Abstract
To realize the full potential of cancer immunotherapy, the latest generation immunotherapeutics are designed to harness the potent tumor-killing capacity of T cells. Thus, to mobilize T cells, new optimized bispecific antibody (BsAb) designs, enabling efficient polyclonal redirection of cytotoxic activity through binding to CD3 and a Tumor Associated Antigen (TAA) and refined genetically-modified T cells have recently expanded the arsenal of available options for cancer treatment. This review presents the current understanding of the parameters crucial to the design of optimal T cell redirecting BsAb and chimeric antigen receptor (CAR)-modified T cells. However, there are additional questions that require thorough elucidation. Both modalities will benefit from design changes that may increase the therapeutic window. One such approach could employ the discrimination afforded by multiple TAA to significantly increase selectivity.
Introduction
The potential of immunotherapies to treat cancer has been recognized since Dr. William Coley’s discovery that immune system stimulation can treat cancer [1]. For the last two decades, monoclonal antibodies have been at the forefront as anti-tumor therapeutics. However, they are unable to engage the most powerful agent of the immune system – T cells. Thus, to mobilize T cells, new optimized bispecific antibody (BsAb) designs, enabling efficient polyclonal redirection of cytotoxic activity through binding to CD3 and a Tumor Associated Antigen (TAA) and refinements of genetically-modified T cells have recently expanded the arsenal of available options for cancer treatment.
In the last six years, two T cell redirecting BsAbs have received regulatory approval: catumaxomab [2] for the treatment of malignant ascites and blinatumomab [3*] for acute lymphoblastic leukemia. Numerous others are undergoing clinical investigation [4*, 5]. This review presents the current understanding of selected parameters crucial to design of a “perfect” T cell redirecting BsAb, which has undergone evolution in recent years; this review will not cover all criteria that may influence the efficacy and potency of T cell redirecting BsAb, nor will it recapitulate recent reviews of the numerous BsAb formats that appear elsewhere [4*, 5].
Similarly, the field of genetically-modified re-directed T cells, particularly those engineered with antibody-based chimeric antigen receptors (CARs), has grown tremendously. In the past 5 years there have been over 800 publications; this figure does not include T cell receptor-transduced T cells or those bearing fusion proteins that are not based on chimeric antigen receptors, which are not a focus of this review. The key clinical observations emerging in the past few years are that CAR T cells can be a highly effective therapy for B cell-derived tumors, and are likely to be effective for other blood-based cancers. As with T cell redirecting BsAb, the parameters for optimal CAR T design are still evolving.
These two modalities, redirection of cytotoxic T cells to tumor cells via BsAb and tumor targeting of CAR T, both capitalize on the cytotoxic activity of these most potent effector cells to treat cancer. In this review, we first survey T cell redirecting BsAb and then therapeutic CAR T.
The rise of bispecific Abs and early T cell redirecting technologies
The first BsAb were produced by various chemical cross-linking protocols from two mono-specific antibodies [6, 7]. In 1983, a modification of the hybridoma technology was employed to produce hybrid-hybridoma (quadroma) from the fusion of two hybridomas [8*]; this technology enabled the cellular production of “hybrid antibodies” as the early BsAb were named. Both of these technologies were suboptimal; large-scale production of homogeneous antibody preparations was challenging since the dominant species were “mispaired” products. Thus, the BsAb field was of little interest to drug developers until the advent of modern antibody engineering technologies allowed the production of full-length and fragment BsAb. However, this did not prevent academic investigation of the polyclonal redirection of T cells to induce potent lysis of cells bearing the second specificity by BsAb [6, 7, 9–12]. Initially, this approach was used to circumvent the major histocompatibility complex (MHC) restriction of the T cell receptor and engage a broad subset of endogenous T cells for the eradication of tumor or infected cells. This seminal work foreshadowed current successes in expanding the clinical application of tumor-targeted T cell redirecting antibodies. In what follows, a discussion of the mechanism-of-action (MOA) of T cell redirecting antibodies will inform a survey of recent contributions directed toward the development of optimal therapeutics.
MOA of T cell redirecting mAbs defines optimal functional characteristics
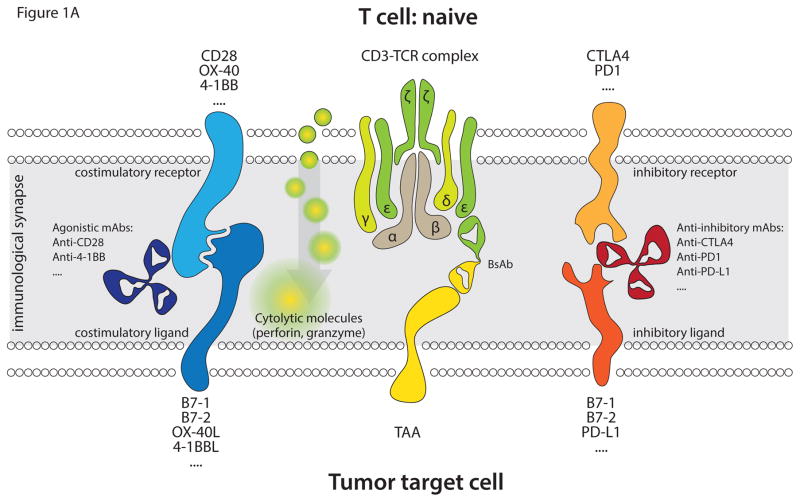
The accepted MOA of T cell redirecting BsAb is via the formation of an immunological synapse [13, 14]; the initial recognition event of this process, and potential modulators of the T cell response, is depicted in Figure 1. This BsAb-mediated cross-linking of CD3 receptor and target cell TAA results in: the activation of T cells, the subsequent release of perforin and granzyme from the cytotoxic granules into the milieu of the immunological synapse, and the ultimate destruction of the target cell by the ensuing apoptosis. In the case of the Bispecific T cell Engager (BiTE), the immunological synapses formed appear indistinguishable from those induced in the course of natural cytotoxic T cell recognition [13]. Since delivery of these apoptotic mediators is accomplished by passive diffusion, the size of the synapse, defined by the distance between the anti-CD3 and anti-TAA moieties of the BsAb, are critical to cytotoxic potency. The distance between the TAA epitope and the target cell membrane determines the activity of the BiTE [15, 16] and may explain the differences in reported cytotoxic activity between different T cell redirecting BsAb formats [17, 18], confirming that when the two cell membranes are in closest proximity the tumor cell lysis is most efficient.
