA= African American
Table 2.
Schizophrenia Subjects Demographics
| Medicated (n=18) | Unmedicated (n=7) | Statistics | |||
|---|---|---|---|---|---|
| Variable | Mean or n | SD or % | Mean or n | SD or % | p-value |
| Age (years) | 35.7 | 10.9 | 32.3 | 12.9 | 0.515 |
| Education (years) | 13.1 | 1.5 | 13.3 | 1.5 | 0.805 |
| BMI | 30.7 | 4.1 | 30.7 | 7.6 | 0.994 |
| NART | 108.4 | 8.1 | 111.9 | 9.6 | 0.377 |
| FTND | 2.2 | 2.7 | 1.4 | 2.4 | 0.527 |
| Injected Activity [mCi] | 13.64 | 3.96 | 15.18 | 2.71 | 0.36 |
| Injected Mass [μg] | 3.84 | 3.29 | 3.25 | 3.23 | 0.69 |
| Specific Activity [mCi/nmol] | 3.40 | 2.78 | 5.01 | 5.17 | 0.32 |
| Free Fraction (fp) | 0.001 | 0.0004 | 0.001 | 0.004 | 0.26 |
| Duration since antipsychotic exposure (months) | N.A | - | 27* | 24 | |
| Tobacco Smoker (No/Yes) | 9/9 | 50/50 | 5/2 | 71/29 | 0.4 |
| Race (White/AA/Other) | 11/6/1 | 61/33/6 | 4/3/0 | 57/43/0 | |
| Ethnicity (Non-Hispanic/Hispanic) | 16/2 | 89/11 | 5/2 | 71/29 | |
| Lifetime Cannabis Exposure** (No/Yes) | 5/12 | 29/71 | 2/4 | 33/66 | 1 |
| Recent Cannabis exposure (No/Yes) | 17/1 | 94.5/0.05 | 7/0 | 100/0 | 0.5 |
Medicated = receiving antipsychotic treatment; Unmedicated = not receiving antipsychotic treatment;
NART = National Adult Reading Test; BMI = Body Mass Index; FTND = Fagerstrom Tobacco Test for Nicotine Dependence; AA = African American
Range (2 months – 60 months); One patient was antipsychotic naïve.
Lifetime cannabis use data are missing in 2 SCZs (neither subject met criteria for any lifetime cannabis use disorder).
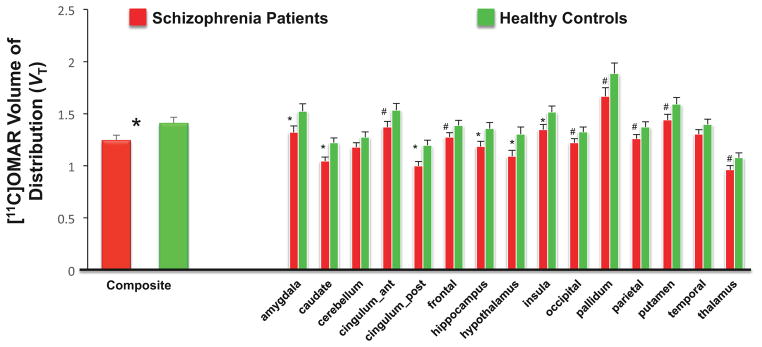
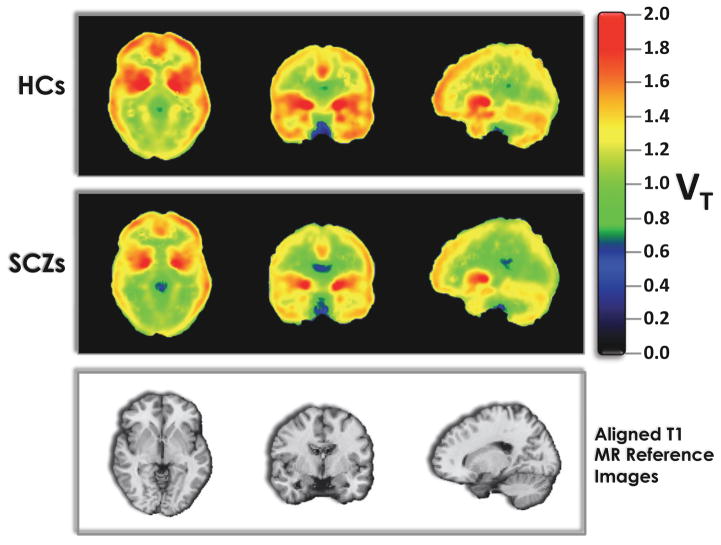
CB1R availability in SCZ vs. HC (Figures 1 and 2)
Figure 1. Grand Averaged CB1R availability in Schizophrenia Patients vs. Healthy Controls.