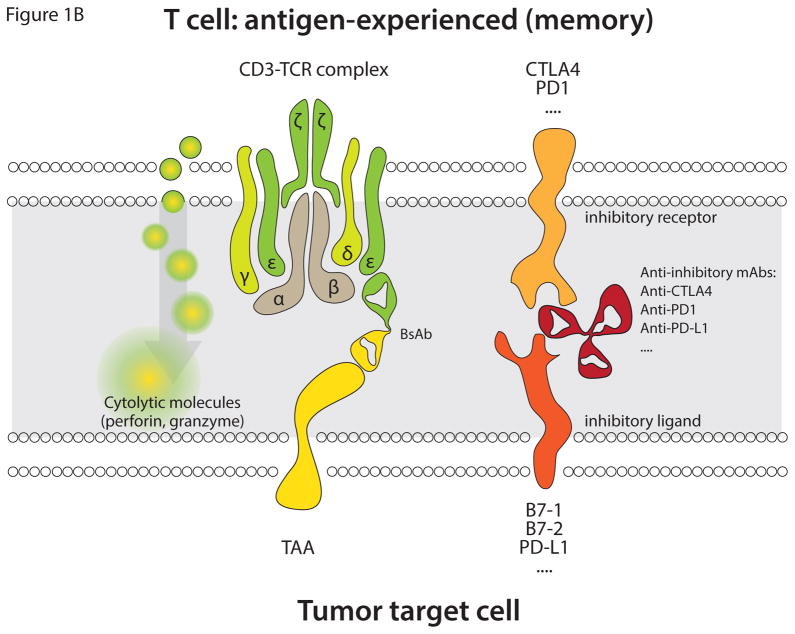
Figure 1.
Depicted is the initial recognition event in the formation of an immunological synapse between T cells and TAA-expressing target cells, mediated by T cell redirecting BsAb. (A) Main interactions in the recognition event involving naïve T cells. A T cell redirecting BsAb (anti-TAA/anti-CD3) binds to CD3 on T cells and to TAA expressed on the cell surface of target cells, leading to immunological synapse formation. This results in activation of polyclonal T cells in a non-MHC-restricted fashion in the presence of appropriate costimulatory signaling. The costimulatory signal is transmitted upon binding of costimulatory receptors (CD28, OX-40, 4-1BB etc) to their cognate ligands (B7-1, B7-2, OX-40, 4-1BBL etc). Antibodies (anti-CD28, anti-4-1BB etc) may also facilitate activation of T cells by triggering costimulatory receptors. The two signals transmitted through CD3 and costimulatory receptors facilitate activation of T cells with their subsequent degranulation and release of perforin and granzyme, leading to target cell apoptosis. In addition to costimulatory receptors T cells also express inhibitory receptors (CTLA4, PD-1, etc), which when bound to their ligands (B7-1, B7-2, PD-L1, etc) on target cells inhibit activation of T cells. Non-activating antibodies, which bind to either coinhibitory receptors (anti-CTLA4, anti-PD-1) or to their cognate ligands (anti-PD-L1), can block inhibitory signaling on T cells. (B) Main interactions in the recognition event leading to the formation of an immunological synapse involving antigen-experienced (memory) T cells. The immunological synapse formed via memory T cells is similar to that of naïve T cells except that the former cells have already been activated previously and only require stimulation via the CD3 signaling pathway to elicit a cytotoxic response. Effector memory cells are major contributors to the anti-tumor activity exhibited by BiTE antibodies.
Further, the activated T cells produce interleukin (IL)-2 and interferon (IFN)-γ that facilitates their proliferation and expansion at tumor sites, making T cells the most potent mediators of the immune response. CD8+ cells are the earliest to proliferate and exert their cytotoxic activity on target cells; however CD4+ cells start, with a short delay, equally contributing to observed cytotoxicity [19–21*].
Additionally, costimulation (e.g. via CD28, CD134, 4-1BB (CD137), etc. pathways) significantly augments proliferation and cytotoxicity of the BsAb redirected T cells [22*, 23]. Moreover, studies [24] suggest that costimulation significantly expands the activation-experienced memory T cell population. T cells having this phenotype, which is costimulation-independent, facilitate the cytotoxicity of BiTEs in the absence of costimulatory signaling [25, 26]. However, the broader role of costimulation has a long and contradictory history in the field of T cell redirecting BsAb that should be further elaborated because of its bearing upon the MOA of both BsAb and CAR T.
The role of costimulatory signaling pathways
Initially, both hybrid hybridoma and chemically linked T cell redirecting BsAb appeared to function independently of costimulation [6, 10] because of the employ of cytotoxic T cell clones as effectors. T cell redirecting experiments conducted later with resting T cells exhibited little cytotoxic activity [27]. Subsequently, it was demonstrated that lytic activity of T cell effectors depended upon either the engagement of costimulatory receptors (CD28) [28] or IL-2 pre-activation of T cells [29, 30]; costimulatory engagement was presumed to be a universal requirement for redirected T cell cytotoxic activity [31–33]. As later generation formats (i.e. BiTE [34], Dual-Affinity Re-Targeting (DART) antibodies [17], Tandem Diabodies (TandAb) [21*], and others [4*, 5]) advanced into pre-clinical and clinical development, they appeared to potently eliminate targets expressing their TAA in the absence of either costimulatory molecules or the presence of IL-2 pre-activated T cells [16, 17, 21*, 25, 35–38*, 39]. As described above, a T cell population independent of costimulatory signaling, those having the CD8+ and CD4+ memory phenotype, is the major contributor to the cytotoxic potency of BiTEs, and this is probably also the case for the other later generation formats (Figure 1). However, more recent findings suggest a nuanced view of these results and the factors necessary for the optimal redirection and activation of T cells for non—MHC-restricted cell lysis.
It has recently been reported that several mechanisms of cancer cell immune evasion (related to programmed death-ligand (PD-L)1, IL-10, transforming growth factor (TGF)β, B cell lymphoma (Bcl)-2, serpin proteinase inhibitor (PI)-9, adenosine, and indoleamine 2,3-dioxigenase (IDO)) did not significantly affect the in vitro cytotoxic activity of a T cell redirecting BiTE targeting epithelial cell adhesion molecule (EpCAM/CD3) [40]. Even combination of multiple mechanisms of immune evasion did not completely neutralize the activity of the T cell redirecting BsAb. However, caution is advised when drawing conclusions based solely upon in vitro data; the clinical experience with BiTEs, at least with hematological malignancies, suggests immune evasion mechanisms may affect therapeutic outcome in patients. Recent analysis of a case of B cell acute lymphoblastic leukemia (B-ALL) resistant to blinatumomab treatment revealed a 20-fold increase in the percentage of PD-L1 positive blasts that resulted in a more than 10-fold reduction of in vitro CD19+ cell lysis [41*]. Similarly, in a solid tumor setting, studies of a full-length HER2/CD3 BsAb revealed that PD-L1 expression on tumor cells limited its in vivo activity, and that the coadministration of anti PD-L1 antibodies could reverse this effect [42]. Finally, recent studies demonstrate that agonism of costimulatory pathways (e.g. CD28 [43*], 4-1BB [23, 44]) or inhibition of inhibitory pathways (e.g. checkpoint inhibitory PD1 [43*] and cytotoxic T-lymphocyte-associated protein (CTLA)4 [45]) can provide significant potentiation of target cell lysis; thus, in some patients resistance to T cell redirecting therapy may be overcome. These latter data suggest that although T cell redirecting BsAb can exhibit substantial anti-tumoral clinical activity as monotherapy, clinical responses may be significantly augmented by either providing positive stimulation or checkpoint blockade to cytotoxic T lymphocytes (CTL).