Top row: HC averaged (n=21) of the distribution volume (VT; Innis et al., 2007) images computed using the multilinear analysis (MA1; Ichise et al., 2002)
Middle row: SCZ averaged (n=25) of the distribution volume (VT; Innis et al., 2007) images computed using the multilinear analysis (MA1; Ichise et al., 2002)
Bottom row: Aligned T1 MR image for anatomical reference
Figure 2. CB1R availability in Schizophrenia Patients vs. Healthy Controls.
The figure shows the mean and standard error bars of [11C]OMAR Volume of Distribution (VT).
Significant differences (p<0.05) between groups are indicated with *.
Trend differences (p<0.06–0.1) between groups are indicated with #.
Composite brain [11C]OMAR VT values were ~12% lower in SCZs relative to HCs (F(1,39)= 5.56, p=.02; effect size (d) = 0.73). In the exploratory analysis including region as a factor, significant group (F(1,39)= 4.51, p=.04), region (F(14,574)= 82.4, p<.0001) and group x region interaction (F(14,574)=1.72, p=.05) effects were observed. Post hoc analyses revealed that SCZs had significantly lower [11C]OMAR VT values compared to HCs in the amygdala, caudate, posterior cingulate cortex, hippocampus, hypothalamus and insula (all p≤ 0.05) with a trend level (0.05 < p <0.1) in the anterior cingulate, frontal, occipital and parietal cortices, pallidum, putamen and thalamus.
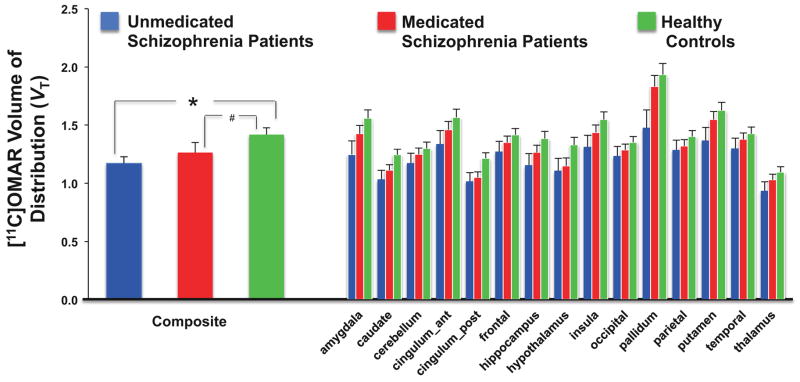
Effects of antipsychotic medication on CB1R availability: CB1R availability in SCZ-UNMED vs. SCZ-MED vs. HCs (Figure 3)
Figure 3. Effects of Antipsychotic Treatment on CB1R availability.
The figure shows the mean and standard error bars of [11C]OMAR Volume of Distribution (VT).
See text for statistically significant differences between 3 groups. *p = 0.025. #p = 0.07.
There was a main effect of group (F (2,38)= 3.16, p=.05) such that composite brain [11C]OMAR VT was HCs> SCZ-MED > SCZ-UNMED (effect size (d)= 0.40). In the exploratory analysis including region as a factor, the region (F (14,38)= 55.92, p<.0001) and group x region interaction (F(28,38)=2.98, p=.001) effects were significant, and there was no main group effect (F(2,38)=2.01, p=0.14). Post hoc analyses revealed significantly lower [11C]OMAR VT (all p < 0.05) in SCZ-UNMED compared to HCs in the amygdala, caudate, posterior cingulate cortex, and pallidum with trend level (0.05 < p <0.1) differences in the hippocampus, hypothalamus, insula, putamen and thalamus. [11C]OMAR VT was significantly (p=0.02) lower in SCZ-MED compared to HCs only in the posterior cingulate cortex with trend (0.05 < p <0.1) level differences in the caudate and hypothalamus. There were no significant differences between SCZ-UNMED and SCZ-MED. Finally, there were no significant correlations between antipsychotic dose (in risperidone equivalents) and [11C]OMAR VT in any brain region.
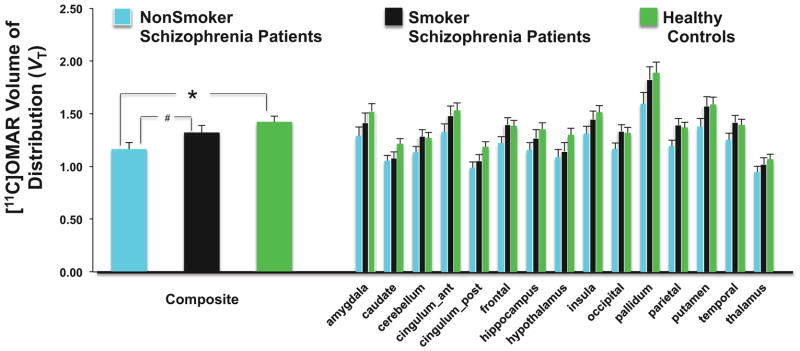
Effect of tobacco smoking on CB1R availability in SCZ: CB1R availability in non-smoker SCZs (n=14) vs. smoker SCZs (n=11) vs. HCs (n=18) (Figure 4)
Figure 4. Effects of Tobacco Smoking on CB1R availability.
The figure shows the mean and standard error bars of [11C]OMAR Volume of Distribution (VT).
See text for statistically significant differences between 3 groups. *p = 0.006. #p = 0.096.
There was a significant main effect of group (F(2,38)= 4.37, p=.02) such that composite brain [11C]OMAR VT was HCs> smoker SCZs > non-smoker SCZs. In the exploratory analysis including region as a factor, the region (F (14,38)= 84.15, p<.0001) and group x region interaction (F(28,38)=3.44, p=.0002) effects were significant and there were trend level (F(2,38)=2.51, p=0.09) group differences. Post hoc analyses revealed significantly lower [11C]OMAR VT (all p < 0.05) in non-smoker SCZs compared to HCs in the amygdala, caudate, hippocampus, hypothalamus, insula, putamen, pallidum and frontal, parietal, occipital and posterior cingulate cortices, and trend level (0.05 < p <0.1) differences in the cerebellum and anterior cingulate and temporal cortices. In contrast, there were no significant differences in [11C]OMAR VT between smoker-SCZs and HCs. Finally, [11C]OMAR VT was significantly (all p < 0.05) lower in non-smoker SCZs vs. smoker SCZs in the parietal and occipital cortices with trend level differences (0.05 < p <0.1) in the cerebellum and frontal and temporal cortices. There was no significant correlation between Fagerstrom Test for Nicotine Dependence scores and composite or regional [11C]OMAR VT.
Free fraction corrected results
There was no significant difference (p = 0.1) in the plasma free fraction (fP) between HCs and SCZs. Nevertheless, for completeness, fP-corrected results are presented in supplementary Table S2 – the results were no different.
Effect of unequal partial volume loss on results
Due to the high resolution of the HRRT PET scanner used in this study, partial volume effects tend to be smaller than those found on conventional PET scanners. However, to address the possibility that the group differences may be driven by partial volume effects, further analyses were conducted.
Supplementary Table S3 lists the results for ROI volume and VT where the AAL regions were masked to only include gray matter. Cortical regions were analyzed because segmentations of gray and white matters were considered reliable only in these regions. SCZs had slightly lower ROI volumes compared to HCs, with a mean group difference of 4.33% (SD= 1.93). In comparison, group difference in [11C]OMAR VT calculated after grey matter masking was much larger, with a mean of 17.93% decrease in SCZs (SD= 3.88). Thus, the magnitude of difference in [11C]OMAR VT between SCZs and HCs cannot be explained by ROI volume difference between the two groups.
Relationship between symptom levels and cannabis exposure with CB1R availability
Overall, there were no group differences in PANSS Total or any PANSS subscale scores between SCZ-UNMED and SCZ-MED. Among SCZ patients, there was a positive association (r = 0.41 – 0.48) observed between PANSS general and total scores and CB1R availability in the hippocampus, hypothalamus and thalamus (all p < 0.05) respectively. There were no significant correlations between cannabis use and [11C]OMAR VT.