In a Phase II clinical study in relapsed and refractory B-ALL patients, complete responses were achieved by treatment with a CD19/CD3 BiTE single agent in 43% of the patients, whereas 48% of patients exhibited no response to therapy; this was attributed to advanced disease and high tumor burden [46]. Given the above data, one may speculate that co-engagement of costimulatory pathways in this context could have redirected a broader population of T cells (including naive) and may have benefited the clinical activity of the molecule.
These examples emphasizing the importance of engaging costimulatory pathways in addition to CD3 engagement are merely a restatement of fundamental T cell immunobiology. One must wonder how it became initially accepted that the new CD3-engaging BsAb obviated the requirement of the second signal to mobilize all possible T cell populations. It is now understood that this is not the case [26], and that memory T cells are the major contributors to the cytotoxic potency of BiTEs and perhaps the other T cell redirecting BsAb; in the future realizing the full clinical potential of these molecules may require inclusion of costimulatory modalities.
The questions of optimal affinity and valency
Similar to the evolution of thinking regarding costimulatory signaling, there has been some evolution of thought regarding the optimal CD3 affinity of T cell redirecting BsAbs and whether bivalent binding to CD3 may negatively affect the function of such therapeutics. Initially, low CD3 affinity (KD ~ 10−7 M and higher) was considered a requirement for avoidance of non—tumor-target-specific activation of T cells [34] It was suggested that high affinity (KD ~10−9 – 10−8 M and lower), bivalent binding to CD3 might lead to non—target-specific T cell activation [16]. However, other reports suggested that non—target-specific triggering of T cells was dependent on cross-linking or immobilization of the antibody by FcγR+ cells, and was not directly a function of bivalency [28, 47–49]. Since higher affinity to CD3 and bivalency were reported to mediate higher T cell proliferation and cytotoxicity [28, Zhukovsky, et al., unpublished], a recent study [21*] revisited these questions. It demonstrated that efficient T cell activation occurs in a target-specific fashion with fragment BsAb that redirect T cells via CD3, and also with anti-CD3 IgG antibodies, such as OKT3, when they cross-link T cells and FcγR+ immune cells via the Fc-domain. No activation of T cells was observed with bivalent anti-CD3 IgG in homogeneous T cell preparations, but T cells were potently activated in the presence of FcγR+ cells [21*, 50]. (Nevertheless, it is plausible that for bivalent anti-CD3 antibodies, e.g. OKT3, at concentrations resulting in saturated binding of cell-surface CD3, signs of T cell activation could be detected, even in homogeneous T cell preparations. At low bivalent anti-CD3 concentrations, at which T cell redirecting BsAb are dosed in the clinic, the preferential binding mode will be cis, i.e. on the same T cell, but as the concentration of bivalent anti-CD3 increases, the binding mode is likely to shift to trans, i.e. crosslinking different T cells; the latter would activate T cells in the absence of TAA- or FcγR+ cells.) Thus, high affinity or bivalent CD3 binding, at clinically-relevant concentrations, did not induce non-target—specific T cell activation, which requires cell-to-cell crosslinking as opposed to bivalent cis antibody engagement of CD3. Although others have observed that bivalent binding to CD3 does not increase T cell cytotoxic potency [51], current [21*, 52, 53] and future T cell redirecting strategies are sure to clinically evaluate whether high affinity CD3 binding results in optimal T cell redirection.
Optimization for solid tumors via costimulation and TAA selection: the next challenge
Two Phase I clinical studies of activated T cells targeting human epidermal growth factor receptor (HER2), which were armed with HER2/CD3 BsAb (produced by chemically cross-linking trastuzumab and OKT-3), were conducted in stage IV breast cancer [54] and castration-resistant prostate cancer [55]. During treatment, patients in both studies received simultaneous low doses of (costimulatory) IL-2 and granulocyte-macrophage colony-stimulating factor (GM-CSF). Multiple infusions were well-tolerated with no dose-limiting toxicities reported. In the breast cancer study, 60% of patients achieved stable disease, and for the entire cohort overall survival was extended relative to historical treatment data. In the prostate cancer trial, one patient achieved partial response, and approximately half exhibited polarization towards Th1 proinflammatory cytokines, which persisted throughout treatment. Both of these results are similarly promising in the realm of solid tumors, but not as spectacular as the initial success of blinatumomab in hematological malignancies [25].
Another approach was recently proposed [56*] for the expansion of the clinical success of T cell redirecting BsAb in hematological malignancies to solid tumors; the goal was optimization of their cytotoxic efficacy for the destruction of solid tumors, which are less likely than hematological tumors to express costimulatory pathway ligands. In that study, a model system was developed combining BsAb-mediated T cell redirection with genetically engineered T cells; the T cells expressed an immune receptor with an intracellular domain constructed from the TCR intracellular domain fused in tandem with that of CD28, similar to second generation CAR T. The difference relative to CAR T is that the extracellular domain of this receptor is constructed from that of folate receptor alpha (FRα). To redirect these genetically modified T cells to tumor cells expressing CD20 or HER2, two BsAb were generated, anti-FRα/CD20 and FRα/HER2. The study reported superior production of pro-inflammatory cytokines by the T cells competent for simultaneous CD28 and TCR signaling, and hypothesized that this would create a favorable environment for redirection and activation of endogenous immune cells, and a more robust anti-tumor response.
Selection of optimal TAAs for employ with T cell redirecting mechanisms is difficult. Due to the high cytotoxic potency of T cells, the therapeutic window of T cell redirecting approaches is rather narrow. Antigens overexpressed on tumor cells are also usually expressed on healthy tissues, albeit at lower density. Many CD3-based T cell redirecting BsAb target hematological malignancies, which express TAA (CD19, CD20) only in the hematological compartment, thus limiting potential side effects; there is accumulated clinical evidence that some blood cell types can be eliminated for extended periods of time without life-threatening side effects [57]. Unfortunately, when targeting TAA on solid tumors the high potency of T cell redirecting approaches is challenging [58] since they are frequently expressed on vital tissues. One option is to employ antigens that are exclusively expressed on tumor cells; such antigens (e.g., the oncogenic variant III mutation of the epidermal growth factor receptor (EGFRvIII)) may arise due to some genetic alterations/lesions and are not present in healthy tissues [59]. An alternative could employ a dual TAA-targeting strategy [60] for the specific optimization of T cell redirecting applications for solid tumors. Targeting a unique “signature” of two TAA, expressed exclusively on tumor cells and absent from healthy cells, may enhance the selectivity and safety of T cell redirecting antibodies and broaden their range of clinical indications. In this approach, the affinity of a trispecific T cell redirecting antibody for each TAA is selected low to minimize binding to healthy tissues expressing a single antigen, whereas the binding to cancer cells, which express both TAAs, is substantially increased due to avidity. For this approach to succeed, the selection of TAAs should be limited to those whose expression is known, or expected, to be in close cell-surface proximity. Finally, this approach may also improve clinical efficacy of T cell redirecting antibodies since targeting two TAA may also reduce tumor escape mechanisms [61*].