Discussion
Male SCZs have reduced CB1R availability as compared to male HCs. The magnitude of the reduction was medium (effect size d = ~0.73) and the pattern of reduction was global rather than localized. Reduced [11C]OMAR VT in SCZs relative to HCs is most likely driven by the diagnosis of SCZ rather than other factors. That differences in CB1R availability between HCs and SCZ-UNMED were larger than those between HCs and SCZ-MED suggests that antipsychotic treatment is not likely an explanation for the observed differences between SCZs and HCs. Similarly, that differences in CB1R availability between SCZ non-smokers and HCs were larger than those between SCZ smokers and HCs suggest that tobacco use is unlikely to explain differences between SCZs and HCs. Finally, since the groups studied had minimal and similar exposure to cannabis, it is further unlikely that the group differences can be explained by cannabis exposure.
The outcome measure used in this study was VT, which includes specific binding and non-displaceable binding (free + non-specific). We have interpreted the global differences seen here as being lower specific CB1R availability in SCZs compared to HCs. However, it is possible that some or all of the difference can be attributed to non-displaceable binding. Blocking studies are generally used to assess the magnitude of specific binding. In nonhuman primates (NHP), rimonabant was able to block more than half of the signal in the regions with high specific binding (39). We had previously assessed the level of nonspecific binding in NHP using low specific activity injections of OMAR (40) and found that the nondisplaceable volume of distribution (VND) represented 40–50% of the VT values in high-binding regions. However, to the best of our knowledge, human blocking studies have not been performed with [11C]OMAR due to the lack of available blocking drug, and thus the portion of non-specific binding in human brain regions remains unknown.
The global pattern of reduced CB1R availability in SCZs is in contrast to the patterns observed in cannabis dependence where the reduction in CB1R availability is greater in cortical compared to subcortical regions (41–45). Furthermore, the findings of reduced CB1R availability in SCZ are in contrast to the findings of studies with the same ligand ([11C]OMAR) and methodology that showed increased CB1R availability in PTSD (46) and in alcohol dependence (47). This suggests that our findings are not merely a nonspecific consequence of chronic neuropsychiatric illness but are specific to SCZ.
The reductions in CB1R availability in chronic SCZ observed in our study may have several interpretations. Decreased or increased eCB levels are associated with up or downregulation of CB1R, respectively. For example, Neumeister et al. reported that low anandamide levels were associated with increased CB1R in PTSD (46). Thus, our findings of lower CB1R availability in SCZs are consistent with the findings of elevated CSF eCB levels in SCZ (7–11). Whether elevated eCB levels drive the reduction in CB1R availability or vice versa remains unknown. Furthermore, while elevated eCB levels reportedly normalize with symptom resolution (7–11), whether the same occurs with CB1R availability is not known.
The relationship between CB1R availability and symptoms remains unclear. While Ceccarini et al. observed that CB1R binding was negatively correlated to negative symptoms and depression, we found modest positive relationships between symptoms (PANSS total scores) and regional CB1R availability. However, our findings should be interpreted with caution given that they were not adjusted for multiple comparisons.
It is tempting to speculate how the eCB hypothesis supported by our observations of reduced CB1R availability in SCZ interacts with the exogenous cannabinoid hypothesis. According to the latter, early and heavy exposure to cannabis increases the risk for SCZ reviewed by(48). Also, exposure to cannabis results in CB1R downregulation (41–45). Hence, CB1R may be reduced de novo and/or secondary to cannabis exposure, thus incorporating both the endogenous and exogenous hypotheses under one overarching hypothesis. Furthermore, and admittedly speculative, if those at risk for SCZ have reduced CB1R to begin with, and if reduced CB1R availability contributes to the pathophysiology of SCZ, then heavy and early exposure to cannabis might further enhance the risk for the development of the illness.
CB1R activation serves to inhibit the release of other neurotransmitters e.g., gamma-amino-butyric acid (GABA), dopamine and glutamate (49), that have been implicated in the pathophysiology of SCZ. Therefore, one possible consequence of reduced CB1R may be the unregulated release of neurotransmitters relevant to psychosis. Alternatively, as suggested by Eggan et al (17), it is conceivable that reduced CB1R may be a compensatory response to abnormalities in other neurotransmitter systems. While the current study does not allow us to determine the consequences of a reduction in CB1R availability, similar small reductions in CB1R availability in cannabis dependence are associated with functional consequences (50–53).
Effects of Antipsychotic treatment on CB1R availability in SCZ
CB1R availability was greater in HC> SCZ-MED > SCZ-UNMED suggestive of a “restorative” effect of antipsychotics on CB1R availability in SCZs. While the preclinical literature on the effects of antipsychotics on CB1R availability are mixed (24–26), our results are consistent with data demonstrating regional increase in CB1R binding associated with treatment with haloperidol (27) and risperidone (28).
Furthermore, our finding of higher CB1R availability in SCZ-MED vs. SCZ-UNMED which may be interpreted as a “normalization” of CB1R availability with antipsychotic treatment, is also consistent with the findings of Leweke et al that elevated CSF eCB levels in acutely ill SCZs “normalized” with antipsychotic treatment (7–11). Future studies with larger samples of medicated and unmedicated SCZs will be necessary to determine the effects, if any, of antipsychotics in general and the effects of specific antipsychotics on CB1R availability.
Effects of Tobacco Smoking on CB1R availability in SCZ
CB1R availability was greater in HC> SCZ smokers > SCZ non-smokers suggesting that tobacco smoking may increase CB1R availability in SCZs. Several lines of evidence suggest interplay between CB1R and nicotinic acetyl cholinergic receptor (nAChR) systems. Rodent studies suggest that intact CB1R is required for the rewarding effects of nicotine and CB1R antagonists have shown some efficacy in treating tobacco dependence (54–56). A recent PET study in rats failed to show an effect of subchronic (2 weeks) nicotine treatment on the binding of the CB1R ligand [18F]MK-9470 (57). However, the authors reported an increase in CB1R only in the cerebellum, a finding that would be consistent with the findings of the current study. Nicotine does not appear to have a direct effect on CB1R but exposure to nicotine may alter eCB levels (58, 59) and elevated eCBs in turn can alter CB1R binding capacity by enhancing affinity (acutely) and increasing receptor density (chronically) (60). Thus, it is conceivable that chronic tobacco smoking is associated with increased CB1R availability as was observed in our study. Since this study did not include smokers without SCZ, it is not clear whether this effect of smoking is limited to SCZ or more generalizable. Finally, it is important to note that the effects of tobacco use in SCZs may have been confounded by medication status given that there were more tobacco users in the medicated group.
Implications for Cannabis Use in SCZ
Cannabis is one of the most commonly used drugs by SCZ patients (61). Whether reduced CB1R has any implications on the risk of cannabis or other drug/alcohol use by this population is not known but warrants further study. Given the large CB1R reserve (62), only a small number of receptors need to be activated to produce psychoactive effects of cannabinoids so it is unlikely that reduced CB1R should have any bearing on the amount of cannabis required to produce effects.
Comparison to extant findings
Our findings are consistent with some but not other postmortem literature on CB1R in SCZ (13–22) (supplementary table S1). For example, Uriguen et al and Eggan et al., reported reduced CB1R mRNA and immunocytochemical receptor protein expression in SCZ (17–19). Our findings are at variance with the in vivo findings of Wong et al and Ceccarini et al., who showed increased CB1R availability in SCZ compared to HCs (31, 32). This may be related to differences in the sample size and characteristics, and/or PET methodology.
Partly due to its high binding affinity, the kinetics of [18F]MK-9470, used in Ceccarini et al., are very slow, which presents difficulties in quantifying the uptake and binding using conventional tracer kinetic modeling methods. Due to its nearly irreversible uptake, reliable measurements of VT cannot be obtained. There is consensus in the neuroreceptor imaging community that reversible-binding tracers provide more meaningful and reliable data (33). The outcome measure used by Ceccarini et al. was a mSUV value, where tracer uptake is normalized by injected dose and an adjusted subject weight. The mSUV approach assumed no group differences in peripheral tracer clearance or metabolism among the groups studied. Factors such as tobacco smoking status, level of smoking, cannabis use status, antipsychotic treatment or diagnosis of SCZ could theoretically impact peripheral metabolism. While SUV measurements can still potentially be useful to demonstrate large differences in receptor binding, e.g., those obtained from a receptor occupancy study (63), smaller between-group differences can easily be confounded by the lack of absolute measurements. As acknowledged by Ceccarini et al. and others there are limitations to the validity of the mSUV technique (34, 35). Given the range of demographic factors in these populations (gender, BMI, smoking, drug-status), we feel that reliable results can only be obtained by measuring the arterial input functions in all subjects, so that the final outcome measure (VT) is no longer affected by tracer availability, which is controlled by peripheral tracer metabolism and clearance from the body. In contrast to Ceccarini et al., we conducted kinetic analysis of [11C]OMAR data using metabolite-corrected arterial input functions in all subjects.