Optimal CD3+ cell subset redirection and proliferation
Current T cell redirecting BsAb target CD3, and thus will mobilize all CD3+ T cells at tumor sites including CD4+ and CD8+ as well as undesirable Tregs, which when localized in target tissues reduce the immune response and suppress CD4+ and CD8+ effector cells by secreting immunosuppressive cytokines and activating inhibitory pathways on CTL [62, 63]. Several groups have reported that isolated Tregs can facilitate cytotoxic activity [64]. However, it has also been demonstrated that the presence of Tregs facilitates in vivo tumor growth during treatment with a T cell redirecting BsAb targeting prostate stem cell antigen (PSCA/CD3) in a xenograft model [62]. Though one report indicates no proliferation of Tregs is observed in human ex-vivo studies of a CD33/CD3 T cell redirecting BsAb [65], exclusive redirection of CTLs may provide therapeutic benefit and further enhance the clinical efficacy of this class of drugs. To this end, a study [66*] demonstrated that a PSCA/CD8 BiTE molecule is capable of eliciting a potent anti-tumor response, albeit only pre-activated CD8+ T cells exhibited cytotoxicity. More studies will be required to enable practical clinical applications of CD3+ subset redirection.
CAR T cells targeting CD19 can mediate rapid clinical responses in B cell malignancies
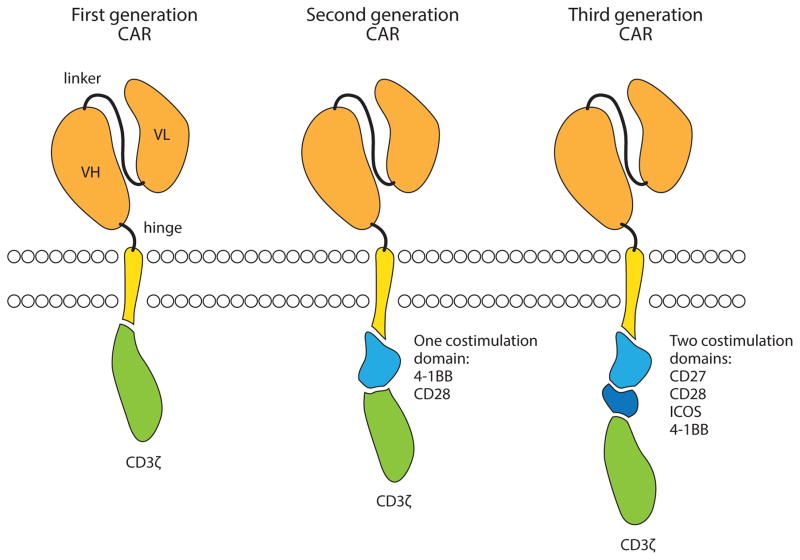
Unlike BsAb, CAR T cells incorporate a single chain variable fragment directly fused to a transmembrane domain and the signaling domains important for T cell activation (Figure 2). The CAR itself is genetically encoded in the T cell genome following vector or plasmid transduction. CAR T cells are therefore “living drugs,” with autonomous cell function and persistence independent of the administration of a pharmacologic molecule. Although CAR T cells were initially developed for solid tumors [67], several factors limited their efficacy at that time, including poor transduction, inadequate costimulation, and limited persistence. Multiple investigators addressed these issues by exploring different forms of gene transfer, including transposon-based systems, retroviral vectors, and lentiviral vectors, and different costimulatory molecules to enhance T cell activation. In 2011–2013, dramatic responses to CAR T cell therapy were reported [68*–70*], mainly with second-generation CAR T cells generated with viral vectors. Larger studies at multiple centers have now extended the findings and demonstrated that this form of therapy is highly effective, particularly in patients with acute lymphoblastic leukemia [71–73]. It can also be effective in chronic lymphocytic leukemia [74*] and other B cell lymphomas [75]. Effective CAR T cell therapy in these tumors is also associated with a generalized cytokine release syndrome, which appears to be manageable in most cases with antibody therapy targeting the IL-6 receptor [76]. However, as with any therapy that targets a single molecule, tumor escape variants have been observed; mutations and splice variants that result in loss of the CAR targeted CD19-binding epitope have been identified [77*].
Figure 2.
Chimeric antigen receptor design. The extracellular portion of a CAR consists of the variable heavy (VH) and light (VL) chains of an antibody, linked together to form a single chain variable fragment (scFv). The scFv is fused to a hinge domain and the transmembrane domain of another molecule such as CD8 or CD28. First generation CARs include the intracellular domain of CD3ζ, which contains 3 ITAM domains (red). Second generation CARs also include the intracellular domain of a costimulatory molecule such as 4-1BB or CD28, whereas Third generation CARs include 2 or more costimulatory domains in addition to CD3ζ.
Based on the success of CAR T cells developed against B cell tumors, CAR T cells are thought to have a high likelihood of efficacy in other hematological malignancies. In the past few years, several investigators have identified suitable targets for the malignant plasma cells of multiple myeloma, including signaling lymphocytic activation molecule family member 7 (SLAMF7/CS1) [78], B cell maturation antigen [79*], and CD44v6 [80]. These are all expressed on mature B cells and plasma cells, but SLAMF7 is also expressed on normal activated T cells, and CD44v6 is expressed on keratinocytes, which could make both of these targets problematic for clinical use. Investigators have also identified other targets for myeloid leukemias, particularly folate receptor beta [81], CD33 [82] and CD123 [83, 84], which are now in clinical trials.
CAR T cells for solid tumors: discovering targets and overcoming the tumor microenvironment
The two main obstacles for delivering on the promise of CAR T cells for solid tumors are the identification of suitable target antigens and overcoming the effects of the tumor microenvironment. Investigators are evaluating several antigens for solid tumors in pre-clinical models and in early human trials; some of the targets include prostate specific membrane antigen and prostate stem cell antigen, glypican 3 for lung and liver cancer, mesothelin [85*] for mesothelioma or ovarian cancers, EGFRvIII [86*], and normal HER2 [87] and wild-type EGFR [88]. Because some of these antigens are expressed on normal tissues, the affinity of the CAR has been reduced in these cases, and this has been shown to be an effective mechanism to target tumor cells versus normal tissues in mouse models.