Using [11C]OMAR, the same tracer as the one used in our study, Wong et al. reported a small increase in CB1R availability significant only in the pons, in a small sample (n=10) of SCZs all of whom were receiving antipsychotic medications (31). The latter is important to consider given our findings suggesting that antipsychotic treatment may increase CB1R availability.
Further, relative to our sample, the chronic SCZs studied by both Wong et al. and Ceccarini et al. were less symptomatic. The mean PANSS total scores in our sample was 73.9 vs. 52.3 in Ceccarini et al’s sample (group means ranged from 45–59), while the sample of Wong et al. had mean BPRS scores of 34.1, suggesting that our sample consisted of more symptomatic chronic SCZs. Psychotic symptoms are inversely correlated with eCB levels and tend to normalize following treatment with antipsychotics (8–10) and with clinical remission (12). Furthermore, eCB levels are related to CB1R availability. Thus, it is conceivable that the higher CB1R availability observed by Wong et al. and Ceccarini et al. may reflect the lower symptoms levels in their study samples.
Conclusions and future directions
This is the first report, to our knowledge, of reduced CB1R availability in males with SCZ. Antipsychotic and tobacco use may affect CB1R availability in this population. These findings provide further evidence supporting the hypothesis that alterations in the eCB system might contribute to the pathophysiology of SCZ.
In light of published data from our center showing higher CB1R availability in women (64), it will be important to study females with SCZ. Attention and memory are well known to be acutely impaired by cannabinoids (65, 66) and to be impaired in SCZ (67–69). Furthermore, SCZ patients are more sensitive to the cognitive impairing effects of cannabinoids (70). Similarly, cannabinoids can induce a range of acute SCZ-relevant behavioral outcomes, and SCZ patients are more sensitive to the psychosis-inducing effects of cannabinoids (66). Thus, future studies that are adequately powered will be necessary to establish the relationship between CB1R availability and cognitive outcomes, core symptoms in SCZ, stage of illness and eCB levels.
Supplementary Material
Acknowledgments
The authors wish to acknowledge support from the National Institute of Mental Health (R21MH094961 to DCD, R01MH096876 to AN, and R25MH071584 supporting RR), the National Institute of Drug Abuse (21DA030702-01A1 to DCD) the Department of Veterans Affairs, the Yale Center for Clinical Investigation (YCCI) for their contributions to the success of this project.
Funding
Rajiv Radhakrishnan has received funding from APIRE/Janssen Pharmaceutical Resident Psychiatric Research Scholar and is supported by R25MH071584. Mohini Ranganathan has in the past three years or currently receives research grant support administered through Yale University School of Medicine from Eli Lilly Inc. Deepak Cyril D’Souza has in the past three years or currently receives research grant support administered through Yale University School of Medicine from Astra Zeneca, Abbott Laboratories, Eli Lilly Inc., Forest Laboratories, Organon, Pfizer Inc., and Sanofi; he is a consultant for Bristol Meyers Squibb and Johnson and Johnson. Yiyun (Henry) Huang has in the past three years or currently receives research support administered through Yale University from Eli Lilly Inc., Pfizer Inc., and UCB Biopharma SPRL. Richard E. Carson has in the past three years or currently receives research support administered through Yale University from Abbvie, Aptuit, Astellas Pharma, Bristol-Myers Squibb, Eli Lilly Inc., Pfizer Inc., Taisho Pharmaceuticals, Theravance Inc., and UCB Pharma SA.
Footnotes
Clinicaltrials.gov registration: Identifier: NCT01730781 https://clinicaltrials.gov/ct2/show/NCT01730781?term=OMAR%2C+schizophrenia&rank=1
Disclosure: All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koethe D, Hoyer C, Leweke FM. The endocannabinoid system as a target for modelling psychosis. Psychopharmacology (Berl) 2009;206:551–561. doi: 10.1007/s00213-009-1591-7. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Espejo E, Viveros MP, Nunez L, Ellenbroek BA, Rodriguez de Fonseca F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology (Berl) 2009;206:531–549. doi: 10.1007/s00213-009-1612-6. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza DC, Sewell RA, Ranganathan M. Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci. 2009;259:413–431. doi: 10.1007/s00406-009-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21:152–162. doi: 10.1080/09540260902782802. [DOI] [PubMed] [Google Scholar]
- 5.Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- 6.Sewell RA, Skosnik PD, Garcia-Sosa I, Ranganathan M, D’Souza DC. Behavioral, cognitive and psychophysiological effects of cannabinoids: relevance to psychosis and schizophrenia. Rev Bras Psiquiatr. 2010;32(Suppl 1):S15–30. [PubMed] [Google Scholar]
- 7.Leweke FM, Giuffrida A, Koethe D, Schreiber D, Nolden BM, Kranaster L, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res. 2007;94:29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- 9.Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 10.Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry. 2009;194:371–372. doi: 10.1192/bjp.bp.108.053843. [DOI] [PubMed] [Google Scholar]
- 11.Leweke FM. Anandamide dysfunction in prodromal and established psychosis. Curr Pharm Des. 2012;18:5188–5193. doi: 10.2174/138161212802884843. [DOI] [PubMed] [Google Scholar]
- 12.De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids in health and disease. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res. 2006;172:556–560. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- 14.Deng C, Han M, Huang XF. No changes in densities of cannabinoid receptors in the superior temporal gyrus in schizophrenia. Neuroscience bulletin. 2007;23:341–347. doi: 10.1007/s12264-007-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28:355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, et al. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2007;114:1055–1063. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- 17.Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uriguen L, Garcia-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casado V, et al. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 2009;206:313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- 20.Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2011;36:1620–1630. doi: 10.1038/npp.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenko KJ, Hirvonen J, Henter ID, Anderson KB, Zoghbi SS, Hyde TM, et al. Binding of a tritiated inverse agonist to cannabinoid CB1 receptors is increased in patients with schizophrenia. Schizophr Res. 2012;141:185–188. doi: 10.1016/j.schres.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 23.Volk DW, Eggan SM, Horti AG, Wong DF, Lewis DA. Reciprocal alterations in cortical cannabinoid receptor 1 binding relative to protein immunoreactivity and transcript levels in schizophrenia. Schizophr Res. 2014;159:124–129. doi: 10.1016/j.schres.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Secher A, Husum H, Holst B, Egerod KL, Mellerup E. Risperidone treatment increases CB1 receptor binding in rat brain. Neuroendocrinology. 2010;91:155–168. doi: 10.1159/000245220. [DOI] [PubMed] [Google Scholar]
- 25.Weston-Green K, Huang XF, Han M, Deng C. The effects of antipsychotics on the density of cannabinoid receptors in the dorsal vagal complex of rats: implications for olanzapine-induced weight gain. Int J Neuropsychopharmacol. 2008;11:827–835. doi: 10.1017/S1461145708008560. [DOI] [PubMed] [Google Scholar]
- 26.Weston-Green K, Huang XF, Deng C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PLoS One. 2012;7:e33548. doi: 10.1371/journal.pone.0033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson M, Terasmaa A, Fuxe K, Stromberg I. Subchronic haloperidol increases CB(1) receptor binding and G protein coupling in discrete regions of the basal ganglia. J Neurosci Res. 2005;82:264–272. doi: 10.1002/jnr.20630. [DOI] [PubMed] [Google Scholar]
- 28.Secher A, Husum H, Holst B, Egerod KL, Mellerup E. Risperidone treatment increases CB1 receptor binding in rat brain. Neuroendocrinology. 2009;91:155–168. doi: 10.1159/000245220. [DOI] [PubMed] [Google Scholar]
- 29.Sundram S, Copolov D, Dean B. Clozapine decreases [3H] CP 55940 binding to the cannabinoid 1 receptor in the rat nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:428–433. doi: 10.1007/s00210-005-1074-2. [DOI] [PubMed] [Google Scholar]
- 30.Horti AG, Van Laere K. Development of radioligands for in vivo imaging of type 1 cannabinoid receptors (CB1) in human brain. Curr Pharm Des. 2008;14:3363–3383. doi: 10.2174/138161208786549380. [DOI] [PubMed] [Google Scholar]