Although T cells can penetrate tissues, the immunosuppressive effect of the tumor microenvironment in solid tumors is hypothesized to form a functional barrier to endogenous T cells, and, by extension, may also inhibit CAR T cells [89]. Some investigators have designed CAR T cells specifically to target the microenvironment, such as CAR T cells direct to fibroblast activation protein [90] or chondroitin sulfate [91]. However, it is not clear that targeting the tumor stroma will have an anti-tumor effect. Alternatively, CAR T cells directly targeting the tumor can also be used as carriers to modulate the tumor environment with certain cytokines or costimulatory molecules, such as IL-12 [92] or CD40 ligand [93]. It will be interesting to determine whether targeting only the tumor microenvironment has anti-tumor effects, or if it sensitizes the tumor to other forms of immunotherapy; the most obvious combinations will include anti-stroma CAR T cells with either anti-tumor CAR T cells or checkpoint blockade to enable endogenous T cell responses. In mouse models, checkpoint blockade antibodies enhance CAR T cell function [94], suggesting that both combinations are worth exploring.
Novel CAR designs and CAR tuning
The first CAR T cells that exhibited clinical effects are comprised of a high-affinity single-chain variable fragment fused to the hinge and transmembrane domains of either CD8 or CD28, and the intracellular domains of the costimulatory molecules 4-1BB or CD28, followed by CD3ζ. However, the rules for optimal CAR design are still emerging. It is now clear that inclusion of at least one costimulation domain is critical to allow the CAR T cells to expand and persist and effectively kill tumor cells in vivo, and some costimulatory molecules, such as 4-1BB, may be better than others [95] in certain respects, such as facilitating long-term persistence; other aspects of CAR design are still being investigated. For example, many solid tumor targets are employing low-affinity single-chain variable fragments, with the hypothesis that low-affinity CAR will only target cells with high levels of target expression. The spacing of where the CAR binds the epitope is also important, where the closer the CAR T cell can get to the target, the better [96*]; spacer domains such as those derived from immunoglobulins may also be independently biologically active [97].
Some CAR engineering techniques aim to expand the possibility of targeting multiple antigens, by using CAR T cells that will recognize two antigens and only become activated by the correct combination [98–100*, 101*]. In one case, CAR T cells were formed to re-capitulate a 2-signal system, whereby one antigen delivers signal 1 with a first generation CAR, and the second antigen delivers signal 2 by engaging a chimeric costimulatory CAR (CCR) encoding only costimulation without CD3ζ. Finally, two highly novel CAR designs have emerged quite recently: one has the backbone of a CAR, but with the extracellular domain of CD16 rather than a single-chain variable fragment. In this case, the antigen-specificity is conferred by binding of a soluble antibody to CD16 [102]. A second design is based on a drug-activated CAR, where the signaling components of the CAR are divided among different proteins that only come together in the presence of a drug that causes them to dimerize and signal [103*].
As new targets and new CAR designs are tested, there is also great interest in developing ways to control CAR T cell function after infusion into a patient, to mitigate either short-term or long-term toxicity. Several investigators have designed “suicide genes,” which can be co-introduced in a bi-cistronic vector along with the CAR. Examples of suicide genes are (1) the coding sequences for herpes simplex virus thymidine kinase (HSV-tk), which will render the transduced T cells susceptible to the drug ganciclovir (but also makes them immunogenic); (2) natural self antigens, such as EGFR or CD20, which can be targeted with available monoclonal antibodies; and (3) fusion proteins, such as inducible caspase 9 (iCaspase 9), which contain a domain that only dimerizes and induces apoptosis in the presence of a specific chemical inducer of dimerization [104].
Alternative sources of T cells
Although CARs are not MHC-restricted, most work on CAR T cells has focused on autologous sources of T cells for adoptive transfer. In cases where patients have already had an allogeneic stem cell transplant, T cells derived from the healthy donor may offer healthier T cells, though these have the potential of causing graft-vs-host disease [105–107]. With new techniques of gene editing, some investigators are generating T cells from alternative sources, which offers the tantalizing possibility of “off-the-shelf” T cell therapy. These T cells can be derived from induced pluripotent stem cells [108] or from mature T cells from allogeneic donors [109, 110].
The optimal subset of T cells to genetically modify is also a matter of investigation. Although most investigators modify bulk, mature, peripheral blood lymphocytes, there is also interest in selecting long-lived central memory T cells as the target population [111], or virus-specific T cells with known antigen-specificity [106].
Concluding remarks
The treatment of cancers was transformed by the introduction of therapeutic antibodies. A large number of more generalized immunotherapeutic approaches for cancer treatment are currently under development. The most promising of these approaches attempt to harness T cells, the most potent tumor-killing effectors. T cell redirection by BsAb and mobilization by CAR T technologies have been key drivers of this progress.
For both of these immunotherapeutic modalities, future effort will focus on optimization of functional design parameters. For both modalities it appears that the TAA binding epitope, and its relationship to the size of the resulting immunological synapse, is key. Similarly, both modalities are impacted by the role of costimulatory pathways, albeit BsAb to a lesser extent. Also, both modalities will benefit from design changes that may increase the therapeutic window. One such approach could employ the discrimination afforded by multiple TAA to significantly increase selectivity, which may be easier to accomplish with CAR T technology. This is because optimized CAR T designs incorporating CCR may afford more reliable tuning to trigger cytolytic activity solely in the presence of the two-component cell-surface signature. T cell redirecting BsAb, on the other hand, must be redesigned as trispecific antibodies wherein the TAA affinities are selected below the threshold necessary to ensure that avidity for targets expressing both TAAs triggers selective cytolysis.
Many of the key parameters governing the optimal employ of T cell redirecting BsAb are well understood. However, there are additional questions that require thorough elucidation. Continuous T cell stimulation may result in anergy in the absence of costimulatory signaling; therefore, are oscillating drug concentrations from periodic infusions preferable to the constant concentrations resulting from continuous infusion? Current BsAb designs span a large range of molecular weight (50 kDa – 150 kDa); would the smaller molecules have better solid tumor penetration properties (neglecting the issue of TAA affinity) or is it possible that the T cell-driven MOA is so potent that larger molecules may be equally efficacious? Is it possible to optimize current BsAb technologies to selectively engage desired cytotoxic CD3+ populations while excluding immunosuppressive Tregs? Can one increase therapeutic efficacy by increasing the available number, and cytotoxic potential, of T cells in immunosuppressed cancer patients?
The optimal employ of CAR T is still in its infancy; we have learned that costimulation increases the expansion and persistence of CAR T cells upon re-infusion into the patients, and that both of these parameters are essential for effective clinical responses. Although CD19-directed CAR T cells are well on their way to widespread clinical use, the largest question in the field is whether this mode of immunotherapy will be effective in other tumors. There are still many elements of the design itself that are being investigated, including: is the single-chain variable fragment the optimal way to bind tumor antigens? Do different scFv’s behave differently? Are other costimulatory domains useful or preferred? Will multi-targeted CAR T cells be useful to target more antigens to enhance safety or to prevent tumor escape? Finally, which combinations with existing therapies will optimize the cure of multiple cancers?