- 31.Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceccarini J, De Hert M, Van Winkel R, Peuskens J, Bormans G, Kranaster L, et al. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage. 2013;79:304–312. doi: 10.1016/j.neuroimage.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 33.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 34.Normandin MD, Zheng M-Q, Ropchan JR, Labaree D, Najafzadeh S, Hull R, et al. Imaging the cannabinoid CB1 receptor in humans with [11C]OMAR: Test-retest reproducibility and gender differences. NeuroImage. 2010;52:S82–S83. doi: 10.1038/jcbfm.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirvonen J, Terry GE, Halldin C, Pike VW, Innis RB. Approaches to quantify radioligands that wash out slowly from target organs. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37:917–919. doi: 10.1007/s00259-010-1426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. British Journal of Psychiatry - Supplementum. 1989:59–67. [PubMed] [Google Scholar]
- 37.Normandin MD, Zheng MQ, Lin KS, Mason NS, Lin SF, Ropchan J, et al. Imaging the cannabinoid CB1 receptor in humans with [11C]OMAR: assessment of kinetic analysis methods, test-retest reproducibility, and gender differences. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22:1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 39.Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP, et al. 11C-JHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J Nucl Med. 2006;47:1689–1696. [PubMed] [Google Scholar]
- 40.Normandin MWD, Ropchan J, Labaree D, Lin K, Mason N, Carson R, D’Souza DC, Neumeister A, Huang Y. Kinetic modeling of CB1 PET tracer [11C]OMAR in rhesus monkeys and humans. The Journal of Nuclear Medicine. 2010;51(supplement 2):216. [Google Scholar]
- 41.McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero J, Garcia-Palomero E, Castro JG, Garcia-Gil L, Ramos JA, Fernandez-Ruiz JJ. Effects of chronic exposure to delta9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Brain Res Mol Brain Res. 1997;46:100–108. doi: 10.1016/s0169-328x(96)00277-x. [DOI] [PubMed] [Google Scholar]
- 43.Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K. [F]MK-9470 PET measurement of cannabinoid CB receptor availability in chronic cannabis users. Addict Biol. 2013 doi: 10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- 46.Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18:1034–1040. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumeister A, Normandin MD, Murrough JW, Henry S, Bailey CR, Luckenbaugh DA, et al. Positron emission tomography shows elevated cannabinoid CB1 receptor binding in men with alcohol dependence. Alcohol Clin Exp Res. 2012;36:2104–2109. doi: 10.1111/j.1530-0277.2012.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 48.Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to Pot - A Review of the Association between Cannabis and Psychosis. Frontiers in psychiatry. 2014;5:54. doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 50.D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranganathan M, Braley G, Pittman B, Cooper T, Perry E, Krystal J, et al. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl) 2009;203:737–744. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, et al. Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Delta(9)-THC in humans. Neuropsychopharmacology. 2012;37:1632–1646. doi: 10.1038/npp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- 54.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 55.Siu EC, Tyndale RF. Non-nicotinic therapies for smoking cessation. Annu Rev Pharmacol Toxicol. 2007;47:541–564. doi: 10.1146/annurev.pharmtox.47.120505.105354. [DOI] [PubMed] [Google Scholar]
- 56.Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 57.Gerard N, Ceccarini J, Bormans G, Vanbilloen B, Casteels C, Goffin K, et al. Influence of chronic nicotine administration on cerebral type 1 cannabinoid receptor binding: an in vivo micro-PET study in the rat using [18F]MK-9470. J Mol Neurosci. 2010;42:162–167. doi: 10.1007/s12031-010-9340-2. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 59.Marco EM, Granstrem O, Moreno E, Llorente R, Adriani W, Laviola G, et al. Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. Eur J Pharmacol. 2007;557:37–43. doi: 10.1016/j.ejphar.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Romero J, Garcia L, Fernandez-Ruiz JJ, Cebeira M, Ramos JA. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1995;51:731–737. doi: 10.1016/0091-3057(95)00023-p. [DOI] [PubMed] [Google Scholar]
- 61.Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36:1115–1130. doi: 10.1093/schbul/sbp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther. 1999;288:478–483. [PubMed] [Google Scholar]
- 63.Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A. 2007;104:9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Normandin MD, Zheng MQ, Lin KS, Mason NS, Lin SF, Ropchan J, et al. Imaging the cannabinoid CB1 receptor in humans with [C]OMAR: assessment of kinetic analysis methods, test-retest reproducibility, and gender differences. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- 66.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 67.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 68.Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 70.D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.