Though both immunotherapeutic modalities are undergoing continuous optimization, it is evident that these powerful tumor-fighting technologies are moving into oncology standard-of-care and shall revolutionize treatment and positively impact the prognoses and lives of numerous patients.
T cell redirection is the most promising immunotherapeutic modality for cancer treatment
Many parameters for optimal activity of T cell-recruiting BsAb and of CAR T are well understood
Costimulatory pathways, TAA-binding epitopes, and antigen affinity are key for both modalities
Both modalities will benefit from improved therapeutic window
Acknowledgments
Dr. Marcela V. Maus is supported by the National Cancer Institute (K08 CA1669039). The authors wish to thank Joost Bakker (Scicomvisuals) for help in preparation of the Figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Coley WB. Contribution to the knowledge of sarcoma. Ann Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskander RN, Tewari KS. Epithelial cell-adhesion molecule-directed trifunctional antibody immunotherapy for symptom management of advanced ovarian cancer. Clin Pharmacol. 2013;5:55–61. doi: 10.2147/CPAA.S45885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Stieglmaier J, Benjamin J, Nagorsen D. Utilizing the BiTE (bispecific T-cell engager) platform for immunotherapy of cancer. Expert Opin Biol Ther. 2015;15:1093–1099. doi: 10.1517/14712598.2015.1041373. Review of clinical experience with blinatumomab. [DOI] [PubMed] [Google Scholar]
- 4*.Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67:95–106. doi: 10.1016/j.molimm.2015.01.003. Comprehensive review of BsAb formats and summary of all programs in clinical development. [DOI] [PubMed] [Google Scholar]
- 5.Kontermann RE, Brinkmann U. Bispecific Antibodies. Drug Discovery Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- 7.Nitta T, Yagita H, Azuma T, Sato K, Okumura K. Bispecific F (ab′)2 monomer prepared with anti-CD3 and anti-tumor monoclonal antibodies is most potent in induction of cytolysis of human T cells. Eur J Immunol. 1989;19:1437–1441. doi: 10.1002/eji.1830190814. [DOI] [PubMed] [Google Scholar]
- 8*.Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305:537–540. doi: 10.1038/305537a0. An old reference whose inclusion illustrates there is a large literature, dating back to the sixties, concerning “hybrid antibodies” as the early bispecifics were called. [DOI] [PubMed] [Google Scholar]
- 9.Laky M, Mota G, Gheţie V. Specific cytotoxic activity of normal T-lymphocytes coated with multivalent hybrid antibody. Mol Immunol. 1982;19:1029–1036. doi: 10.1016/0161-5890(82)90311-x. [DOI] [PubMed] [Google Scholar]
- 10.Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985;316:354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- 11.Staerz UD, Bevan MJ. Hybrid hybridoma producing a bispecific monoclonal antibody that can focus effector T-cell activity. Proc Natl Acad Sci U S A. 1986;83:1453–1457. doi: 10.1073/pnas.83.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzavecchia A, Scheidegger D. The use of hybrid hybridomas to target human cytotoxic T lymphocytes. Eur J Immunol. 1987;17:105–111. doi: 10.1002/eji.1830170118. [DOI] [PubMed] [Google Scholar]
- 13.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Nagorsen D, Baeuerle PA. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp Cell Res. 2011;317:1255–1260. doi: 10.1016/j.yexcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, Kufer P. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother. 2010;59:1197–1209. doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, Itin C, Prang N, Baeuerle PA. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 17.Moore PA, Zhang W, Rainey GJ, Burke S, Li H, Huang L, Gorlatov S, Veri MC, Aggarwal S, Yang Y, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117:4542–4551. doi: 10.1182/blood-2010-09-306449. [DOI] [PubMed] [Google Scholar]
- 18.Moore GL, Bautista C, Pong E, Nguyen DT, Jacinto J, Eivazi A, Muchhal US, Karki S, Chu SY, Lazar GA. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs. 2011;3:546–557. doi: 10.4161/mabs.3.6.18123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbüse R, Schlereth B, Kufer P, Baeuerle PA. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214:441–45316. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann A, Arndt C, Töpfer K, Stamova S, Krone F, Cartellieri M, Koristka S, Michalk I, Lindemann D, Schmitz M, et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J Immunol. 2012;189:3249–3259. doi: 10.4049/jimmunol.1200341. [DOI] [PubMed] [Google Scholar]
- 21*.Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss SH, Fucek I, Eser M, McAleese F, Molkenthin V, Gall FL, et al. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. MAbs. 2015;7:584–604. doi: 10.1080/19420862.2015.1029216. Targeting of tumor cells with bivalent high affinity T cell redirecting BsAb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Hornig N, Reinhardt K, Kermer V, Kontermann RE, Müller D. Evaluating combinations of costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. Cancer Immunol Immunother. 2013;62:1369–1380. doi: 10.1007/s00262-013-1441-7. T cell redirecting molecules benefit from costimulation by a second bispecific antibody-ligand fusion protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arndt C, Feldmann A, von Bonin M, Cartellieri M, Ewen E, Koristka S, Michalk I, Stamova S, Berndt N, Gocht A, et al. Costimulation improves the killing capability of T cells redirected to tumor cells expressing low levels of CD33: description of a novel modular targeting system. Leukemia. 2014;28:59–69. doi: 10.1038/leu.2013.243. [DOI] [PubMed] [Google Scholar]
- 24.Hornig N, Kermer V, Frey K, Diebolder P, Kontermann RE, Müller D. Combination of a bispecific antibody and costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. J Immunother. 2012;35:418–429. doi: 10.1097/CJI.0b013e3182594387. [DOI] [PubMed] [Google Scholar]
- 25.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 26.Wong R, Pepper C, Brennan P, Nagorsen D, Man S, Fegan C. Blinatumomab induces autologous T-cell killing of chronic lymphocytic leukemia cells. Haematologica. 2013;98:1930–1938. doi: 10.3324/haematol.2012.082248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung G, Honsik CJ, Reisfeld RA, Müller-Eberhard HJ. Activation of human peripheral blood mononuclear cells by anti-T3: killing of tumor target cells coated with anti-target-anti-T3 conjugates. Proc Natl Acad Sci U S A. 1986;83:4479–4483. doi: 10.1073/pnas.83.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung G, Freimann U, Von Marschall Z, Reisfeld RA, Wilmanns W. Target cell-induced T cell activation with bi- and trispecific antibody fragments. Eur J Immunol. 1991;21:2431–2435. doi: 10.1002/eji.1830211020. [DOI] [PubMed] [Google Scholar]
- 29.Tsoukas CD, Landgraf B, Bentin J, Valentine M, Lotz M, Vaughan JH, Carson DA. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. J Immunol. 1985;135:1719–1723. [PubMed] [Google Scholar]
- 30.Perez P, Hoffman RW, Titus JA, Segal DM. Specific targeting of human peripheral blood T cells by heteroaggregates containing anti-T3 crosslinked to anti-target cell antibodies. J Exp Med. 1986;163:166–178. doi: 10.1084/jem.163.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohlen H, Hopff T, Manzke O, Engert A, Kube D, Wickramanayake PD, Diehl V, Tesch H. Lysis of malignant B cells from patients with B-chronic lymphocytic leukemia by autologous T cells activated with CD3 x CD19 bispecific antibodies in combination with bivalent CD28 antibodies. Blood. 1993;82:1803–1812. [PubMed] [Google Scholar]
- 32.Haagen IA. Performance of CD3xCD19 bispecific monoclonal antibodies in B cell malignancy. Leuk Lymphoma. 1995;19:381–393. doi: 10.3109/10428199509112195. [DOI] [PubMed] [Google Scholar]
- 33.Reusch U, Le Gall F, Hensel M, Moldenhauer G, Ho AD, Little M, Kipriyanov SM. Effect of tetravalent bispecific CD19xCD3 recombinant antibody construct and CD28 costimulation on lysis of malignant B cells from patients with chronic lymphocytic leukemia by autologous T cells. Int J Cancer. 2004;112:509–518. doi: 10.1002/ijc.20417. [DOI] [PubMed] [Google Scholar]
- 34.Brischwein K, Parr L, Pflanz S, Volkland J, Lumsden J, Klinger M, Locher M, Hammond SA, Kiener P, Kufer P, et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30:798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 35.Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmüller G, Dörken B, Bargou RC. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 36.Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, Kufer P, Riethmuller G, Bargou R, Baeuerle PA. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 37.Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, Horst H, Raff T, Viardot A, Schmid M, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 38*.Topp MS, Gökbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, Viardot A, Marks R, Diedrich H, Faul C, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. Principal clinical study that led to the accelerated approval of blinatumomab by the FDA. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, Gou L, Guo Z, Liu H, Wang J, Zhou S, Yang J, Li X. Fully human HER2/cluster of differentiation 3 bispecific antibody triggers potent and specific cytotoxicity of T lymphocytes against breast cancer. Mol Med Rep. 2015;12:147–154. doi: 10.3892/mmr.2015.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deisting W, Raum T, Kufer P, Baeuerle PA, Münz M. Impact of Diverse Immune Evasion Mechanisms of Cancer Cells on T Cells Engaged by EpCAM/CD3-Bispecific Antibody Construct AMG 110. PLoS One. 2015;10:e0141669. doi: 10.1371/journal.pone.0141669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Köhnke T, Krupka C, Tischer J, Knösel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8:111. doi: 10.1186/s13045-015-0213-6. The potent blinatumomab may have found its match in treatment-resistant tumor cells that overexpress PD-L1; authors argue for patient stratification and a potential combinatorial treatment approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res. 2014;74:5561–5571. doi: 10.1158/0008-5472.CAN-13-3622-T. [DOI] [PubMed] [Google Scholar]
- 43*.Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG 330. Blood Cancer J. 2015;5:e340. doi: 10.1038/bcj.2015.68. Demonstration that BiTE activity benefits from the engagement of costimulatory pathways with an insightful discussion of the results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aliperta R, Cartellieri M, Feldmann A, Arndt C, Koristka S, Michalk I, von Bonin M, Ehninger A, Bachmann J, Ehninger G, et al. Bispecific antibody releasing-mesenchymal stromal cell machinery for retargeting T cells towards acute myeloid leukemia blasts. Blood Cancer J. 2015;5:e348. doi: 10.1038/bcj.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yano H, Thakur A, Tomaszewski EN, Choi M, Deol A, Lum LG. Ipilimumab augments antitumor activity of bispecific antibody-armed T cells. J Transl Med. 2014;12:191. doi: 10.1186/1479-5876-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 47.Tax WJ, Hermes FF, Willems RW, Capel PJ, Koene RA. Fc receptors for mouse IgG1 on human monocytes: polymorphism and role in antibody-induced T cell proliferation. J Immunol. 1984;133:1185–1189. [PubMed] [Google Scholar]
- 48.Ceuppens JL, Bloemmen FJ, Van Wauwe JP. T cell unresponsiveness to the mitogenic activity of OKT3 antibody results from a deficiency of monocyte Fc gamma receptors for murine IgG2a and inability to cross-link the T3-Ti complex. J Immunol. 1985;135:3882–3886. [PubMed] [Google Scholar]
- 49.Smith KG, Austyn JM, Hariri G, Beverley PC, Morris PJ. T cell activation by anti-T3 antibodies: comparison of IgG1 and IgG2b switch variants and direct evidence for accessory function of macrophage Fc receptors. Eur J Immunol. 1986;16:478–486. doi: 10.1002/eji.1830160503. [DOI] [PubMed] [Google Scholar]
- 50.Verwilghen J, Baroja ML, Van Vaeck F, Van Damme J, Ceuppens JL. Differences in the stimulating capacity of immobilized anti-CD3 monoclonal antibodies: variable dependence on interleukin-1 as a helper signal for T-cell activation. Immunology. 1991;72:269–276. [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y, Axup JY, Ma JSY, Wang RE, Choi S, Tardif V, Lim RKV, Pugh HM, Lawson BR, Welzel G, et al. Multiformat T-cell-engaging bispecific antibodies targeting human breast cancers. Angew Chem Int Ed Engl. 2015;54:7022–7027. doi: 10.1002/anie.201500799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedrich M, Raum T, Lutterbuese R, Voelkel M, Deegen P, Rau D, Kischel R, Hoffmann P, Brandl C, Schuhmacher J, et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther. 2012;11:2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- 53.Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17:385–392. doi: 10.1016/j.cbpa.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 54.Lum LG, Thakur A, Al-Kadhimi Z, Colvin GA, Cummings FJ, Legare RD, Dizon DS, Kouttab N, Maizel A, Colaiace W, et al. Targeted T-cell Therapy in Stage IV Breast Cancer: A Phase I Clinical Trial. Clin Cancer Res. 2015;21:2305–2314. doi: 10.1158/1078-0432.CCR-14-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaishampayan U, Thakur A, Rathore R, Kouttab N, Lum LG. Phase I Study of Anti-CD3 x Anti-Her2 Bispecific Antibody in Metastatic Castrate Resistant Prostate Cancer Patients. Prostate Cancer. 2015;2015:285193. doi: 10.1155/2015/285193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Urbanska K, Lynn RC, Stashwick C, Thakur A, Lum LG, Powell DJJ. Targeted cancer immunotherapy via combination of designer bispecific antibody and novel gene-engineered T cells. J Transl Med. 2014;12:347. doi: 10.1186/s12967-014-0347-2. Demonstration of the utility of T cells, genetically-engineered to express a signaling adapter-binding moiety, in combination with a BsAb “adapter” targeting a specific TAA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen DR, Cohen PL. Living life without B cells: is repeated B-cell depletion a safe and effective long-term treatment plan for rheumatoid arthritis? Int J Clin Rheumtol. 2012;7:159–166. doi: 10.2217/ijr.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiedler WM, Wolf M, Kebenko M, Goebeler M, Ritter B, Quaas A, Vieser E, Hijazi Y, Patzak I, Friedrich M, et al. A phase I study of EpCAM/CD3-bispecific antibody (MT110) in patients with advanced solid tumors. J Clin Oncol. 2012;30(suppl):abst 2504. [Google Scholar]
- 59.Choi BD, Kuan C, Cai M, Archer GE, Mitchell DA, Gedeon PC, Sanchez-Perez L, Pastan I, Bigner DD, Sampson JH. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci U S A. 2013;110:270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karan D, Dubey S, Van Veldhuizen P, Holzbeierlein JM, Tawfik O, Thrasher JB. Dual antigen target-based immunotherapy for prostate cancer eliminates the growth of established tumors in mice. Immunotherapy. 2011;3:735–746. doi: 10.2217/imt.11.59. [DOI] [PubMed] [Google Scholar]
- 61*.Arndt C, Feldmann A, Koristka S, Cartellieri M, Dimmel M, Ehninger A, Ehninger G, Bachmann M. Simultaneous targeting of prostate stem cell antigen and prostate-specific membrane antigen improves the killing of prostate cancer cells using a novel modular T cell-retargeting system. Prostate. 2014;74:1335–1346. doi: 10.1002/pros.22850. Novel strategy utilizing a dual-targeting approach to prevent tumor escape. [DOI] [PubMed] [Google Scholar]
- 62.Koristka S, Cartellieri M, Theil A, Feldmann A, Arndt C, Stamova S, Michalk I, Töpfer K, Temme A, Kretschmer K, et al. Retargeting of human regulatory T cells by single-chain bispecific antibodies. J Immunol. 2012;188:1551–1558. doi: 10.4049/jimmunol.1101760. [DOI] [PubMed] [Google Scholar]
- 63.Koristka S, Cartellieri M, Arndt C, Bippes CC, Feldmann A, Michalk I, Wiefel K, Stamova S, Schmitz M, Ehninger G, et al. Retargeting of regulatory T cells to surface-inducible autoantigen La/SS-B. J Autoimmun. 2013;42:105–116. doi: 10.1016/j.jaut.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Choi BD, Gedeon PC, Herndon JE2, Archer GE, Reap EA, Sanchez-Perez L, Mitchell DA, Bigner DD, Sampson JH. Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol Res. 2013;1:163. doi: 10.1158/2326-6066.CIR-13-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krupka C, Kufer P, Kischel R, Zugmaier G, Bögeholz J, Köhnke T, Lichtenegger FS, Schneider S, Metzeler KH, Fiegl M, et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood. 2014;123:356–365. doi: 10.1182/blood-2013-08-523548. [DOI] [PubMed] [Google Scholar]
- 66*.Michalk I, Feldmann A, Koristka S, Arndt C, Cartellieri M, Ehninger A, Ehninger G, Bachmann MP. Characterization of a novel single-chain bispecific antibody for retargeting of T cells to tumor cells via the TCR co-receptor CD8. PLoS One. 2014;9:e95517. doi: 10.1371/journal.pone.0095517. An example of selective redirection of (preactivated) CD8+ cells to tumor cells while avoiding Treg redirection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68*.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. First paper showing dramatic effects of CAR T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70*.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. First paper demonstrating cytokine release syndrome and target loss effected by CAR T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. Long term persistence of CAR T cells decribed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77*.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. Mechanism of CD19 target loss in CAR T cell-treated patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, Yi L, Kwon CH, Wang QE, Devine SM, et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clin Cancer Res. 2014;20:3989–4000. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT, Kochenderfer JN. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. In depth paper describing new and one of the most promising targets for multiple myeloma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni M, Bernardi M, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 81.Lynn RC, Poussin M, Kalota A, Feng Y, Low PS, Dimitrov DS, Powell DJ., Jr Targeting of folate receptor beta on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood. 2015;125:3466–3476. doi: 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter DL, Carroll M, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29:1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, Hoffman L, Aguilar B, Chang WC, Bretzlaff W, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, Danet-Desnoyers G, Scholler J, Grupp SA, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85*.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. Pre-clinical description of CAR T toward solid tumor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86*.Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, Nace AK, Dentchev T, Thekkat P, Loew A, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7:275ra222. doi: 10.1126/scitranslmed.aaa4963. In depth evaluation of a novel CAR T cell target for solid tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, et al. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caruso HG, Hurton LV, Najjar A, Rushworth D, Ang S, Olivares S, Mi T, Switzer K, Singh H, Huls H, et al. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Pure E, Milone MC, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lo A, Wang LC, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DJ, Clendenin C, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beard RE, Zheng Z, Lagisetty KH, Burns WR, Tran E, Hewitt SM, Abate-Daga D, Rosati SF, Fine HA, Ferrone S, et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J Immunother Cancer. 2014;2:25. doi: 10.1186/2051-1426-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pegram HJ, Purdon TJ, van Leeuwen DG, Curran KJ, Giralt SA, Barker JN, Brentjens RJ. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29:415–422. doi: 10.1038/leu.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, van Leeuwen DG, Purdon T, Pegram HJ, Brentjens RJ. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–778. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 95.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96*.Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, FitzGerald DJ, Barrett DM, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. Novel target described and distance of binding between target and CAR shown to be important. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ., Jr Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100*.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. Novel inhibitory CAR to make T cells recognize two antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101*.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. Dual-targeting CAR T cells described in a pre-clinical model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res. 2014;74:93–103. doi: 10.1158/0008-5472.CAN-13-1365. [DOI] [PubMed] [Google Scholar]
- 103*.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. CAR T cell function is controlled by small molecule drug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou X, Di Stasi A, Brenner MK. iCaspase 9 Suicide Gene System. Methods Mol Biol. 2015;1317:87–105. doi: 10.1007/978-1-4939-2727-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jacoby E, Yang Y, Qin H, Chien CD, Kochenderfer JN, Fry TJ. Murine allogeneic CD19 CAR T-cells harbor potent anti-leukemic activity but have the potential to mediate lethal GVHD. Blood. 2015 doi: 10.1182/blood-2015-08-664250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poirot L, Philip B, Schiffer-Mannioui C, Le Clerre D, Chion-Sotinel I, Derniame S, Potrel P, Bas C, Lemaire L, Galetto R, et al. Multiplex Genome-Edited T-cell Manufacturing Platform for “Off-the-Shelf” Adoptive T-cell Immunotherapies. Cancer Res. 2015;75:3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 110.Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovitch B, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119:5697–5705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, Riddell SR. Chimeric antigen receptor-modified T cells derived from defined CD8(+) and CD4(+) subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]